Abstract
The presence of Epstein-Barr virus (EBV) in the tumor cells of some EBV-associated malignancies may facilitate selective killing of these tumor cells. We show that treatment of an EBV+ Burkitt's lymphoma cell line with 5-azacytidine led to a dose-dependent induction of EBV lytic antigen expression, including expression of the viral thymidine kinase (TK) and phosphotransferase (PT). Azacytidine treatment for 24 h modestly sensitized the cell line to all nucleosides tested. To better characterize EBV TK with regard to various nucleoside analogues, we expressed EBV TK in stable cell clones. Two EBV TK-expressing clones were moderately sensitive to high doses of acyclovir and penciclovir (PCV) (62.5 to 500 μM) and to lower doses of ganciclovir (GCV) and bromovinyldeoxyuridine (BVdU) (10 to 100 μM) compared to a control clone and were shown to phosphorylate GCV. Similar experiments in a transient overexpression system showed more killing of cells transfected with the EBV TK expression vector than of cells transfected with the control mutant vector (50 μM GCV for 4 days). A putative PT was also studied in the transient transfection system and appeared similar to the TK in phosphorylating GCV and conferring sensitivity to GCV, but not in BVdU- or PCV-mediated cell killing. Induction of EBV kinases in combination with agents such as GCV merits further evaluation as an alternative strategy to gene therapy for selective killing of EBV-infected cells.
Epstein-Barr virus (EBV), a human gammaherpesvirus, is associated with several malignancies, including AIDS-associated primary central nervous system lymphoma, nasopharyngeal carcinoma, nasal lymphoma, a subset of Hodgkin's disease, posttransplant B-cell lymphoproliferative disease, and African Burkitt's lymphoma (BL) (31, 38, 51, 52, 54, 55). The presence of viral genomes in malignancies offers unique opportunities for novel and specific approaches to therapy. The herpesvirus prodrug-converting enzymes thymidine kinase (TK) and phosphotransferase (PT) phosphorylate nucleoside analogues, converting these drugs into intermediates able to inhibit critical cellular processes (13, 14, 25, 34, 46). For example, the nucleoside analogue ganciclovir (GCV) is very efficiently phosphorylated by the herpes simplex virus type 1 (HSV-1) TK but is less efficiently phosphorylated by cellular enzymes (10). The phosphorylated compound inhibits the cellular DNA polymerase, leading to cell death (16, 41). Gene therapy studies illustrate the possible utility of herpesvirus prodrug-converting enzymes in mediating selective cell killing. The HSV-1 TK gene has been introduced into brain tumor cells using retroviral vectors so that these transfected tumor cells might be targeted by GCV (11). Similarly, allogeneic lymphocytes used in adoptive immunotherapy programs have been marked with a retroviral vector encoding HSV-1 TK so that if graft-versus-host disease develops, the infused cells can be selectively destroyed by treating with GCV (4).
EBV encodes a TK that shows sequence and functional homology with HSV-1 TK (22, 24, 26, 27, 53). The EBV TK is larger than the HSV-1 TK and encodes a 243-amino-acid N terminus whose function is unknown (22, 26). The EBV protein, like its HSV-1 homologue, but unlike the homologues in HSV-2 and varicella-zoster virus, has both TK and thymidylate kinase activity (6, 19). The substrate specificity of the EBV TK with regard to GCV has been the subject of conflicting reports, although there is general agreement that GCV inhibits EBV lytic replication (19, 24). In addition to EBV TK, EBV also encodes a second kinase. The open reading frame in BGLF4 encodes a protein that is homologous to other herpesvirus PTs (5, 47). The EBV protein autophosphorylates and phosphorylates viral protein substrates, including the EBV early antigen EA-D and a DNA polymerase accessory factor (8).
In EBV-associated malignancies, there is little expression of lytic cycle genes, including the TK gene. Studies from several laboratories, including our own, however, have shown that CpG methylation of the episome plays an important role in the regulation of EBV gene expression. Viral genomes are methylated in a variety of EBV-associated tumors, including BL, Hodgkin's disease, nasopharyngeal carcinoma, and a subset of posttransplant lymphomas (15, 23, 35, 43, 49). In vitro, inhibitors of DNA methyltransferase lead to lytic induction in some BL cell lines (3, 35, 39). We sought to determine whether azacytidine would activate expression of viral kinases and thus sensitize EBV+ tumor cells to killing by antiviral nucleoside analogues such as GCV.
MATERIALS AND METHODS
Chemicals.
5-Azacytidine, (E)-5-bromovinyldeoxyuridine (BVdU), and acyclovir (ACV) were purchased from Sigma (St. Louis, Mo.). S-BVdU was a gift from Erik De Clerq (Katholieke Universiteit Leuven, Leuven, Belgium). GCV and penciclovir (PCV) were purchased from Hoffmann-La Roche Inc. (Nutley, N.J.) and SmithKline Beecham Pharmaceuticals (Philadelphia, Pa.), respectively. Azidodeoxythymidine (AZT) was purchased from Calbiochem (La Jolla, Calif.). Hypoxanthine-aminopterin-thymidine (HAT) was purchased from Life Technologies (Gaithersburg, Md.) as a 100× lyophilized supplement and diluted in water to a 10× working concentration. HAT diluted in water was added diluted 1:10 to media.
Plasmids.
The plasmid pEBVTK was generated by subcloning the BamHI fragment of pUCX (from J. R. Arrand, Christie Hospital and Holt Radium Institute, Manchester, United Kingdom) (24) into the BamHI site of the mammalian expression vector pcDNA3 (Invitrogen, Carlsbad, Calif.). In the recombinant plasmid, the EBV TK is expressed from a cytomegalovirus promoter. The pcDNA3 parent was used as the vector control.
The plasmid pEBVPT was generated by PCR amplification of the EBV PT gene using Pfu polymerase (Stratagene, La Jolla, Calif.), followed by cloning into pcDNA3 at the BamHI site. The EBV PT is encoded by the open reading frame within the BGLF4 fragment of EBV. Genomic DNA isolated from EBV+ B95.8 cells was used as template in the PCR amplification. The primers used for amplification of the 1.3-kb BGLF4 insert were 5′, 5′-AGTCAGATCTATGGATGTGAATATGGCTGCGGA-3′, and 3′, 5′-AATCAGATCTTCCTCGAGCTCATCCACGTCG-3′.
The plasmid pEBVmutTK was generated by site-directed mutagenesis using the QuickChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions, with minor changes. The plasmid was amplified using 25 ng of pEBVTK as template and complementary oligonucleotides, in which the sequences, with the exception of a single point mutation, are identical to that of the wild-type EBV TK, as primers. The sequences of the primers used to generate pEBVmutTK were 5′-CCATTTGCTGTCGACCTCCGTGGTTTTCCC-3′ and 5′-GGGAAAACCACGGAGGTCGACAGCAAATGG-3′. Input plasmid DNA was digested with DpnI at 37°C for 1 h. The remaining plasmid DNA containing a point mutation in the EBV TK gene was transformed into Epicurian Coli XL1-Blue competent cells (Stratagene).
The plasmid pHSV1TK was generated by PCR amplification of the HSV-1 TK gene from plasmid pHSV-106 (from G. Hayward, Johns Hopkins University) containing the HSV-1 gene (tk) inserted at the BamHI site of pBR322 (Life Technologies). Amplification was performed using Pfu polymerase and the following primers: 5′, 5′-TTAGGATCCCGTATGGCTTCGTAC-3′, and 3′, 5′ ACTGGATCCGTTTCAGTTAGCCTC-3′. The amplified HSV-1 TK gene was then cloned into the BamHI site of pcDNA3.
Cloned sequences were confirmed by complete sequencing of both strands.
Cell culture and transfection.
Rael and CA46 are EBV+ and EBV− BL cell lines, respectively (42). These were maintained in 1640 RPMI (Gibco BRL) with 10% fetal bovine serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 100 mM l-glutamine. The cell line 143b is derived from a human osteosarcoma and was passaged in 15 μg of bromodeoxyuridine (Sigma)/ml to maintain the cellular TK− phenotype (33). 293T cells, a cellular TK+ cell line, are derived from human kidney epithelial cells transformed with adenovirus E1A and E1B as well as the simian virus 40 T antigen (40). These cells were obtained from W. Burns (Medical College of Wisconsin). 143b and 293T cells were maintained in Dulbecco's modified essential medium supplemented with 10% fetal calf serum, 100 mM nonessential amino acids, 100 U of penicillin/ml, and 250 μg of streptomycin/ml.
To create a stable cell line expressing the EBV TK, 143b cells were transfected with pEBVTK. Cells were seeded at 2 × 105/ml into six-well plates and allowed to adhere overnight. After adherence, cells were transfected using 2 μg of plasmid DNA and 6 μl of Lipofectin reagent (Life Technologies). For stable cell clones, selection was carried out in growth medium containing 400 μg of G418/ml. Colonies were isolated and expanded. Colonies derived from cells transfected with the pEBVTK plasmid were selected in 1× HAT for 1 week before experimental use (TK143b.1 and TK143b.2). Colonies derived from cells transfected with vector alone (pcDNA3) were maintained in G418 and 15 μg of bromodeoxyuridine/ml (V143b.1).
Transient transfections were performed with 293T cells using the Lipofectamine-PLUS reagent (Life Technologies) according to the manufacturer's protocol. Cells were seeded into the wells of six-well plates at a concentration of 4 × 105/ml in culture medium without antibiotics and allowed to grow until the confluency was approximately 80%. Cells were transfected with 2 μg of DNA, 14 μl of PLUS reagent, and 7 μl of Lipofectamine per well in the absence of serum and antibiotics. Twenty-four hours after transfection, cells were trypsinized, counted, and prepared for immunohistochemistry and cell proliferation and phosphorylation assays. To assay for gene expression and transfection efficiency, cells were cytospun onto microscope slides for immunohistochemical analysis. Transfection efficiency was determined by counting the number of EBV TK-transfected cells expressing the EBV TK. The percentage of antigen-positive cells was determined by averaging the number of cells expressing antigen in two high-power fields relative to the total number of cells in those fields.
For analysis of cell proliferation and phosphorylation, the transiently transfected cells were seeded into 96-well plates for 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (Sigma) cell proliferation assays and/or were seeded into six-well plates for determination of GCV phosphorylation by high-performance liquid chromatography (HPLC). After adherence overnight, the transfected cells were treated with the appropriate concentrations of cold nucleoside analogues and, where appropriate, [3H]GCV. MTT analysis was performed after incubation with GCV for 4 days, while cells were extracted for phosphorylation analysis 36 h after incubation with cold GCV and [3H]GCV.
Analysis of EBV TK and PT expression.
The expression of EBV TK in TK143b cells was monitored by [3H]thymidine incorporation and by immunoblotting. For [3H]thymidine incorporation, cells were first seeded at 2 × 104/well into 96-well plates, pulsed for 48 h with 1 μCi of [3H]thymidine/well, and then harvested onto glass fiber filters. [3H]thymidine incorporation was measured by scintillation counting. For immunoblotting, proteins were separated by sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose filters (Schleicher & Schuell, Inc., Keene, N.H.). These were blocked overnight in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10.1 mM Na2PO4, and 1.8 mM KPO4) with 5% dry milk–0.1% Tween 20 and then incubated for 1 h with a polyclonal rabbit antiserum (diluted 1:1,000 in PBS with 5% dry milk–0.1% Tween 20) from a rabbit immunized with a synthetic EBV TK peptide (GRHESGLDAGYLKSVNDAC). After washing, the filters were incubated with goat anti-rabbit antibody conjugated to horseradish peroxidase (Bio-Rad, Hercules, Calif.) (diluted 1:3,000 in PBS–0.1% Tween 20). The filters were washed and the proteins were detected by ECL chemiluminescence (Amersham, Arlington Heights, Ill.).
The EBV PT was detected by Northern blot analysis. Rael and CA46 cells were treated with 1 μM 5-azacytidine overnight. RNA was extracted from treated and untreated cells using Trizol (Life Technologies). Five micrograms of RNA was electrophoresed through a 1% agarose–2 M formaldehyde gel. Following electrophoresis, the RNA was transferred to a Hybond-N+ nylon filter (Amersham Pharmacia Biotech Limited) in 20× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate). A probe specific for the EBV PT was generated from the BamHI fragment of pEBVPT using [α-32P]dCTP and the Ready-To-Go DNA labeling kit (Pharmacia Biotech, Buckinghamshire, United Kingdom). Prehybridization and hybridization were carried out at 42°C in a mixture of 50% formamide, 1% bovine serum albumin, 5% SDS, 0.5 M sodium phosphate, and 100 μg of salmon sperm DNA/ml. After hybridization, the membrane was washed once in 3× SSPE (1× SSPE is 0.18 M NaCl, 1 mM EDTA, 10 mM NaH2PO4)–0.1% SDS at 65°C, once in 0.3× SSPE–0.1% SDS at 60°C, and once in 0.1× SSPE–0.1% SDS at 42°C. Detection of the EBV PT was performed by exposing the membrane to Kodak XAR-5 film at −70°C with screens.
In vitro viability assays.
Stable cell clones were seeded into 96-well plates, cultured overnight, and incubated with the indicated nucleoside analogue for 7 days. Transiently transfected 293T cells were seeded 24 h after transfection and incubated with GCV for 4 days. Rael (EBV+) and CA46 (EBV−) cells were seeded into 96-well microtiter plates and treated with 0.5 μM 5-azacytidine for 24 h. After 24 h, the plates were centrifuged to pellet the cells, the medium was removed, and cells were resuspended in medium containing the indicated nucleoside analogue. After drug treatment, cell viability was measured using the tetrazolium salt MTT in a colorimetric 96-well-plate assay (37). MTT was dissolved in PBS at a concentration of 1 mg/ml. Cells were incubated with the MTT solution for 4 h at 37°C. The formazan crystals were solubilized in 0.04 N acid isopropanol. Plates were assayed at 560 nm using a microplate reader (Cambridge Technologies, Inc., Watertown, Mass.). Absorbance readings were standardized on untreated cells. Each experiment was repeated three times, with each MTT absorbance value representing replicates of six wells. Each bar in the figures represents the average of three experiments. Error bars represent the standard errors of the means.
Immunohistochemistry.
Lytic antigen expression was assessed in Rael cells by immunochemical analysis of cytospin preparations from treated or untreated cells. Cells (200 μl) diluted to 3 × 105 to 5 × 105 /ml in PBS were cytospun onto positively charged microscope slides, fixed at −20°C in acetone-methanol (1:1) for 10 min, and stored dry at −20°C. Before use, slides were hydrated in distilled H2O, and endogenous peroxidase activity was blocked. Slides were then incubated with monoclonal antibodies specific for either EBV Zta (1:200; Dako, Carpinteria, Calif.) or EBV VCA (1:500; Chemicon International, Inc., Temecula, Calif.). In transient transfection analysis, EBV TK expression was detected using a polyclonal antiserum from a rabbit immunized with a synthetic EBV TK peptide (described above; 1:200 dilution). Detection was performed by standard indirect detection methods. The peroxidase-based Dako Duet system (Dako) and 0.5 mg of diaminobenzidine (Sigma)/ml were used for detection of Zta and VCA. The alkaline phosphatase-based Vectastain ABC-AP kit (Vector Laboratories, Burlingame, Calif.) was used for detection of the EBV TK.
Extraction of phosphorylated GCV and HPLC analysis.
TK143b.1, TK143b.2, and V143b.1 cells (2 × 106 to 3 × 106) were seeded into wells of six-well plates and incubated with [3H]GCV (14.6 Ci/mmol; Moravek Biochemicals, Brea, Calif.) and 2 to 16 μM GCV (final specific activity, 0.5 to 4 Ci/mmol) for 60 h at 37°C. Transfected 293T cells (1.5 × 106 to 2 × 106) were seeded into wells of six-well plates incubated with [3H]GCV (14.6 Ci/mmol; Moravek Biochemicals) and 8 μM GCV for 36 h at 37°C. Following incubation, the cells were trypsinized, washed three times in PBS, and counted using a hemacytometer. [3H]GCV was extracted by lysing the cells in 60% methanol at −80°C for 18 h. Cell lysates were centrifuged at 13,000 × g for 10 min at 4°C to remove cell debris and dried in a speed vacuum. Dried extracts were stored at −80°C until analysis.
Phosphorylated forms of GCV were separated using HPLC with a strong-anion-exchange column (Whatman Partisil 10-SAX) according to a previously described procedure (14, 45), with minor modifications. Cell extracts were reconstituted in 200 μl of HPLC-grade water and centrifuged at 13,000 × g for 5 min at 4°C, and the supernatant was injected. Nucleotides were eluted with a gradient of KH2PO4 buffer (pH 3.5) at a flow rate of 0.5 ml/min (0.02 M KH2PO4 [pH 3.5] for 10 min, followed by a linear gradient to 1 M KH2PO4 [pH 3.5] over 45 min and a final 15 min at 1 M KH2PO4 [pH 3.5]). Fractions were collected every 1 min using an ISCO fraction collector (Lincoln, Nebr.). Radioactivity was counted using Hydrofluor scintillation fluid (National Diagnostics, Atlanta, Ga.) and a Beckman 5000TD scintillation counter (Fullerton, Calif.). Picomoles of phosphorylated and nonphosphorylated drug were determined by measuring the disintegrations per minute per picomole of GCV of the extract and of the fractions after separation. Relative peak retention times for GCV metabolites were as follows: GCV monophosphate (GCV-MP), 27 to 29; GCV diphosphate (GCV-DP), 41 to 45; and GCV triphosphate (GCV-TP), 61 to 65. This procedure had a detection limit of >0.02 pmol of [3H]GCV.
RESULTS
Induction of EBV lytic antigen expression and activity of the viral TK.
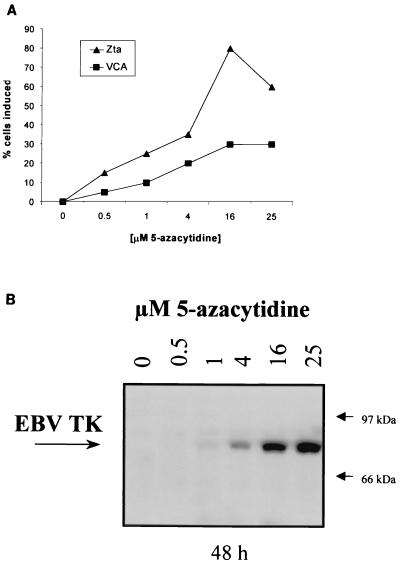
Induction of lytic viral antigen expression was evaluated after exposure of the EBV+ BL cell line Rael to various concentrations of 5-azacytidine for 6 h. Expression of EBV Zta, VCA, and TK was dose dependent. Treatment with 15 μM 5-azacytidine induced expression of the immediate early antigen Zta in 80% of the cells and expression of the late lytic antigen VCA in 20% of the cells at 48 h (Fig. 1A). Immunoreactivity with antiserum to an EBV TK peptide was detected in induced cells as a 70-kDa protein (Fig. 1B), consistent with previous reports of EBV TK detection by immunoblotting (19, 22, 24). The antiserum worked well for the immunoblot format but not for immunohistochemical assays, and therefore it was not possible to directly assess the percentage of cells expressing TK in induction experiments.
FIG. 1.
Induction of lytic antigens by 5-azacytidine. (A) The EBV+ Burkitt's cell line Rael was incubated with the indicated concentrations of 5-azacytidine for 6 h, washed three times, and resuspended in complete medium. Cells were fixed in acetone-methanol 48 h after incubation with 5-azacytidine. Zta and VCA were detected by immunohistochemistry. The percentage of antigen-positive cells was determined by averaging the number of cells expressing antigen in two high-power fields relative to the total number of cells in those fields. (B) Detection of EBV TK protein in 5-azacytidine-induced Rael cells by immunoblot. Rael cells were treated with the indicated concentrations of 5-azacytidine for 6 h and washed three times, and total cellular protein was isolated 48 h later. Fifty micrograms of protein per lane was separated by SDS–7.5% PAGE.
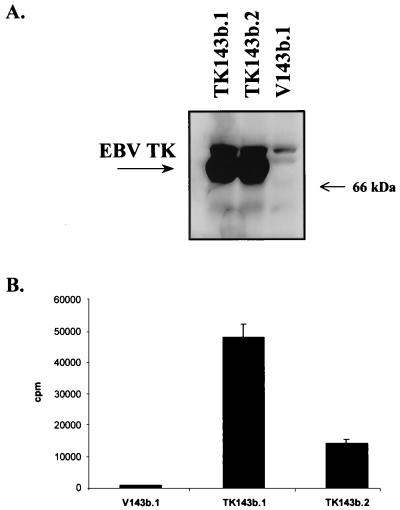
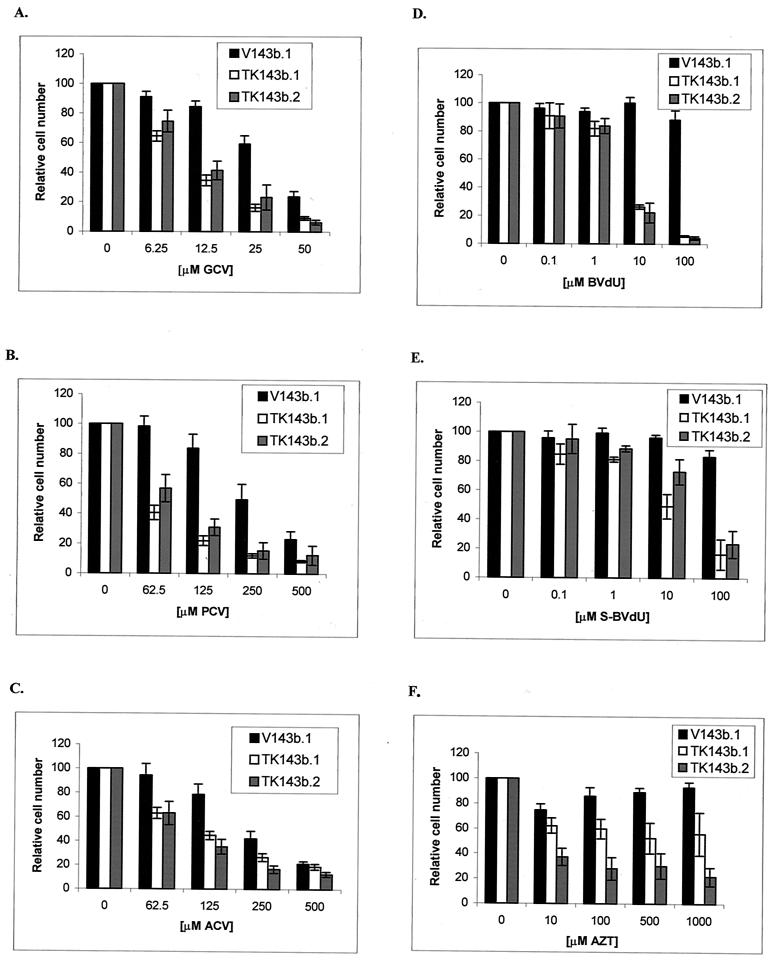
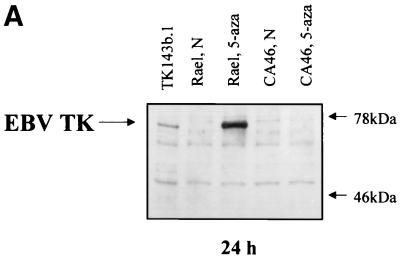
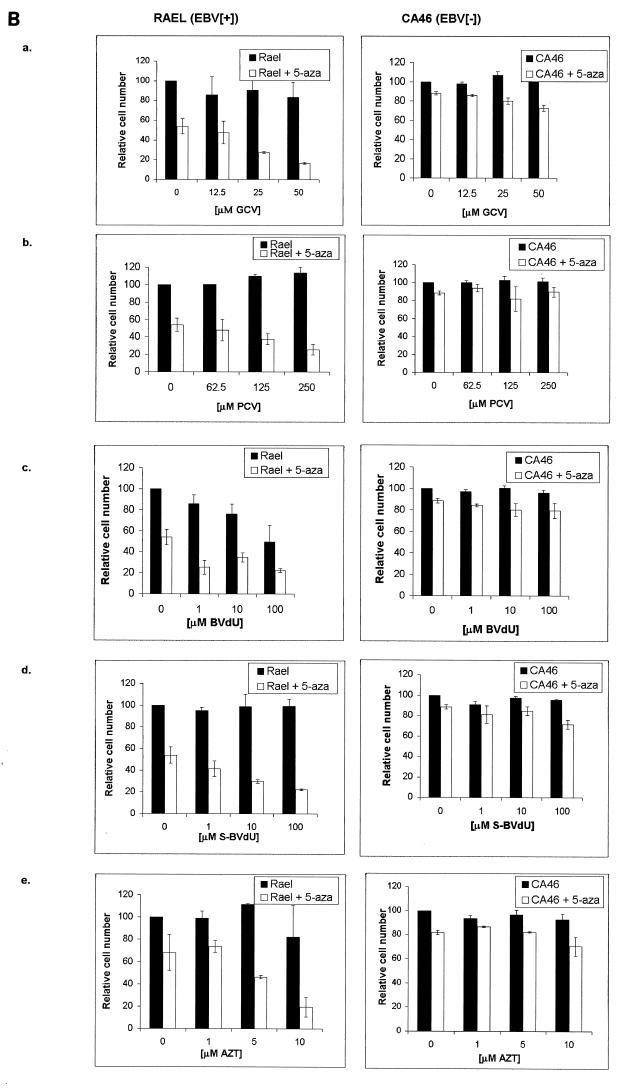
To determine whether the expression of EBV TK might sensitize cells to nucleoside analogues, we transfected the TK− 143b osteosarcoma cell line with an EBV TK expression vector and a control vector as described in Materials and Methods. An immunoblot showed reactivity in the TK-transfected clones (TK143b.1 and TK143b.2) but not in the control clone (V143b.1) (Fig. 2A). EBV TK is functional in these cell clones, as evidenced by the ability to grow in HAT and by [3H]thymidine incorporation (Fig. 2B). We measured the sensitivity of EBV TK-expressing cells and control cells to several nucleoside analogues. TK143b cell clones were moderately more sensitive to high doses of ACV and PCV (62.5 to 500 μM) and to lower doses of GCV and BVdU (10 to 100 μM) than was the control (V143b.1 [TK−]) clone (Fig. 3). Some sensitivity to AZT was found, but there was no dose-response relationship. This result is consistent with a previous report that EBV TK sensitizes NIH 3T3 cells to BVdU, but poorly sensitizes them to ACV and GCV (28).
FIG. 2.
(A) Immunoblot detection of EBV TK expression in EBV TK-expressing cell clones (TK143b.1 and TK143b.2). One hundred micrograms of total cell protein per lane was separated by SDS–7.5% PAGE. (B) Incorporation of [3H]thymidine in TK-expressing (TK143b.1 and TK143b.2) and control (V143b.1) cell clones. Incorporation into V143b.1 cells is similar to background (medium alone without cells).
FIG. 3.
Treatment of EBV TK-expressing cells and control cells with nucleoside analogues in vitro. Cells were seeded into 96-well microtiter plates at 103 cells/well. After allowing cells to adhere overnight, cells were incubated with GCV (A), PCV (B), ACV (C), BVdU (D), S-BVdU (E), or AZT (F) for 7 days. A colorimetric assay measuring the conversion of MTT to formazan was used to determine the fraction of cells surviving relative to untreated controls (100% viable). Each experiment was repeated three times, with each MTT absorbance value representing replicates of six wells. Each bar represents the average of three experiments, with error bars representing the standard errors of the means.
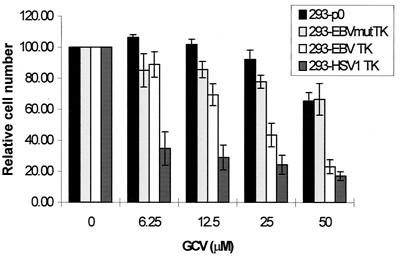
We carried out further experiments to exclude the possibility that our results were an artifact of clonal selection. The sensitivity to GCV was also assessed by the transient transfection of cellular TK+ 293T cells with pEBVTK. Historically, high transfection efficiencies can be achieved using this system, and no selection is employed. The sensitivity of cellular TK+ 293T cells transfected with pEBVTK, but not empty control vector DNA, to GCV (Fig. 4) supports the hypothesis that the difference in sensitivity to nucleoside analogues between EBV TK-expressing cells and control cells is due specifically to the presence of an overexpressed protein. Previous investigators have reported various conflicting results in this regard (6, 19, 24, 28, 53). We sought to determine whether the sensitivity of EBV TK-expressing cells to GCV reflected the specific enzymatic activity of EBV TK or reflected a nonspecific cellular toxicity, possibly associated with foreign protein overexpression, or cellular activation of GCV. To this end, we introduced a point mutation (A398T) into EBV TK in the nucleotide-nucleoside binding site inferred on the basis of homology with HSV-1 TK (18). Cells transfected with this plasmid (pEBVmutTK) qualitatively expressed TK, as assessed by immunohistochemistry (not shown), but were not sensitized to GCV (Fig. 4). Cells transfected with HSV-1 TK (pHSV1TK) showed more sensitivity to GCV than those transfected with EBV TK, consistent with data from other laboratories (6, 20, 28). Note that at high levels of GCV (50 μM) both the EBV and HSV-1 TKs result in the killing of ∼70% of the cells in the transfection experiment, while at lower levels of GCV (6.25 μM), EBV TK leads to little or no killing and HSV-1 TK leads to the killing of ∼60% of the cells, consistent with a transfection efficiency of ∼70 to 75% (Fig. 4).
FIG. 4.
Sensitivity of EBV TK-expressing, cellular TK+ 293T cells to GCV. 293T cells were transfected with pEBVTK (293-EBV TK), pHSV1TK (293-HSV1 TK), pEBVmutTK (293-EBVmutTK), or control vector DNA (293-p0 cells). Following transfection, cells were seeded into a 96-well plate and treated with GCV for 4 days, and viability was determined by the MTT assay. For each experiment, concentrations were analyzed in replicates of six wells. Bars represent the averages of three experiments. Error bars show the standard errors of the means.
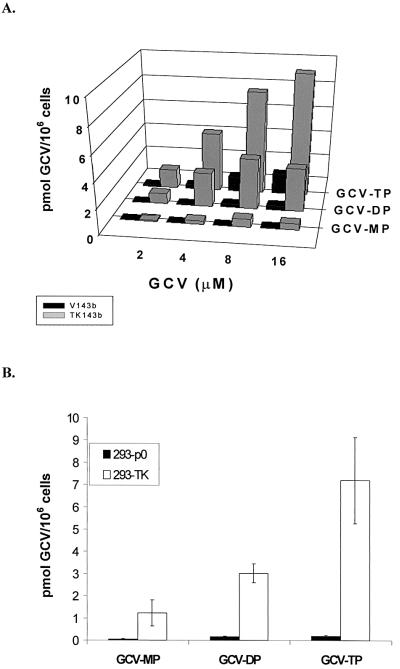
To directly confirm that EBV TK expression resulted in the phosphorylation of GCV, we performed HPLC analyses on lysates of EBV TK-expressing 143b cells and control cells incubated with [3H]GCV. Lysates from EBV TK-expressing cells had 1 to 9 pmol of GCV-TP/106 cells, which was four- to fivefold more than non-EBV TK-expressing V143b.1 control cells, when exposed to 2 to 16 μM GCV for 60 h (Fig. 5A). This phosphorylation was dose dependent, consistent with cell killing due to GCV-TP accumulation at higher concentrations of GCV. Similarly, in the transient transfection of 293T cells with pEBVTK, phosphorylation of GCV was confirmed by HPLC (Fig. 5B). In further experiments, transfection with HSV-1 TK was associated with higher levels of GCV phosphates (15, 94, and 450 pmol of GCV/106 cells detected for GCV-MP, -DP, and -TP, respectively) than EBV TK, while transfection with EBVmutTK was associated with negligible levels (data not shown). Thus, the results of these experiments parallel the killing experiments.
FIG. 5.
(A) Phosphorylation of GCV in TK143b and V143b cells as determined by HPLC. Cells were incubated with 2 to 16 μM GCV for 60 h. [3H]GCV was added as a tracer, and phosphorylated products were separated by HPLC. (B) Phosphorylation of GCV in 293T cells transfected with pEBVTK. Following transfection, cells were treated with 8 μM GCV using 3[H]GCV as a tracer, incubated for 36 h at 37°C, and analyzed for GCV phosphorylation by HPLC. Bars represent the averages of three experiments. Error bars show the standard errors of the means.
A second EBV GCV kinase.
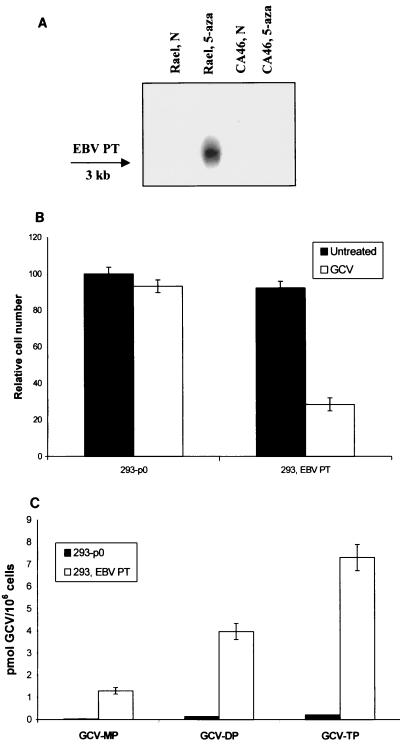
Herpesviruses such as cytomegalovirus, while lacking a TK, are nonetheless able to phosphorylate GCV (21, 48). The cytomegalovirus GCV kinase (UL97) has been identified as a PT with homologues throughout the herpesvirus family (1, 47). Recently we have shown that human herpesvirus 8 open reading frames with homology to herpesvirus TKs and PTs both phosphorylate GCV (5). In order to determine whether the EBV PT homologue, BGLF4, also sensitizes cells to GCV, we transiently transfected 293T cells with an EBV PT expression vector as described in Materials and Methods. EBV PT RNA can be induced by 5-azacytidine in Rael cells (Fig. 6A). Though expression from pEBVPT was not assessed, as shown in Fig. 6 transient transfection of the plasmid pEBVPT into 293T cells was associated with phosphorylation of GCV and sensitization to GCV-mediated cell killing (25 μM GCV for 4 days, compared with an empty plasmid control). A comparison with UL97 was not performed. In contrast to pEBVTK, the putative EBV PT did not sensitize cells to BVdU or, interestingly, to PCV (data not shown).
FIG. 6.
EBV PT induction and activity. (A) The EBV PT transcript was detected in Rael cells by Northern blotting after exposure to 1 μM 5-azacytidine overnight. (B) Expression of EBV PT in 293T cells sensitizes cells to GCV cell killing (25 μM for 4 days), as shown by MTT analysis. (C) Phosphorylation of GCV as shown by HPLC analysis. 293T cells expressing EBV PT were treated with 8 μM GCV using [3H]GCV as a tracer as described in Materials and Methods. Bars represent the averages of three experiments. Error bars represent the standard errors of the means.
To determine whether induction of the kinases led to increased sensitivity to antiviral nucleoside analogues in EBV+ BL cell lines, we compared the effects of 5-azacytidine on an EBV+ and an EBV− Burkitt's cell line in the presence and absence of various antiviral nucleoside analogues. EBV+ Rael cells treated with 0.5 μM 5-azacytidine are induced to express EBV TK, as demonstrated by an immunoblot (Fig. 7A). EBV+ Rael cells were more sensitive than EBV− CA46 cells to the cytotoxic effects of 5-azacytidine, perhaps as a result of the induction of lytic viral antigen expression, reducing cell numbers by half in the absence of nucleosides and hampering evaluation of nucleoside effects. The data suggested that 5-azacytidine sensitized an EBV+, but not an EBV−, BL cell line to killing by GCV, PCV, BVdU, S-BVdU, and AZT (Fig. 7B). The effects were not as pronounced as in the transfected cells (Fig. 3), and with the exception of AZT, high concentrations of drug were necessary to see an effect of 50% or greater. No nucleoside that did not sensitize cells was identified.
FIG. 7.
Sensitivity of 5-azacytidine-treated cells to nucleoside analogues. In separate experiments, Rael (EBV+) and CA46 (EBV−) cells were treated with 0.5 μM 5-azacytidine for 24 h. (A) Detection of EBV TK protein in 5-azacytidine-induced Rael cells by immunoblot analysis. Cells were treated and washed three times, and total cellular protein was isolated. Five micrograms of protein per lane was separated by SDS–7.5% PAGE. (B) Cells were seeded into 96-well microtiter plates at 104 cells/well. Cells were then treated with 0.5 μM 5-azacytidine for 24 h. After 24 h, the plates were centrifuged to pellet the cells, the medium was removed, and cells were resuspended in medium containing GCV (a), PCV (b), BVdU (c), S-BVdU (d), or AZT (e). Plates were incubated for an additional 7 days. Viability was determined by the MTT assay. The values of cells treated with drug are presented as the fractions of cells surviving relative to untreated controls (100% viable). Experiments for panels a to d were performed at the same time, while the experiment for panel e was performed at a later date. Each experiment was repeated three times, with each MTT absorbance value representing replicates of six wells. Each bar represents the average of three experiments, with error bars representing the standard errors of the means.
DISCUSSION
The investigations presented here suggest that pharmacologic activation of viral kinase gene expression may render cells carrying EBV genomes selectively sensitive to high doses of several nucleoside antiviral agents. EBV TK appeared to sensitize cells to killing by GCV, PCV, BVdU, and AZT. A putative PT sensitized cells to GCV but not to BVdU or PCV.
The ability of EBV TK to sensitize cells to killing by GCV has been the subject of recent conflicting reports (6, 19, 24, 28, 53). Four previous studies examined the activity (phosphorylation) and substrate specificity (competition) of purified or partially purified EBV TK expressed as fusion proteins in bacteria, and all documented TK function. Three studies further examined GCV: two of the studies showed no significant thymidine competition by nor phosphorylation of GCV, whereas one study showed GCV to compete 10-fold more efficiently for EBV TK than for HSV-1 TK, a result that has not been generally confirmed. Because it was possible that purified EBV TK lost its ability to selectively phosphorylate GCV, though not thymidine or thymidine analogues, when compared with EBV TK protein expressed in cells, we examined GCV phosphorylation as well as sensitization to various nucleoside analogues. Our results parallel those of Loubiere et al., who calculated a selectivity index as the ratio of the drug concentrations required to reduce thymidine incorporation by 50% in untransfected parental and EBV TK-transfected NIH 3T3 cells (28). The selectivity index was 4 for ACV, 12 for GCV, and 1,375 for BVdU. Although the thymidine analogue was >1,000 times more selective sensitization to purine analogues was suggested. The goal of this study was to compare two efficient TKs for gene therapy, with EBV TK serving as a negative control. Since comparisons were made to a parental line that was neither transfected nor selected with toxic drug and in the absence of an analysis of protein expression or GCV phosphorylation, these results are less conclusive. Gustafson et al. showed that lysates from an EBV TK+ clone of 143b TK− cells phosphorylated GCV twice as well as a TK− control clone but ∼1,000 times less well than an HSV-1 TK-expressing clone analyzed under the same conditions. However, they did not see sensitization to GCV or ACV, although they used a system closely parallel to that used in our studies (19, 20). Some of the discrepancies might be explained by a loss of viral TK expression, with partial reversion of cells to a TK+ phenotype, if periodic assessment were not performed. Such reversion has sometimes been noted in HAT-selected lines (2, 17, 32). To help prevent this problem, our EBV TK-expressing cell clones were selected in HAT for only 1 week prior to experimentation. Periodic immunoblotting of total TK143b cell protein with a polyclonal rabbit antiserum from a rabbit immunized with an EBV TK peptide confirmed that the EBV TK was being expressed in TK143b cell clones over time (data not shown). Our sensitization results using two stable (cellular TK−) clones were parallel to those of a transient (cellular TK+) transfection system that overexpressed EBV TK. However, we were unable to determine transfection efficiencies, and sensitization was modest (50% at 50 μM GCV for 4 days when EBV TK and EBVmutTK were compared). Our HPLC analysis of a cell clone that constitutively expressed the EBV TK and of a transiently transfected cell line overexpressing EBV TK compared with an empty vector control suggested that the EBV TK does phosphorylate GCV. Our experiments further raised the possibility that, as suggested by Gustafson et al. (19), another EBV kinase, the EBV PT, might phosphorylate and sensitize cells to GCV. Though overall activity was low, together these experiments suggest that the presence of viral enzymes could contribute to phosphorylation of and sensitization of cells to GCV.
Is the use of a combination of a lytio-inducing agent and an antiviral prodrug appropriate for consideration in the clinical setting in view of the high doses of antiviral drug that may be required? The susceptibility of EBV-associated disease in vivo to the combination of an EBV-inducing agent and GCV has been reported (36; H. Oettle, F. Wilborn, C. A. Schmidt, and W. Siegert, Letter, Blood 82:2257–2258, 1993), supporting the hypothesis that EBV-associated malignancies can be targeted with an inducing agent in combination with a prodrug. Although the levels of GCV required for killing in combination with 5-azacytidine in vitro are higher than those achieved with standard doses of these drugs in vivo, those standard doses are based on regimens that involve chronic administration. 5-Azacytidine-induced EBV+ cells were sensitive to GCV when treated with concentrations of GCV at 25 to 50 μM for several days. Peak plasma concentrations of GCV can reach 30 to 40 μM after an intravenous dose of 5 mg of GCV/kg of body weight (12). The limiting toxicity associated with chronic GCV administration is myelotoxicity; neutropenia has been associated with peak plasma levels of GCV exceeding 30 to 50 μM in patients treated for cytomegalovirus pneumonia (44). GCV administered at very high doses in a short-term regimen, however, might be associated with tolerable myelotoxicity when used in combination with myeloid growth factors. Alternative cancer therapies are consistently associated with neutropenia. With regard to PCV (active metabolite of the parent compound famciclovir), no dose-limiting toxicity has been defined in any regimen and substantially higher plasma levels might be achieved than are associated with current regimens.
A variety of lytic inducers have been studied in the laboratory. These include phorbol esters, anti-immunoglobulin antibodies, butyrate, and DNA methyltransferase inhibitors (3, 30, 50, 56). For the studies described here, we chose to study a DNA methyltransferase inhibitor because there is a large amount of clinical experience with this agent in other settings (7, 9, 29). 5-Azacytidine has been used clinically in the treatment of leukemia, myelodysplasia, and hemoglobinopathies for more than two decades at doses that achieve concentration ranges similar to the concentrations studied. In a recent clinical trial, 5-azacytidine administered at a dose of 75 mg/m2 per day for 5 to 7 days was associated with demethylation of EBV genomes in an EBV+ AIDS lymphoma and in nasopharyngeal carcinoma as assessed by biopsy within 72 h after treatment (Ambinder, unpublished data). This suggests that administration of agents that impact on patterns of gene expression in vitro may also impact on viral gene regulation in vivo.
To implement this therapy in vivo, a large percentage of EBV+ tumor cells need to be induced. As shown in Fig. 1, approximately 60% of Rael cells were induced by 5-azacytidine (25 μM) to express the immediate early lytic protein Zta. The ability of 5-azacytidine to induce lytic EBV infection is not limited to one particular cell line. The Burkitt's-derived Akata cell line and the nasopharyngeal carcinoma C666 cell line also show lytic activation following treatment with this agent (unpublished data). Greater levels of induction are likely to be required in order to target tumors. Other inducing agents or a combination of inducing agents, such as the combination of a methyltransferase inhibitor and a histone deacetylase inhibitor, may be able to induce levels of EBV TK closer to 100%. Further investigation to optimize induction strategies for specific EBV-associated tumors is underway.
The principle that EBV-infected cells can be selectively killed by the combination of an inducer of lytic cycle viral gene expression with an antiviral nucleoside analogue merits further evaluation. In contrast to gene therapy approaches for treating EBV+ tumors, the approach we suggest eliminates the requirement for the introduction of foreign genes. The demonstration that the EBV TK and PT can be pharmacologically induced in EBV+ tumor cells and that these induced cells are sensitive to antiviral nucleoside analogues suggests a pharmacologic approach which specifically targets EBV-associated tumor cells.
ACKNOWLEDGMENT
This work was supported by NIH grant P01 CA81400.
REFERENCES
- 1.Ansari A, Emery V C. The U69 gene of human herpesvirus 6 encodes a protein kinase which can confer ganciclovir sensitivity to baculoviruses. J Virol. 1999;73:3284–3291. doi: 10.1128/jvi.73.4.3284-3291.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin M B, Potter H, Yandell D W, Little J B. A system for assaying homologous recombination at the endogenous human thymidine kinase gene. Proc Natl Acad Sci USA. 1991;88:6652–6656. doi: 10.1073/pnas.88.15.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Sasson S A, Klein G. Activation of the Epstein-Barr virus genome by 5-aza-cytidine in latently infected human lymphoid lines. Int J Cancer. 1981;28:131–135. doi: 10.1002/ijc.2910280204. [DOI] [PubMed] [Google Scholar]
- 4.Bordignon C, Bonini C, Verzeletti S, Nobili N, Maggioni D, Traversari C, Giavazzi R, Servida P, Zappone E, Benazzi E, et al. Transfer of the HSV-tk gene into donor peripheral blood lymphocytes for in vivo modulation of donor anti-tumor immunity after allogeneic bone marrow transplantation. Hum Gene Ther. 1995;6:813–819. doi: 10.1089/hum.1995.6.6-813. [DOI] [PubMed] [Google Scholar]
- 5.Cannon J S, Hamzeh F, Moore S, Nicholas J, Ambinder R F. Human herpesvirus 8-encoded thymidine kinase and phosphotransferase homologues confer sensitivity to ganciclovir. J Virol. 1999;73:4786–4793. doi: 10.1128/jvi.73.6.4786-4793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cazaux C, Tiraby M, Loubiere L, Haren L, Klatzmann D, Tiraby G. Phosphorylation and cytotoxicity of therapeutic nucleoside analogues: a comparison of alpha and gamma herpesvirus thymidine kinase suicide genes. Cancer Gene Ther. 1998;5:83–91. [PubMed] [Google Scholar]
- 7.Charache S, Dover G, Smith K, Talbot C C J, Moyer M, Boyer S. Treatment of sickle cell anemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation of DNA around the gamma-delta-beta-globin gene complex. Proc Natl Acad Sci USA. 1983;80:4842–4846. doi: 10.1073/pnas.80.15.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen M R, Chang S J, Huang H, Chen J Y. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J Virol. 2000;74:3093–3104. doi: 10.1128/jvi.74.7.3093-3104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chitambar C R, Libnoch J A, Matthaeus W G, Ash R C, Ritch P S, Anderson T. Evaluation of continuous infusion low-dose 5-azacytidine in the treatment of myelodysplastic syndromes. Am J Hematol. 1991;37:100–104. doi: 10.1002/ajh.2830370207. [DOI] [PubMed] [Google Scholar]
- 10.Crumpacker C S. Ganciclovir. N Engl J Med. 1996;335:721–729. doi: 10.1056/NEJM199609053351007. [DOI] [PubMed] [Google Scholar]
- 11.Culver K W, Van Gilder J, Link C J, Carlstrom T, Buroker T, Yuh W, Koch K, Schabold K, Doornbas S, Wetjen B. Gene therapy for the treatment of malignant brain tumors with in vivo tumor transduction with the herpes simplex thymidine kinase gene/ganciclovir system. Hum Gene Ther. 1994;5:343–379. doi: 10.1089/hum.1994.5.3-343. [DOI] [PubMed] [Google Scholar]
- 12.Douglas R., Jr . Antimicrobial agents. In: Gilman A G, et al., editors. Goodman and Gilman's the pharmacological basis of therapeutics. New York, N.Y: Pergamon Press; 1990. pp. 1182–1201. [Google Scholar]
- 13.Elion G B. The biochemistry and mechanism of action of acyclovir. J Antimicrob Chemother. 1983;12(Suppl. B):9–17. doi: 10.1093/jac/12.suppl_b.9. [DOI] [PubMed] [Google Scholar]
- 14.Elion G B, Furman P A, Fyfe J A, De Miranda P, Beauchamp L, Schaeffer H J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci USA. 1977;74:5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernberg I, Falk K, Minarovits J, Busson P, Tursz T, Masucci M G, Klein G. The role of methylation in the phenotype-dependent modulation of Epstein-Barr nuclear antigen 2 and latent membrane protein genes in cells latently infected with Epstein-Barr virus J. Gen Virol. 1989;70:2989–3002. doi: 10.1099/0022-1317-70-11-2989. . (Erratum, 71:499, 1990.) [DOI] [PubMed] [Google Scholar]
- 16.Faulds D, Heel R C. Ganciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs. 1990;39:597–638. doi: 10.2165/00003495-199039040-00008. [DOI] [PubMed] [Google Scholar]
- 17.Goring D R, Gupta K, DuBow M S. Analysis of spontaneous mutations in a chromosomally located HSV-1 thymidine kinase (TK) gene in a human cell line. Somat Cell Mol Genet. 1987;13:47–56. doi: 10.1007/BF02422298. [DOI] [PubMed] [Google Scholar]
- 18.Graham D, Larder B A, Inglis M M. Evidence that the ‘active centre’ of the herpes simplex virus thymidine kinase involves an interaction between three distinct regions of the polypeptide. J Gen Virol. 1986;67:753–758. doi: 10.1099/0022-1317-67-4-753. [DOI] [PubMed] [Google Scholar]
- 19.Gustafson E A, Chillemi A C, Sage D R, Fingeroth J D. The Epstein-Barr virus thymidine kinase does not phosphorylate ganciclovir or acyclovir and demonstrates a narrow substrate specificity compared to the herpes simplex virus type 1 thymidine kinase. Antimicrob Agents Chemother. 1998;42:2923–2931. doi: 10.1128/aac.42.11.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustafson E A, Schinazi R F, Fingeroth J D. Human herpesvirus 8 open reading frame 21 is a thymidine and thymidylate kinase of narrow substrate specificity that efficiently phosphorylates zidovudine but not ganciclovir. J Virol. 2000;74:684–692. doi: 10.1128/jvi.74.2.684-692.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Z, He Y S, Kim Y, Chu L, Ohmstede C, Biron K K, Coen D M. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J Virol. 1997;71:405–411. doi: 10.1128/jvi.71.1.405-411.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holton R H, Gentry G A. The Epstein-Barr virus genome encodes deoxythymidine kinase activity in a nested internal open reading frame. Intervirology. 1996;39:270–274. doi: 10.1159/000150528. [DOI] [PubMed] [Google Scholar]
- 23.Jansson A, Masucci M, Rymo L. Methylation of discrete sites within the enhancer region regulates the activity of the Epstein-Barr virus BamHI W promoter in Burkitt lymphoma lines. J Virol. 1992;66:62–69. doi: 10.1128/jvi.66.1.62-69.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Littler E, Arrand J R. Characterization of the Epstein-Barr virus-encoded thymidine kinase expressed in heterologous eucaryotic and procaryotic systems. J Virol. 1988;62:3892–3895. doi: 10.1128/jvi.62.10.3892-3895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Littler E, Stuart A D, Chee M S. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992;358:160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- 26.Littler E, Zeuthen J, McBride A A, Trost Sorensen E, Powell K L, Walsh-Arrand J E, Arrand J R. Identification of an Epstein-Barr virus-coded thymidine kinase. EMBO J. 1986;5:1959–1966. doi: 10.1002/j.1460-2075.1986.tb04450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu M Y, Pai C Y, Shieh S M, Hsu T Y, Chen J Y, Yang C S. Cloning and expression of a cDNA encoding the Epstein-Barr virus thymidine kinase gene. J Virol Methods. 1992;40:107–118. doi: 10.1016/0166-0934(92)90012-3. [DOI] [PubMed] [Google Scholar]
- 28.Loubiere L, Tiraby M, Cazaux C, Brisson E, Grisoni M, Zhao-Emonet J, Tiraby G, Klatzmann D. The equine herpes virus 4 thymidine kinase is a better suicide gene than the human herpes virus 1 thymidine kinase. Gene Ther. 1999;6:1638–1642. doi: 10.1038/sj.gt.3300993. [DOI] [PubMed] [Google Scholar]
- 29.Lowrey C H, Nienhuis A W. Treatment with azacitidine of patients with end-stage β-thalassemia. N Engl J Med. 1993;329:845–848. doi: 10.1056/NEJM199309163291205. [DOI] [PubMed] [Google Scholar]
- 30.Luka J, Kallin B, Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979;94:228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- 31.MacMahon E M E, Glass J D, Hayward S D, Mann R B, Becker P S, Charache P, McArthur J C, Ambinder R F. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet. 1991;338:969–973. doi: 10.1016/0140-6736(91)91837-k. [DOI] [PubMed] [Google Scholar]
- 32.Manjunath G S, Dufresne M J. Plasmid DNA mediated transfer of the herpes simplex virus thymidine kinase gene to a new bromodeoxyuridine resistant variant of human primary lung carcinoma cells. Biochem Int. 1988;16:149–156. [PubMed] [Google Scholar]
- 33.Manservigi R, Gualandri R, Negrini M, Albonici L, Milanesi G, Cassai E, Barbanti-Brodano G. Constitutive expression in human cells of herpes simplex virus type 1 glycoprotein B gene cloned in an episomal eukaryotic vector. Virology. 1988;167:284–288. doi: 10.1016/0042-6822(88)90080-3. [DOI] [PubMed] [Google Scholar]
- 34.Mar E C, Chiou J F, Cheng Y C, Huang E S. Inhibition of cellular DNA polymerase alpha and human cytomegalovirus-induced DNA polymerase by the triphosphates of 9-(2-hydroxyethoxymethyl) guanine and 9-(1,3-dihydroxy-2-propoxymethyl)guanine. J Virol. 1985;53:776–780. doi: 10.1128/jvi.53.3.776-780.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masucci M G, Contreras-Salazar B, Ragnar E, Falk K, Minarovits J, Ernberg I, Klein G. 5-Azacytidine up regulates the expression of Epstein-Barr virus nuclear antigen 2 (EBNA-2) through EBNA-6 and latent membrane protein in the Burkitt's lymphoma line Rael. J Virol. 1989;63:3135–3141. doi: 10.1128/jvi.63.7.3135-3141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mentzer S J, Fingeroth J, Reilly J J, Perrine S P, Faller D V. Arginine butyrate-induced susceptibility to ganciclovir in an Epstein-Barr-virus-associated lymphoma. Blood Cells Mol Dis. 1998;24:114–123. doi: 10.1006/bcmd.1998.0178. [DOI] [PubMed] [Google Scholar]
- 37.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 38.Murray P G, Swinnen L J, Constandinou C M, Pyle J M, Carr T J, Hardwick J M, Ambinder R F. BCL-2 but not its Epstein-Barr virus-encoded homologue, BHRF1, is commonly expressed in posttransplantation lymphoproliferative disorders. Blood. 1996;87:706–711. [PubMed] [Google Scholar]
- 39.Nonkwelo C B, Long W K. Regulation of Epstein-Barr virus BamHI-H divergent promoter by DNA methylation. Virology. 1993;197:205–215. doi: 10.1006/viro.1993.1581. [DOI] [PubMed] [Google Scholar]
- 40.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reardon J E. Herpes simplex virus type 1 and human DNA polymerase interactions with 2′-deoxyguanosine 5′-triphosphate analogues. Kinetics of incorporation into DNA and induction of inhibition. J Biol Chem. 1989;264:19039–19044. [PubMed] [Google Scholar]
- 42.Robertson K D, Hayward D J, Ling P D, Samid D, Ambinder R F. Transcriptional activation of the EBV latency C promoter following 5-azacytidine treatment: evidence that demethylation at a single CpG site is crucial. Mol Cell Biol. 1995;15:6150–6159. doi: 10.1128/mcb.15.11.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson K D, Manns A, Swinnen L J, Zong J C, Gulley M L, Ambinder R F. CpG methylation of the major Epstein-Barr virus latency promoter in Burkitt's lymphoma and Hodgkin's disease. Blood. 1996;88:3129–3136. [PubMed] [Google Scholar]
- 44.Shepp D H, Dandliker P S, De Miranda P, Burnette T C, Cederberg D M, Kirk L E, Meyers J D. Activity of 9-[2-hydroxy-1-(hydroxymethyl)ethoxymethyl]guanine in the treatment of cytomegalovirus pneumonia. Ann Intern Med. 1985;103:368–373. doi: 10.7326/0003-4819-103-3-368. [DOI] [PubMed] [Google Scholar]
- 45.Slusher J T, Kuwahara S K, Hamzeh F M, Lewis L D, Kornhauser D M, Lietman P S. Intracellular zidovudine (ZDV) and ZDV phosphates as measured by a validated combined high-pressure liquid chromatography-radioimmunoassay procedure. Antimicrob Agents Chemother. 1992;36:2473–2477. doi: 10.1128/aac.36.11.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smee D F, Boehme R, Chernow M, Binko B P, Matthews T R. Intracellular metabolism and enzymatic phosphorylation of 9-(1,3-dihydroxy-2-propoxymethyl)guanine and acyclovir in herpes simplex virus-infected and uninfected cells. Biochem Pharmacol. 1985;34:1049–1056. doi: 10.1016/0006-2952(85)90608-2. [DOI] [PubMed] [Google Scholar]
- 47.Smith R S, Smith T F. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J Virol. 1989;63:450–455. doi: 10.1128/jvi.63.1.450-455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sullivan V, Talarico C L, Stanat S C, Davis M, Coen D M, Biron K K. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992;358:162–164. doi: 10.1038/358162a0. . (Errata, 359:85, 1992, and 366:756, 1993.) [DOI] [PubMed] [Google Scholar]
- 49.Takacs M, Myohanen S, Altiok E, Minarovits J. Analysis of methylation patterns in the regulatory region of the latent Epstein-Barr virus promoter BCR2 by automated fluorescent genomic sequencing. Biol Chem. 1998;379:417–422. doi: 10.1515/bchm.1998.379.4-5.417. [DOI] [PubMed] [Google Scholar]
- 50.Takada K. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines. Int J Cancer. 1984;33:27–32. doi: 10.1002/ijc.2910330106. [DOI] [PubMed] [Google Scholar]
- 51.Tao Q, Ho F C, Loke S L, Srivastava G. Epstein-Barr virus is localized in the tumour cells of nasal lymphomas of NK, T or B cell type. Int J Cancer. 1995;60:315–320. doi: 10.1002/ijc.2910600306. [DOI] [PubMed] [Google Scholar]
- 52.Tao Q, Robertson K D, Manns A, Hildesheim A, Ambinder R F. Epstein-Barr virus (EBV) in endemic Burkitt's lymphoma: molecular analysis of primary tumor tissue. Blood. 1998;91:1373–1381. [PubMed] [Google Scholar]
- 53.Tung P P, Summers W C. Substrate specificity of Epstein-Barr virus thymidine kinase. Antimicrob Agents Chemother. 1994;38:2175–2179. doi: 10.1128/aac.38.9.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiss L M, Movahed L A, Warnke R A, Sklar J. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin's disease. N Engl J Med. 1989;320:502–506. doi: 10.1056/NEJM198902233200806. [DOI] [PubMed] [Google Scholar]
- 55.Wu T C, Mann R B, Charache P, Hayward S D, Staal S, Lambe B C, Ambinder R F. Detection of EBV gene expression in Reed-Sternberg cells of Hodgkin's disease. Int J Cancer. 1990;46:801–804. doi: 10.1002/ijc.2910460509. [DOI] [PubMed] [Google Scholar]
- 56.ZurHausen H, O'Neill F J, Freese U K, Hecher E. Persisting oncogenic herpesvirus induced by tumor promoter TPA. Nature. 1978;272:373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]