Abstract
A 23-year-old previously healthy male presented to the hospital with symptoms of heart failure. He was diagnosed with pericarditis and found to have a reduced left ventricular ejection fraction of 25%. He was noted to have mediastinal lymphadenopathy. Pulmonary and abdominal sampling were non-diagnostic for infection, autoimmune disease, or malignancy. A QuantiFERON Gold returned positive. After a thorough travel history and detailed exam, the patient was diagnosed with disseminated tuberculosis after the discovery of a cutaneous gumma that was found to have acid-fast bacilli present on biopsy with Fite’s stain. 18F-FDG PET CT and cardiac MRI were pursued given that pericardial and myocardial biopsy could not be safely performed due to the patient’s hemodynamics. 18F-FDG PET CT and cardiac MRI did not demonstrate any myocardial pathology responsible for the left ventricular ejection fraction. This case highlights that pulmonary involvement is not necessary for disseminated TB, Fite’s stain may be used to identify M. tuberculosis, and that cardiac MRI and 18F-FDG PET CT may be useful to delineate myocardial involvement in high-risk situations.
INTRODUCTION/BACKGROUND
Prior to the development of the SARS-CoV-2 virus, Mycobacterium tuberculosis (TB) was responsible for more deaths globally than any other infectious disease, accounting for over 1.3 million deaths in 2017 alone.1 Though the lungs are the primary site of TB infection, approximately 20% of disseminated TB cases within the United States (US) lack pulmonary findings.2 Despite non-pulmonary TB constituting a significant portion of disseminated TB cases, diagnosis is often difficult given the paucity of typical findings. We present a case of disseminated TB complicated by constrictive pericarditis and cutaneous TB without evidence of pulmonary involvement. Understanding the spectrum of extra-pulmonary TB manifestations, including cutaneous and cardiac, is important for establishing the diagnosis. This case also demonstrates the value of obtaining a thorough travel and exposure history.
CLINICAL CASE
A 23-year-old immunocompetent male with recently diagnosed heart failure was admitted to the hospital with 2 weeks of progressive dyspnea, abdominal distention, and bilateral lower extremity edema after running out of his prescribed medications. Three months earlier, the patient was on vacation with his family in Hawaii where he experienced cough, subjective fevers, and nasal congestion. Shortly thereafter, he developed dyspnea, lower extremity edema, and abdominal distension. He presented to an emergency department in Hawaii where he was presumptively diagnosed with heart failure due to myocarditis, though no confirmatory cardiac magnetic resonance imaging (cMRI) or endomyocardial biopsy (EMB) was obtained. He was discharged home on guideline-directed medical therapy (GDMT) and a diuretic.
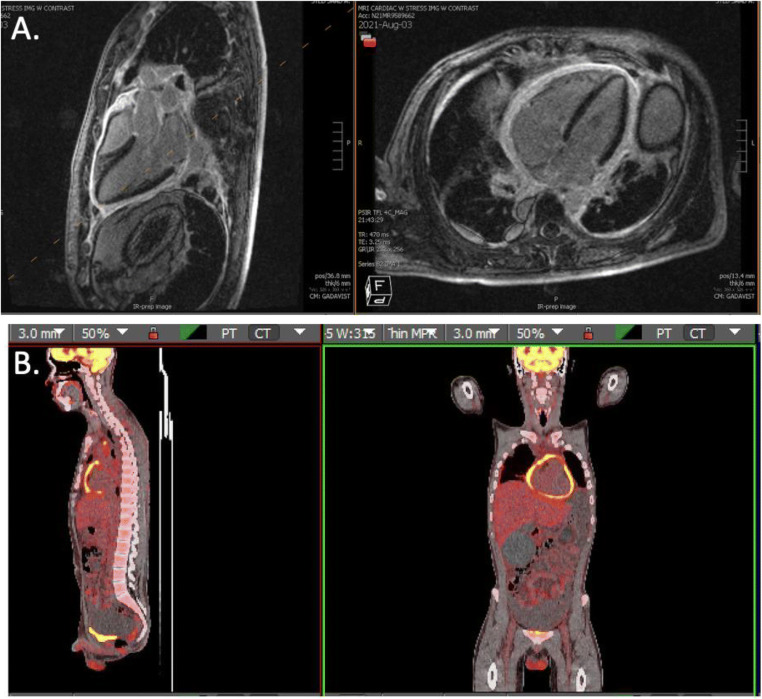
At the time of his subsequent presentation, he did not endorse cough, sputum production, fever, chills, or night sweats. Initial vital signs were notable for heart rate 111 bpm, blood pressure 116/94 mmHg, respiratory rate 20/min, and oxygen saturation of 98% on room air. Clinical examination demonstrated lower extremity edema, abdominal distention, and elevated jugular venous pressure confirming the diagnosis of heart failure. Evaluation with transthoracic echocardiogram demonstrated a reduced left ventricular ejection fraction (LVEF) of 25%, small pericardial effusion, septal bounce, and significant respiratory flow reversal across the mitral valve concerning for constrictive pericarditis. Computerized tomography (CT) of the chest revealed enlarged anterior mediastinal lymph nodes along with a complex, dense pericardial effusion. Subsequent cMRI demonstrated mild-to-moderate pericardial thickening with diffuse enhancement and interventricular dependence consistent with a diagnosis of constrictive pericarditis (Fig. 1). At this time, a QuantiFERON gold obtained on admission returned positive.
Figure 1.
A Cardiac MRI demonstrating pericardial thickening with diffuse enhancement throughout the pericardium. B 18F-FDG PET CT demonstrating hypermetabolic circumferential pericardial thickening, consistent with actively inflamed constrictive pericarditis.
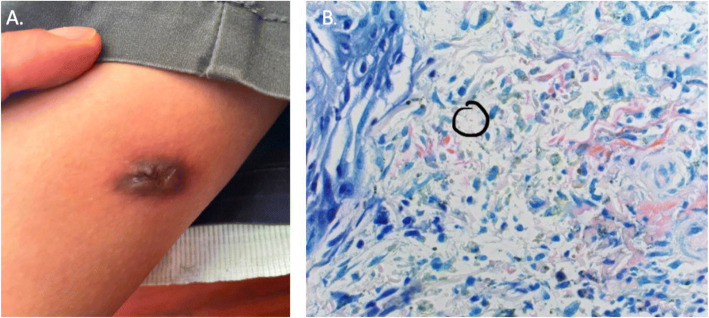
Further history revealed that he had been born in Vietnam, emigrated to the US as a child, and had traveled back to Vietnam three years prior. While travelling in the country, he visited and resided in rural areas. He had never previously been diagnosed with TB and had no known contact with anyone undergoing treatment for TB. Sputum acid-fast bacilli (AFB) cultures were obtained without organism growth. Transbronchial biopsy of the enlarged anterior mediastinal lymph nodes demonstrated non-caseating granulomas; however, AFB cultures did not grow and AFB stain did not demonstrate organisms. Bronchoalveolar lavage and paracentesis of abdominal ascitic fluid were performed, on which TB polymerase chain reaction (PCR) tests were negative and AFB cultures did not grow from either sample. Detailed re-examination of the patient demonstrated a right medial thigh plaque with central ulceration (Fig. 2). The thigh plaque had been present for one year prior to presentation and had been progressively increasing in size. Skin biopsy of the plaque revealed suppurative granulomatous inflammation along with a rod-shaped organism on Fite’s stain (Fig. 2). Given his epidemiological risk factors, positive QuantiFERON, and skin biopsy suggestive of a TB gumma, he was diagnosed with disseminated TB complicated by constrictive pericarditis. Anti-TB medications (rifampicin, isoniazid, pyrazinamide, ethambutol) were prescribed. Two weeks after discharge, AFB culture from the skin biopsy isolated Mycobacterium tuberculosis. The Department of Health was notified, and contact tracing was performed.
Figure 2.
A Medial right thigh gumma that had been increasing in size over the 6 months prior to presentation. AFB cultures obtained from the lesion would later grow M. tuberculosis. B Fite’s stain performed on the skin biopsy demonstrating a positive rod-shaped organism.
After discharge, the patient’s reduced ejection fraction was further investigated with an 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET CT) scan to assess for myocardial inflammation. Hypermetabolic circumferential pericardial thickening consistent with active inflammation within the pericardium was seen; however, no active inflammation within the myocardium was detected (Fig. 1). Given there was no myocardial inflammation seen on 18F-FDG PET CT or myocardial fibrosis identified on cMRI, myocarditis or other inflammatory cardiomyopathies were ruled out. Repeat outpatient echocardiogram demonstrated continued constrictive pericarditis, but the LVEF improved to 35%. The patient is tolerating anti-TB medications well to date. GDMT and oral prednisone have since been prescribed to treat heart failure and constrictive pericarditis, respectively. He is currently symptom-free from a heart failure standpoint.
DISCUSSION
This case highlights two important concepts regarding disseminated TB infection: pulmonary findings are not a prerequisite for the diagnosis of disseminated TB, and a high index of suspicion for TB should be present for any returning traveler from an endemic area. Though the incidence of TB in the USA has decreased over the last several decades, the percentage of US cases that occur among foreign-born persons is increasing.3 In 2020, 71% of all TB cases in the USA were from foreign-born individuals who had emigrated from countries with high rates of endemic TB.4 In concert with this, disseminated TB is more frequent in patients from low-income countries, with the most common extrapulmonary sites for TB being lymphatic (36.9%), pleural (16.9%), bone or joint (9.6%), and peritoneal (5.8%) involvement.5 Approximately 2% of all TB diagnoses consist of extra-pulmonary TB and up to 20% of these extrapulmonary TB cases have no evidence of pulmonary involvement.6
Constrictive pericarditis is one of the more severe sequelae of extra-pulmonary TB and is present in 30–60% of patients diagnosed with TB pericarditis.7,8 The presentation of constrictive pericarditis due to TB is often variable and non-specific. The most common symptoms are cough, chest pain, and dyspnea. Some patients may also experience symptoms classically associated with TB such as night sweats, weight loss, fatigue, and fever.9 Depending on the degree of constriction, patients may present insidiously with heart failure or acutely with cardiac tamponade.10 TB pericarditis was previously believed to be a paucibacillary condition with symptoms stemming from an exaggerated immune response; however, high burdens of TB bacilli have been found in pericardial fluid, suggesting that the pericarditis is likely due to direct infection.11 TB most commonly enters the pericardium by hematogenous dissemination, but rarely can be inoculated directly via contiguous spread from nearby infected tissue such as the lungs.10
The diagnosis of TB pericarditis remains challenging. While most sites of both pulmonary and extrapulmonary TB infection have well-established testing methods, such as tissue or fluid microscopy, AFB smear, culture, and PCR, these methods have poor yield for TB pericarditis.12 Pericardial fluid AFB cultures have a sensitivity of 53–75%; despite this, pericardiocentesis is still recommended in all patients with TB and pericardial effusions >1 cm.13 In our patient’s case, the pericardial effusion was too small to safely perform pericardiocentesis. Investigative biopsy of the pericardium is reserved for difficult cases when the aforementioned tests are unrevealing or pericardiocentesis is not feasible. However, pericardial biopsy is rarely performed due to its invasive and hazardous nature. Our patient’s hemodynamics were precarious throughout his admission with borderline hypotension and persistent tachycardia. A multidisciplinary team including cardiology and cardiothoracic surgery determined pericardiectomy during this admission would be too high risk and elected to pursue conservative management instead. Though a definitive diagnosis can only be made with the demonstration of bacilli in the pericardial fluid or from a sample of pericardium itself, a “probable” diagnosis can be made when there is evidence of TB elsewhere in a patient with unexplained pericarditis.8 The confirmation of cutaneous TB in our patient led to the presumed diagnosis of TB pericarditis. This was further supported by the active pericardial inflammation seen on 18F-FDG PET CT and the improvement in his heart failure symptoms with initiation of anti-TB therapy.
Cutaneous TB is rare and is present in only 1–1.5% of extrapulmonary tuberculosis cases.14 TB has a wide array of cutaneous manifestations that can masquerade as a multitude of other diagnoses. Cutaneous TB can be classified broadly into two distinct categories: paucibacillary tuberculids and true cutaneous TB.15,16 Tuberculids are a hypersensitivity reaction to M. tuberculosis that occur in patients with a moderate-to-high level of immunity against the organism.17 Tuberculids are characterized by granulomatous inflammation on histopathology with the absence of M. tuberculosis on tissue stain or culture, while true cutaneous TB will identify organisms on stain or culture.18 True cutaneous tuberculosis can be further subdivided into 3 categories based on the mode of infection: exogenous inoculation, contiguous, or hematogenous spread.17 Hematogenous spread from the primary infection can lead to the development of metastatic TB abscesses, also known as gummas. TB gummas typically present as nontender, fluctuant, subcutaneous nodules that eventually penetrate the skin and result in the formation of an ulcer or draining sinus. TB gummas usually occur in children or immunosuppressed adults.18 In an immunocompetent host, gummas are usually an isolated single lesion—such as the singular right thigh gumma that was seen in our patient. The diagnosis is made by the presence of the bacillus on culture, smear, or biopsy of the lesion, as opposed to the absence of organisms in tuberculids.18 Identifying an organism with AFB stain is often difficult given its low sensitivity. The complexity is even more pronounced in the setting of extrapulmonary TB where there may be minimal organism burden. Though Fite’s stain is more commonly used to detect other mycobacteria such as Mycobacterium leprae, it has been suggested that Fite’s stain may be more sensitive than the traditional Ziehl-Neelsen AFB stain for M. tuberculosis, and was beneficial in identifying the causal organism in this case.19
Treatment of disseminated TB is typically with the standard four-drug anti-TB regimen (rifampicin, isoniazid, pyrazinamide, and ethambutol) for a minimum of 6 months, unless the organism is known or strongly suspected to be resistant to first-line therapies.20 Prior studies have demonstrated poor penetration of TB drugs into the pericardium.21 Despite this, the standard anti-TB regimen is still globally accepted as the standard of care. Recent evidence has shown that up to 75% of patients diagnosed with TB constrictive pericarditis had resolution of their pericardial constriction with anti-TB medications alone. This benefit persisted at follow-up 10 years later,22 suggesting that pericardiectomy at the time of diagnosis may not be necessary. It is unclear if steroids add benefit to the treatment of TB constrictive pericarditis. In a randomized control trial of 143 patients with TB constrictive pericarditis, the addition of oral steroids to anti-TB therapy reduced the need for pericardiectomy (relative risk, 0.66; confidence interval 95%, 0.33–1.29; P = 0.29); however, 20% of patients were excluded from the analysis due to non-compliance or loss to follow-up, and the result was non-significant.23 Given the ongoing inflammation on our patient’s 18FDG-PET CT as well as a continued constriction on repeat echocardiogram, he was started on oral prednisone.
CONCLUSIONS
This case highlights the importance of recognizing disseminated TB with two a typical extra-pulmonary manifestations. In the case presented, the diagnosis was raised by a positive QuantiFERON test, became more evident with a thorough travel history and physical exam, and was confirmed by histopathologic stain of a TB gumma. A high index of suspicion should be maintained for the diagnosis of TB in travelers returning from endemic regions, even in absence of pulmonary symptoms. Once concern for TB is raised, communication with histopathologists is critical to allow proper diagnosis utilizing specialized stains, such as Fite’s stain, on biopsied lesions. Given the difficulty in obtaining definitive histopathologic diagnosis in extrapulmonary TB and the beneficial impact of early treatment, it is not unreasonable to trial anti-TB therapy when clinical suspicion is high and pathology is negative or equivocal. This case also highlights the use of 18FDG-PET CT and cMRI to evaluate myocardial involvement of TB when pericardial and myocardial biopsy are too high risk to safely obtain.
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. Aids. 2015;29:1987–2002. doi: 10.1097/QAD.0000000000000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 2018. https://www.cdc.gov/tb/statistics/reports/2018/national_data.htm. Accessed 2022.
- 3.Scott C, Kirking HL, Jeffries C, Price SF, Pratt R. Tuberculosis trends--United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:265–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Deutsch-Feldman M, Pratt RH, Price SF, Tsang CA, Self JL. Tuberculosis - United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:409–14. doi: 10.15585/mmwr.mm7012a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72:1761–8. [PubMed] [Google Scholar]
- 6.Sharma SK, Mohan A, Sharma A, Mitra DK. Miliary tuberculosis: new insights into an old disease. Lancet Infect Dis. 2005;5:415–30. doi: 10.1016/S1473-3099(05)70163-8. [DOI] [PubMed] [Google Scholar]
- 7.Schrire V. Experience with pericarditis at Groote Schuur Hospital, Cape Town-an analysis of one hundred and sixty cases studied over a six-year period. S Afr Med J. 1959;33:810–7. [PubMed] [Google Scholar]
- 8.Sagristà-Sauleda J, Permanyer-Miralda G, Soler-Soler J. Tuberculous pericarditis: Ten year experience with a prospective protocol for diagnosis and treatment. J Am Coll Cardiol. 1988;11:724–8. doi: 10.1016/0735-1097(88)90203-3. [DOI] [PubMed] [Google Scholar]
- 9.Lin HC, Lu CW, Lin MW, et al. Tuberculous Pericarditis. Circulation. 2015;132:1154–6. doi: 10.1161/CIRCULATIONAHA.115.015311. [DOI] [PubMed] [Google Scholar]
- 10.Isiguzo G, Du Bruyn E, Howlett P, Ntsekhe M. Diagnosis and Management of Tuberculous Pericarditis: What Is New? Curr Cardiol Rep. 2020;22:2. doi: 10.1007/s11886-020-1254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasipanodya JG, Mubanga M, Ntsekhe M, et al. Tuberculous Pericarditis is Multibacillary and Bacterial Burden Drives High Mortality. EBioMedicine. 2015;2:1634–9. doi: 10.1016/j.ebiom.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee VY, Wong JT, Fan HC, Yeung VT. Tuberculous pericarditis presenting as massive haemorrhagic pericardial effusion. BMJ Case Rep. 2012;2012:bcr0320125967. doi: 10.1136/bcr.03.2012.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler Y, Charron P, Imazio M, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) G Ital Cardiol (Rome). 2015;16:702–38. doi: 10.1714/2088.22592. [DOI] [PubMed] [Google Scholar]
- 14.Khadka P, Koirala S, Thapaliya J. Cutaneous Tuberculosis: Clinicopathologic Arrays and Diagnostic Challenges. Dermatol Res Pract. 2018;2018:7201973. doi: 10.1155/2018/7201973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, Chen W, Hao F. Cutaneous tuberculosis: A great imitator. Clin Dermatol. 2019;37:192–9. doi: 10.1016/j.clindermatol.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Brown AE, Ibraheim MK, Petersen E, Swaby MG, Pinney SS. An evolving presentation of cutaneous tuberculosis. Dermatol Online J. 2020;26:13030/qt55d3f43c. [PubMed] [Google Scholar]
- 17.Bravo FG, Gotuzzo E. Cutaneous tuberculosis. Clin Dermatol. 2007;25:173–80. doi: 10.1016/j.clindermatol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Barbagallo J, Tager P, Ingleton R, Hirsch RJ, Weinberg JM. Cutaneous tuberculosis: diagnosis and treatment. Am J Clin Dermatol. 2002;3:319–28. doi: 10.2165/00128071-200203050-00004. [DOI] [PubMed] [Google Scholar]
- 19.Fukunaga H, Murakami T, Gondo T, Sugi K, Ishihara T. Sensitivity of acid-fast staining for Mycobacterium tuberculosis in formalin-fixed tissue. Am J Respir Crit Care Med. 2002;166:994–7. doi: 10.1164/rccm.2111028. [DOI] [PubMed] [Google Scholar]
- 20.Naicker K, Ntsekhe M. Tuberculous pericardial disease: a focused update on diagnosis, therapy and prevention of complications. Cardiovasc Diagn Ther. 2020;10:289–95. doi: 10.21037/cdt.2019.09.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shenje J, Ifeoma Adimora-Nweke F, Ross IL, et al. Poor Penetration of Antibiotics Into Pericardium in Pericardial Tuberculosis. EBioMedicine. 2015;2:1640–9. doi: 10.1016/j.ebiom.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strang JIG, Nunn AJ, Johnson DA, Casbard A, Gibson DG, Girling DJ. Management of tuberculous constrictive pericarditis and tuberculous pericardial effusion in Transkei: results at 10 years follow-up. QJM: Int J Med. 2004;97:525–35. doi: 10.1093/qjmed/hch086. [DOI] [PubMed] [Google Scholar]
- 23.Strang J, Gibson D, Nunn A, Kakaza H, Girling D, Fox W. Controlled trial of prednisolone as adjuvant in treatment of tuberculous constrictive pericarditis in Transkei. Lancet. 1987;330:1418–22. doi: 10.1016/S0140-6736(87)91127-5. [DOI] [PubMed] [Google Scholar]