Abstract
Human language, a signature of our species, derives its power from its links to human cognition. For centuries, scholars have been captivated by the link between language and cognition. In this chapter, we shift this focus. Adopting a developmental lens, our goal is to identify the origin of this link and how it unfolds in human infants. We identify joint contributions of infants’ innate capacities and their exquisite sensitivity to experience. By 3 months of age, infants have begun to link language and cognition. Initially this link is broad, encompassing the vocalizations of both humans and nonhuman primates. Within months, infants tune this link specifically to human vocalizations. Moreover, we identify two routes by which infants establish this link. Tracing the language-cognition link in infancy reveals a dynamic cascade of increasingly precise links, each providing the foundation for the next, and offers insights into its ontogenetic and phylogenetic origins.
Keywords: infancy, language acquisition, conceptual development, categorization, developmental plasticity, developmental tuning
INTRODUCTION
Language is a signature of our species and our most powerful cultural and cognitive tool. The power of language derives not from the exquisite detail of its signals or the precision of its grammatical rules, but from its intricate and inextricable link to human cognition. This link, unparalleled elsewhere in the animal kingdom, serves as the conduit through which we share with others the contents of our minds. It enables us to move beyond the exigencies of the “here and now,” to represent the past and the future, to build upon one another’s knowledge and beliefs, and to consider different perspectives on the very same phenomena. Through human language, we can essentially “hijack” one another’s minds, working collectively to invent history and time, to promote religious beliefs and scientific theories, and to create the arts.
George Miller (1990), a father of cognitive psychology, described this uniquely human link eloquently:
Human language is the happy result of bringing together two systems that all higher organisms must have: a representational system and a communication system… A representational system is necessary if an organism is going to move around purposefully in its environment; a communication system is necessary if an organism is going to interact with others of its own kind. Presumably, some of the historical disagreements over the importance of language for our understanding of human cognition arose because different protagonists identified language with different parts of this combination. It is certainly true that human beings are not the only animals capable of a complex representational intelligence, nor are they the only animals that communicate. But human beings do seem to be the only animals in which a single system serves both of these functions (p. 12).
Questions concerning this relation between human language and cognition have long captivated attention within all fields currently considered under the umbrella of “cognitive science” (Fodor 1975, Gleitman & Papafragou 2005, Whorf 1956). Decades, if not centuries, of lively debate have illuminated several issues at the crossroads of human language and cognition, but until recently a more fundamental question has been left in the shadows: How do infants begin to forge a link between language and cognition in the first place?
In this chapter, our goal is to shed light on the developmental origins of this uniquely human link and to trace how it unfolds in infancy. Focusing primarily (but not exclusively) on the first year of life, well before infants can say their own names, we peel back the layers to reveal the foundation of this uniquely human interface. We propose that shifting the focus to infants changes the questions we ask. The question is no longer whether language and cognition are linked, but what initial capacities (if any) support infants as they first forge this link and how these capacities are then sculpted by the forces of maturation and experience.
Three recurring themes
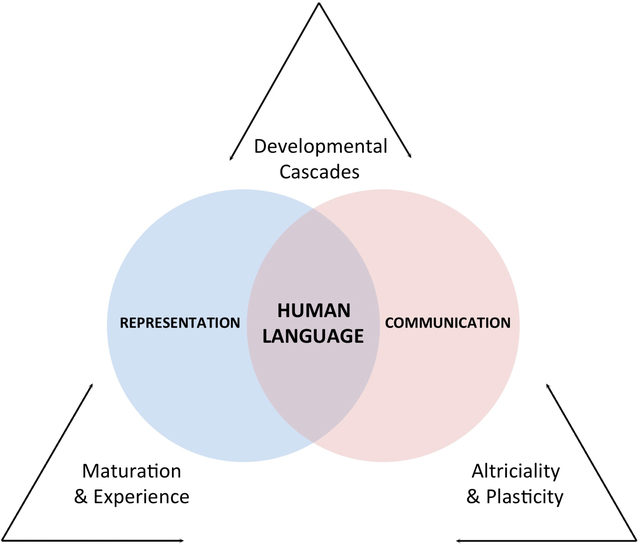
Three interrelated themes, central to the developmental sciences, are woven throughout (Figure 1). These themes, built on an assumption that human infants are endowed with an innate capacity to acquire human language (Chomsky 1986), go on to ask how the process of language acquisition unfolds. The first theme concerns the joint, bidirectional contributions of maturation and experience. We examine how these twin engines of human development guide infants to establish increasingly precise links between language and cognition. The second theme amplifies the view that human development is best characterized as a series of cascading effects that occur over developmental time, with each point along the developmental continuum setting constraints upon the unfolding of the next (Werker & Hensch 2014). The third theme, articulated below, highlights the importance of two other signatures of our species—our altricial status at birth and our developmental plasticity.
Figure 1.
Human language occupies the intersection of our systems for representation and communication. Interactions between “nature and nurture”—reflected in the sculpting forces of maturation and experience and in the synergy between altriciality and plasticity—provide our foundation for language. Developmental cascades, in turn, characterize our acquisition of language.
Human infants are born altricial—neurally and behaviorally immature—compared to our closest evolutionary relatives. For example, the human newborn’s brain is less than 30% the size of an adult’s, a ratio that is substantially lower than that observed in other primate species (DeSilva & Lesnik 2006). Moreover, while infants of other species are endowed at birth with the behavioral tools to maintain proximity with their caregivers, either by walking alongside them or clinging to their fur, human infants have no such endowment. This altriciality brings with it a developmental imperative: It falls to human caregivers to keep their infants in close contact, and to do so for an extraordinarily protracted period. But this altriciality also unlocks an incomparable degree of developmental plasticity (for review, see Kuzawa & Bragg 2012)—exquisite sensitivity to postnatal experience—that in turn enables us to link our minds together in a way that no other species can. Human infants’ exceptionally long period of dependency, coupled with their innate capacity to learn a human language and exquisite sensitivity to postnatal experience, is “advantageous for a species whose major specialization is its capacity for learning and whose basic invention is culture” (Gleitman et al. 2010, p. 588.) And, we add, a species whose signature is the link between language and cognition.
The advantages of adopting a developmental lens
Approaching the relation between language and cognition from a developmental lens is especially compelling. Although an infant from a remote village in the Chaco rain forest and an infant from an urban Chicago neighborhood grow up amidst objects and events that the other has never seen, immersed in daily practices that the other has not witnessed, and listening to language that the other cannot understand, there are strong convergences in their acquisition of fundamental conceptual and linguistic capacities (Schieffelin & Ochs 1986, Waxman & Lidz 2006). Within their first year, infants across the world’s communities spontaneously form concepts that capture commonalities across the diverse objects they encounter, and they use their representations of these concepts as a basis for learning about new objects. Also within their first year, infants across the world’s communities begin to acquire language. Moreover, infants’ early linguistic and conceptual advances do not proceed independently, but are instead powerfully and implicitly linked (for reviews, see Ferguson & Waxman, 2016a, Swingley, 2012).
This precocious link, remarkable in its own right, raises a new question: What is the developmental origin of this link and how does it unfold? Adopting a distinctly developmental approach to this question reveals that this link unfolds dynamically beginning in the first months of life. Uncovering these developmental changes—as well as the factors that drive them—has major implications for advancing our understanding of the developmental and evolutionary origins of human language.
WORDS AS INVITATIONS TO FORM CATEGORIES: EVIDENCE FROM 6- TO 24-MONTH-OLD INFANTS
Most of the research investigating the dawning of this language-cognition link has focused on word learning. This seemingly straightforward achievement stands at the intersection between linguistic and conceptual development. After all, to learn the meaning of a word, learners must identify a portion of the ongoing stream of speech (a “problem” for the communication system to solve), must identify a referent for that word (a “problem” for the representational system to solve), and must establish a mapping between them. Most of the word learning research with infants and young children has, in turn, focused primarily on one cognitive capacity in particular—object categorization—because it is a building block of cognition (for review, see Waxman, 1998).
An object category (e.g., animal) is a mental representation that honors what is common among a set of distinct objects (e.g., a dog, horse, duck). The focus on object categorization in developmental work stems from two sources. First, from a cognitive vantage point, categorization is a core conceptual capacity with far-reaching impact on virtually all aspects of learning and cognition. Categorization supports memory and reasoning, guiding our predictions about the likely behaviors and properties of new objects—even those we have yet to encounter (Gelman 2004, Medin & Ortony 1989, Waxman & Gelman 2009). Second, from a linguistic vantage point, the words that children learn first are predominately names of objects, including not only key individuals in their lives (e.g., mama, dada), but also objects and object categories (e.g., doggie, bottle) (for discussion about the universality of this early predominance of nouns, see Chan et al. 2011, Tardif et al. 2008, Waxman et al. 2013). Roger Brown, the father of the modern study of child language and a contemporary of George Miller, famously argued that “words are invitations to form categories” (1958). In articulating this point of view, Brown’s sights were set on preschool-aged children. But more recent evidence reveals that words invite infants to form categories even before they say their first words.
A foundational experimental paradigm
The developmental evidence for the earliest links between language and cognition comes from a robust behavioral paradigm, elegant in its simplicity. It is essentially an object categorization task with two phases. During the familiarization phase, infants view a series of discriminably different objects (e.g., dog, horse, duck) from a given object category (e.g., animal). Next, during the test phase, infants view two new objects—one a member of the now-familiar category (e.g., a cat) and the other a member of an entirely different category (e.g., an apple). The two test objects are presented side-by-side in silence. Infants’ looking time during the test phase serves as an index of categorization. The logic of this task is straightforward: If infants detect the category-based commonalities among the familiarization objects, then they should distinguish the novel test image from the familiar; if they fail to detect these category-based commonalities, then they should perform at chance (Aslin 2007, Colombo 2002, Golinkoff et al. 1987). A key feature of this paradigm is that it permits researchers to hold constant the objects infants view while manipulating, systematically, the amount and kind of auditory information that infants hear. As a result, this design can elucidate whether and how infants’ ability to form an object category is influenced by the amount and kind of auditory information with which the objects have been paired (for review, see Waxman & Lidz 2009).
Links between language and cognition in 6- to 12-month-old infants
This paradigm has yielded considerable evidence for the power of language on infants’ object categorization. In the first demonstration of its kind, Waxman and Markow (1995) introduced 12-month-old infants to a series of objects from a single category during a familiarization phase; at test infants viewed a new member of the now-familiar category and a member of an entirely novel category. When each familiarization object was introduced in conjunction with the same novel word embedded in a naming phrase (e.g., “Look at the blick”), infants successfully formed the object category, exhibiting a reliable preference for the novel test object. But when precisely the same familiarization objects were presented under different auditory conditions, infants failed to form object categories. First, the presence of a novel word is instrumental: When infants are directed to the same familiarization objects but with no novel word (e.g., “Look at that!”), they fail to form categories (Waxman & Markow 1995). Second, the consistency with which novel words is applied is also instrumental: When the same novel word is consistently applied to all familiarization objects, infants successfully form categories, but when different novel words are applied to each familiarization object (e.g., “Look at the blick!; Look at the toma!; Look at the modi!”), they fail (Ferguson et al. 2015, Waxman & Braun 2005, Waxman & Markow 1995, Xu 2002). Third, for infants as young as 6 months, the link to categorization is specific to language: Other signals, even if they are consistently applied to the familiarization objects, fail to promote object categorization. For example, when the familiarization objects were paired with sine-wave tone sequences, matched precisely to the naming condition for mean pitch, amplitude, duration, and pause length, infants failed to form categories (Balaban & Waxman 1997, Fulkerson & Waxman 2007).
Thus, by 12 months, infants are well on their way to linking language to the core cognitive process of object categorization. They successfully cull novel words from the ongoing stream of speech, track whether the same (or different) words have been applied to a set of objects, and expect that objects named by the same word (but not those named by different words) are members of the same object category. Notice that because this link is in place by 12 months, it cannot be a result of word learning, as some have claimed (Smith et al. 1996, 2003); rather, it is available to very young infants in the service of building their earliest lexicon (Booth & Waxman 2002, Booth et al. 2005, Waxman & Booth 2003). Notice, too, that this link cannot be accounted for by appealing to simple associative mechanisms alone (Waxman & Gelman 2009). For example, in the paradigm featured here, tone sequences (and other signals) were paired just as precisely with the familiarization objects as were novel labels. Yet only in the context of hearing consistently applied novel labels do infants successfully form object categories.
The claim here is not that an infant’s ability to form an object category depends entirely on listening to language. This is clearly false: Preverbal infants and nonhuman animals successfully form object categories, even in the absence of listening to language (for review, see Mareschal & Quinn 2001). Instead, we offer an interpretation that is both more measured and more precise: Even before infants produce words on their own, words are invitations to form categories, effectively highlighting commonalities among objects that may otherwise have gone unnoticed.
The conceptual consequences of naming at 12 months are far-reaching. First, the power of novel labels extends beyond the particular objects that have been named. Labels presented during a familiarization phase influence infants’ attention to the new, and as-yet unnamed, objects presented at test. Second, naming not only influences whether infants form an object category, but it also guides them to identify the location of category boundaries for objects that fall along a perceptual continuum. For example, like adults, when infants view a series of individuals that fall along a perceptual continuum, they can either construe the individuals as members of a single overarching category or as two or more distinct categories. If the same name is applied to individuals along the continuum, then infants form a single category. But if one name is applied to individuals at one end of the continuum and a different name applied to individuals at the other end, then infants form two categories, using the distinct names to demarcate the category boundary (Graham et al. 2013, Havy & Waxman 2016, Westermann & Mareschal 2014). Third, the relation between naming and object categories is bi-directional. Infants more successfully discriminate minimal language pairs (e.g., bin vs din) if each is presented as a name for a perceptually different object (Werker & Yeung 2005).
Summarizing to this point, the evidence shows that even before infants begin to produce words on their own, they are guided by an expectation for linking novel words to commonalities among objects. This expectation, however skeletal at the start, is important because it supports infants’ establishment of their first words. It also supports fundamental conceptual and logical capacities, including notions of categories and individuals. Finally, this expectation sets the stage for infants’ subsequent discovery of more precise links between language and cognition.
More precise links between language and cognition emerge in 12- to 24-month-old infants
Human language, of course, involves more than naming objects and object categories. A fundamental feature of human language is that it is comprised of distinct kinds of words (or grammatical forms; e.g., noun, adjective, verb) that can be linked to distinct kinds of meaning (e.g., categories of objects, properties, and events, respectively). Over the second year of life, infants establish increasingly more precise links that reflect this feature of language, and these unfold in a cascading fashion (for reviews, see Ferguson & Waxman 2016a, Waxman & Lidz 2006).
For infants in their first year, any consistently applied novel word, whether it is presented as a noun (e.g., “Look at the blick!”) or an adjective (“Look at the blick one!”), highlights a wide range of commonalities, including those based on object categories (e.g., a green dog and a red horse) and on object properties like color or texture (e.g., a green dog and a green chair). At roughly 13 months, more precise mappings begin to emerge. First, infants tease apart the nouns from the other grammatical forms and link them specifically to categories of objects (e.g., dog), but not to properties of objects (e.g., green) (Waxman 1999). To forge this more precise noun-object category link, infants must attend not only to novel words themselves, but also to their position relative to other linguistic elements in the surrounding sentence (e.g., determiners, arguments). With this noun-category link in place, infants go on to establish more precise links for the predicates, linking novel words presented as adjectives primarily to properties of objects (e.g., color, texture) (Booth & Waxman 2009) and novel words presented as verbs primarily to events and relations among objects (Waxman et al. 2009). Thus, over the second year, infants tease apart distinct kinds of words (e.g., nouns, adjectives, verbs) and expect that each will map to a distinct kind of meaning.
Because infants’ increasingly precise links unfold in a cascading fashion, the conceptual consequences of naming will vary systematically as a function of development. By 6 months, listening to language itself promotes object categorization. Until roughly 12 months, any kind of novel word directs infants’ attention to any kind of commonality among objects. Beginning at roughly 13 months, novel nouns in particular focus infants on category-based commonalities; novel adjectives and verbs likewise direct infants’ attention to different kinds of relations. Thus, the signal value of listening to a novel word changes rapidly over development, depending upon an infant’s position in the developmental cascade.
Although infants’ increasing precision in linking language and cognition is impressive, it cannot answer our more fundamental question: How does a link between language and cognition emerge in the first place? What capacities—perhaps innately endowed or evolutionarily specified—are available to infants from the very start?
BORN ALTRICIAL, BUT NOT “BLANK SLATES”: EARLY PERCEPTION AND TUNING OF COMMUNICATIVE SIGNALS
Human infants are certainly born altricial, but they are just as certainly not born as “blank slates.” Instead, within hours of their birth, infants demonstrate strong perceptual preferences and perceptual discriminatory capacities. Most importantly, although newborns are exposed to a wide range of sights and sounds, they prefer certain signals to others. These preferences—evident in auditory, visual, and cross-modal perception—are initially broad and then rapidly tuned on the basis of infants’ postnatal experience in the domain-general developmental process known as perceptual narrowing (for reviews, see Maurer & Werker 2014, Scott et al. 2007).
Perceptual narrowing
At birth, infants’ perceptual preferences and capacities in the auditory domain are broad. Newborns equally prefer listening to human and nonhuman vocalizations over other sounds (Vouloumanos et al. 2010), and they can distinguish among sounds across human languages (Werker & Tees 1984) and nonhuman primate vocalizations (Friendly et al. 2013a). Within their first months, infants’ broad preferences narrow to specifically human language (Vouloumanos et al. 2010). Similarly, within their first year, their ability to discriminate among sounds within their native language becomes sharpened, while their discriminatory capacities for nonnative languages and nonhuman primate vocalizations become concomitantly decreased (Friendly et al. 2013a, Werker & Tees 1984). These perceptual narrowing processes, well-documented in infants’ behavioral preferences and discriminatory capacities, are also evident in their neural activity (Kuhl & Rivera-Gaxiola 2008, Shultz et al. 2014). Moreover, the same path of perceptual narrowing is also evident in infants’ behavioral and neural responses to faces and to intermodal correspondences (for review, see Maurer & Werker 2014). Homing in on voices, faces, and the correspondences between familiar voices and faces results in the integration of multisensory systems (Lewkowicz & Ghazanfar 2009), which further enhances infants’ language perception and their ability to identify individuals by their voices and faces.
Across diverse perceptual systems, then, infants’ initially broad preferences and discriminatory capacities become increasingly specialized for processing certain signals, chief among them the voices and faces of members of their own communities. Tuning processes like these increase the signal-to-noise ratio for the communicative signals of their communities, and because they proceed with no conscious effort on the part of the learner (Kraus & Slater 2016), infants can establish increasingly precise, efficient, and integrated systems across perceptual modalities even before they begin to speak. Two factors—infants’ postnatal experience and their maturational status—mutually interact in the process of perceptual narrowing.
Experience guides perceptual narrowing
Evidence for perceptual narrowing showcases the dynamic interplay between infants’ impressive perceptual abilities at birth and their exquisite sensitivity to postnatal experience. Especially compelling evidence for the force of experience comes from “exposure studies.” By manipulating infants’ postnatal experience, these studies reveal that when infants are provided with sufficient exposure at key developmental junctures, infants can maintain or reinstate their developmentally prior, broad perceptual abilities. For instance, a classic experiment by Werker and Tees (1984) demonstrated that the language to which infants are exposed shapes their sensitivity to the sounds of that language. Similarly, in the visual domain, Bar-Haim et al. (2006) showed that the ethnicity of the faces to which infants are exposed shapes their perceptual preference for faces of that ethnicity. Other studies have adopted a more direct approach, systematically manipulating infants’ exposure to various stimuli. For example, providing infants with exposure to nonnative phonemes (Maye et al. 2002), nonnative musical rhythms (Hannon & Trehub 2005), monkey vocalizations (Friendly et al. 2013b), monkey faces (Fair et al. 2012, Pascalis et al. 2005, Scott & Monesson 2009), or other-race faces (Heron-Delaney et al. 2011) enables them to reinstate their developmentally prior sensitivity to distinctions among these signals, even months after they had tuned their perception specifically to signals in their native environment. Combined, this evidence highlights infants’ perceptual plasticity and the powerful effects of exposure at the behavioral and neural levels.
Maturation constrains the effects of experience
Yet infants’ experience is not the only factor guiding their perceptual narrowing. In addition, infants’ maturational status plays an important role. In essence, the effects of experience are tempered by infants’ maturational status. Evidence for this interplay between the relative contributions of maturation and experience comes from investigations comparing the timing of key developmental milestones in fullterm and preterm infants. For example, Peña and her colleagues (Peña et al. 2010, 2012) focused on infants’ increasing attunement to the rhythmic and phonemic properties of their native language. By comparing fullterm and preterm infants’ performance in the same speech perception tasks, these researchers discovered that the shaping role of postnatal exposure is itself constrained by infants’ maturational status. Because preterm infants are born early, they are exposed to language (outside the womb) earlier than are fullterm infants of the same maturational age. However, this additional exposure does not accelerate preterm infants’ pace in tuning to the sounds of their native language.
Comparisons like these between fullterm and preterm infants, which have been instrumental in disentangling the relative contributions of experience and maturation across perceptual domains, paint a nuanced picture. For instance, while some aspects of language tuning, such as rhythmic and phonemic perception, appear to depend upon infants’ maturational status (Peña et al. 2010, 2012), others, such as phonotactic acquisition, appear to emerge primarily in response to infants’ postnatal experience (Gonzalez-Gomez & Nazzi 2012). Findings like these have implications for understanding how developmental advances in one arena might have consequences on subsequent development, and how subtle differences in developmental timing may amplify later differences.
Tuning processes confer human infants with an adaptive edge
Throughout the animal kingdom, tuning processes are ubiquitous (Lorenz 1937). But in humans, the amount of tuning dramatically surpasses that observed in other species (Zangenehpour et al. 2009). Our altricial status, coupled with our prolonged period of dependency and exceptional ability to learn, unlocks an unparalleled degree of developmental plasticity and responsiveness to postnatal exposure. Importantly, this confers an adaptive advantage, ensuring that human infants direct their attention increasingly toward the signals of their species, and especially to those who serve as their communicative and pedagogical partners (Vouloumanos & Waxman 2014, Vouloumanos et al. 2009).
Crucially, however, acquiring a human language requires more than narrowing in on the vocalizations and the faces of its speakers. The power of human language derives from its links to cognition. Thus, infants’ advances in speech perception alone are mute when it comes to identifying whether and when infants first begin to link the sounds of the language they hear to the objects and events they observe in the world.
LANGUAGE EXERTS A HIDDEN FORCE: EARLY LINKS BETWEEN LANGUAGE AND COGNITION
When poet Rita Mae Brown wrote, “Language exerts a hidden force, like the moon on the tides,” she captured the power of language on cognition—especially in infants’ first months.
Infants link language and cognition by 3 months
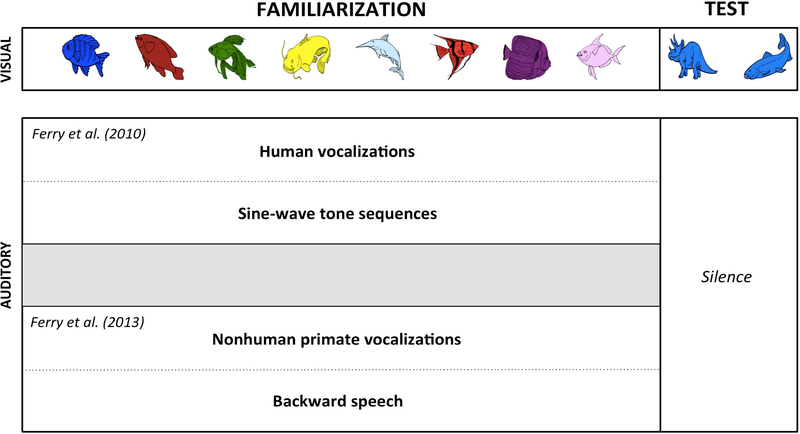
To discover when infants first forge a link between language and cognition, and to trace how this link unfolds, researchers have harnessed the foundational categorization task described earlier. To accommodate infants as young as 3 months of age, Ferry et al. (2010) adapted a version of the task (Figure 2) used previously with infants at 11 (Balaban & Waxman 1997) and 6 months of age (Fulkerson & Waxman 2007). During familiarization, the images (here, different fish) were presented sequentially. During test, two new images were presented in silence: one a new member of the now-familiar category (here, another fish) and the other a member of a novel category (here, a dinosaur). What varied across infants was the type of auditory information they heard while viewing the familiarization images. For half of the infants, each image was presented in conjunction with human language; for the others, each was accompanied by a sine-wave tone sequence, created to carefully match the language stimuli in mean pitch, duration, and pause length.
Figure 2.
A foundational experimental paradigm, designed to investigate the relation between language and the core cognitive process of object categorization in infants. In this paradigm, infants in all conditions view the same visual materials. What varies is the auditory information that accompanies each familiarization object. Shown here are representative stimuli from Balaban and Waxman (1997), Ferry et al. (2010, 2013) and Fulkerson and Waxman (2007).
The results documented a surprisingly early link between cognition and language. Listening to language boosted object categorization in infants as young as 3 and 4 months of age. In sharp contrast, infants listening to tone sequences failed to form object categories, as in prior experiments (Ferry et al. 2010). Interestingly, infants listening to language revealed a systematic and tightly timed developmental shift: at 3 months infants exhibited a preference for the familiar object (the fish in the example above), but by 4 months they shifted to a preference for the novel object (the dinosaur in the example above)—a preference that infants maintain thereafter (Balaban & Waxman 1997, Fulkerson & Waxman 2007, Waxman & Markow 1995). Shifts like this, from familiarity to novelty preferences, are well-documented in the infancy literature (for review, see Rose et al. 2004). Although the precise mechanism underlying this the shift is not yet fully specified, decades of psychophysical evidence suggest that familiarity-to-novelty shifts likely reflect neural maturation and advances in processing efficiency, and they tend to occur in tasks involving complex stimuli (recall that infants in the current paradigm must integrate an ongoing stream of visual and acoustic information) (for reviews, see Aslin 2007, Colombo 2002, Reynolds & Romano 2016).
Thus, by 3 months, infants are not only tuned to the communicative signals of their partners, but also to a principled and surprisingly early link between these signals and the fundamental cognitive process of categorization. What remained unclear, however, was why listening to language engendered this effect in infants so young. It is unlikely that 3- and 4-month-old infants identified individual words in the ongoing speech stream (Bortfeld et al. 2005, Jusczyk & Aslin 1995, Seidl et al. 2015); it is also unlikely that they understood the meaning of the words and phrases presented (Frank et al. 2016). For these reasons, Ferry et al. (2010) speculated that listening to language may promote categorization in very young infants by engaging basic attentional and behavioral processes, rather than word learning per se. To illustrate this idea, Ferry et al. (2010) drew an analogy from Vergne and Mathevon’s (2008) ethological investigation of the Nile crocodile. Just before they hatch, Nile crocodile hatchlings produce high-pitched vocalizations that can be heard by the mother and neighboring hatchlings in the nest. Vergne and Mathevon (2008) played recordings of hatchling vocalizations to identify what effect, if any, these vocalizations had on mothers and neighboring hatchlings. The effect was clear: Listening to hatchling vocalizations engendered specific behavioral responses, motivating mothers to open their nests and surrounding hatchlings to scratch open their shells. By analogy, for human infants at 3 and 4 months, listening to language may engage infants in attentional and cognitive behaviors that happen to boost object categorization. Over developmental time, as infants begin to cull individual words from fluent speech, to distinguish among individual words and kinds of words, and to map those words to meaning, the effects of language become more refined.
If basic attentional mechanisms can account for the advantageous cognitive effect of listening to language at 3 and 4 months, then might other sounds—beyond human vocalizations—also support their object categorization? Ferry et al. (2013) addressed this question using the same task, this time accompanying each familiarization image with the vocalization of a blue-eyed Madagascar lemur (Eulemur macaco flavifrons) (Figure 2). They selected this vocalization because while it differs from human speech and is entirely unfamiliar to the young participants, its acoustic properties (e.g., duration, mean pitch) fall naturally within the range of infant-directed human speech—a type of speech that infants find especially engaging (for review, see Saint-Georges et al. 2013).
The results were striking. At 3 and 4 months, infants’ responses to lemur vocalizations were identical to their responses to human vocalizations. Listening to both human and nonhuman primate vocalizations supported 3- and 4-month-old infants’ object categorization, and both elicited precisely the same shift from familiarity to novelty preferences. But by 6 months, lemur vocalizations no longer promoted object categorization; infants had tuned their link specifically to human vocalizations. In a control condition, Ferry et al. (2013) ruled out the possibility that the facilitative effects of lemur (and human) vocalizations were merely a consequence of the acoustic complexity of these vocalizations, especially as compared to simple sine-wave tone sequences. Another group of infants participated in the same categorization paradigm, but listened to backward speech (the segment of infant-directed speech from prior experiments, played backwards) in conjunction with each familiarization image. This provided a powerful comparison: Although backward speech and forward speech are identical in their temporal and spectral complexity, backward speech violates fundamental acoustic properties of forward speech (Ramus 2000) and is processed in different brain regions than forward speech from birth (Peña et al. 2003). If the cognitive advantage conferred by lemur (and human) vocalizations stemmed from their acoustic complexity, then backward speech should also promote categorization. But this was not the case, as infants listening to backward speech failed to form object categories, performing at chance at 3, 4, and 6 months of age.
Together, these findings offer several insights. First, a link between human vocalizations and object categorization, evident at 3 months, derives from a broader template that initially encompasses vocalizations of both humans and nonhuman primates. Second, this initially broad template cannot derive solely on the basis of experience. By 3 months, infants have enjoyed rich exposure to language and virtually no exposure to lemur vocalizations, yet both signals confer the same cognitive advantage. Third, by 6 months, infants tune their initially broad link specifically to human vocalizations. This is adaptive: It supports infants as they focus increasingly on the signals (language) that will ultimately constitute the foundations of meaning.
TUNING THE LANGUAGE-COGNITION LINK: JOINT CONTRIBUTIONS OF EXPERIENCE AND MATURATION
What factors support infants as they tune the link between language and cognition? Certainly, both experience and maturation are instrumental for perceptual tuning. But how do maturation and experience guide infants as they tune the link between language and cognition?
Experience guides the developing link between language and cognition
Perszyk and Waxman (2016) reasoned that infants’ rich and consistent exposure to human language (and their paucity of exposure to nonhuman primate vocalizations) might be a guiding force as infants sculpt the increasingly precise link between human language and cognition. If this is the case, then exposing infants to nonhuman primate vocalizations might enable them to reinstate or maintain their initial link between this signal and cognition.
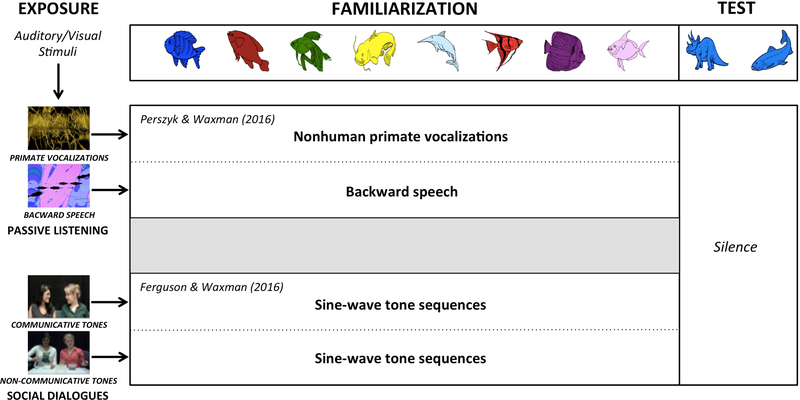
To test this, they designed a paradigm that systematically manipulated young infants’ exposure to lemur vocalizations or backward speech and tested its effect on infants’ cognition. They focused on infants at 6 and 7 months, a developmental point at which infants no longer link lemur vocalizations to cognition (Ferry et al. 2013). Infants participated in the classic categorization task, but this time, immediately before the task, the experimenters provided infants with exposure to either lemur vocalizations or backward speech (Figure 3). To accomplish this, the experimenters created a 10-minute soundtrack of instrumental music (a Mozart quartet). In the lemur soundtrack, eight distinct lemur vocalizations were interspersed into this music at irregular intervals (providing a total of two minutes of exposure to lemur vocalizations); none of the vocalizations in the exposure track were presented later in the categorization task. The backward speech soundtrack was identical, except that the eight lemur vocalizations were replaced with eight distinct segments of backward speech. The instrumental music provided an engaging vehicle for exposing infants to lemur vocalizations or backward speech. Importantly, however, neither the lemur vocalizations nor the backward speech were linked to a communicative exchange of any kind; instead, they were simply part of infants’ ambient acoustic environment.
Figure 3.
Design of exposure studies. In Perszyk and Waxman (2016) (top), infants first passively listened to either two minutes of nonhuman primate vocalizations or backward speech, embedded within a 10-minute soundtrack of classical music, while watching an iTunes visualizer. Immediately after this exposure phase, infants participated in the categorization task with either nonhuman primate vocalizations or backward speech. In Ferguson and Waxman (2016b) (bottom), infants first observed a two-minute social dialogue with sine-wave tone sequences that were either embedded within communicative or a non-communicative context. Immediately after this exposure phase, infants participated in the categorization task with sine-wave tone sequences.
Consider first the effect of providing infants with brief exposure. In one experiment, infants listened to this soundtrack in the lab, just once, immediately before participating in the categorization task. Even this brief exposure to lemur vocalizations had a dramatic effect: In sharp contrast to 6-month-old infants who received no exposure (Ferry et al. 2013), infants who first listened to two minutes of lemur vocalizations in the lab successfully formed the object categories while listening to lemur vocalizations in the subsequent categorization task. This shows that brief exposure to lemur vocalizations enables infants to reinstate their earlier link between this signal and cognition. In contrast, exposure to backward speech had no effect: Infants exposed to backward speech failed to form object categories in the subsequent categorization task. Thus, exposure does not permit infants to create, de novo, a link between an otherwise inert signal and cognition.
In the next experiment, Perszyk and Waxman (2016) delved deeper. Perhaps the effect of brief exposure in the first experiment was nothing more than a fleeting phenomenon, one that permitted infants to reinstate the link between lemur vocalizations and cognition for only a few moments. In other words, perhaps this effect, like a priming effect, is tightly tied to time or place (experience in the laboratory). But perhaps exposing infants to lemur vocalizations (a signal that was part of their initial template) exerts a more profound, longer-lasting effect. To address this possibility, they recruited infants at 4.5 months—when lemur vocalizations still promote categorization (Ferry et al. 2013)—and assigned them to either the lemur or backward speech condition. Parents were instructed to play their infants the musical soundtrack (either with lemur vocalizations or backward speech) at home for a period of 6 weeks, imposing a tightly-controlled exposure paradigm that mirrored the tapering exposure paradigms in the perceptual narrowing literature (cf., Friendly et al. 2013b, Pascalis et al. 2005). After 6 weeks of exposure, infants (who were now 6 months old) and their parents were invited into the lab. At the time of their visit, infants had not listened to their respective soundtracks for days, and they simply participated in the categorization task immediately upon their arrival. The results were clear: Once again, 6-month-old infants listening to lemur vocalizations successfully formed object categories, demonstrating that exposure permits them to maintain their link between this signal and cognition. Thus, exposing infants to lemur vocalizations has a robust and long-lasting effect. In contrast, exposing infants to backward speech has no such effect.
This illustrates that infants’ experience with the signals available in their ambient environment guides them to forge increasingly precise links between the sounds they hear and the core cognitive processes that will ultimately serve as the foundations of meaning. But does experience alone guide this tuning, or might the effects of experience be gated by infants’ maturational status?
Maturation constrains the effects of experience in tuning the link
To answer this question, Perszyk et al. (2016) capitalized upon the precisely timed developmental shift from familiarity (at 3 months) to novelty preferences (at 4 months and thereafter) observed in fullterm infants. To uncouple the effects of experience and maturation, they compared fullterm and preterm infants’ object categorization, asking whether and when preterm infants might also exhibit a familiarity-to-novelty shift in response to listening to language. They recruited healthy “late preterm infants,” born no more than one month before their due dates (Boyle & Boyle 2013). If infants’ postnatal experience listening to language is instrumental in guiding infants’ expression of this early language-cognition link, then preterm infants should shift from familiarity to novelty preferences when they are between 3 and 4 months post birth, just as their fullterm counterparts. If the effect of experience is instead gated by infants’ maturational status, then preterm infants should exhibit this familiarity-to-novelty shift when they are maturationally equivalent to their fullterm counterparts. Because these infants were born roughly one month early, they should show the shift when they are between 4 and 5 months post birth.
The results underscored the importance of infants’ maturational status. For preterm infants, the timing of the familiarity-to-novelty shift mirrored precisely that of maturationally equivalent fullterm infants. Thus, preterm infants’ additional postnatal exposure to speech conferred no advantage in the timing of their developmental shift from familiarity to novelty preferences.
In sum, (1) a link between language and categorization, evident as early as 3 months of age (Ferry et al. 2010), emerges as part of a broad initial template that encompasses not only the vocalizations of humans, but also those of nonhuman primates (Ferry et al. 2013). At 3 and 4 months, listening to lemur vocalizations confers precisely the same cognitive advantage as listening to human speech. Although 3-month-old infants have had ample exposure to human vocalizations and little (if any) to lemur vocalizations, both signals initially confer the same cognitive advantage, suggesting that infants’ initial template of sounds that support their cognition is innately specified. (2) By 6 months, lemur vocalizations no longer confer a cognitive advantage; infants have tuned the link specifically to human vocalizations (Ferry et al. 2013). (3) Still, if infants are exposed to lemur vocalizations during this period of developmental tuning, they can either reinstate or maintain the early link to lemur vocalizations (Perszyk & Waxman 2016). Yet exposure alone cannot create, de novo, a link to cognition for any kind of signal (Ferguson & Waxman 2016b, Perszyk & Waxman 2016), highlighting a vital interplay between infants’ innate capacities and the shaping role of experience. (4) Finally, maturation constrains the effects of experience as infants establish links between language and cognition.
HARNESSING INFANTS’ ADVANCES IN LANGUAGE AND COGNITION TO SOCIAL-COMMUNICATIVE CUES
Infants’ advances as they link language and cognition do not occur in a vacuum. Instead, a signature of human development is that infants and their caregivers interact in a richly social, communicative context. From infants’ first days of life, infant-caregiver interactions are reciprocally social, filled with face-to-face communication, turn-taking, and other communicative cues (e.g., eye-gaze, intonation, pointing). Social exchanges like these, especially engaging for infants, are the stage upon which language and conceptual development unfold (for reviews, see Csibra & Gergely 2009, Kuhl 2007, Vouloumanos & Waxman 2014).
How do infants harness their exquisite sensitivity to social communicative cues in the service of acquiring language and discovering meaning? A hallmark of human communication is our remarkably flexible capacity to infuse otherwise non-linguistic signals, like tone sequences (e.g., Morse code) and smoke signals, with communicative status. Does this flexible appropriation of new signals rest upon a fully developed system of language, or is it available in infancy? Recall that infants fail to form object categories when listening to backward speech or tone sequences (Ferry et al. 2010, 2013): Merely exposing infants to these inert signals does not permit infants to create a new link to object categories (Ferguson & Waxman 2016b, Perszyk & Waxman 2016). But what if these otherwise inert signals were embedded within an explicitly social, communicative interchange? Would infants then interpret them as communicative and link them to the core cognitive process of categorization?
Infants link communicative signals to cognition
Ferguson and Waxman (2016b) demonstrated that 6-month-old infants can, in fact, establish a link between non-linguistic signals and cognition, given exposure to such signals within a social context. They adapted the standard categorization task, presenting the familiarization images in conjunction with sine-wave tone sequences—a signal that consistently fails to promote object categorization throughout the first year (Balaban & Waxman 1997, Ferry et al. 2010, Fulkerson & Waxman 2007). Importantly, before the categorization task, infants participated in an exposure phase in which tone sequences were embedded within a videotaped dialogue (Figure 3). In this dialogue, one actor spoke normally and the other responded by “beeping” in sine-wave tone sequences. The tone sequences, which cannot be produced by humans, were dubbed into the dialogues. Notice that in contrast to the passive exposure period in Perszyk and Waxman’s (2016) paradigms with lemur vocalizations, Ferguson and Waxman (2016b) embedded the tone sequences in a decidedly social, communicative exchange between two people. This was deliberate: The goal was to assess both whether embedding tones within a rich social exchange would enable infants to infuse them with communicative status and whether tones (like language) might then boost infants’ object categorization.
Embedding this otherwise null signal in a communicative exchange had a dramatic effect. After observing this two-minute dialogue, 6-month-old infants successfully formed object categories while listening to tone sequences. This was not the case when the very same tone sequences were uncoupled from the communicative exchange and presented simply as part of the ambient listening environment. Thus, 6-month-old infants can flexibly imbue an otherwise inert acoustic signal (tone sequence) with communicative intent if it is embedded within a social-communicative exchange. Their flexibility in appropriating an otherwise null signal may shed light on the contribution of social factors as humans broaden their communicative reach. This outcome offers converging evidence for the power of communicative signals on infants’ learning, memory, and ability to interpret the intentions of others (e.g., Chen & Waxman 2013, Csibra & Gergely 2009, Gliga et al. 2009, Wu et al. 2014, Yoon et al. 2008).
Multiple routes for linking language and cognition?
Considered together, the results from these two distinct kinds of exposure paradigms—(1) exposing infants to signals passively, as part of their ambient surroundings and uncoupled from any communicative signaling (Perszyk & Waxman 2016), and (2) embedding signals within a rich social exchange (Ferguson & Waxman 2016b)—suggest an intriguing possibility. Perhaps there are (at least) two routes by which young infants forge a connection between communicative signals and cognition. For signals that are part of infants’ initial template (e.g., human and nonhuman primate vocalizations), mere exposure is sufficient to either maintain or reinstate a developmentally prior link between these signals and cognition. In contrast, for signals that fall outside infants’ initial template (e.g., sine-wave tone sequences, backward speech), mere exposure cannot, on its own, enable infants to establish a link between these signals and cognition. Instead, a different route is required: Infants appear to link otherwise arbitrary signals to cognition only if they are embedded within a rich social-communicative interchange (Ferguson & Waxman 2016b, May & Werker 2014, Namy & Waxman 1998, Woodward & Hoyne 1999).
This possibility—that infants have at their disposal two distinct routes for linking signals to core cognitive capacities—brings to mind recent neuroscientific claims for dual-pathways underlying various communication systems. For example, Ackermann et al. (2014) propose a dual-pathway model for human acoustic communication, arguing that speech production engages two separate neuroanatomic channels. One channel, shared among primates, reflects subcortical mechanisms that support affective vocalizations (e.g., for nonhumans, warning or mating calls; for humans, affective intonation). A second channel, specific to humans, reflects cortical mechanisms that support articulate speech. Similarly, Senju and Johnson (2009) propose a dual-pathway model of human eye-gaze communication. They argue that a subcortical route, which may have served the evolutionary function of signaling social rank (Gobel et al. 2015), is augmented in humans by a more elaborate cortical route—a “social brain” network—which may serve the more precise, human-specific function of identifying communicative or pedagogical intent (Senju & Csibra 2008, Senju & Johnson 2009).
Whether there are parallels between these dual-pathway models and the developmental evidence we have described here is, at best, speculative. Perhaps infants’ initially broad link—which includes both human and nonhuman primate vocalizations—corresponds to an “ancestral route” that confers its cognitive advantage via primate-general affective and attentional neural systems. And perhaps infants’ link between more explicitly communicative signals and cognition—such as tones embedded within social-communicative dialogues and, eventually, more precise links between kinds of words and kinds of meaning—correspond to a “human-specific route,” built upon the “ancestral route,” that confers its cognitive advantage via more recently evolved neural systems.
FUTURE DIRECTIONS AND CONCLUSIONS
Here we have considered the earliest evidence that infants link the sounds of language with object categorization, a building block of cognition. This work heralds a new research agenda, one aimed at advancing our understanding of the ontogenetic and phylogenetic origins of human language.
Bridging infant behavior and developmental cognitive neuroscience
A goal for future work will be to bring the behavioral evidence summarized here into closer alignment with new evidence from developmental cognitive neuroscience. For example, is it possible to distinguish infants’ neural responses to the signals that are included in their initially broad template from those that are not? Can we trace whether and how infants’ neural responses to these signals change as infants tune this link specifically to human vocalizations?
Certainly, infants’ neural responses to both language and objects have received considerable attention (e.g., Csibra et al. 2000, Grossmann et al. 2009, Kaufman et al. 2003, 2005; Kuhl & Rivera-Gaxiola 2008, Quinn et al. 2006, Southgate et al. 2008). More recently, developmental cognitive neuroscience has sought to identify neural signatures underlying the language-cognition interface in infants. Although this work is itself in its infancy, there are hints that infants’ neural responses converge with the behavioral evidence for the effects of naming on cognition. For example, by 9–12 months infants’ neural responses to objects vary systematically as a function of whether these are named correctly (Friedrich & Friederici 2010, Parise & Csibra 2012). Gliga et al. (2009) provide more direct neural evidence for a link between naming and object representation: At 12 months, infants’ neural responses to objects vary as function of whether they know a name for the objects—even when they are viewing those objects in absence of their names. This outcome mirrors precisely the behavioral evidence that naming objects (presented consistently during familiarization) influences infants’ attention to new, and as-yet unnamed, objects (presented in silence at test).
Currently, the earliest neural evidence concerning the language-cognition link comes from infants at 9 months. Therefore, it cannot yet shed light on the neural processes underlying infants’ earliest links between language and cognition or how they unfold in the first 6 months. By harnessing the now considerable behavioral evidence to state-of-the-art techniques in developmental cognitive neuroscience, researchers may illuminate how infants’ brain systems interact in their first months and how they are sculpted by experience. In our view, the most exciting frontier along these lines is one that will bring the rich theoretical framework—thus far focusing exclusively on adults (for review, see Giraud & Poeppel 2012)—to investigations of infant development. If the past is a prologue to the future, we suspect that focusing on cascading neural oscillatory activity (Goswami 2011, 2016) may be an ideal avenue for identifying the developmental origins and unfolding of the links between language and cognition in young infants.
Range of signals: Beyond primates?
Another goal will be to identify the range of signals that initially promote core cognitive capacities in human infants, and how these are tuned over the first year of life. For example, is infants’ template initially broad enough to include all animal vocalizations (e.g., including birds and non-primate mammals), or does it include only vocalizations produced by primates? With this evidence in hand, it may be possible to identify which acoustic features, present in infants’ initially broad set but absent in others, are instrumental as infants first link signals to core cognitive capacities.
Range of cognitive capacities: Beyond object categorization?
It is striking that by 3 months, listening to language serves as an invitation to form object categories. But what other core cognitive capacities are also shaped by language in infancy? Perhaps the advantageous effect of listening to language is specific to infant object categorization. Yet a growing body of evidence suggests that listening to human language may have more far-reaching cognitive consequences (for review, see Vouloumanos & Waxman 2014). Language appears to support other core cognitive processes in infants, including abstract rule-learning (Ferguson & Lew-Williams 2014, Marcus et al. 2007), detecting contingencies in the environment (Reeb-Sutherland et al. 2011), understanding the communicative nature of social exchanges (Martin et al. 2012, Vouloumanos et al. 2014), and reasoning about the minds and intentions of others (Vouloumanos et al. 2012). Identifying the range of cognitive capacities that are boosted by listening to language will bring greater precision to theories about the impact of human language on the developing mind.
Range of infants: Vulnerable populations?
This basic research, focused thus far on typically developing infants, may ultimately be a foundation for promoting positive developmental outcomes in vulnerable infants. For example, individuals with autism show enhanced processing of pure tones relative to more complex sounds, and they do not show typical preferences for listening to language over other sounds (for review, see O’Connor 2012). Perhaps embedding tones in communicative episodes could serve as a beneficial bridge for establishing language skills. In addition, for infants at risk for language delays and disorders, perhaps additional language exposure early on would be advantageous. More generally, one goal for future work will be to assess vulnerable infants’ entry points in establishing a language-cognition link in the first months of life, to trace how it unfolds, and to examine their responses to either passive listening exposure or social-communicative exposure.
Summary
In this chapter, we have illuminated the developmental origins of a link between language and cognition and how it unfolds. This link, evident by 3 months of age (Ferry et al. 2010), emerges as part of a broader initial template that encompasses not only the vocalizations of humans, but also those of nonhuman primates (Ferry et al. 2013). This suggests an important role for capacities inherent in the infant: Although 3-month-old infants have acquired considerable exposure to human language and virtually no exposure to lemur vocalizations, both confer the same cognitive advantage. By 6 months, infants have tuned this link to human vocalizations alone (Ferry et al. 2013). Yet at this point in development, infants remain sensitive to experience: With sufficient exposure to lemur vocalizations, infants can maintain or reinstate a link between this signal and cognition (Perszyk & Waxman 2016). But for signals that fall outside of infants’ initial template (backward speech, sine-wave tone sequences), passive exposure alone is insufficient to create a new link to cognition (Ferguson & Waxman 2016b, Perszyk & Waxman 2016). Instead, signals like these must be embedded within a social-communicative context (Ferguson & Waxman 2016b). Together, these two pieces of evidence—one showing the effects of passive exposure for initially privileged signals and the other showing the effects of social-communicative contexts for otherwise arbitrary signals—suggest two routes by which infants can link signals to cognition in their first year of life. These early links set the stage for the increasingly precise links between distinct kinds of words and distinct kinds of meaning that unfold in infants’ second year of life.
Perhaps most importantly from a developmental vantage point, we have argued that the relation between language and cognition is not a steady state. Whatever information an infant gleans from listening to language will vary as a function of her developmental status and the precision of her language-cognition link. Initially, simply listening to language boosts cognition. Within a few months, the consequences of listening to language become considerably more nuanced and more powerful, as infants discover the increasingly precise links between language and concepts that are a hallmark of our species. In human infants, a prolonged period of dependency, exquisite sensitivity to language and to postnatal experience, and powerful learning strategies collectively spark a cascade of developmental change whose ultimate result is the acquisition of human language.
LITERATURE CITED
- Ackermann H, Hage SR, Ziegler W. 2014. Brain mechanisms of acoustic communication in humans and nonhuman primates: an evolutionary perspective. Behav. Brain Sci 37:529–604 [DOI] [PubMed] [Google Scholar]
- Aslin RN. 2007. What’s in a look? Dev. Sci 10(1):48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban MT, Waxman SR. 1997. Do words facilitate object categorization in 9-month-old infants? J. Exp. Child Psychol 64:3–26 [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Ziv T, Lamy D, Hodes RM. 2006. Nature and nurture in own-race face processing. Psychol. Sci 17(2):159–63 [DOI] [PubMed] [Google Scholar]
- Booth AE, Waxman SR. 2002. Word learning is “smart”: evidence that conceptual information affects preschoolers’ extension of novel words. Cognition. 84(1):B11–22 [DOI] [PubMed] [Google Scholar]
- Booth AE, Waxman SR. 2009. A horse of a different color: Specifying with precision infants’ mappings of novel nouns and adjectives. Child Dev. 80(1):15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth AE, Waxman SR, Huang YT. 2005. Conceptual information permeates word learning in infancy. Dev. Psychol 41(3):491–505 [DOI] [PubMed] [Google Scholar]
- Bortfeld H, Morgan JL, Golinkoff RM, Rathbun K. 2005. Mommy and me: Familiar names help launch babies into speech-stream segmentation. Psychol. Sci 16(4):298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle JD, Boyle EM. 2013. Born just a few weeks early: Does it matter? Arch. Dis. Child. Fetal Neonatal Ed 98:F85–88 [DOI] [PubMed] [Google Scholar]
- Brown R 1958. Words and Things: An Introduction to Language. Glencoe, IL: The Free Press [Google Scholar]
- Chan CCY, Tardif T, Chen J, Pulverman RB, Zhu L, Meng X. 2011. English- and Chinese-learning infants map novel labels to objects and actions differently. Dev. Psychol 47(5):1459–71 [DOI] [PubMed] [Google Scholar]
- Chen ML, Waxman SR. 2013. “Shall we blick?” Novel words highlight actors’ underlying intentions for 14-month-old infants. Dev. Psychol 49(3):426–31 [DOI] [PubMed] [Google Scholar]
- Chomsky N 1986. Knowledge of Language: Its Nature, Origin, and Use. Santa Barbara, CA: Greenwood Publishing Group [Google Scholar]
- Colombo J 2002. Infant attention grows up: The emergence of a developmental cognitive neuroscience perspective. Curr. Dir. Psychol. Sci 11(6):196–200 [Google Scholar]
- Csibra G, Davis G, Spratling MW, Johnson MH. 2000. Gamma oscillations and object processing in the infant brain. Science. 290:1582–85 [DOI] [PubMed] [Google Scholar]
- Csibra G, Gergely G. 2009. Natural pedagogy. Trends Cogn. Sci 13(4):148–53 [DOI] [PubMed] [Google Scholar]
- DeSilva J, Lesnik J. 2006. Chimpanzee neonatal brain size: Implications for brain growth in Homo erectus. J. Hum. Evol 51(2):207–12 [DOI] [PubMed] [Google Scholar]
- Fair J, Flom R, Jones J, Martin J. 2012. Perceptual learning: 12-month-olds’ discrimination of monkey faces. Child Dev. 83(6):1996–2006 [DOI] [PubMed] [Google Scholar]
- Ferguson B, Havy M, Waxman SR. 2015. The precision of 12-month-old infants’ link between language and categorization predicts vocabulary size at 12 and 18 months. Front. Psychol 6:1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B, Lew-Williams C. 2014. Communicative signals promote abstract rule learning by 7-month-old infants. Sci. Rep 6:25434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B, Waxman SR. 2016a. Linking language and categorization in infancy. J. Child Lang [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B, Waxman SR. 2016b. What the [beep]? Six-month-olds link novel communicative signals to meaning. Cognition. 146:185–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry AL, Hespos SJ, Waxman SR. 2010. Categorization in 3- and 4-month-old infants: An advantage of words over tones. Child Dev. 81(2):472–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry AL, Hespos SJ, Waxman SR. 2013. Nonhuman primate vocalizations support categorization in very young human infants. Proc. Natl. Acad. Sci. U. S. A 110(38):15231–15235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor JA. 1975. The Language of Thought. Cambridge, MA: Havard University Press [Google Scholar]
- Frank MC, Braginsky M, Yurovsky D, Marchman VA. 2016. Wordbank: an open repository for developmental vocabulary data. J. Child Lang [DOI] [PubMed] [Google Scholar]
- Friedrich M, Friederici AD. 2010. Maturing brain mechanisms and developing behavioral language skills. Brain Lang. 114(2):66–71 [DOI] [PubMed] [Google Scholar]
- Friendly RH, Rendall D, Trainor LJ. 2013a. Learning to differentiate individuals by their voices: Infants’ individuation of native- and foreign-species voices. Dev. Psychobiol 56(2):228–37 [DOI] [PubMed] [Google Scholar]
- Friendly RH, Rendall D, Trainor LJ. 2013b. Plasticity after perceptual narrowing for voice perception: reinstating the ability to discriminate monkeys by their voices at 12 months of age. Front. Psychol 4:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulkerson AL, Waxman SR. 2007. Words (but not tones) facilitate object categorization: Evidence from 6- and 12-month-olds. Cognition. 105(1):218–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman SA. 2004. Psychological essentialism in children. Trends Cogn. Sci 8(9):404–9 [DOI] [PubMed] [Google Scholar]
- Giraud A-L, Poeppel D. 2012. Cortical oscillations and speech processing: emerging computational principles and operations. Nat. Neurosci 15(4):511–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleitman H, Gross J, Reisberg D. 2011. Psychology. New York, NY: W.W. Norton & Company. 8th ed. [Google Scholar]
- Gleitman L, Papafragou A. 2005. Language and thought. In Cambridge Handbook of Thinking and Reasoning, Vol. 9, eds. Holyoak K, Morrison R, pp. 633–61. Cambridge: Cambridge University Press [Google Scholar]
- Gliga T, Volein A, Csibra G. 2009. Verbal labels modulate perceptual object processing in 1-year-old infants. J. Cogn. Neurosci 22(12):2781–89 [DOI] [PubMed] [Google Scholar]
- Gobel MS, Kim HS, Richardson DC. 2015. The dual function of social gaze. Cognition. 136:359–64 [DOI] [PubMed] [Google Scholar]
- Golinkoff RM, Hirsh-Pasek K, Cauley KM, Gordon L. 1987. The eyes have it: lexical and syntactic comprehension in a new paradigm. J. Child Lang 14(1):23–45 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gomez N, Nazzi T. 2012. Phonotactic acquisition in healthy preterm infants. Dev. Sci 15(6):885–94 [DOI] [PubMed] [Google Scholar]
- Goswami U 2011. A temporal sampling framework for developmental dyslexia. Trends Cogn. Sci 15(1):3–10 [DOI] [PubMed] [Google Scholar]
- Goswami U 2016. Educational neuroscience: Neural structure-mapping and the promise of oscillations. Curr. Opin. Behav. Sci 10:89–96 [Google Scholar]
- Graham SA, Keates J, Vukatana E, Khu M. 2013. Distinct labels attenuate 15-month-olds’ attention to shape in an inductive inference task. Front. Psychol 3(586):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Gliga T, Johnson MH, Mareschal D. 2009. The neural basis of perceptual category learning in human infants. J. Cogn. Neurosci 21(12):2276–86 [DOI] [PubMed] [Google Scholar]
- Hannon EE, Trehub SE. 2005. Tuning in to musical rhythms: infants learn more readily than adults. Proc. Natl. Acad. Sci. U. S. A 102(35):12639–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PH. 1985. Life history variation in primates. Evolution. 39(3):559–81 [DOI] [PubMed] [Google Scholar]
- Havy M, Waxman SR. 2016. Naming influences 9-month-olds’ identification of discrete categories along a perceptual continuum. Cognition. 156:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron-Delaney M, Anzures G, Herbert JS, Quinn PC, Slater AM, et al. 2011. Perceptual training prevents the emergence of the other race effect during infancy. PLoS One. 6(5):e19858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusczyk PW, Aslin RN. 1995. Infants’ detection of the sound patterns of words in fluent speech. Cogn. Psychol 29:1–23 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Csibra G, Johnson MH. 2003. Representing occluded objects in the human infant brain. Proc. R. Soc. London B 270:S140–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Csibra G, Johnson MH. 2005. Oscillatory activity in the infant brain reflects object maintenance. Proc. Natl. Acad. Sci. U. S. A 102(42):15271–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus N, Slater J. 2016. Beyond words: How humans communicate through sound. Annu. Rev. Psychol 67:83–103 [DOI] [PubMed] [Google Scholar]
- Kuhl P, Rivera-Gaxiola M. 2008. Neural substrates of language acquisition. Annu. Rev. Neurosci 31:511–34 [DOI] [PubMed] [Google Scholar]
- Kuhl PK. 2007. Is speech learning “gated” by the social brain? Dev. Sci 10(1):110–20 [DOI] [PubMed] [Google Scholar]
- Lancaster JB, Lancaster CS. 1983. Parental investment: The hominid adaptation. In How Humans Adapt: A Biocultural Odyssey, ed. Ortner D, pp. 33–66. Washington: Smithsonian Institution Press [Google Scholar]
- Lewkowicz DJ, Ghazanfar AA. 2009. The emergence of multisensory systems through perceptual narrowing. Trends Cogn. Sci 13(11):470–78 [DOI] [PubMed] [Google Scholar]
- Lorenz K 1937. On the formation of the concept of instinct. Nat. Sci 25:289–300 [Google Scholar]
- Marcus GF, Fernandes KJ, Johnson SP. 2007. Infant rule learning facilitated by speech: Research report. Psychol. Sci 18(5):387–91 [DOI] [PubMed] [Google Scholar]
- Mareschal D, Quinn PC. 2001. Categorization in infancy. Trends Cogn. Sci 5(10):443–50 [DOI] [PubMed] [Google Scholar]
- Martin A, Onishi KH, Vouloumanos A. 2012. Understanding the abstract role of speech in communication at 12months. Cognition. 123:50–60 [DOI] [PubMed] [Google Scholar]
- Maurer D, Werker JF. 2014. Perceptual narrowing during infancy: a comparison of language and faces. Dev. Psychobiol 56(2):154–78 [DOI] [PubMed] [Google Scholar]
- May L, Werker JF. 2014. Can a click be a word?: Infants’ learning of non-native words. Infancy. 19(3):281–300 [Google Scholar]
- Maye J, Werker JF, Gerken LA. 2002. Infant sensitivity to distributional information can affect phonetic discrimination. Cognition. 82:B101–11 [DOI] [PubMed] [Google Scholar]
- Medin D, Ortony A. 1989. Psychological essentialism. In Similarity and Analogical Reasoning, eds. Vosniadou S, Ortony A, pp. 179–95. Cambridge: Cambridge University Press [Google Scholar]
- Miller GA. 1990. The place of language in a scientific psychology. Psychol. Sci 1(1):7–14 [Google Scholar]
- Namy LL, Waxman SR. 1998. Words and gestures: infants’ interpretations of different forms of symbolic reference. Child Dev. 69(2):295–308 [PubMed] [Google Scholar]
- O’Connor K 2012. Auditory processing in autism spectrum disorder: A review. Neurosci. Biobehav. Rev 36(2):836–54 [DOI] [PubMed] [Google Scholar]
- Parise E, Csibra G. 2012. Electrophysiological evidence for the understanding of maternal speech by 9-month-old infants. Psychol. Sci 23(7):728–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascalis O, Scott LS, Kelly DJ, Shannon RW, Nicholson E, et al. 2005. Plasticity of face processing in infancy. Proc. Natl. Acad. Sci. U. S. A 102(14):5297–5300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña M, Maki A, Kovacić D, Dehaene-Lambertz G, Koizumi H, et al. 2003. Sounds and silence: an optical topography study of language recognition at birth. Proc. Natl. Acad. Sci. U. S. A 100(20):11702–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña M, Pittaluga E, Mehler J. 2010. Language acquisition in premature and full-term infants. Proc. Natl. Acad. Sci. U. S. A 107(8):3823–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña M, Werker JF, Dehaene-Lambertz G. 2012. Earlier speech exposure does not accelerate speech acquisition. J. Neurosci 32(33):11159–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perszyk DR, Ferguson B, Waxman SR. 2016. Maturation constrains the effect of exposure in linking language and thought: Evidence from healthy preterm infants. Dev. Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perszyk DR, Waxman SR. 2016. Listening to the calls of the wild: The role of experience in linking language and cognition in young infants. Cognition. 153:175–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PC, Westerlund A, Nelson CA. 2006. Neural markers of categorization in 6-month-old infants. Psychol. Sci 17(1):59–66 [DOI] [PubMed] [Google Scholar]
- Ramus F 2000. Language discrimination by human newborns and by cotton-top tamarin monkeys. Science. 208:349–51 [DOI] [PubMed] [Google Scholar]
- Reeb-Sutherland BC, Fifer WP, Byrd DL, Hammock EAD, Levitt P, Fox NA. 2011. One-month-old human infants learn about the social world while they sleep. Dev. Sci 14(5):1134–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Romano AC. 2016. The development of attention systems and working memory in infancy. Front. Syst. Neurosci 10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. 2004. Infant visual recognition memory. Dev. Rev 24:74–100 [DOI] [PubMed] [Google Scholar]
- Saint-Georges C, Chetouani M, Cassel R, Apicella F, Mahdhaoui A, et al. 2013. Motherese in interaction: at the cross-road of emotion and cognition? (A systematic review). PLoS One. 8(10):e78103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieffelin B, Ochs E. 1986. Language socialization. Annu. Rev. Anthropol 15:163–91 [Google Scholar]
- Scott LS, Monesson A. 2009. The origin of biases in face perception. Psychol. Sci 20(6):676–80 [DOI] [PubMed] [Google Scholar]
- Scott LS, Pascalis O, Nelson CA. 2007. A domain-general theory of the development of perceptual discrimination. Curr. Dir. Psychol. Sci 16(4):197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl A, Tincoff R, Baker C, Cristia A. 2015. Why the body comes first: Effects of experimenter touch on infants’ word finding. Dev. Sci 18(1):155–64 [DOI] [PubMed] [Google Scholar]
- Senju A, Csibra G. 2008. Gaze following in human infants depends on communicative signals. Curr. Biol 18:668–71 [DOI] [PubMed] [Google Scholar]
- Senju A, Johnson MH. 2009. The eye contact effect: mechanisms and development. Trends Cogn. Sci 13(February):127–34 [DOI] [PubMed] [Google Scholar]
- Shultz S, Vouloumanos A, Bennett RH, Pelphrey K. 2014. Neural specialization for speech in the first months of life. Dev. Sci. 17(5):766–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LB, Jones SS, Landau B. 1996. Naming in young children: a dumb attentional mechanism? Cognition. 60(2):143–71 [DOI] [PubMed] [Google Scholar]
- Smith LB, Jones SS, Yoshida H, Colunga E. 2003. Whose DAM account? Attentional learning explains Booth and Waxman. Cognition. 87:209–13 [DOI] [PubMed] [Google Scholar]
- Southgate V, Csibra G, Kaufman J, Johnson MH. 2008. Distinct processing of objects and faces in the infant brain. J. Cogn. Neurosci 20:741–49 [DOI] [PubMed] [Google Scholar]
- Swingley D 2012. Cognitive development in language acquisition. Lang. Learn. Dev 8:1–3 [Google Scholar]
- Tardif T, Fletcher P, Liang W, Zhang Z, Kaciroti N, Marchman VA. 2008. Baby’s first 10 words. Dev. Psychol 44(4):929–38 [DOI] [PubMed] [Google Scholar]
- Vergne AL, Mathevon N. 2008. Crocodile egg sounds signal hatching time. Curr. Biol 18(12):513–14 [DOI] [PubMed] [Google Scholar]
- Vouloumanos A, Druhen MJ, Hauser MD, Huizink AT. 2009. Five-month-old infants’ identification of the sources of vocalizations. Proc. Natl. Acad. Sci. U. S. A 106(44):18867–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouloumanos A, Hauser MD, Werker JF, Martin A. 2010. The tuning of human neonates’ preference for speech. Child Dev. 81(2):517–27 [DOI] [PubMed] [Google Scholar]
- Vouloumanos A, Martin A, Onishi KH. 2014. Do 6-month-olds understand that speech can communicate? Dev. Sci 17(6):872–79 [DOI] [PubMed] [Google Scholar]
- Vouloumanos A, Onishi KH, Pogue A. 2012. Twelve-month-old infants recognize that speech can communicate unobservable intentions. Proc. Natl. Acad. Sci 109(32):12933–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouloumanos A, Waxman SR. 2014. Listen up! Speech is for thinking during infancy. Trends Cogn. Sci 18(12):642–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S, Fu X, Arunachalam S, Leddon E, Geraghty K, Song H. 2013. Are nouns learned before verbs? Infants provide insight into a long-standing debate. Child Dev. Perspect 7(3):155–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SR. 1998. Linking object categorization and naming: Early expectations and the shaping role of language. In The Psychology of Learning and Motivation, Vol. 38, ed. Medin DL, pp. 249–491. San Diego: Academic Press [Google Scholar]
- Waxman SR. 1999. Specifying the scope of 13-month-olds’ expectations for novel words. Cognition. 70(3): [DOI] [PubMed] [Google Scholar]
- Waxman SR, Booth AE. 2003. Bringing theories of word learning in line with the evidence. Cognition. 87:215–18 [DOI] [PubMed] [Google Scholar]
- Waxman SR, Braun I. 2005. Consistent (but not variable) names as invitations to form object categories: new evidence from 12-month-old infants. Cognition. 95(3):B59–68 [DOI] [PubMed] [Google Scholar]
- Waxman SR, Gelman SA. 2009. Early word-learning entails reference, not merely associations. Trends Cogn. Sci 13(6):258–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SR, Lidz JL. 2006. Early word learning. In Handbook of Child Psychology, Vol. 2, eds. Kuhn D, Siegler R, pp. 299–335. Hoboken NJ: Wiley. 6th ed. [Google Scholar]
- Waxman SR, Lidz JL, Braun IE, Lavin T. 2009. Twenty four-month-old infants’ interpretations of novel verbs and nouns in dynamic scenes. Cogn. Psychol. 59:67–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SR, Markow DB. 1995. Words as invitations to form categories: Evidence from 12- to 13-month-old infants. [DOI] [PubMed]
- Werker JF, Hensch TK. 2014. Critical Periods in Speech Perception: New Directions. Annu. Rev. Psychol 66:173–96 [DOI] [PubMed] [Google Scholar]
- Werker JF, Tees RC. 1984. Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behav. Dev 7:49–63 [Google Scholar]
- Werker JF, Yeung HH. 2005. Infant speech perception bootstraps word learning. Trends Cogn. Sci 9(11):519–27 [DOI] [PubMed] [Google Scholar]
- Westermann G, Mareschal D. 2014. From perceptual to language-mediated categorization. Philos. Trans. R. Soc. B 369:20120391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorf BL. 1956. Language, Thought and Reality. Cambridge, MA: MIT Press [Google Scholar]
- Woodward AL, Hoyne KL. 1999. Infants’ learning about words and sounds in relation to objects. Child Dev 70(1):65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Tummeltshammer KS, Gliga T, Kirkham NZ. 2014. Ostensive signals support learning from novel attention cues during infancy. Front. Psychol 5:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F 2002. The role of language in acquiring object kind concepts in infancy. Cognition. 85(3):223–50 [DOI] [PubMed] [Google Scholar]
- Yoon JMD, Johnson MH, Csibra G. 2008. Communication-induced memory biases in preverbal infants. Proc. Natl. Acad. Sci. U. S. A 105(36):13690–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangenehpour S, Ghazanfar AA, Lewkowicz DJ, Zatorre RJ. 2009. Heterochrony and cross-species intersensory matching by infant vervet monkeys. PLoS One. 4(1):e4302. [DOI] [PMC free article] [PubMed] [Google Scholar]