Regulatory T (Treg) cells expressing the transcription factor Foxp3 are a distinct subset of CD4+ helper T cells (1). Treg cells are indispensable for the establishment of immune homeostasis but can also inhibit effective antitumor immunity (2). Additionally, Treg cells play an important role in the prevention of immunopathogenic reactions to infection. During chronic infection, limiting Treg cell number boosts immune responses mediated by CD8+ T cells (cytotoxic T lymphocytes, or CTLs), leading to improved control of the infection (3, 4). In the context of acute infection, Treg cells inhibit the accumulation and cytotoxicity of CTL responses, associated with diminished pathogen clearance in multiple models (5, 6). However, Treg cells also have beneficial effects for the host during acute infection. For instance, in an acute infection model via the Armstrong strain of lymphocytic choriomeningitis virus, Treg cells produce interleukin (IL)-10 that promotes the maturation of memory T cells (7). Thus, Treg cells play complex roles during acute infections, and the mechanisms underlying these discrete functions of Treg cells are largely unresolved. In PNAS, Dolina et al. (8) demonstrate that distinct Treg cell populations arise during acute Listeria monocytogenes (Lm) infection, with discrete effects at regulating CD8+ T cell responses during the priming versus contraction phases (Fig. 1).
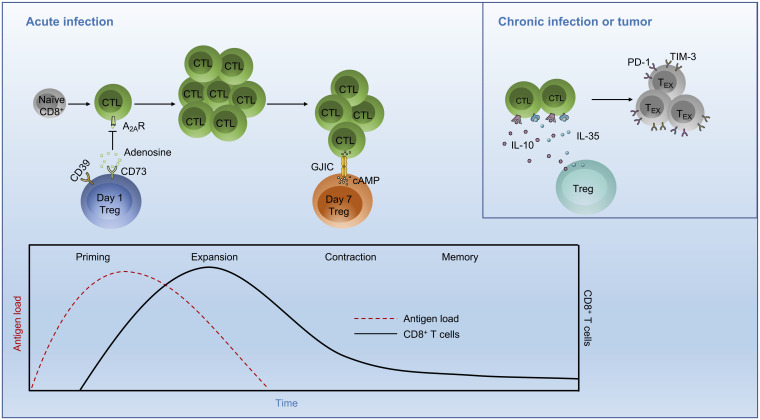
Fig. 1.
Temporal effects of Treg cells on CD8+ T cell responses in infection. During the priming phase of an acute infection, the first wave of Treg cell expansion occurs at day 1 postinfection. These Treg cells are capable of cell contact-independent suppressive function mediated by rapid generation of adenosine, which inhibits A2AR-expressing CD8+ T cell function. A second wave of Treg cell expansion occurs at day 7 postinfection, associated with the stage of peak CTL expansion. This Treg cell population is distinct from cells arising on day 1 postinfection and suppresses CTL proliferation by cell contact-dependent transfer of cAMP to CTLs via GJIC. (Inset) In a chronic infection or tumor, the immunosuppressive cytokines IL-10 and IL-35 are secreted by Treg cells and promote CD8+ T cell exhaustion, which is a progressive process characterized by loss of effector function and up-regulation of inhibitory receptors, such as PD-1 and TIM-3. A2AR, adenosine A2A receptor; GJIC, gap junction intracellular communication; TEX, exhausted T cells.
During acute infection, naïve CD8+ T cells are primed and undergo clonal expansion to generate CTLs (9). After antigen clearance, these effector cells experience contraction where 90 to 95% of effector cells are eliminated. However, the dynamics of Treg cell responses during acute infection remains poorly defined. To investigate the role of Treg cells in regulating the adaptive immune response against acute infection, Dolina et al. (8) infected C57BL/6 mice with Lm ΔactA-Ova (an attenuated Listeria strain with deletion of the virulence factor actA and expression of chicken ovalbumin [Ova]), which could induce distinct Treg responses according to low or high dose of bacterial challenge (10). They found that there were two distinct waves of Treg cell responses that occurred at days 1 and 7 after Listeria infection (called day 1 and day 7 Treg cells, respectively). Dolina et al. (8) next performed lineage tracing assay by labeling day 1 and 7 Treg cells from Foxp3-eGFP reporter mice with EdU (5-ethynyl-2′-deoxyuridine) and BrdU (bromodeoxyuridine), respectively. They found that most labeled populations were either single positive for EdU or BrdU, suggesting that day 1 and day 7 Treg cells may not follow the same differentiation trajectory. Dolina et al. (8) also performed hemisplenectomy to isolate Treg cells from the same host at these two time points and then compared the T cell receptor repertoires of day 1 and day 7 Treg cells. The authors found that these two Treg subpopulations were clonally distinct. These results collectively show that day 1 and day 7 Treg cells are likely to be generated from different progenitors.
Analysis of in vitro suppressive activity using Treg cells isolated from Foxp3-eGFP reporter mice revealed that day 1 Treg cells had suppressive capacity comparable to Treg cells isolated from uninfected mice (called day 0 Treg cells), while day 7 Treg cells up-regulated their suppressive activity. Based on these results, Dolina et al. (8) next explored the underlying mechanisms by RNA sequencing (RNA-seq). Using unbiased hierarchical clustering, they found that Treg cell signature genes were grouped into three gene clusters, with differential expression patterns in days 0, 1, and 7 Treg cells. Moreover, the gene expression profiles of day 1 and day 7 Treg cells were largely distinct based on principal component analysis. Further, pathway enrichment analysis showed that day 1 Treg cells were enriched for pathways related to innate immune signaling, protein kinase A signaling, and p53 signaling, while in day 7 Treg cells adenylyl cyclase-linked pathways, including cAMP-mediated signaling, were up-regulated. Thus, day 1 and day 7 Treg cells have distinct suppressive activities and gene expression programs.
Furthermore, Dolina et al. (8) found that day 1 Treg cells had increased expression of CD73, a cell-surface molecule that aids in the conversion of extracellular ATP to adenosine in cooperation with CD39. Indeed, high-performance liquid chromatography analysis revealed that day 1 Treg cells had increased enzymatic activity of CD73 to convert 5′-AMP to adenosine compared to day 0 and day 7 Treg cells. CD73 is important for the suppressive capacity of Treg cells to inhibit intratumoral CD8+ T cell function (11). Using a transwell assay and CD8+ T cells from Adora2a−/− mice (germline deletion of A2AR, an adenosine receptor), the authors found that day 1 Treg cells regulated CTL priming via a cell contact-independent mechanism but instead were dependent upon the generation of adenosine that inhibits CD8+ T cell function via A2AR. Moreover, CD73 antibody blockade administered early (days 0 to 2) after Listeria infection was associated with increased antigen-specific CTL numbers. In contrast to day 1 Treg cells, day 7 Treg cells had increased intracellular cAMP level, which was consistent with the up-regulation of cAMP-associated signatures as revealed by RNA-seq and pathway enrichment analysis. cAMP suppresses the activation of responding cells via a cell contact-dependent mechanism called gap junction intercellular communication (GJIC) (12). Gap27204–214, the connexin mimetic peptide, is reported to inhibit the formation and stability of gap junctions and impede GJIC (12). Indeed, coculture with CD8+ T cells in vitro in the presence of Gap27204–214 markedly reduced the suppressive capacity of day 7 Treg cells but had no effects on day 1 Treg cells. Thus, day 7 Treg cells influence the contraction phase of the CTL response via cell contact-dependent regulation, namely cAMP transfer to CD8+ T cells via GJIC. Altogether, the results from Dolina et al. (8) indicate that developmentally distinct Treg cell populations regulate CTL priming and contraction phases in acute infection via different suppressive mechanisms (Fig. 1).
In PNAS, Dolina et al. demonstrate that distinct Treg cell populations arise during acute Listeria monocytogenes (Lm) infection, with discrete effects at regulating CD8+ T cell responses during the priming versus contraction phases.
Treg cells are important for controlling CD8+ T cell responses in infection and cancer (3–6, 13). The study from Dolina et al. (8) advances our understanding of the dynamic interplay between Treg cells and CD8+ T cells in acute infection. Specifically, during the priming phase of an acute infection, Treg cells exert their suppressive capacity by rapid generation of adenosine, which may prevent immunopathological damage. In contrast, day 7 Treg cells accumulate cAMP that is transferrable to effector CD8+ T cells via GJIC to suppress CD8+ T cell function, which likely restores immune homeostasis during the contraction phase. After priming, expansion, and contraction, CD8+ T cells can also differentiate into long-lived memory T cells, and Treg cell-derived IL-10 facilitates this process through the suppression of proinflammatory cytokine production by dendritic cells (DCs) (7). Moreover, during chronic infection or in the tumor microenvironment, continuous exposure to antigens can trigger CD8+ T cell exhaustion, which limits immune-mediated clearance of pathogens or antitumor immunity (14). In this scenario, Treg cells produce immunoregulatory cytokines such as IL-10 and IL-35, which program CD8+ T cells to lose effector function and become exhausted (15). Thus, Treg cells utilize multiple mechanisms for the regulation of CD8+ T cell fate and function, which may be tuned by immunological context (e.g., infection versus tumor), microenvironment, and inflammatory state.
This study raises important questions that warrant further investigation. First, after the first wave of Treg cell expansion at day 1 postinfection during the priming phase, Treg cells rapidly reduce suppressive capacity and decline in number (8), but the underlying mechanisms remain to be established. Of note, the collapse of Treg cells is dependent on the deprivation of IL-2 and local DCs during acute Toxoplasma gondii infection (16), suggesting that environmental cues may contribute to the dynamic regulation of Treg cell population in acute Listeria infection. Second, after activation by DCs in respiratory syncytial virus infection, the stability and function of Treg cells are partially dependent upon epigenetic reprogramming (17–19). Therefore, it remains to be addressed whether DCs are involved in the differentiation of these distinct Treg cell populations in Listeria infection, and the role of epigenetic modification also requires additional investigation. Third, recent studies have revealed that cell-extrinsic and -intrinsic metabolic factors contribute to the differentiation and function of T cells including Treg cells (20, 21). Indeed, Dolina et al. (8) showed that extracellular ATP could be converted into immunosuppressive adenosine by CD39 and CD73 expressed on the surface of Treg cells, which impacts CD8+ T cell function during the priming phase. As day 1 and day 7 Treg cell populations differentiate from separate progenitor cells and exhibit distinct suppressive mechanisms, it will be interesting to explore whether and how metabolic programs, such as oxidative phosphorylation and mTOR signaling that are essential for the establishment of Treg cell suppressive activity (22), orchestrate these Treg cell populations in acute infection.
Collectively, the exciting findings from Dolina et al. (8) provide insight into Treg cell-dependent regulation of CD8+ T cell responses in acute infection, and this dynamic process is mediated by developmentally distinct Treg cell populations. Strategies to manipulate Treg cell number or function should have high therapeutic potential, as depletion of Treg cells results in enhanced immune responses in infection models. However, systemically targeting Treg cells may not be applicable in the clinic for the treatment of infections, as this strategy increases the risk of autoimmune disorders or uncontrolled immunopathology. Therefore, the suppressive mechanisms of distinct Treg cell populations revealed by Dolina et al. (8) present opportunities of targeted manipulation of Treg cell functions while minimizing such risks.
Acknowledgments
This work was supported by American Lebanese Syrian Associated Charities; NIH Grants AI105887, AI131703, AI140761, AI150241, AI150514, and CA253188; and a Lupus Research Alliance Grant (to H.C.). This work is the sole responsibility of the authors and does not reflect the official views of the NIH.
Footnotes
Competing interest statement: H.C. is a consultant for Kumquat Biosciences, Inc.
See companion article, “Developmentally distinct CD4+ Treg lineages shape the CD8+ T cell response to acute Listeria infection,” 10.1073/pnas.2113329119.
References
- 1.Saravia J., Chapman N. M., Chi H., Helper T cell differentiation. Cell. Mol. Immunol. 16, 634–643 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plitas G., Rudensky A. Y., Regulatory T cells in cancer. Annu. Rev. Cancer Biol. 4, 459–477 (2020). [Google Scholar]
- 3.Dietze K. K., et al. , Transient depletion of regulatory T cells in transgenic mice reactivates virus-specific CD8+ T cells and reduces chronic retroviral set points. Proc. Natl. Acad. Sci. U.S.A. 108, 2420–2425 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers L., Messer R. J., Carmody A. B., Hasenkrug K. J., Tissue-specific abundance of regulatory T cells correlates with CD8+ T cell dysfunction and chronic retrovirus loads. J. Immunol. 183, 1636–1643 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zelinskyy G., et al. , The regulatory T-cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T-cell response. Blood 114, 3199–3207 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Zelinskyy G., Dietze K., Sparwasser T., Dittmer U., Regulatory T cells suppress antiviral immune responses and increase viral loads during acute infection with a lymphotropic retrovirus. PLoS Pathog. 5, e1000406 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laidlaw B. J., et al. , Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat. Immunol. 16, 871–879 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolina J. S., et al. , Developmentally distinct CD4+ Treg lineages shape the CD8+ T cell response to acute Listeria infection. Proc. Natl. Acad. Sci. U.S.A. 119, 10.1073/pnas.2113329119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar B. V., Connors T. J., Farber D. L., Human T cell development, localization, and function throughout life. Immunity 48, 202–213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolina J. S., et al. , TLR9 sensing of self-DNA controls cell-mediated immunity to Listeria infection via rapid conversion of conventional CD4+ T cells to Treg. Cell Rep. 31, 107249 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider E., et al. , CD73-mediated adenosine production by CD8 T cell-derived extracellular vesicles constitutes an intrinsic mechanism of immune suppression. Nat. Commun. 12, 5911 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bopp T., et al. , Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J. Exp. Med. 204, 1303–1310 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim S. A., et al. , Lipid signalling enforces functional specialization of Treg cells in tumours. Nature 591, 306–311 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collier J. L., Weiss S. A., Pauken K. E., Sen D. R., Sharpe A. H., Not-so-opposite ends of the spectrum: CD8+ T cell dysfunction across chronic infection, cancer and autoimmunity. Nat. Immunol. 22, 809–819 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawant D. V., et al. , Adaptive plasticity of IL-10+ and IL-35+ Treg cells cooperatively promotes tumor T cell exhaustion. Nat. Immunol. 20, 724–735 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldenhove G., et al. , Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31, 772–786 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ting H. A., et al. , Notch ligand Delta-like 4 promotes regulatory T cell identity in pulmonary viral infection. J. Immunol. 198, 1492–1502 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ting H. A., et al. , Notch ligand Delta-like 4 induces epigenetic regulation of Treg cell differentiation and function in viral infection. Mucosal Immunol. 11, 1524–1536 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charbonnier L. M., Wang S., Georgiev P., Sefik E., Chatila T. A., Control of peripheral tolerance by regulatory T cell-intrinsic Notch signaling. Nat. Immunol. 16, 1162–1173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman N. M., Chi H., Metabolic adaptation of lymphocytes in immunity and disease. Immunity 55, 14–30 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman N. M., Boothby M. R., Chi H., Metabolic coordination of T cell quiescence and activation. Nat. Rev. Immunol. 20, 55–70 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Shi H., Chi H., Metabolic control of Treg cell stability, plasticity, and tissue-specific heterogeneity. Front. Immunol. 10, 2716 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]