Abstract
The pharmacodynamic parameters of peak serum drug concentration/MIC (peak/MIC) ratio and the area under the curve (AUC)/MIC ratio have been used to characterize in vivo drug exposure and its relationship to bacterial killing for the fluoroquinolones. Our study objectives were to describe the pharmacodynamic relationship between gatifloxacin exposure and outcome as assessed by bacterial density and survival in an immunocompromised murine thigh model of pneumococcal infection and to assess the relationship between drug exposure and these outcomes in an immunocompetent host. ICR mice were rendered neutropenic, and thigh infection was induced by intramuscular administration of 0.1 ml of 105 to 107 CFU of Streptococcus pneumoniae/ml. Mice received 1 to 5 mg of uranyl nitrate/kg of body weight at day −3 and were randomized to receive 10 to 80 mg of gatifloxacin/kg every 6 to 24 h orally, starting at 2 h postinoculation. Bacterial density studies were completed 24 h after initiation of therapy, and survival was assessed after 4 days of treatment. MICs for clinical isolates (n = 8) ranged from 0.25 to 1.0 μg/ml. Correlations were assessed between the change in bacterial density, as well as survival, and the AUC/MIC ratio, peak/MIC ratio, and the duration of time that serum drug concentration remained above the MIC. The best predictor of bacterial response was the AUC/MIC ratio for both outcome measures. There was greater efficacy, as measured by a decrease in log change in CFU as well as by survival data, in the immunocompetent mice compared to the immunocompromised mice. These data demonstrate (i) the appropriateness of the AUC/MIC ratio as a dynamic predictor of response to pneumococcal infection for the fluoroquinolones, (ii) that gatifloxacin AUC/MIC ratios of 30 to 40 appear to optimize bactericidal activity and survival in this model, and (iii) that immunocompetency of the host plays a role in efficacy.
Considerable controversy exists regarding the most appropriate way to administer antibiotics to maximize bacterial killing and minimize toxicity. In the case of the fluoroquinolones, debate exists as to not only the best pharmacodynamic parameter (i.e., peak serum drug concentration/MIC [peak/MIC] ratio or area under the curve [AUC]/MIC ratio) to characterize bactericidal activity but also the magnitude of the required interaction to optimize therapeutic outcome (10, 13).
The current study was undertaken to investigate the pharmacodynamic relationship between gatifloxacin exposure and outcome as assessed by bacterial density and survival in a murine thigh model of pneumococcal infection. Additionally, the influence of the host on these outcomes was assessed in an immunocompetent model.
MATERIALS AND METHODS
Antimicrobial test agents.
Gatifloxacin analytical grade standard was obtained for in vitro testing from Bristol-Myers Squibb, Princeton, N.J. For all in vivo studies, laboratory standard grade gatifloxacin (lot no. 9D07329; expiration date, Sept. 2000) was obtained from the manufacturer, reconstituted based on weight, and administered via oral gavage, using a feeding tube.
Bacterial isolates and susceptibilities.
Eight clinical isolates of Streptococcus pneumoniae were included in this study. Organisms were selected to represent a range of susceptibilities to gatifloxacin, thus allowing the opportunity for pharmacodynamic modeling over a larger range for the parameters of AUC/MIC ratio, peak/MIC ratio, and duration of time that serum drug concentration remained above the MIC (T > MIC). The MIC of gatifloxacin was determined in duplicate using the microdilution method according to NCCLS guidelines (9). The MICs were determined in cation-adjusted Mueller-Hinton broth (20 to 25 mg of calcium/liter, 10 to 12.5 mg of magnesium/liter) with 5% lysed horse blood in ambient air. Trypticase soy agar with 5% sheep blood was used as the growth medium.
Protein binding studies.
The protein binding studies were done in triplicate using an ultrafiltration method. An aqueous stock solution of gatifloxacin was prepared, and dilutions were made in fresh ICR mouse serum to yield final concentrations of 1, 10, and 100 μg/ml. All spiked serum samples were placed in a 37°C shaking water bath for 10 min. Then, 0.9-ml aliguots of the serum samples were transferred to Centrifree Micropartition devices (Millipore, Bedford, Mass.; 30,000 molecular weight cut-off filter). These filters were spun at 1,000 × g at 10°C for 15 min. Protein binding studies were conducted in triplicate at each concentration. Concentrations of gatifloxacin in both initial serum solutions and filtrates were determined by a validated high-performance liquid chromatography (HPLC) method.
Thigh infection model.
Specific-pathogen-free ICR mice weighing approximately 25 g were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, Ind.). Mice were rendered transiently neutropenic by injecting cyclophosphamide intraperitoneally (i.p.) on days −4 (150 mg/kg of body weight) and −1 (100 mg/kg) before treatment began. This regimen has been shown to induce neutropenia in the animals for 5 days (1, 5, 12). In addition, renal impairment was produced by a single i.p. injection of uranyl nitrate on day −3 prior to the initiation of antimicrobial therapy (1, 12).
Isolates to be inoculated into the thighs were previously frozen at −80°C in skim milk. From each fresh isolate approximately 10 colonies were transferred to tubes containing 12 ml of cation-adjusted Mueller-Hinton broth with 5% lysed horse blood. The tubes were placed into an incubator at 37°C for 10 h to obtain logarithmic growth of a 107 to 109 CFU/ml suspension and subsequently diluted to an inoculum of 105 to 107 CFU/ml. Final inoculum concentrations were confirmed by serial dilution and plating techniques. Thigh infection with each of the test isolates was produced by intramuscular injection of 0.1 ml of the inoculum into each thigh of the mice 2 h prior to the initiation of antimicrobial therapy. In addition, similar inoculum ranging studies were performed without cyclophosphamide-induced neutropenia in a second mouse species, CBA/J, with one pneumococcal isolate. These mice received uranyl nitrate in a manner previously described for the ICR mice. This second murine strain is susceptible to pneumococcal infection in the presence of this host defense (14).
Pharmacokinetic studies.
Studies were undertaken to determine the pharmacokinetic profile of gatifloxacin in infected ICR mice. The mice received i.p. injections of cyclophosphamide, as described above. Three days prior to the pharmacokinetic study, ICR mice received a single i.p. injection of 1, 2.5, or 5 mg of uranyl nitrate/kg, resulting in various degrees of renal impairment. Mice were administered a single oral gatifloxacin dose of 10, 25, or 40 mg/kg, utilizing a method previously described. Animals were euthanatized by CO2 exposure followed by cervical dislocation, and blood was obtained through intracardiac puncture in groups containing three to five mice at 0.25, 0.5, 1, 2, 4, 6, 12, and 24 h following drug administration. The blood was centrifuged at 10,000 × g for 10 min; the serum was transferred into a polypropylene tube and stored at −80°C until analyzed. Abbreviated pharmacokinetic studies were completed using the CBA/J species with gatifloxacin (25 mg/kg) and uranyl nitrate (2.5 mg/kg).
Concentrations of gatifloxacin in murine sera were determined using a validated HPLC procedure. Sample treatment involved the addition of 100 μl of a 10-μg/ml solution of internal standard (moxifloxacin; Bayer Pharmaceuticals) and 800 μl of acetonitrile to a 100-μl sample contained in a polypropylene tube. After vortexing and centrifuging the mixture, the acetonitrile layer was evaporated to dryness and reconstituted with 200 μl of 0.01 N HCL. The aqueous layer was injected into the HPLC system using a Waters autosampler (model 717 plus; Waters Associates, Milford, Mass.) coupled to a Waters chromatographic pump (model M515). Chromatographic separations for both drugs were achieved on a reversed-phase 10-μm C18 column (250 by 4.6 nm; Nucleosil; Allteck Associates Inc., Deerfield, Ill.). Sample detection was done with a fluorescence detector (model 980; Applied Biosystems, Ramsey, N.J.) with the excitation and emission wavelengths set at 295 and 418 nm, respectively. Chromatograms were registered on an integrator (EZChrom Elite chromatography data system; Scientific Software, San Ramon, Calif.). The mobile phase was delivered at a flow rate of 1.4 ml/min and consisted of buffer, methanol, and acetonitrile (66.8:15.1:18.1 [vol/vol/vol]). The buffer was prepared with triethylamine, phosphoric acid, and HPLC-grade water at a ratio of 0.37:0.30:99.3 (vol/vol/vol).
The assay was linear over a range of 0.1 to 30 μg/ml. Intraday coefficients of variation for the low (0.5 μg/ml) and high (25 μg/ml) check samples were 3.6 and 3.2%, respectively. Interday coefficients of variation for the low and high check samples were 1.3 and 1.4%, respectively.
Pharmacokinetic analysis was conducted with WinNonlin Pro (version 3.0; Pharsight Corporation, Mountain View, Calif.) using a compartmental method. A one-compartment model with first-order absorption and first-order elimination was used to characterize the disposition of gatifloxacin in infected mice. Pharmacokinetic parameters determined included the terminal-phase elimination rate constant, elimination half-life, apparent volume of the central compartment, apparent steady-state volume of distribution, area under the serum drug concentration-time curve, and total body clearance. Model selection was based on Akaike criteria.
Efficacy as assessed by bacterial density.
Six S. pneumoniae isolates were used in these studies: 53, 84, 59, 21, 77, and 75. Once the animals had been prepared as above and inoculated, treatment with gatifloxacin was initiated at 2 h post-thigh infection in a method previously described. The concentration and treatment regimen of gatifloxacin as well as the concentration of uranyl nitrate were varied to simulate a range of drug exposures. Final dosage range of gatifloxacin was varied from 10 to 80 mg/kg every 6 to 24 h. Control animals received water orally at the same volume and schedule as gatifloxacin-treated animals. Groups of untreated control mice were sacrificed at the initiation of therapy and after 24 h. Treated mice were sacrificed after 24 h of therapy.
After sacrifice, both thighs were removed and individually homogenized in normal saline. Serial dilutions were plated on Trypticase soy agar with 5% sheep blood for CFU determinations. Efficacy (change in bacterial density) was calculated by subtracting the mean log CFU per thigh of the control mice, obtained 2 h postbacterial inoculation, from the log CFU per thigh of the gatifloxacin-treated or untreated control mice at the end of therapy (24 h).
Efficacy as assessed by survival.
Six S. pneumoniae isolates were used in these studies: 59, 84, 21, 77, 85, and 63. Groups of mice were similarly infected with each test strain for evaluation of survival during 96 h of therapy. Gatifloxacin therapy was initiated at a time corresponding to 2 h post-thigh inoculations. Similarly to density studies, gatifloxacin was administered in various regimens, from 10 to 80 mg/kg every 6 to 24 h for 96 h of therapy. Control animals received water orally at the same volume and schedule as gatifloxacin. The cumulative mortality was calculated during 96 h of therapy. Although death has historically been used as an end point for studies of this type, this end point is no longer suitable in the current era of animal research. Therefore, our study methodology has been modified to contemporary standards, which have been recently employed in studies conducted at our institution (4). The term mortality has been used as an end point for this study; however, it should be clearly understood that when possible every attempt was made to minimize pain and suffering of the animals. Animals were euthanatized prior to naturally succumbing to infection if symptoms of impending death were observed, including substantial alterations in posture (e.g., abnormal posture or head tucked into abdomen), coat, exudate around eyes and/or nose, and breathing or movement. For the purposes of this study, whether an animal died due to the natural process or was euthanatized, both were considered the same end point for experimental and statistical purposes.
Data analysis.
Spearman's rank correlation coefficient was used to evaluate the relationship between mortality and T > MIC, the peak/MIC ratio, and the AUC/MIC ratio, for gatifloxacin. This test was also used to evaluate the relationship between change in CFU after 24 h of therapy and the three pharmacodynamic parameters listed above. Additionally, the goodness of fit for each of these relationships was characterized and evaluated using the sigmoid Emax model (WinNonlin Pro, version 3.0; Pharsight Corporation).
RESULTS
Eight clinical isolates of S. pneumoniae were utilized; the MICs of gatifloxacin for these isolates are displayed in Table 1.
TABLE 1.
Gatifloxacin susceptibility of S. pneumoniae isolates
| Isolate no. | MIC (μg/ml) |
|---|---|
| 59 | 0.25 |
| 63 | 0.25 |
| 84 | 0.25 |
| 85 | 0.25 |
| 53 | 0.5 |
| 75 | 0.5 |
| 77 | 0.5 |
| 21 | 1.0 |
Protein binding and pharmacokinetic studies.
The percent protein bound (mean ± standard deviation) for ICR mice measured at concentrations of 1, 10, and 100 μg/ml was 22.5% ± 1.8%, 26.6% ± 5.3%, and 17.2% ± 0.7%, respectively. An abbreviated study was initiated for the CBA/J strain, and a concentration of 10 μg/ml demonstrated 11.1% ± 2.2% protein binding, which was less than half that observed with the ICR mice. Published data on human protein binding in serum in volunteers indicate results of approximately 20% binding (Tequin [gatifloxacin] tablets/injection prescribing information, doc. no. E5-A002, Bristol-Myers Squibb, Dec. 1999).
The resultant gatifloxacin pharmacokinetic parameter estimates for several gatifloxacin and uranyl nitrate dosages are presented in Table 2. These data reveal the dose proportionality related to the maximum concentration and the influence of uranyl nitrate in substantially altering the clearance of gatifloxacin in this infection model, allowing for varied drug exposures. An abbreviated pharmacokinetic study was conducted with CBA/J mice using the 25-mg/kg gatifloxacin and 2.5-mg/kg uranyl nitrate regimen. At time points of 1 and 6 h postdosing the CBA/J mice demonstrated concentrations of 1.49 ± 0.12 μg/ml and 0.22 ± 0.08 μg/ml, respectively. These results are similar to those observed with ICR mice, which had gatifloxacin concentrations of 1.60 ± 1.13 μg/ml and 0.24 ± 0.03 μg/ml, respectively, at the same time points postdosing.
TABLE 2.
Pharmacokinetics of gatifloxacin in infected neutropenic micea
| Gatifloxacin (mg/kg) | Uranyl nitrate (mg/kg) | Cmax (μg/ml) | Tmax (h) | AUC (h · μg/ml) | Half-life (h) |
|---|---|---|---|---|---|
| 10 | 1 | 0.41 (0.06) | 0.94 (0.20) | 1.42 (0.21) | 1.62 (0.64) |
| 2.5 | 0.65 (0.11) | 0.96 (0.26) | 3.45 (1.16) | 2.92 (1.76) | |
| 5 | 0.44 (0.11) | 0.27 (1.53) | 2.99 (0.88) | 4.52 (1.85) | |
| 25 | 1 | 1.28 (0.13) | 0.90 (0.13) | 4.28 (0.32) | 1.56 (0.23) |
| 2.5 | 1.48 (0.15) | 0.72 (0.16) | 5.04 (0.39) | 1.79 (0.25) | |
| 5 | 0.90 (0.39) | 0.27 (2.79) | 6.67 (2.15) | 4.97 (2.44) | |
| 40 | 1 | 1.63 (0.87) | 0.69 (0.62) | 4.91 (2.23) | 1.52 (0.80) |
| 2.5 | 1.71 (0.51) | 0.79 (0.80) | 6.67 (1.30) | 2.08 (1.17) | |
| 5 | 1.60 (0.98) | 0.26 (3.05) | 9.85 (2.10) | 4.07 (0.99) |
Data are presented as mean (standard error).
Bacterial density studies.
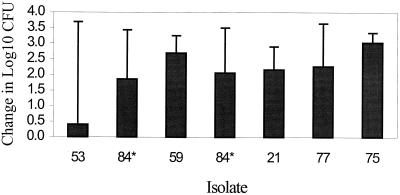
ICR mice received 0.1 ml of a 105 to 107 CFU/ml suspension of S. pneumoniae in each thigh. Time zero cultures at 2 h post-thigh inoculation (prior to initiation of therapy) revealed good bacterial recovery from tissue, as the median log CFU was 5.08 (range, 4.04 to 5.67). Organisms also grew well in untreated control animals over the 24-h study period, as the median log change in CFU for control animals was 2.21 (range, 0.4 to 3.03) (Fig. 1). Isolate 84 was utilized in two experimental runs using differing gatifloxacin exposures, and growth controls were similar in each run, demonstrating reproducibility of growth kinetics within the strain (Fig. 1).
FIG. 1.
Growth of S. pneumoniae in the thighs of infected mice not receiving gatifloxacin (controls). Values represent means ± standard deviations. ∗, isolate 84 was utilized twice.
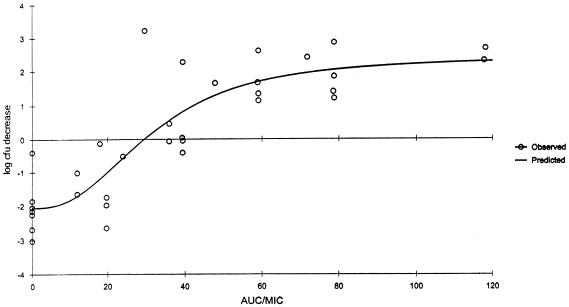
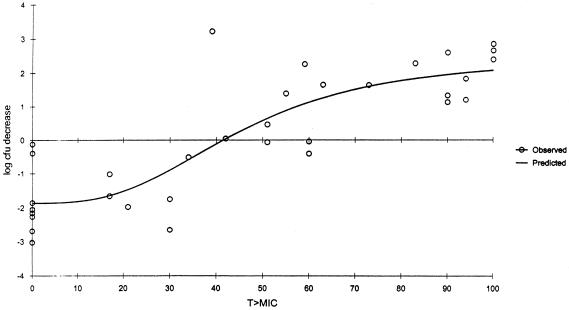
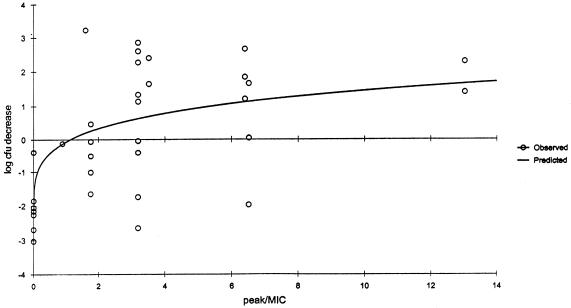
Significant correlations (Spearman's rank correlation coefficient P ≤ 0.001) were observed for the relationships between the change in CFU and AUC/MIC ratio (r2 = 0.8647); the change in CFU and percent T > MIC (r2 = 0.8445); and the change in CFU and peak/MIC ratio (r2 = 0.6428) for gatifloxacin (Fig. 2, 3, and 4). While all three pharmacodynamic parameters were correlated, the strongest correlation was found to exist between the change in CFU and AUC/MIC ratio. A bacteriostatic effect appeared to occur at an AUC/MIC ratio of approximately 30, while maximum bactericidal effects were observed when the AUC/MIC ratios approached approximately 40.
FIG. 2.
Decrease in log CFU at 24 h versus AUC/MIC ratio for neutropenic (ICR) mice. Each data point represents the mean of six to eight thighs. r2 = 0.8647.
FIG. 3.
Decrease in log CFU at 24 h versus percent T > MIC of neutropenic (ICR) mice. Each data point represents the mean of six to eight thighs. r2 = 0.8445.
FIG. 4.
Decrease in log CFU at 24 h versus peak/MIC ratio for neutropenic (ICR) mice. Each data point represents the mean of six to eight thighs. r2 = 0.6428.
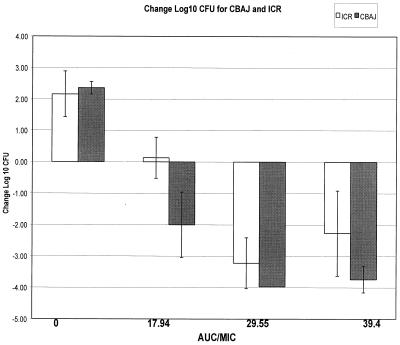
A comparison of the efficacy of gatifloxacin, as assessed by change in bacterial density, was undertaken in ICR (neutropenic) and CBA/J (nonneutropenic) mice using the same treatment regimens with isolate 21. Regardless of the immunocompetency of the mice, greater than 2 log growth was noted in control animals at 24 h (Fig. 5). All nonneutropenic groups demonstrated a significantly (P ≤ 0.05) enhanced killing of the isolate compared with that in the ICR mice that were correspondingly treated with the same dosing regimen of gatifloxacin which produced AUC/MIC ratios of 17.94, 29.55, and 39.4. As might be expected, the most profound effect of the immunocompetency of the CBA/J mice was noted when the ICR bacterial density was near static conditions, corresponding to an AUC/MIC ratio of approximately 15 to 20 (Fig. 5).
FIG. 5.
Comparison of ICR (neutropenic) and CBA/J (nonneutropenic) species for the relationship between log change CFU and AUC/MIC ratio. Each group contains data from eight thighs. Data were derived using one isolate, 21.
Survival studies.
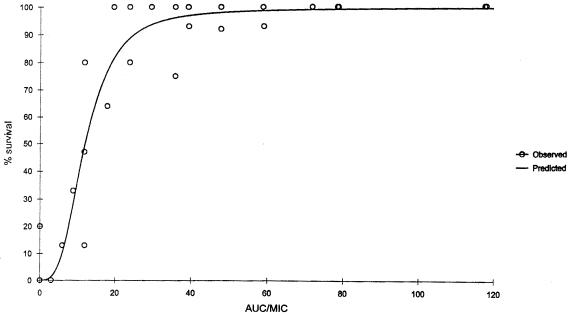
Overall mortality in untreated control mice injected with the pneumococcal isolates was 97%. As noted for bacterial density, a significant correlation (P ≤ 0.001) was noted for the relationships between percent survival and AUC/MIC ratio (r2 = 0.9640); percent survival and peak/MIC ratio (r2 = 0.9265); and percent survival versus percent T > MIC (r2 = 0.9199) for gatifloxacin. Although all three pharmacodynamic parameters examined were correlated, the strongest correlation was found to exist between the percent survival and AUC/MIC ratio (Fig. 6). In the neutropenic model, the maximum effect on survival appeared to occur at AUC/MIC ratios between 30 and 40.
FIG. 6.
Percent survival versus AUC/MIC ratio for neutropenic (ICR) mice. Each data point represents 10 to 15 mice. r2 = 0.9640.
A comparison of percent survival between neutropenic and nonneutropenic mice using isolate 21 demonstrated increased efficacy of gatifloxacin in the immunocompetent group, especially at AUC/MIC ratios between 6 and 30 (Table 3). While the neutropenic mice demonstrated an increasing percent survival that was proportional to AUC/MIC ratios over the range of 6 to 40, 100% of the immunocompetent mice survived even when AUC/MIC ratios were <10.
TABLE 3.
Cumulative survival of ICR (neutropenic) and CBA/J (nonneutropenic) mice after infection with S. pneumoniae isolate 21 and 4 days of gatifloxacin therapya
| AUC/MIC ratio | % Survival
|
|
|---|---|---|
| ICR | CBA/J | |
| 6 | 13 | 100 |
| 9 | 33 | 100 |
| 12 | 47 | 100 |
| 18 | 64 | 100 |
| 30 | 100 | 100 |
| 40 | 93 | 100 |
100% mortality was observed in all untreated control animals in both strains.
DISCUSSION
Since their introduction in clinical practice, numerous studies have investigated the most appropriate way to administer fluoroquinolone antibiotics to maximize bacterial killing and minimize toxicity. Currently available pharmacodynamic data on this class of compounds suggest that the AUC/MIC ratio is the best predictor of success. However, while this pharmacodynamic parameter is generally accepted, considerable controversy still exists as to the required magnitude of exposure to obtain maximum effectiveness (2, 3, 10, 13, 15).
In the current study, we evaluated the pharmacodynamic relationship between gatifloxacin exposure and outcome as assessed by the change in bacterial density and survival in the pneumococcal thigh infection model over 24 and 96 h of therapy, respectively. S. pneumoniae isolates selected for study included a range of susceptibilities to gatifloxacin and, when combined with the dosage regimens utilized, provided a study strategy which enabled a broad view of the compound's dynamic profile. As demonstrated in Fig. 1, differences in the growth control are inherent in in vivo studies, due to natural variations between isolates, and similar growth differences have been published previously (11). Results obtained through analyses of these current gatifloxacin data reveal that all the pharmacodynamic parameters were correlated; however, the AUC/MIC ratio was the best predictor of success for both outcome parameters studied. Similar results demonstrating multiple correlates, with the strongest correlation associated with the AUC/MIC ratio, have been previously reported (3).
Additionally, our results are in accordance with previously reported in vivo findings which suggested the appropriateness of gatifloxacin AUC/MIC ratios as a dynamic predictor of response using a similar neutropenic infection model (D. Andes and W. A. Craig, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. P-0191, 1999). While the eight pneumococcal isolates utilized in this study were different from those used in the previous report, the 24-h AUC/MIC ratio exposures required to produce both a static and bactericidal effect in the infected thighs were similar. In our study gatifloxacin AUC/MIC ratios of approximately 40 appeared to optimize the dynamic response, as judged by the bactericidal activity of the compound within infected thighs.
Our in vivo findings reflect those obtained in recent in vitro models which have evaluated the influence of drug exposure on both the kill and regrowth profiles of pneumococci (6–8). Each of these in vitro studies determined that an AUC/MIC ratio between 30 and 64 for pneumococci was sufficient to result in rapid and sustained bactericidal activity.
We have also demonstrated a similar significant correlation between data obtained from the survival studies and AUC/MIC exposures. Using this outcome parameter it appears that AUC/MIC ratios between approximately 30 and 40 produce maximum survival in immunocompromised hosts infected with lethal inocula of S. pneumoniae.
In the current study, a comparison of bacterial density determinations between the immunocompromised (ICR mice) and the immunocompetent (CBA/J mice) host using the same pneumococcal strain demonstrates greater treatment efficacy in the immunocompetent species. As might be expected, the most notable contribution of the host defenses was observed when static doses (i.e., AUC/MIC ratios of 15 to 20) were administered. Similar to improved bactericidal activity of gatifloxacin in the presence of functional white blood cells, the percent survival for animals infected with a lethal inoculum was 100% despite AUC/MIC ratios of less than 10. While a slight increase in free drug was noted for the CBA/J mice (90%, versus 80% for ICR species) during the protein binding studies, this profound difference in observed survival is most likely related to the presence of white blood cells, since it is doubtful that this relatively small difference in free drug would account for such dramatic changes in treatment outcome.
In conclusion, our data demonstrate the appropriateness of the gatifloxacin AUC/MIC ratio as a dynamic predictor of response to lethal pneumococcal disease and show that AUC/MIC ratios of at least 40 appear to optimize the dynamic response to infection. Lastly, these data further display the importance of the immune status of the host and the need for maximal effective in vivo drug exposures when these systems are compromised.
ACKNOWLEDGMENTS
We thank Jeff Mather for his statistical consultation.
This study was supported by a grant from Bristol-Myers Squibb, Princeton, N.J. C. H. Nightingale has financial relationships with Bristol-Myers Squibb.
REFERENCES
- 1.Andes D, Craig W A. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob Agents Chemother. 1998;42:2375–2379. doi: 10.1128/aac.42.9.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drusano G L, Johnson D E, Rosen M, Standiford H C. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas sepsis. Antimicrob Agents Chemother. 1993;37:483–490. doi: 10.1128/aac.37.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forrest A, Nix D E, Ballow C H, Gross T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamm T E. Proposed institutional animal care and use committee guidelines for death as an end point in rodent studies. AALAS Contemp Top. 1995;34:69–71. [Google Scholar]
- 5.Joly-Guillou M-L, Wolff M, Pocidalo J-J, Walker F, Carbon C. Use of a new mouse model of Acinetobacter baumannii pneumonia to evaluate the postantibiotic effect of imipenem. Antimicrob Agents Chemother. 1997;41:345–351. doi: 10.1128/aac.41.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacy M K, Lu W, Xu X, Tessier P R, Nicolau N P, Quintiliani R, Nightingale C H. Pharmacodynamic comparisons of levofloxacin, ciprofloxacin, and ampicillin against Streptococcus pneumoniae in an in vitro model of infection. Antimicrob Agents Chemother. 1999;43:672–677. doi: 10.1128/aac.43.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lister P D, Sanders C C. Pharmacodynamics of levofloxacin and ciprofloxacin against Streptococcus pneumoniae. J Antimicrob Chemother. 1999;43:79–86. doi: 10.1093/jac/43.1.79. [DOI] [PubMed] [Google Scholar]
- 8.Lister P D, Sanders C C. Pharmacodynamics of trovafloxacin, ofloxacin, and ciprofloxacin against Streptococcus pneumoniae in an in vitro pharmacokinetic model. Antimicrob Agents Chemother. 1999;43:1118–1123. doi: 10.1128/aac.43.5.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 10.Nicolau D P. Fluoroquinolone pharmacokinetics, pharmacodynamics, and drug interactions. Formulary. 1998;33(3 Suppl.):S27–S33. [Google Scholar]
- 11.Nicolau D P, Onyeji C O, Zhong M, Tessier P R, Banevicius M A, Nightingale C H. Pharmacodynamic assessment of cefprozil against Streptococcus pneumoniae: implications for breakpoint determinations. Antimicrob Agents Chemother. 2000;44:1291–1295. doi: 10.1128/aac.44.5.1291-1295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onyeji C O, Bui K Q, Owens R C, Jr, Nicolau D P, Quintiliani R, Nightingale C H. Comparative efficacies of levofloxacin and ciprofloxacin against Streptococcus pneumoniae in a mouse model of experimental septicaemia. Int J Antimicrob Agents. 1999;12:107–114. doi: 10.1016/s0924-8579(98)00087-9. [DOI] [PubMed] [Google Scholar]
- 13.Quintiliani R, Nicolau D P, Nightingale C H. Pharmacokinetic and pharmacodynamic principles in antibiotic usage. In: Johnson J T, Yu V L, editors. Infectious diseases and antimicrobial therapy of the ears, nose and throat. W. B. Philadelphia, Pa: Saunders; 1997. pp. 48–55. [Google Scholar]
- 14.Tateda K, Takashima K, Miyazaki H, Matsumoto T, Hatori T, Yamaguchi K. Noncompromised penicillin-resistant pneumococcal pneumonia CBA/J mouse model and comparative efficacies of antibiotics in this model. Antimicrob Agents Chemother. 1996;40:1520–1525. doi: 10.1128/aac.40.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas J K, Forrest A, Bhavnani S M, Hyatt J M, Cheng A, Ballow C H, Schentag J J. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob Agents Chemother. 1998;42:521–527. doi: 10.1128/aac.42.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]