Abstract
Four sensory systems (vestibular, lateral line, electroreception, auditory) are unique and project exclusively to the brainstem of vertebrates. All sensory neurons depend on a common set of genes (Eya1, Sox2, Neurog1, Neurod1) that project to a dorsal nucleus and an intermediate nucleus, which differentiate into the vestibular ear, lateral line and electroreception in vertebrates. In tetrapods, a loss of two sensory systems (lateral line, electroreception) leads to the development of a unique ear and auditory system in amniotes. Lmx1a/b, Gdf7, Wnt1/3a, BMP4/7 and Atoh1 define the lateral line, electroreception and auditory nuclei. In contrast, vestibular nuclei depend on Neurog1/2, Ascl1, Ptf1a and Olig3, among others, to develop an independent origin of the vestibular nuclei. A common origin of hair cells depends on Eya1, Sox2 and Atoh1, which generate the mechanosensory cells. Several proteins define the polarity of hair cells in the ear and lateral line. A unique connection of stereocilia requires CDH23 and PCDH15 for connections and TMC1/2 proteins to perceive mechanosensory input. Electroreception has no polarity, and a different system is used to drive electroreceptors. All hair cells function by excitation via ribbons to activate neurons that innervate the distinct target areas. An integrated perspective is presented to understand the gain and loss of different sensory systems.
Keywords: neurons, brainstem nuclei, hair cells, bHLH genes, Sox2, Eya1, Lmx1a/b
1. Introduction
Sensory maps depend on the specific sensory modality and the relevant information to be extracted by them. Beyond primary sensory maps, central map formation underlies the integration of various sensory modalities, namely the ear, lateral line and electroreception. The four primary sensory maps of vertebrates have unique features and seemingly use distinct molecular cues, cell cycle exit and activity combinations during development, regeneration and plasticity. The evolution of chordates is comparable with the organization of the dorsal spinal cord and brainstem, which is associated with neurons and hair cells in 71,000 vertebrates. On the other hand, we have limited support for the two chordates associated with the neural crest and placodes, hair cells and central brainstem in 31 species of lancelets and 3100 species of ascidians. Fossils appeared approximately 540 million years ago (Mya), and all major bilaterian phyla presented by 500 Mya [1].
The brainstem of vertebrates is organized into rhombomeres (r0–11) that superficially resemble other chordates, lancelet and ascidians [2–4]. A dorsal part of the brainstem expresses a continuation to the spinal cord in vertebrates [5] which is absent in a true brainstem in other chordates. Partial similarity is found in ‘dorsal root ganglia’ in ascidians that resembles the spinal cord in vertebrates, which is absent in lancelets [2,6,7]. Adding these differences in chordates, gene duplication [8], followed by diversification [9,10], is the basis for the unique brainstem, neurons and hair cells that developed in vertebrates [11]. The unique formation of mechano- and electroreception evolved in four distinct sensory inputs that are partially similar with the lateral line of ascidians [6,12–14], The progression must start with the sensory neurons that connect all neurons with the brainstem and reach out the peripheral sensory hair cells.
Neurons depend upon Eya1 [15], Sox2 [16], Neurog1 [17] and Neurod1 [18]. In contrast to Neurog1 null mice, which showed a complete loss of neurons [19], Neurod1 null mice showed residual neurons extending centrally to smaller vestibular and cochlear nuclei [20,21] that reached the ear [22,23]. It is worth noting that the lateral line and electroreception are separate for the vertebrate ear that is lost in most tetrapods to generate novel cochlear neurons, the spiral ganglion neurons (Figure 1).
Figure 1.
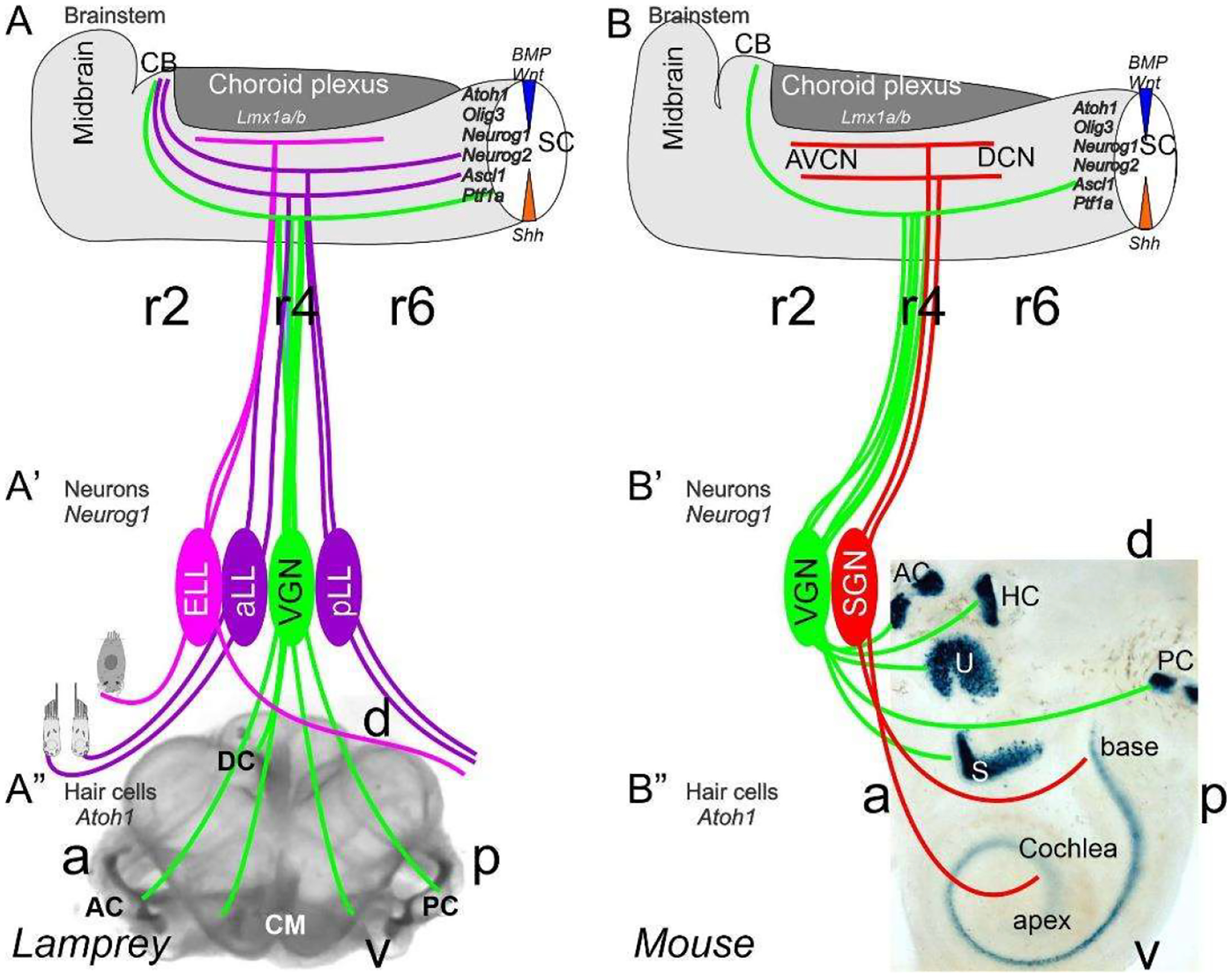
Inner ear, Lateral line and electroreception revealed. Neurons (Neurog1; A′) form vestibular ganglia (VGN) to reach out 4 hair cell organs in lampreys (A″). A separate lateral line (LL) and electroreceptor neurons (ELL) that innervate hair cells project more dorsal in lampreys. Central projection depends on Atoh1 to receive LL and ELL fibers, whereas several bHLH genes (Neurog1/2, Olig3, Ascla1, Ptf1a) receive all VGN (A). In the absence of ELL and LL development in amniotes, mammals develop separate spiral ganglion neurons (SGN; B′) that extend from the cochlea (B″) and end in a topological central projection that depends on Atoh1 (B). The formation of VGNs (Neurog1; B′) reach the 5 hair cells (B″) to extend the distribution of bHLH genes. Note that certain areas are lost or gained which enter central projections near r4. Images are shown by miR-183 ISH (A″) and Atoh1-LacZ (B″). AC, anterior crista; AVCN, anteroventral cochlear neurons; CB, cerebellum; aLL, pLL, anterior/posterior lateral line neurons; CM, common macula; DC, dorsal crista; DCN, dorsal cochlear neurons; HC, horizontal crista; PC, posterior crista; r2/4/6, rhombomeres; S, saccule; SC, spinal cord; U, utricle. Modified after [11,30,31].
The brainstem is a continuation of the spinal cord (SC; [11,24,25]) that develops into rhombomeres and differentiates into nuclei, namely the vestibular, lateral line and electroreception nuclei in basal vertebrates (Figure 1). Loss of the lateral line and electroreception leads to the development of cochlear nuclei in tetrapods [26,27]. All dorsal expression of the brainstem depends on Lmx1a/b [28] and Gdf7 [29], which drive the choroid plexus (Figure 1). Combined, Lmx1a/b and Gdf7 regulate the formation of Wnt1/3a, BMP4/7 and Atoh1. This formation is likely reduced or absent in Neurog1/2, Ascl1, Ptf1a and Olig3, among others (Figure 1).
Mechanosensory and electrosensory hair cells (Figure 1) depend on Eya1, Sox2 and Atoh1 to initiate the cell cycle and to differentiate into vestibular, cochlear, lateral line and electrosensory hair cells [22,32,33]. Planar cell polarity (PCP) depends on the formation of shifting the central projection of the kinocilium into a lateral position. PCP extends the length of the stereocilia to develop the staircase of tip links of the vestibular, cochlear and lateral line hair cells [34–36]. The next step involves the development of the tip links to allow the connections between CDH23 and PCDH15 to open up the channel to form a mechanosensory hair cell [37,38], with opposing polarity in most of the ear and lateral line [34,39–41]. TMC1/2 provides a major function that seems to interact with additional channel proteins (TMHS, TMIE), forming a complex interaction [37,42–44]. In contrast, while the electroreception forms next to lateral line hair cells [22,23,45], these hair cells lack any polarity organization, and certain ampullary hair cells are dependent on Cav1.3 [46].
This review will compare the three neurosensory components that form the neurons which, on the one hand, connect to the brainstem for input, and, on the other hand, receive the hair cells for sensory input. Gene regulation of neurons, central nuclei and hair cells is driven by gene duplication and diversifies after chordates diverge from vertebrates [10], leading to the gain and loss of three sensory systems (lateral line, electroreception, auditory). Gene regulation explains the diversification of the vestibular system from three hair cells up to nine hair cell populations, including the cochlea of mammals [3,47],
2. Neurons Depend upon Eya1, Sox2, Neurog1 and Neurod1
The ear, lateral line and electroreception neurons depend on genes that, collectively, define their development. Upstream of bHLH genes, which initiate the proliferation of neurons, is the expression of Eya1, which interacts with Brg1 to initiate pro-neurosensory development [15,48,49]. In the absence of Eya1, there is no neuronal development that allows ear formation, and neither neurons nor hair cells differentiate [15]. Evolving neurons start in the lancelet, which lack dorsal root ganglia. The dorsal root ganglia show partial expression of Neurog inside the spinal cord (Figure 2), which lacks an Atoh gene [50,51]. In contrast, at least a smaller set of bHLH genes are partially characterized in the developing ascidian, Ciona [52], which have at least six bHLH genes driving neuron development: Ptf1a, Tcf3, Atoh, Ascl and Neurog [7,12]. A detailed serial section analysis shows the innervation of sensory cells (Atoh) from fibers of the neurons (bipolar tail neurons; Figure 2) that can trace to reach the anterior motor ganglion [13]. Neither the full expression of Eya nor Sox2 outside the neural plate are unclear in the lancelet and tunicates [2,52].
Figure 2.
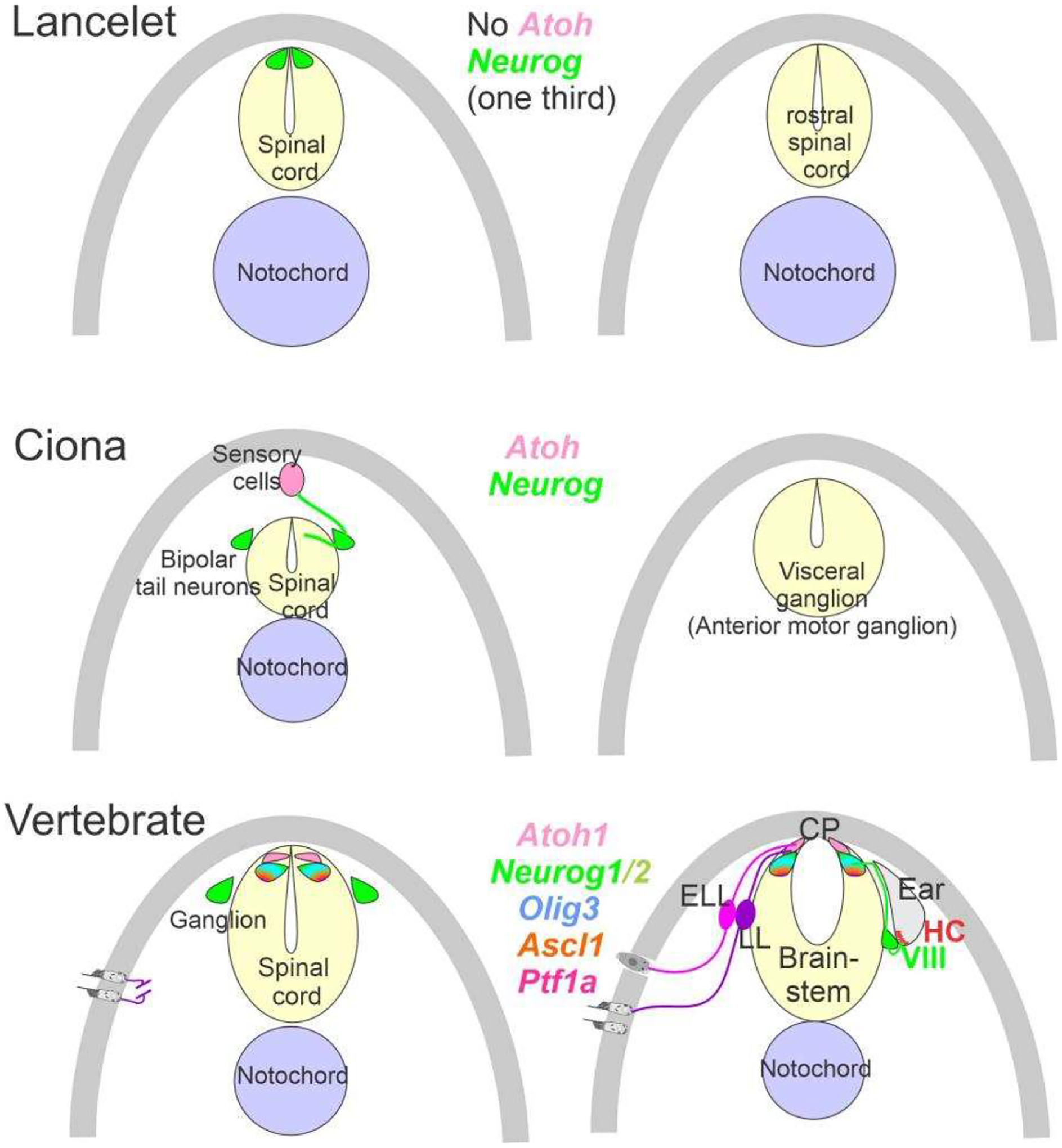
Neurons require Neurog expression. Lancelets have a limited description of bHLH genes that are characterized in the more caudal spinal cord, which is positive for Neurog. Note that the lancelet has no Atoh bHLH gene. Ciona has at least 6 bHLH genes expressed in sensory cells that are innervated by bipolar tail neurons which extend to reach the visceral ganglion for interactions. Atoh and Neurog genes are described in Ciona associated with the spinal cord. Vertebrates have dorsal root ganglia that depend on Neurog1/2, which is also expressed in Atoh1 and Neurog1 of the spinal cord. The brainstem is innervated by electroreceptor (ELL) and lateral line fibers (LL) that extend to innervate migration populations of LL and some ELL). The ear is unique in vertebrates, which give rise to the VIII ganglia that innervate more ventral nuclei compared to LL and ELL projections to reach Atoh1. CP, choroid plexus. Modified after [2,7,12,23,24].
A crucial next step is the initiation of Sox2, which is needed to upregulate Neurog1 [53–55]. In fact, Sox2 delays certain neuron development in bony fish [56], and in the presence of Sox2 is unclear the sequence of gene regulation in the lamprey and hagfish [57]. There is a distinct effect of the loss of early genes in the vestibular ganglion, which initially differentiates in the absence of Sox2 and Neurog1 (Figures 1 and 2) and does not develop in the auditory neurons [16]. A loss of all auditory neurons, and partial loss of vestibular neurons, are known for Pax2 [58], Gata3 [59], Lmx1a/b [28], Fgfr2 [60], Shh [61] and Dicer [62]. Partial loss of some vestibular neurons are known for Fgf10 [63] and Foxg1 [64,65], indicating a limited loss of sensory hair cells and/or neurons. Unfortunately, the details of the lateral line and electroreception (Figures 1–3) are not as fully genetically characterized [22,23,27,33]. The lateral line and electroreception likely depend on neuronal development (Figures 1 and 2), including the development of spinal ganglia neurons [66] and trigeminal neurons [67–69]. A separate placode is derived from neurons that develop from Neurog1 in mammals [68,70]. In birds, this placode is driven by Neurog1 [71,72]. Furthermore, separate amniotic paratympanic placodal neurons innervate separate hair cells that partially integrate into the central vestibular projection [72].
Figure 3.
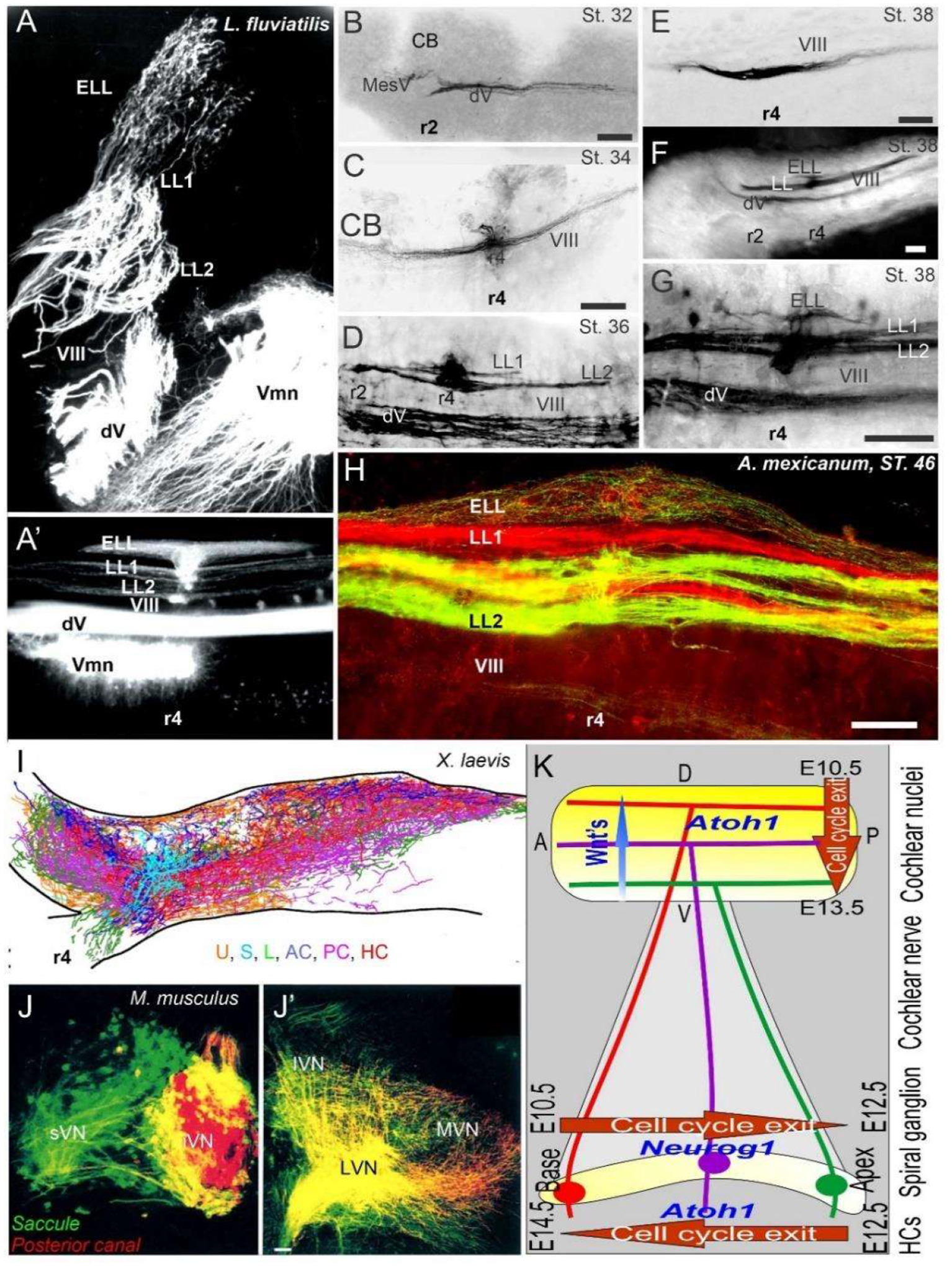
Central projections form afferents to distinct innervation. The lateral line of 2 or more branches form, whereas electroreception receives the short dorsal projection in lampreys (A,A′) and salamanders (B-H). Vestibular projection forms after the trigeminal central projection, followed by the lateral line and electroreception (B-H). Central projection in a frog (I) and mammal (J,J′) show the incomplete distribution of distinct neurons (J) that overlap and incompletely segregate the vestibular projection (I,J′). Spiral ganglia (K) proliferate neurons in a base to apex progression (E10.5–12.5) that reach the central projection to form a topology from dorsal to ventral cochlear nuclei (E10.5–13.5), depending on Wnt expression. Later, hair cells proliferate from apex to base (E12.5–14.5) that reach the afferents. AC, anterior crista; dV, trigeminal afferents; ELL, electroreception; HC, horizontal crista; LL1/2; lateral line; L, lagena; LVN, lateral vestibular nuclei; IVN, inferior vestibular nuclei; iVN, inferior vestibular neurons; MVN, medial vestibular nuclei; PC, posterior crista; S, saccule; sVN, superior vestibular neurons; U, utricle; Vmn, trigeminal motoneurons; VIII, vestibular projections. Modified after [3,23,67,123].
In addition to directly initiating the formation of neurons by Eya1, Sox2, Pax2 and Neurog1/2, another set of genes are regulated to differentiate into Neurod1 [18,20,21,71,73], followed by Isl1, Foxg1, Pou4f1 and Phox2b [71,74–76], which interact with Shh, BMPs and Wnts to define neurons [77,78]. Regional regulation of the distinct vestibular, lateral line, electroreception and auditory neurons are sorted out by downstream genes regulating the distinct innervation. For example, the expression of Calbindin, Calretinin, Pou4f1 and Peripherin is required to sort out the innervation from the inner and outer hair cells [79–82]. In Sox10 null mice, an interaction showed disorganized cochlear neurons, whereas the development of vestibular neurons was near normal [83]. This interaction is consistent with the loss of Erb2 of nearly all cochlear neurons, as well as reduced vestibular neurons [84]. The concept of having multiple sources of neurons from the placode and neural crest is likely due to a misinterpretation [3,83,85–87].
Downstream of gene development, the expression of TrkB (Ntrk2) and TrkC (Ntrk3) has a reduction and loss in vestibular and cochlear neurons. Vestibular neurons are mostly dependent on TrkB [88,89] whereas the cochlear neurons are mostly dependent on TrkC [90,91]. Loss of both neurotrophin receptors causes the early loss of all neurons [92–94]. Limited expression is characterized in some ascidians which are unknown in the lancelet [1]. The comparable expression of the lateral line and electroreception are unclear due to the multiplication of neurotrophins in bony fish [95,96].
The proliferation of neurons and hair cells depend on MycN [97,98], which drives the division of the Gl, S and G2 phases with a set of genes that interactions with cell cycle regulation [53,99–101]. Detailed characterization and proliferation have been described in the ear and brainstem, clarifying cell cycle progression in mice and rats [102–104]. Sox2 and Neurog1 are in negative feedback, which allows proliferation and initiates differentiation. This differentiation interacts with retinoblastoma (Rb), Hes/Hey and IDs to regulate the cyclin-dependent kinases (CDKs), cross-react with e-proteins and define whether a cell cycle is progressing [98,100,105,106]. In the end, continuation depends on either knocking out Rb to continue proliferation or upregulating of Sox2 to jumpstart proliferation [107,108].
In various vertebra, the central projection has been described to show the projection of the vestibular, lateral line, electroreception, and cochlea [3,67,87,109–111]. Three sets of central projections are known in vertebrates that develop a loss of the lateral line, electroreception and added cochlear nuclei [23,26,112]. For electroreception, these central projections always have a single set of an anterior ganglia (Figures 1 and 3) that adds variably the electroreception in bony fish [27,113]. Lateral line neurons (Figures 1–3) can be split into an anterior and posterior branch that diversify the neuromasts to innervate all lateral line hair cells (Figure 3; [114–116]). Vestibular neurons have two neuron populations in hagfish [57], while lampreys and jawed vertebrates have a single vestibular ganglion [111,117,118]. At least 4–5 distinct innervations are described in lampreys [119,120], whereas most gnathostomes have at least five and up to nine branches of vestibular and auditory connections (Figures 1 and 3): three canal cristae, utricle, saccule, lagena, basilar papilla, amphibian papilla and neglecta [121,122]. Branches of discrete neurons are known for an anterior and a posterior (superior) nucleus that innervates two canal cristae (anterior and horizontal cristae), the utricle and part of the saccule (Figure 3). The remaining part of the utricle provides a posterior canal and the branch of the saccule (Figures 1 and 3) in mammals [123]. The development of central projections follows a simple layout. First, the trigeminal and epibranchial neurons develop. Then, central projection follows. Subsequently, vestibular, lateral line and electroception develop, if present (Figure 3; [3,124]). Different developmental patterns exist in neuronal proliferation: nearly all neurons continue proliferation for a long time or lifetime, whereas mammals have an early production of neurons that ends proliferation very early [67,125,126]. The topology of peripheral neurons of the vestibular, lateral line and electroreceptors is unclear, suggesting an overlap with an incomplete segregation of neurons that is well known for the vestibular neurons (Figure 3 [123]).
A long-term proliferation of the vestibular, lateral line and electroreception is followed by a delayed formation of cochlear neurons, the spiral ganglia neurons (SGN), which follow vestibular neurons in mammals (vestibular neurons: E9–11; SGN: E10–12 [125,127]). A unique topological development is known among mammals [128], first showing the basal turn neurons (Figure 3), which reach the anteroventral, posteroventral, and dorsal cochlear nuclei (AVCN, PVCN, DCN). The development of these neurons is followed, with delay, by the apical neurons [67,87,110,129]. Interestingly, there are central projections that can form independently to reach the formation of cochlear nuclei [130]. In the absence of target hair cell development [92,131], cochlear neurons develop and largely proliferate prior to cochlear nuclei and cochlear hair cells (Figure 3). Central cochlea require the expression of Neurod1, Wnts, Fzd, Npr2 and Ephrins for targeted central projections [21,129,132,133].
In contrast to the topology of the cochlear nuclei [11,128], the central vestibular neurons have an incomplete central segregation (Figure 3) that shows both segregation and overlap from different vestibular neurons [3,123,134]. Lateral line central projections can be segregated in certain vertebrates but show an overlap in other vertebrates [3,23]. For electroreception, multiple central topological projections in certain bony fish [27,135] show an overlap in lampreys and salamanders (Figure 3 [23,109]). The vestibular, lateral line, electroreception and cochlea independently reach hair cells that form prior to neurons [23,136], consistent with the same pattern of neurons that develop first, followed by the central axon to the brainstem, and later followed by the hair cell innervation [3,109,134,137]. This is obvious in cases where hair cells are not formed, such as in Atoh1 null mice, which show a near-normal central projection [131,138]. A similar central projection forms after the loss of hair cells in Pou4f1 null mice [139]. Loss of formation of a specific set of hair cells is demonstrated in the posterior canal that projects normally, despite the absence of Fgf10 [63], which degenerates later.
In summary, the neurons of the ear, lateral line and electroreception are generated by a set of genes that act downstream of Neurog1 to initiate the cell cycle. Neurons develop independently of central axons and reach innervate the hair cells shortly after proliferation. Segregation of central projections can be topologically organized in the auditory central projection of most tetrapods, and present two lateral line neurons that segregated in many vertebrates. Some central topology found in some, but not all, lateral line and electroreceptors, show an incomplete segregation for the vestibular neurons.
3. The Brainstem Is Transformed from the Spinal Cord
The spinal cord and rhombomeres (r0–11) of the brainstem [140,141] are basically identical in terms of the distribution of overall gene expression [24,25]. The distribution of gene expression in the spinal cord and rhombomeres differentiates into a unique population of r0–7 [142–145]. The earliest genes— Gemini (Gmnn), Zic and Foxd4 [146–148]—define the neural ectoderm, which cooperates with Smarca/Brg-related genes to induce neural ectoderm. Certain interactions can become more complicated and can, for example, be downstream from Zic1 by Wnt1 and cooperate with Fgf, Noggin/Chordin and Nodal, which counteract with BMPs while Dkk/Cerberus counteracts Wnt. Interestingly enough, certain aspects of Wnt are independently regulated from Wnt3a, defining more variations among the large family of Wnts [149,150]. A major role for the invaginating of neuroectoderm depends on Shh and Gli to induce ventral formation, which counteracts with BMPs and Wnts to define the dorsal part of the brainstem and induces the motoneurons [4,151,152].
Recent work has shown that a unique formation of the choroid plexus in the brainstem depends, at least, on two genes: Lmx1a/b and Gfp7 [29,153,154]. In the absence of Lmx1a/b double-null mice, the choroid plexus disappears (Figure 4), transforming the dorsal part of the brainstem and cerebellum into a continuation from spinal cord to the midbrain [11,28].
Figure 4.
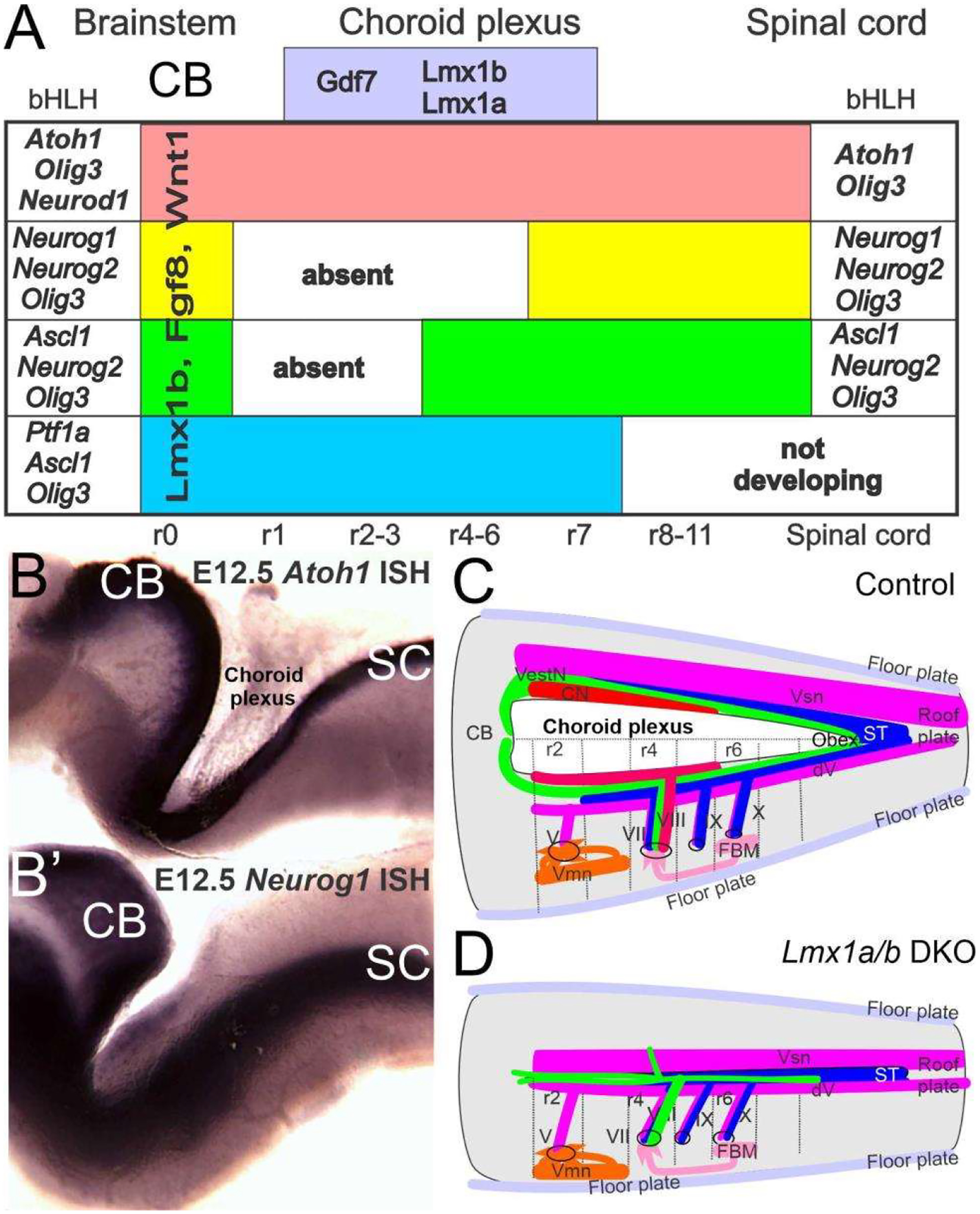
The brainstem depends on Lmx1a/b, Gdf7 and bHLH genes. The choroid plexus is unique, forming in the brainstem. The choroid plexus depends on Lmx1a/b and Gdf7 (A), and is replaced for the roof plate in the spinal cord (C,D). Downstream are bHLH genes that have been identified in the Atoh1 (A,B), Neurog1 (A,B′), Neurog2, Neurod1, Ascl1, Olig3 and Ptf1a. Certain expression is unique for the vestibular and auditory nuclei: Ptf1a is a duplication of ventral genes that are replaced by more rostral genes (Neurog1, Ascl1). Lmx1b, Fgf8 and Wnt1 are common cerebellums (CB) of r0. In the absence of Lmx1a/b and choroid plexus, no cochlear nuclei form and vestibular, trigeminal and solitary tract interact across the roof plate (C,D). dV, trigeminal fibers; FBM, facial branchial motoneurons; ST, solitary tract; V, VII, VIII, IX, X, afferent fibers; Vmn, trigeminal motoneurons; Vsm, trigeminal nucleus. Modified after [28,82,157].
Gene expression of Eya1 [74,155], followed by Sox2 [15,53,54], is needed to upregulate proneuronal formation. In addition, a set of bHLH genes [5,24,25] is required to initiate the formation of neurons. Only two bHLH genes, Atoh1 and Olig3, are expressed throughout the spinal cord and brainstem [5,25,156] that is diversified in the more rostral part of the brainstem into the cerebellum and auditory nuclei [157]. The formation of all neurons that depend on Atoh1/Oligi shows complete loss of all Atoh1 expression genes [158].This formation has been demonstrated using Wnt1-cre upstream of Atoh1, leaving only the choroid plexus in Atoh1 null genes [130,156]. In contrast, some AtoM-positive cells develop in Olig3 null mice that have changed the definition of the effect without Olig3 [145,159]. Loss of Gdf7 [29] and Lmx1a/b double-null mice [154] abolishes Atoh1 expression, Olig3 remains that may or may not expressed in Gdf7/Lmx1a/b mice (Figure 4).
A complex interaction is generated by feedback loops. J Johnson showed the cross-repression of Atoh1-Neurog1 in a reciprocal interaction to sharpen the boundaries of Atoh1 and Neurog1/2 in the spinal cord [24]. Different expression levels define (from roof plate) Atoh1, Neurog1/2, Ascl1 and Ptf1a. In addition, roof plate is regulated by Gdf7 and Lmx1a/b to follow a gradient of high levels of BMP and Wnt. Atoh1-Neurog1/2 is not only repressed, but is also expanded by Ascl1. This expansion defines most ventral fate and expresses Neurog1/2 adjacent to the same expression. Ptf1a is, again, a repression interaction with Ascl1 and defines a subdomain in the spinal cord [24] and brainstem.
In comparison to the spinal cord, certain gains and losses of domains are clear. For example, another unique step is driven by an apparent Ptf1a duplication in the brainstem [25], which results in Ptfila null mice, a specification of more dorsal into a different state of r0–7 [142,143,145]. More complex loss of Neurog1/2 in r1–6 and part of Ascl1 in r1–3 replaces the more dorsal expression of Ptf1a [25,143]. A more rostral reduction of these two domains requires additional research to explain the distinct effects of Ptf1a null mice [142,143]. In essence, the spinal cord has six identical domains (A1–3, B1–3) that differ from the rhombencephalon, showing the differential gains and losses of two domains (dA2, dA3). The spinal cord has the ability to develop two additional domains, for a total of eight domains, (A1–4, B1–4) which highlights the gains and loss of selective bHLH genes [25].
In addition to this cross-interaction, the spinal cord is further expanded by another bHLH set of genes, the Hes/Her genes [53,160] and the ID genes [9,99,161]. Starting with Sox2 expression, the neurosensory precursor cells are self-renewing and are driven by the Hes, ID and Myc genes to enhance proliferation [105]. The expansion changes by an oscillation to interact with Hes/Ascl1, for example. It is important to understand that the Notch interaction allows neurons to differentiate while precursors remain as neural stem cells. In the dorsal part of the spinal cord and brainstem, the genes interact with Atoh1, Neurog1/2, Olig3, Ascl1 and Ptf1a among proneuronal bHLH genes. Diversity is driven by distinct ways to generate astrocytes. In contrast to a downregulation of Hes/Id/Myc, Sox2 is essential for neurosensory cell formation to differentiate in astrocytes that remain in Hes, Id and Sox9, among others [54]. In contrast, oligodendrocytes are equally downregulated, such as in neuronal differentiating cells through upregulation by Olig1/2 and Sox10.
Atoh1, Neurog1/2, Olig3, Neurod1 and Ptf1a, among others [145,157], define the cerebellum (Figure 4). A delayed expression of Neurod1 adds to the interaction by providing negative feedback for the cerebellum of at least Atoh1 [157,162], which expands along the auditory nuclei for feedback. Likewise, identical expression in the hindbrain shows a near-equal expression of Atoh1 (rostral) and Neurod1 (caudal). However, in the adult system, a different level of Atoh1, which shows a much higher level of expression in the auditory nuclei, supposedly counteracts with Neurod1 out of two nuclei, particularly the dorsal cochlear nucleus [157]. In summary, the cerebellum depends on multiple genes (Olig3, Atoh1, Neurod1, Ptf1a, among others), and the exact genes are unclear in lamprey and hagfish [145,157,163].
Lmx1a/b, Fgf8 and Wnt1 delineate the cerebellum [141,152,153]. In the absence of Lmx1a/b, fibers branch to reach unusual central projections of vestibular fibers that receive fibers from the trigeminal and the solitary tract, crossing the nearly closed roof plate (Figure 4). Consistent projections receive the innervation from the vestibular neurons or can expand to reach lateral line fibers in vertebrates (Figures 1 and 4). Neither the electroreception nor the cochlear fibers expand to reach the cerebellum that do not expand beyond r2 (Figures 1 and 4). Certain changes in the auditory fibers can transiently trace to reach the cerebellum in certain mutations [129,164] that never directly reach the electroreceptors [27,135].
Higher projection to the midbrain and telencephalon is known among auditory, vestibular, lateral line and electrorections. However, this topic is out of the scope of this review [3,26,27].
In summary, the four dorsal nuclei depend on bHLH genes that define a complex interaction by the gain and loss of other bHLH genes that cross-correlate, for example, Atoh1 and Neurod1 in the cerebellum and auditory nuclei. Without Lmx1a/b, there is a loss of the choroid plexus, as well as the loss of Atoh1 and likely other more dorsal brainstem genes (Neurog1, Neurog2, Neurod1, Olig3, Ascl1 and Ptf1a).
4. Hair Cells Depend on Eya1, Sox2 and Atoh1
Mechanosensory hair cells are shared among the vestibular, cochlear, lateral line, electroreceptor and Merkel cells, a unique late addition to trigeminal sensory information [3,11,135,165]. Hair cells and Merkel cells depend on Atoh1 for differentiation [166,167]. Evidence suggests that hair cells evolved from single-cell organisms, called choanoflagellates [32,47], which transformed a single kinocilium surrounded by villi (Figure 5) into distinct hair cells, the mechano- and electrosensory hair cells. In addition to vestibular hair cells, the inner ear forms a set of 3–9 patches of hair cells, including the cochlear hair cells (Figure 1; [117,122]). Lateral line hair cells distribute from small clusters of hair cells, referred to as neuromasts (Figure 5), to form a large set of hair cells in sharks [23,115]. Electroreception can subdivide into the ampullary organs of basic vertebrates, various additional bony fish have evolved several sets of ‘electroreceptors’ (Figure 5; [22,27,46]).
Figure 5.
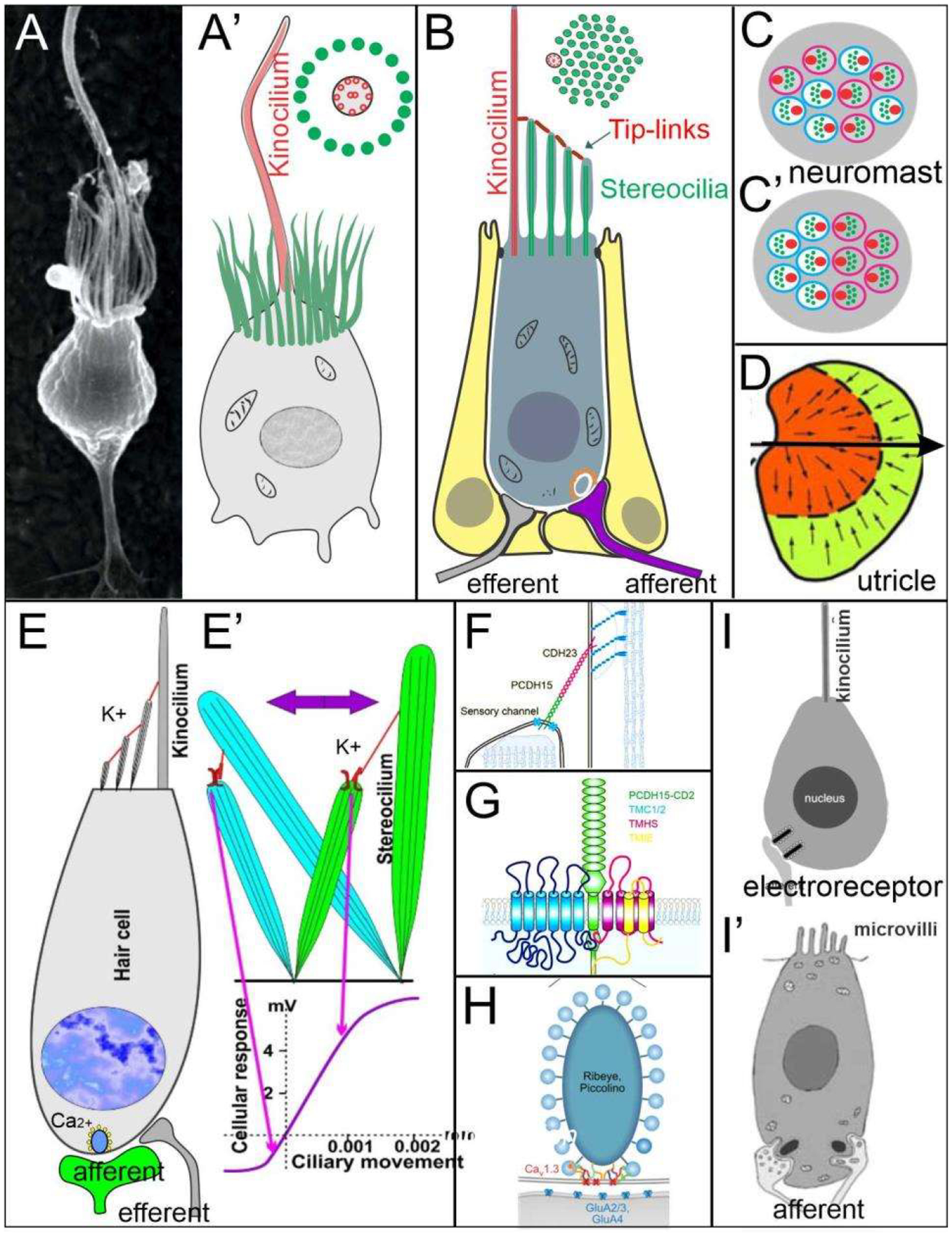
Mechanosensory hair cells evolve from single-cell organisms. Choanoflagellate (A,A′) are the basis of animals that evolved from a kinocilium surrounded by microvilli into an asymmetric staircase of mechanosensory hair cells (B,C,E) that forms the mechanoelectrical transduction channels (MET) of the lateral line (B,C,C′) and vestibular hair cells (E). The lateral line (C,C′) and some vestibular hair cells (D) are bipolar, whereas canal cristae and most auditory organs are polarized in 1 direction. Tip links depend on CDH23 and PCDH15 (F) that interact with Tmc and others (G) to open up the channel (E′) to allow K+ entrance. Ca2+ interactions with t ribbons to allow the release of glutamate (E,H). Electroreceptors are unpolarized and resemble Choanoflagellate that either show microvilli (I′) or only a central kinocilium (I). Modified after [22,37,47,196,211,212].
The vestibular ear requires a set of transcription genes to initiate the placode formation, starting with Foxi3 [168] and Fgf3/10 [63,169,170]. Downstream are Eya1/Six1 [49,171], Pax2/8 [58,172], Shh [78,173], BMPs [174,175] and Wnt’s [176–178] to form the otocyst, among other necessary genes [179], where they interact to define the dorso/ventral, anterior/posterior and lateral/medial divisions to develop the otocyst [180,181]. Further downstream is the expression for Sox2 upregulation [16,182]. Sox2 upregulation sets up the differentiation into hair cells, which depends on the cross-interaction of Atoh1 with Neurod1 [21,183], Pou4f3 [139,184,185], Gfi1 [184,186], Srrm/Rest [187,188] and Barhl1 [189,190], among others, which differ in efferent and afferent innervation [191–194].
Vestibular hair cells form maculae for gravistatic reception and canal cristae for angular receptions [47,195,196]. Polarity depends on function, but the distribution of hair cells differs. Only maculae have opposing maculae (Figure 5), whereas canal cristae are uniform in their polarity [117,191,196]. Canal cristae are also present in most auditory hair cells [122,197]. Sensory hair cells form Type I and Type II hair cells in amniotes have a common organization. All vertebrate hair cells have stereocilia organized in a staircase pattern, displaying distinct apical polarities for stimuli to open mechanoelectrical transduction channels (METs) by tip links using PCDH15 and CDH23 (Figure 5), permitting endolymphatic potassium to enter the HCs and change their resting potential [37,197,198]. The mammalian mechanosensory channel is, in part, formed by the transmembrane proteins Tmc1 and Tmc2 [38,199]. Other interactions are known, but these interactions require additional work for the MET formation (Figure 5). A unique formation of vertebrate hair cells is found in the Tmc1/2 single gene in cyclostomes [43]. Tmc1/2 is separated from the closely related gene, Tmc3. However, the function of Tmc3 is unclear nearly all animals, including basic animals, for which there is no information regarding its function.
Planar cell polarity (PCP) genes depend on Frizzled, Prickle, Disheveled, Van Gogh, Diego and Flamingo for normal development [200,201]. Polarization depends on Emx2 [41], which eliminates the contralateral organization in the utricle by converting it into a single polarity [202,203]. In addition, retinoic acid (RA) sets up various gradients [204]. Saccule and lagena have a different polarity. Instead of polarizing each other again in the utricle, they flip to organize in the saccule and lagena [191]. A distinct pattern of the utricle and saccule have a separate innervation from the cerebellum to reach one polarity (Figure 5D) and receive a descending branch of the caudal vestibular neurons [21,134,205] to end up in a different innervation (Figure 5D).
The functional unit of the lateral line system is the neuromast, which physically couples hair cells to the surrounding medium [206]. Within a neuromast, the hair cells are organized in two opposing polarities that are either randomly distributed within a neuromast or occur in a regularized counter-organization (Figure 5). The transduction from the mechanical stimulus requires an eccentric kinocilium and shorter stereocilia [207]. The absence of Tmc1, Tmc2 or TMIE disrupt stereocilia development [208]. It seems possible that the neurons giving rise to the two afferents, and possibly also the two opposing hair cell populations, are separated by different birthdates in teleosts [124,209]. In zebrafish, it was further shown that, while early-born afferent neurons connect hair cells to the Mauthner cell, those occurring later only project to the central nucleus [210].
The opposing polarity of hair cells and their selective innervation by afferent nerves is determined through the combined action of transcription factor Emx2 [40,41,213–215]. Ectopic expression of Emx2 drives all hair cells to organize their kinocilia in a caudal position, while broadly activating the Notch pathway results in the inhibition of Emx2 expression. Thus, all kinocilia are positioned rostrally [40,116,215]. It appears that a bistable situation then determines of Emx2 in the rostral sibling through Notch-mediated lateral inhibition, which then determines the caudal position for the kinocilium of the rostral sibling and the emergence of the opposing polarity [23].
Auditory hair cells are unipolar in mammals and depend on Vangl2, Dvl1, Celsr1 and Gal2 from the PCP pathway [35,216]. Emx2 and Jag1 are both needed for the development of OHCs, which increases the IHC [41,217,218]. Electroreceptors show no polarity in either single kinocilium or multiple microvilli [22] which use nonmechanical sensation [46,219]. Efferents have been found in vertebrates, and vertebrates that receive the vestibular, lateral line and auditory efferents have shown an absence of electroreceptions [193,220].
In summary, hair cells evolved from single Choanoflagellate to evolve into Atoh1 dependent hair cells of vertebrates. Mechanoreception depends on polarity for the inner ear and lateral line, which may counteract of some vestibular and lateral line hair cells or organize unipolarity of canal cristae and most auditory hair cells. Tip links form between stereocilia to open the channel depending on the evolution of Tmc1/2. Electroreception does not evolve into mechanotransduction and has no polarity, comparable to Choanoflagellates.
5. Conclusions
Choanoflagellates are the basis of animals that evolved approximately 800 million years ago. Apical kinocilia surrounded by microvilli resemble the electroreceptor hair cells, having either a central kinocilium or microvilli [22,23,27]. In contrast, the lateral line, vestibular and cochlear hair cells develop a polarity for a mechanosensory transduction channel for its function [37,44]. Tmc1 and/or Tmc2 are an essential connection of mechanotransduction [42], which can be traced to Choanoflagellates [43]. Further work is needed to understand all the functions of various Tmc forms. For example, the sequence of mechanosensory hair cells is likely expressed by Tmc1/2, which is unique in cyclostomes and splits into two Tmc genes in gnathostomes.
The lateral line, ear and electroreception differentiate into hair cells (Atoh1) that innervate vestibular neurons (Neurog1). In contrast to a simple critical dependency (Atoh1 define hair cells, Neurog1 define neurons), centrally nuclei of the brainstem depend on Atoh1 (LL, ELL, replaced by auditory nuclei in amniotes [26]), Neurog1/2, Olig3, Ascl1 and Ptf1a (VN; [25]). For the brainstem, Shh diffuses from ventral floor plate (Figure 6), whereas the dorsal aspect of the roof plate/choroid plexus depends on Lmx1a/b, BMPs and Wnts [11,28,153]. In the absence of Lmx1a/b, the dorsal formation does not form into a choroid plexus and lacks central nuclei, including Atoh1 (Figure 6). The reduction of Shh and Gli may depend on the feedback between the dorsal and ventral interaction with Lmx1a/b. A similar interaction between Shh defines the cochlear hair cells [173], which interact with Pax2, Lmx1a/b, Sox2 and Gata3 [16,28,58,59] to eliminate cochlear hair cells, suggesting a unique interaction between Shh and cochlear development [11,78]. Interestingly enough, the partial formation of some vestibular hair cells in Shh, Pax2, Lmx1a/b and Gata3 with a near-normal development for central vestibular nuclei (Figure 6) are downstream of Eya1 [15].
Figure 6.
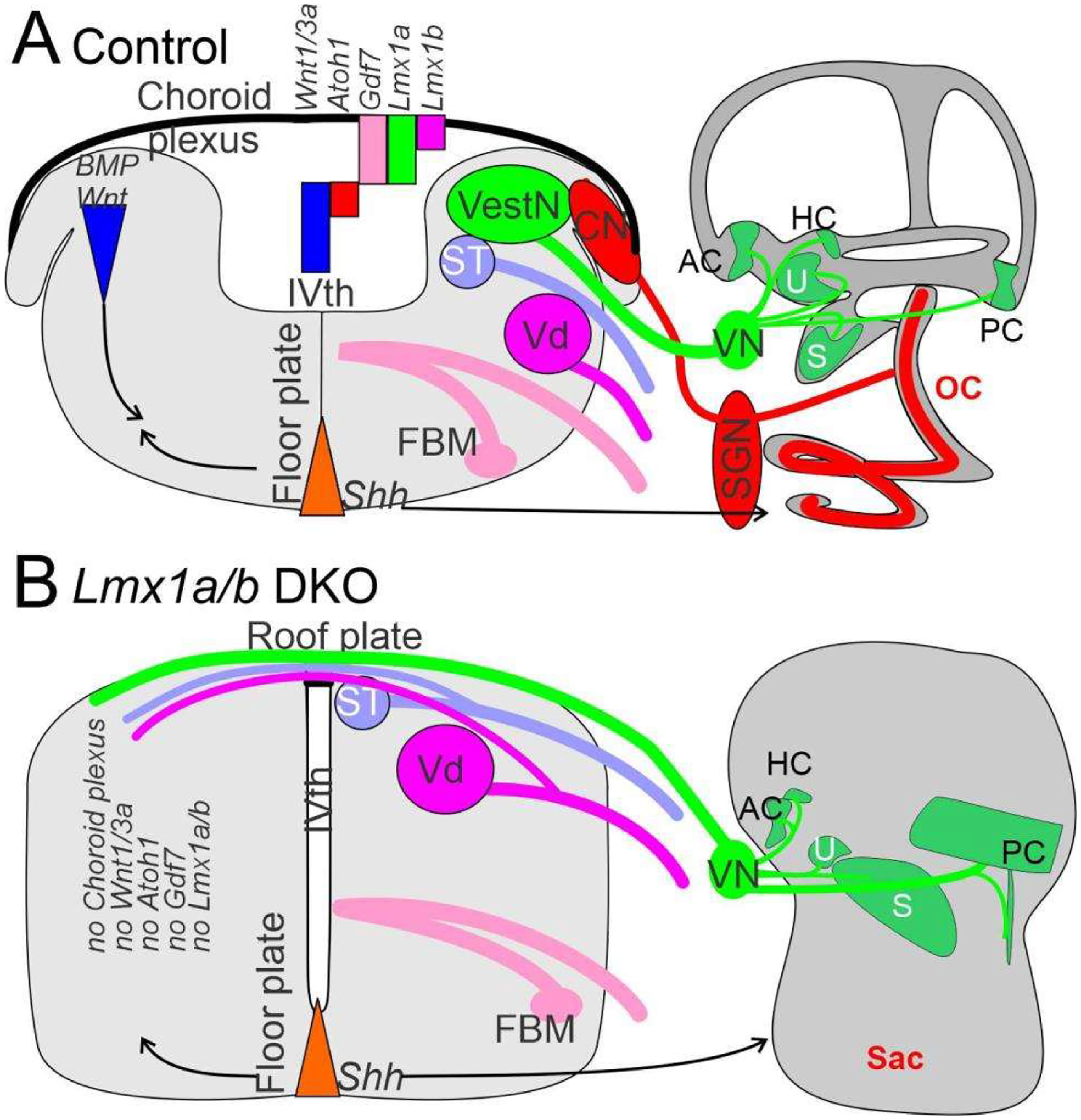
Central projections of the ear depend on the brainstem. Vestibular neurons project dorsally in the hindbrain in control and Lmx1a/b DKO mice (VUI; A,B). In Lmx1a/b DKO mice, central cochlear projections never develop as they do in controls (A,B). In addition, in Lmx1a/b DKO mice, vestibular projections interconnect across the roof plate, whereas vestibular fibers are normally separated by the choroid plexus (A,B). In addition to the loss of the cochlea and spiral ganglion neurons, the cochlear nucleus does not form in Lmx1a/b DKO mice (B). Furthermore, in Lmx1a/b DKO mice, Atoh1, Gdf7 and Wnt1/3a expressions are absent (A,B). The signal of Shh drives both the ventral brainstem and ventral cochlea (arrows), which are altered without dorsal interaction and lack cochlear neurons in Lmx1a/b DKO mice (A,B). AC, HC, PC, anterior, horizontal, posterior cristae; CN, cochlear nuclei; FBM, facial branchial motoneurons; S, saccule; SGN, spiral ganglion neurons; ST, solitary tact; U, utricle; Vd, trigeminal; VestN, vestibular nuclei; VN, vestibular neurons. Modified after [11,28,153].
Obviously, there is a formation of the lateral line and electroreception in most vertebrates, whereas amniotes lose the two sensory neurons, brainstem and hair cells, instead evolving an auditory system [26,122]. Lmx1a/b null mice showed a loss of cochlear hair cells, cochlear neurons and cochlear nuclei (Figure 6). Unfortunately, the expression of Lmx1a/b is required for the dorsal part of the hindbrain, which has not been analyzed in the lateral line and electroreception in gnathostomes. It is possible that the lateral line and electroreception may play a role in Lmx1a/b expression to help the transformation of amniotes after the loss of peripheral hair cells and associated nuclei and central projections. Recent evidence has shown that cyclostomes have a different organization of Lmx [153], but their expression of Lmx1a/b is unclear. Moreover, the two groups of teleosts that have evolved an electroreception have a unique expansion among all gnathostomes [27,135]. This expansion mimics the auditory system of amniotes [26], for which information on Lmx1a/b expression is lacking.
Acknowledgments:
I thank my colleagues for discussing some aspects of the research, particularly V.V. Chizhikov, K.L. Elliott, J. Kersigo, G. Pavlinkova and E.N. Yamoah.
Funding:
This research was funded by NIH/NIA, grant number R01 AG060504.
Footnotes
Conflicts of Interest: The author declares no conflict of interest.
References
- 1.Heger P; Zheng W; Rottmann A; Panfilio KA; Wiehe T The genetic factors of bilaterian evolution. eLife 2020, 9, e45530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland L Invertebrate origins of vertebrate nervous systems. In Evolutionary Neuroscience; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–73. [Google Scholar]
- 3.Chagnaud BP; Engelmann J; Fritzsch B; Glover JC; Straka H Sensing external and self-motion with hair cells: A comparison of the lateral line and vestibular systems from a developmental and evolutionary perspective. Brain Behav. Evol 2017, 90, 98–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fritzsch B; Elliott KL; Glover JC Gaskell revisited: New insights into spinal autonomies necessitate a revised motor neuron nomenclature. Cell Tissue Res. 2017, 370, 195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermingham NA; Hassan BA; Wang VY; Fernandez M; Banfi S; Bellen HJ; Fritzsch B; Zoghbi HY Proprioceptor pathway development is dependent on Mathl. Neuron 2001, 30, 411–422. [DOI] [PubMed] [Google Scholar]
- 6.Kim K; Gibboney S; Razy-Krajka F; Lowe EK; Wang W; Stolfi A Regulation of neurogenesis by FGF signaling and Neurogenin in the invertebrate chordate Ciona. Front. Cell Dev. Biol 2020, 8, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stolfi A; Ryan K; Meinertzhagen IA; Christiaen L Migratory neuronal progenitors arise from the neural plate borders in tunicates. Nature 2015,527,371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holland LZ; Daza DO A new look at an old question: When did the second whole genome duplication occur in vertebrate evolution? Genome Biol. 2018, 19, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritzsch B; Jahan I; Pan N; Elliott KL Evolving gene regulatory networks into cellular networks guiding adaptive behavior: An outline how single cells could have evolved into a centralized neurosensory system. Cell Tissue Res. 2015, 359, 295–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritzsch B; Elliott KL Gene, cell, and organ multiplication drives inner ear evolution. Dev. Biol 2017, 431, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott KL; Pavlínková G; Chizhikov VV; Yamoah EN; Fritzsch B Development in the Mammalian Auditory System Depends on Transcription Factors. Int. J. Mol. Sci 2021, 22, 4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang WJ; Chen JS; Zeller RW Transcriptional regulation of the peripheral nervous system in Ciona intestinalis. Dev. Biol 2013, 378, 183–193. [DOI] [PubMed] [Google Scholar]
- 13.Ryan K; Lu Z; Meinertzhagen IA The peripheral nervous system of the ascidian tadpole larva: Types of neurons and their synaptic networks. J. Comp. Neurol 2018, 526, 583–608. [DOI] [PubMed] [Google Scholar]
- 14.Manni L; Anselmi C; Burighel P; Martini M; Gasparini F Differentiation and induced sensorial alteration of the coronal organ in the asexual life of a tunicate, Integr. Comp. Biol 2018, 58, 317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J; Li J; Zhang T; Jiang H; Ramakrishnan A; Fritzsch B; Shen L; Xu PX Chromatin remodelers and lineage-specific factors interact to target enhancers to establish proneurosensory fate within otic ectoderm. Proc. Natl. Acad. Sci. USA 2021, 118, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dvorakova M; Macova I; Bohuslavova R; Anderova M; Fritzsch B; Pavlinkova G Early ear neuronal development, but not olfactory or lens development, can proceed without SOX2. Deo. Biol 2020, 457, 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Q; Chen Z; del Barco Barrantes I; de la Pompa JL; Anderson DJ Neurogeninl is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron 1998,20,469–482. [DOI] [PubMed] [Google Scholar]
- 18.Kim W-Y; Fritzsch B; Serls A; Bakel LA; Huang EJ; Reichardt LF; Barth DS; Lee JE NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development 2001, 128, 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Q; Anderson DJ; Fritzsch B Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J. Assoc. Res. Otolaryngol 2000, 1, 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahan I; Kersigo J; Pan N; Fritzsch B Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell Tissue Res. 2010, 341, 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macova I; Pysanenko K; Chumak T; Dvorakova M; Bohuslavova R; Syka J; Fritzsch B; Pavlinkova G Neurod1 is essential for the primary tonotopic organization and related auditory information processing in the midbrain. J. Neurosci 2019, 39, 984–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker CV The Development and Evolution of Lateral Line Electroreceptors: Insights from Comparative Molecular Approaches. In Electroreception: Fundamental Insights from Comparative Approaches; Springer: Berlin/Heidelberg, Germany, 2019; pp. 25–62. [Google Scholar]
- 23.Elliott KL; Fritzsch B Evolution and development of lateral line and electroreception: An integrated perception ofneurons, hair cells and brainstem nuclei. In The Senses; Fritzsch B, Bleckmann H, Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 7, pp. 95–115. [Google Scholar]
- 24.Lai HC; Seal RP; Johnson JE Making sense out of spinal cord somatosensory development. Development 2016, 143, 3434–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemandez-Miranda LR; Müller T; Birchmeier C The dorsal spinal cord and hindbrain: From developmental mechanisms to functional circuits. Dev. Biol 2017, 432, 34–42. [DOI] [PubMed] [Google Scholar]
- 26.Grothe B; Carr CE; Casseday JH; Fritzsch B; Köppl C The evolution of central pathways and their neural processing patterns. In Evolution of the Vertebrate Auditory System; Springer: Berlin/Heidelberg, Germany, 2004; pp. 289–359. [Google Scholar]
- 27.Wullimann MF; Grothe B The central nervous organization of the lateral line system. In The Lateral Line System; Springer: Berlin/Heidelberg, Germany, 2013; pp. 195–251. [Google Scholar]
- 28.Chizhikov VV; Iskusnykh IY; Fattakhov N; Fritzsch B Lmx1a and Lmx1b are Redundantly Required for the Development of Multiple Components of the Mammalian Auditory System. Neuroscience 2021, 452, 247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KJ; Dietrich P; Jessell TM Genetic ablation reveals that the roof plate is essential for dorsal intemeuron specification. Nature 2000, 403, 734–740. [DOI] [PubMed] [Google Scholar]
- 30.Pierce ML; Weston MD; Fritzsch B; Gabel HW; Ruvkun G; Soukup GA MicroRNA—183 family conservation and ciliated neurosensory organ expression. Evol. Dev 2008, 10, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichols DH; Pauley S; Jahan I; Beisel KW; Millen KJ; Fritzsch B Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008, 334, 339–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arendt D; Musser JM; Baker CV; Bergman A; Cepko C; Erwin DH; Pavlicev M; Schlosser G; Widder S; Laubichler MD The origin and evolution of cell types. Nat. Rev. Genet 2016, 17, 744–757. [DOI] [PubMed] [Google Scholar]
- 33.Schlosser G A short history of nearly every sense—The evolutionary history of vertebrate sensory cell types. Integr. Comp. Biol 2018, 58, 301–316. [DOI] [PubMed] [Google Scholar]
- 34.Tarchini B; Lu X New insights into regulation and function of planar polarity in the inner ear. Neurosci. Lett 2019, 709, 134373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montcouquiol M; Rachel RA; Lanford PJ; Copeland NG; Jenkins NA; Kelley MW Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 2003, 423, 173–177. [DOI] [PubMed] [Google Scholar]
- 36.Ghimire SR; Deans MR Frizzled3 and Frizzled6 Cooperate with Vangl2 to Direct Cochlear Innervation by type II Spiral Ganglion Neurons. J. Neurosci 2019, 39, 8013–8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu X; Müller U Mechanically gated ion channels in mammalian hair cells. Front. Cell. Neurosci 2018, 12, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibata SB; Ranum PT; Moteki H; Pan B; Goodwin AT; Goodman SS; Abbas PJ; Holt JR; Smith RJ RNA interference prevents autosomal-dominant hearing loss. Am. J. Hum. Genet 2016, 98, 1101–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fritzsch B; López-Schier H Evolution of polarized hair cells in aquatic vertebrates and their connection to directionally sensitive neurons. In Flow Sensing in Air and Water; Springer: Berlin/Heidelberg, Germany, 2014; pp. 271–294. [Google Scholar]
- 40.Kozak EL; Palit S; Miranda-Rodriguez JR; Janjic A; Bottcher A; Lickert H; Enard W; Theis FJ; Lopez-Schier H Epithelial Planar Bipolarity Emerges from Notch-Mediated Asymmetric Inhibition of Emx2. Curr. Biol 2020, 30, 1142–1151.e6. [DOI] [PubMed] [Google Scholar]
- 41.Jiang T; Kindt K; Wu DK Transcription factor Emx2 controls stereociliary bundle orientation of sensory hair cells. eLife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan B; Akyuz N; Liu XP; Asai Y; Nist-Lund C; Kurima K; Derfler BH; Gyorgy B; Limapichat W; Walujkar S; et al. TMC1 Forms the Pore of Mechanosensory Transduction Channels in Vertebrate Inner Ear Hair Cells. Neuron 2018, 99, 736–753.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erives A; Fritzsch B A Screen for Gene Paralogies Delineating Evolutionary Branching Order of Early Metazoa. G3 Genes Genom. Genet 2020, 10, 811–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwander M; Kachar B; Müller U The cell biology of hearing. J. Cell Biol 2010, 190, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Northcutt RG; Brändle K; Fritzsch B Electroreceptors and mechanosensory lateral line organs arise from single placodes in axolotls. Dev. Biol 1995, 168, 358–373. [DOI] [PubMed] [Google Scholar]
- 46.Leitch DB; Julius D Electrosensory Transduction: Comparisons Across Structure, Afferent Response Properties, and Cellular Physiology. In Electroreception: Fundamental Insights from Comparative Approaches; Springer: Berlin/Heidelberg, Germany, 2019; pp. 63–90. [Google Scholar]
- 47.Fritzsch B; Straka H Evolution of vertebrate mechanosensory hair cells and inner ears: Toward identifying stimuli that select mutation driven altered morphologies. J. Comp. Physiol. A 2014, 200, 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu PX; Adams J; Peters H; Brown MC; Heaney S; Maas R Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat. Genet 1999, 23, 113–117. [DOI] [PubMed] [Google Scholar]
- 49.Zou D; Silvius D; Fritzsch B; Xu P-X Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development 2004, 131, 5561–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holland ND; Somorjai IM The sensory peripheral nervous system in the tail of a cephalochordate studied by serial blockface scanning electron microscopy. J. Comp. Neurol 2020, 528, 2569–2582. [DOI] [PubMed] [Google Scholar]
- 51.Fritzsch B; Northcutt G Cranial and spinal nerve organization in amphioxus and lampreys: Evidence for an ancestral craniate pattern. Cells Tissues Organs 1993, 148, 96–109. [DOI] [PubMed] [Google Scholar]
- 52.Negrón-Piñeiro LJ; Wu Y; Di Gregorio A Transcription Factors of the bHLH Family Delineate Vertebrate Landmarks in the Nervous System of a Simple Chordate. Genes 2020, 11, 1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kageyama R; Shimojo H; Ohtsuka T Dynamic control of neural stem cells by bHLH factors. Neurosci. Res 2019, 138, 12–18. [DOI] [PubMed] [Google Scholar]
- 54.Reiprich S; Wegner M From CNS stem cells to neurons and glia: Sox for everyone. Cell Tissue Res. 2015, 359, 111–124. [DOI] [PubMed] [Google Scholar]
- 55.Riddiford N; Schlosser G Dissecting the pre-placodal transcriptome to reveal presumptive direct targets of Six1 and Eya1 in cranial placodes. eLife 2016, 5, e17666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Millimaki BB; Sweet EM; Riley BB Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev. Biol 2010, 338, 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Higuchi S; Sugahara F; Pascual-Anaya J; Takagi W; Oisi Y; Kuratani S Inner ear development in cyclostomes and evolution of the vertebrate semicircular canals. Nature 2019, 565, 347–350. [DOI] [PubMed] [Google Scholar]
- 58.Bouchard M; de Caprona D; Busslinger M; Xu P; Fritzsch B Pax2 and Pax8 cooperate in mouse inner ear morphogenesis and innervation. BMC Dev. Biol 2010, 10, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duncan JS; Fritzsch B Continued expression of GATA3 is necessary for cochlear neurosensory development. PLoS ONE 2013, 8, e62046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pirvola U; Spencer-Dene B; Xing-Qun L; Kettunen P; Thesleff I; Fritzsch B; Dickson C; Ylikoski J FGF/FGFR-2 (IIIb) signaling is essential for inner ear morphogenesis. J. Neurosci 2000, 20, 6125–6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riccomagno MM; Martinu L; Mulheisen M; Wu DK; Epstein DJ Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002, 16, 2365–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kersigo J; D’Angelo A; Gray BD; Soukup GA; Fritzsch B The role of sensory organs and the forebrain for the development of the craniofacial shape as revealed by Foxg1-cre-mediated microRNA loss. Genesis 2011, 49, 326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pauley S; Wright TJ; Pirvola U; Omitz D; Beisel K; Fritzsch B Expression and function of FGF10 in mammalian inner ear development. Dev. Dyn. Off. Publ. Am. Assoc. Anat 2003, 227, 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pauley S; Lai E; Fritzsch B Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev. Dyn. Off. Publ. Am. Assoc. Anat 2006, 235, 2470–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang CH; Simeone A; Lai E; Wu DK Foxg1 is required for proper separation and formation of sensory cristae during inner ear development. Dev. Dyn. Off. Publ. Am. Assoc. Anat 2009, 238, 2725–2734. [DOI] [PubMed] [Google Scholar]
- 66.Ma Q; Fode C; Guillemot F; Anderson DJ Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Deo. 1999, 13, 1717–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fritzsch B; Elliott KL; Pavlinkova G Primary sensory map formations reflect unique needs and molecular cues specific to each sensory system. F1000Research 2019, 8, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dennis DJ; Han S; Schuurmans C bHLH transcription factors in neural development, disease, and reprogramming. Brain Res. 2019, 1705, 48–65. [DOI] [PubMed] [Google Scholar]
- 69.Erzurumlu RS; Murakami Y; Rijli FM Mapping the face in the somatosensory brainstem. Nat. Rev. Neurosci 2010, 11, 252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fode C; Gradwohl G; Morin X; Dierich A; Le Meur M; Goridis C; Guillemot F The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron 1998, 20, 483–494. [DOI] [PubMed] [Google Scholar]
- 71.Alsina B Mechanisms of cell specification and differentiation in vertebrate cranial sensory systems. Curr. Opin. Cell Biol 2020, 67, 79–85. [DOI] [PubMed] [Google Scholar]
- 72.O’Neill P; Mak S-S; Fritzsch B; Ladher RK; Baker CV The amniote paratympanic organ develops from a previously undiscovered sensory placode. Nat. Commun 2012, 3, 1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sapede D; Dyballa S; Pujades C Cell lineage analysis reveals three different progenitor pools for neurosensory elements in the otic vesicle. J. Neurosci 2012, 32, 16424–16434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moody SA; La Mantia A-S Transcriptional regulation of cranial sensory placode development. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 111, pp. 301–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dykes IM; Tempest L; Lee S-I; Turner EE Bm3a and Islet1 act epistatically to regulate the gene expression program of sensory differentiation. J. Neurosci 2011, 31, 9789–9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang EJ; Liu W; Fritzsch B; Bianchi LM; Reichardt LF; Xiang M Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development 2001, 128, 2421–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mackowetzky K; Yoon KH; Mackowetzky EJ; Waskiewicz AJ Development and evolution of the vestibular apparatuses of the inner ear. J. Anat 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muthu V; Rohacek AM; Yao Y; Rakowiecki SM; Brown AS; Zhao Y-T; Meyers J; Won K-J; Ramdas S; Brown CD; et al. Genomic architecture of Shh-dependent cochlear morphogenesis. Development 2019, 146, dev181339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petitpré C; Wu H; Sharma A; Tokarska A; Fontanet P; Wang Y; Helmbacher F; Yackle K; Silberberg G; Hadjab S Neuronal heterogeneity and stereotyped connectivity in the auditory afferent system. Nat. Commun 2018, 9, 3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shrestha BR; Chia C; Wu L; Kujawa SG; Liberman MC; Goodrich LV Sensory neuron diversity in the inner ear is shaped by activity. Cell 2018, 174, 1229–1246.el7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun S; Babola T; Pregemig G; So KS; Nguyen M; Su S-SM; Palermo AT; Bergles DE; Bums JC; Müller U Hair cell mechanotransduction regulates spontaneous activity and spiral ganglion subtype specification in the auditory system. Cell 2018, 174, 1247–1263.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Elliott KL; Kersigo JM; Lee JH; Jahan I; Pavlinkova G; Fritzsch B; Yamoah EN Changes in Peripherin-eGFP expression in spiral ganglion neurons; from development to maturity. Front. Cell. Neurosci 2021, 15, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mao Y; Reiprich S; Wegner M; Fritzsch B Targeted deletion of Sox10 by Wnt1-cre defects neuronal migration and projection in the mouse inner ear. PLoS ONE 2014, 9, e94580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morris JK; Maklad A; Hansen LA; Feng F; Sorensen C; Lee K-F; Macklin WB; Fritzsch B A disorganized innervation of the inner ear persists in the absence of ErbB2. Brain Res. 2006, 1091, 186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Freyer L; Aggarwal V; Morrow BE Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Development 2011, 138, 5403–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Billings SE; Myers NM; Quiruz L; Cheng AG Opposing effects of Wnt/β-catenin signaling on epithelial and mesenchymal cell fate in the developing cochlea. Development 2021, 148, dev199091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goodrich LV Early development of the spiral ganglion. In The Primary Auditory Neurons of the Mammalian Cochlea; Springer: Berlin/Heidelberg, Germany, 2016; pp. 11–48. [Google Scholar]
- 88.Fritzsch B; Tessarollo L; Coppola E; Reichaidt LF Neurotrophins in the ear: Their roles in sensory neuron survival and fiber guidance. Prog. Brain Res 2004, 146, 265–278. [DOI] [PubMed] [Google Scholar]
- 89.Bianchi LM; Conover JC; Fritzsch B; De Chiara T; Lindsay RM; Yancopoulos GD Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Development 1996, 122, 1965–1973. [DOI] [PubMed] [Google Scholar]
- 90.Fritzsch B; Kersigo J; Yang T; Jahan I; Pan N Neurotrophic factor function during ear development: Expression changes define critical phases for neuronal viability. In The Primary Auditoryneurons of the Mammaliancochlea; Dabdoub A, Fritzsch B, Popper A, Fay R, Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 52, pp. 49–84. [Google Scholar]
- 91.Fariñas I; Jones KR; Tessarollo L; Vigers AJ; Huang E; Kirstein M; De Caprona DC; Coppola V; Backus C; Reichardt LF Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J. Neurosci 2001, 21, 6170–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kersigo J; Fritzsch B Inner ear hair cells deteriorate in mice engineered to have no or diminished innervation. Front. Aging Neurosci 2015, 7, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fritzsch B; Sarai P; Barbacid M; Silos-Santiago I Mice with a targeted disruption of the neurotrophin receptor trkB lose their gustatory ganglion cells early but do develop taste buds. Int. J. Dev. Neurosci 1997,15,563–576. [DOI] [PubMed] [Google Scholar]
- 94.Silos-Santiago I; Fagan AM; Garber M; Fritzsch B; Barbacid M Severe sensory deficits but normal CNS development in newborn mice lacking TrkB and TrkC tyrosine protein kinase receptors. Eur. J. Neurosci 1997, 9, 2045–2056. [DOI] [PubMed] [Google Scholar]
- 95.Hallböök F; Wilson K; Thomdyke M; Olinski RP Formation and evolution of the chordate neurotrophin and Trk receptor genes. Brain Behav. Evol 2006, 68, 133–144. [DOI] [PubMed] [Google Scholar]
- 96.Bothwell M Ngf, bdnf, nt3, and nt4. In Neurotrophic Factors; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–15. [DOI] [PubMed] [Google Scholar]
- 97.Kopecky B; Santi P; Johnson S; Schmitz H; Fritzsch B Conditional deletion of N-Myc disrupts neurosensory and non-sensory development of the ear. Dev. Dyn 2011, 240, 1373–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schimmang T; Pirvola U Coupling the cell cycle to development and regeneration of the inner ear. Semin. Cell Dev. Biol 2013, 24, 507–513. [DOI] [PubMed] [Google Scholar]
- 99.Sakamoto S; Tateya T; Omori K; Kageyama R Id genes are required for morphogenesis and cellular patterning in the developing mammalian cochlea. Dev. Biol 2020, 460, 164–175. [DOI] [PubMed] [Google Scholar]
- 100.Chen S-D; Yang J-L; Lin Y-C; Chao A; Yang D-I Emerging Roles of Inhibitor of Differentiation-1 in Alzheimer’s Disease: Cell Cycle Reentry and Beyond. Cells 2020, 9, 1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baker NE; Brown NL All in the family: Proneural bHLH genes and neuronal diversity. Development 2018, 145, dev159426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pierce ET Histogenesis of the dorsal and ventral cochlear nuclei in the mouse. An autoradiographic study. J. Comp. Neurol 1967, 131,27–53. [DOI] [PubMed] [Google Scholar]
- 103.Chou S-W; Chen Z; Zhu S; Davis RW; Hu J; Liu L; Fernando CA; Kindig K; Brown WC; Stepanyan R A molecular basis for water motion detection by the mechanosensory lateral line of zebrafish. Nat. Commun 2017, 8, 2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Altman J; Bayer SA Atlas Of Prenatal Rat Brain Development; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- 105.Kopecky B; Fritzsch B The Myc road to hearing restoration. Cells 2012, 1, 667–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alonso MBD; Hernandez IL; de la Fuente MA; Garcia-Sancho J; Giraldez F; Schimmang T Transcription factor induced conversion of human fibroblasts towards the hair cell lineage. PLoS ONE 2018, 13, e0200210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mantela J; Jiang Z; Ylikoski J; Fritzsch B; Zacksenhaus E; Pirvola U The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development 2005, 132, 2377–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamoah EN; Li M; Shah A; Elliott KL; Cheah K; Xu PX; Phillips S; Young SM Jr.; Eberl DF; Fritzsch B Using Sox2 to alleviate the hallmarks of age-related hearing loss. Ageing Res. Rev 2020, 59, 101042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fritzsch B; Gregory D; Rosa-Molinar E The development of the hindbrain afferent projections in the axolotl: Evidence for timing as a specific mechanism of afferent fiber sorting. Zoology 2005, 108, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang T; Kersigo J; Jahan I; Pan N; Fritzsch B The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hear. Res 2011, 278, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pflieger JF; Dubuc R Relationship between vestibular primary afferents and vestibulospinal neurons in lampreys. J. Comp. Neurol 2000, 427, 255–273. [DOI] [PubMed] [Google Scholar]
- 112.Duncan JS; Cox BC Anatomy and Development of the Inner Ear. In The Senses; Fritzsch B, Grothe B, Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 253–276. [Google Scholar]
- 113.Bell CC; Han V; Sawtell NB Cerebellum-like structures and their implications for cerebellar function. Annu. Rev. Neurosci 2008, 31, 1–24. [DOI] [PubMed] [Google Scholar]
- 114.Northcutt RG The phylogenetic distribution and innervation of craniate mechanoreceptive lateral lines. In The Mechanosensory Lateral Line; Springer: Berlin/Heidelberg, Germany, 1989; pp. 17–78. [Google Scholar]
- 115.Webb JE Morphology of the mechanosensory lateral lien system of fishes. In The Senses; Fritzsch B, Bleckmann H, Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 7, pp. 29–46. [Google Scholar]
- 116.Chitnis AB Development of the zebrafish posterior lateral line system. In The Senses; Fritzsch B, Bleckmann H, Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 7, pp. 66–84. [Google Scholar]
- 117.Fritzsch B; Beisel K; Jones K; Farinas I; Maklad A; Lee J; Reichardt L Development and evolution of inner ear sensory epithelia and their innervation. J. Neurobiol 2002, 53, 143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pombal MA; Megías M Development and functional organization of the cranial nerves in lampreys. Anat. Rec 2019, 302, 512–539. [DOI] [PubMed] [Google Scholar]
- 119.Fritzsch B Evolution of the vestibulo-ocular system. Otolaryngol. Head Neck Surg 1998,119,182–192. [DOI] [PubMed] [Google Scholar]
- 120.Fritzsch B; Signore M; Simeone A Otxl null mutant mice show partial segregation of sensory epithelia comparable to lamprey ears. Deo. Genes Evol 2001, 211, 388–396. [DOI] [PubMed] [Google Scholar]
- 121.Fritzsch B; Wake M The inner ear of gymnophione amphibians and its nerve supply: A comparative study of regressive events in a complex sensory system (Amphibia, Gymnophiona). Zoomorphology 1988, 108, 201–217. [Google Scholar]
- 122.Fritzsch B; Pan N; Jahan I; Duncan JS; Kopecky BJ; Elliott KL; Kersigo J; Vang T Evolution and development of the tetrapod auditory system: An organ of Corti-centric perspective. Evol. Dev 2013, 15, 63–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maklad A; Fritzsch B Development of vestibular afferent projections into the hindbrain and their central targets. Brain Res. Bull 2003, 60, 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zecca A; Dyballa S; Voltes A; Bradley R; Pujades C The order and place of neuronal differentiation establish the topography of sensory projections and the entry points within the hindbrain. J. Neurosci 2015, 35, 7475–7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Matei V; Pauley S; Kaing S; Rowitch D; Beisel K; Morris K; Feng F; Jones K; Lee J; Fritzsch B Smaller inner ear sensory epithelia in Neurogl null mice are related to earlier hair cell cycle exit. Dev. Dyn 2005, 234, 633–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rubel EW; Fritzsch B Auditory system development: Primary auditory neurons and their targets. Annu. Rev. Neurosci 2002, 25, 51–101. [DOI] [PubMed] [Google Scholar]
- 127.Ruben RJ Development of the inner ear of the mouse: A radioautographic study of terminal mitoses. Acta Otolaryngol. 1967, 220, 1–44. [PubMed] [Google Scholar]
- 128.De No RL The Primary Acoustic Nuclei; Raven Press: New York, NY, USA, 1981. [Google Scholar]
- 129.Schmidt H; Fritzsch B Npr2 null mutants show initial overshooting followed by reduction of spiral ganglion axon projections combined with near-normal cochleotopic projection. Cell Tissue Res. 2019, 378, 15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Elliott KL; Kersigo J; Pan N; Jahan I; Fritzsch B Spiral Ganglion Neuron Projection Development to the Hindbrain in Mice Lacking Peripheral and/or Central Target Differentiation. Front. Neural Circuits 2017, 11, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fritzsch B; Matei V; Nichols D; Bermingham N; Jones K; Beisel K; Wang V Atohl null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev. Dyn. Off. Publ. Am. Assoc. Anat 2005, 233, 570–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Milinkeviciute G; Cramer KS Development of the ascending auditory pathway. In The Senses; Fritzsch B, Grothe B, Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2, pp. 337–353. [Google Scholar]
- 133.Duncan JS; Fritzsch B; Houston DW; Ketchum EM; Kersigo J; Deans MR; Elliott KL Topologically correct central projections of tetrapod inner ear afferents require Fzd3. Sci. Rep 2019, 9, 10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Maklad A; Kamel S; Wong E; Fritzsch B Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell Tissue Res. 2010, 340, 303–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bell CC; Maler L Central neuroanatomy of electrosensory systems in fish. In Electroreception; Springer: Berlin/Heidelberg, Germany, 2005; pp. 68–111. [Google Scholar]
- 136.Millimaki BB; Sweet EM; Dhason MS; Riley BB Zebrafish atohl genes: Classic proneural activity in the inner ear and regulation by Fgf and Notch. Development 2007, 134, 295–305. [DOI] [PubMed] [Google Scholar]
- 137.Northcutt RG Distribution and innervation of lateral line organs in the axolotl. J. Comp. Neurol 1992, 325, 95–123. [DOI] [PubMed] [Google Scholar]
- 138.Pan N; Jahan I; Kersigo J; Duncan JS; Kopecky B; Fritzsch B A novel Atohl “self-terminating” mouse model reveals the necessity of proper Atoh1 level and duration for hair cell differentiation and viability. PLoS ONE 2012, 7, e30358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xiang M; Maklad A; Pirvola U; Fritzsch B Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation. BMC Neurosci. 2003, 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nieuwenhuys R; Puelles L Towards a New Neuromorphology; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- 141.Watson C; Shimogori T; Puelles L Mouse Fgf8-Cre-LacZ lineage analysis defines the territory of the postnatal mammalian isthmus. J. Comp. Neurol 2017, 525, 2782–2799. [DOI] [PubMed] [Google Scholar]
- 142.Iskusnykh IY; Steshina EY; Chizhikov VV Loss of Ptf1a leads to a widespread cell-fate misspecification in the brainstem, affecting the development of somatosensory and viscerosensory nuclei. J. Neurosci 2016, 36, 2691–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yamada M; Seto Y; Taya S; Owa T; Jhoue YU; Inoue T; Kawaguchi Y; Nabeshima Y.-i.; Hoshino M Specification of spatial identities of cerebellar neuron progenitors by ptf1a and atoh1 for proper production of GABAergic and glutamatergic neurons. J. Neurosci 2014, 34, 4786–4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fujiyama T; Yamada M; Terao M; Terashima T; Hioki H; Inoue YU; Inoue T; Masuyama N; Obata K; Yanagawa Y Inhibitory and excitatory subtypes of cochlear nucleus neurons are defined by distinct bHLH transcription factors, Ptf1a and Atoh1. Development 2009, 236, 2049–2058. [DOI] [PubMed] [Google Scholar]
- 145.Lowenstein ED; Rusanova A; Stelzer J; Hemaiz-Llorens M; Schroer AE; Epifanova E; Bladt F; Isik EG; Buchert S; Jia S; et al. Olig3 regulates early cerebellar development. eLife 2021, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sankar S; Yellajoshyula D; Zhang B; Teets B; Rockweiler N; Kroll KL Gene regulatory networks in neural cell fate acquisition from genome-wide chromatin association of Gerninin and Zic1. Sci. Rep 2016, 6, 37412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee H-K; Lee H-S; Moody SA Neural transcription factors: From embryos to neural stem cells. Mol. Cells 2014, 37, 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aruga J; Hatayama M Comparative genomics of the Zic family genes. In Zic Family; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–26. [DOI] [PubMed] [Google Scholar]
- 149.Merzdorf CS; Sive HL The zic1 gene is an activator of Wnt signaling. Int. J. Dev. Biol 2004, 50, 611–617. [DOI] [PubMed] [Google Scholar]
- 150.Merzdorf C; Forecki J Amphibian Zic Genes. In Zic Family; Springer: Berlin/Heidelberg, Germany, 2018; pp. 107–140. [DOI] [PubMed] [Google Scholar]
- 151.Litingtung Y; Dahn RD; Li Y; Fallon JF; Chiang C Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature 2002, 418, 979–983. [DOI] [PubMed] [Google Scholar]
- 152.Jahan I; Kersigo J; Elliott KL; Fritzsch B Smoothened overexpression causes trochlear motoneurons to reroute and innervate ipsilateral eyes. Cell Tissue Res. 2021, 384, 59–72. [DOI] [PubMed] [Google Scholar]
- 153.Glover JC; Elliott KL; Erives A; Chizhikov VV; Fritzsch B Wilhelm His’ lasting insights into hindbrain and cranial ganglia development and evolution. Dev. Biol 2018, 444, S14–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mishima Y; Lindgren AG; Chizhikov VV; Johnson RL; Millen KJ Overlapping function of Lmx1a and Lmx1b in anterior hindbrain roof plate formation and cerebellar growth. J. Neurosci 2009, 29, 11377–11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang T; Xu J; Xu PX. Eya2 expression during mouse embryonic development revealed by Eya2(lacZ) knockin reporter and homozygous mice show mild hearing loss. Dev. Dyn. Off. Publ. Am. Assoc. Anat 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fritzsch B; Pauley S; Feng F; Matei V; Nichols D The molecular and developmental basis of the evolution of the vertebrate auditory system. Int. J. Comp. Psychol 2006, 19, 1–25. [Google Scholar]
- 157.Pan N; Jahan I; Lee JE; Fritzsch B Defects in the cerebella of conditional Neurod1 null mice correlate with effective Tg (Atoh1-ere) recombination and granule cell requirements for Neurod1 for differentiation. Cell Tissue Res. 2009, 337, 407–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang VY; Rose MF; Zoghbi HY Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron 2005, 48, 31–43. [DOI] [PubMed] [Google Scholar]
- 159.Storm R; Cholewa-Waclaw J; Reuter K; Brohl D; Sieber M; Treier M; Müller T; Birchmeier C The bHLH transcription factor Olig3 marks the dorsal neuroepithelium of the hindbrain and is essential for the development of brainstem nuclei. Development 2009, 136, 295–305. [DOI] [PubMed] [Google Scholar]
- 160.Imayoshi I; Kageyama R bHLH factors in self-renewal, multipotency, and fate choice of neural progenitor cells. Neuron 2014, 82, 9–23. [DOI] [PubMed] [Google Scholar]
- 161.Jahan I; Pan N; Elliott KL; Fritzsch B The quest for restoring hearing: Understanding ear development more completely. Bioessays 2015, 37, 1016–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kersigo J; Gu L; Xu L; Pan N; Vijayakuma S; Jones T; Shibata SB; Fritzsch B; Hansen MR Effects of Neurodl Expression on Mouse and Human Schwannoma Cells. Laryngoscope 2021, 131, E259–E270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fritzsch B; Sonntag R; Dubuc R; Ohta Y; Grillner S Organization of the six motor nuclei innervating the ocular muscles in lamprey. J. Comp. Neurol 1990, 294, 491–506. [DOI] [PubMed] [Google Scholar]
- 164.Oury F; Murakami Y; Renaud J-S; Pasqualetti M; Chamay P; Ren S-Y; Rijli FM Hoxa2-and rhombomere-dependent development of the mouse facial somatosensory map. Science 2006, 313, 1408–1413. [DOI] [PubMed] [Google Scholar]
- 165.Abraira VE; Ginty DD The sensory neurons of touch. Neuron 2013, 79, 618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bermingham NA; Hassan BA; Price SD; Vollrath MA; Ben-Arie N; Eatock RA; Bellen HJ; Lysakowski A; Zoghbi HY Math1: An essential gene for the generation of inner ear hair cells. Science 1999, 284, 1837–1841. [DOI] [PubMed] [Google Scholar]
- 167.Maricich SM; Xia A; Mathes EL; Wang VY; Oghalai JS; Fritzsch B; Zoghbi HY Atohl-lineal neurons are required for hearing and for the survival of neurons in the spiral ganglion and brainstem accessory auditory nuclei. J. Neurosci 2009, 29, 11123–11133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Birol O; Ohyama T; Edlund RK; Drakou K; Georgiades P; Groves AK The mouse Foxi3 transcription factor is necessary for the development of posterior placodes. Dev. Biol 2016, 409, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wright TJ; Mansour SL Fgf3 and Fgf10 are required for mouse otic placode induction. Development 2003, 130, 3379–3390. [DOI] [PubMed] [Google Scholar]
- 170.Urness LD; Paxton CN; Wang X; Schoenwolf GC; Mansour SL FGF signaling regulates otic placode induction and refinement by controlling both ectodermal target genes and hindbrain Wnt8a. Dev. Biol 2010, 340, 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ahmed M; Xu J; Xu P-X EYA1 and SDQ drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear. Development 2012, 139, 1965–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Burton Q; Cole LK; Mulheisen M; Chang W; Wu DK The role of Pax2 in mouse inner ear development. Dev. Biol 2004, 272, 161–175. [DOI] [PubMed] [Google Scholar]
- 173.Riccomagno MM; Takada S; Epstein DJ Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005, 19, 1612–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ohyama T; Basch ML; Mishina Y; Lyons KM; Segil N; Groves AK BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J. Neurosci. Off. J. Soc. Neurosci 2010, 30, 15044–15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chang W; Lin Z; Kulessa H; Hebert J; Hogan BL; Wu DK Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet. 2008, 4, e1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Munnamalai V; Fekete DM Wnt signaling during cochlear development. Semin. Cell Dev. Biol 2013, 24, 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wright KD; Rogers AAM; Zhang J; Shim K Cooperative and independent functions of FGF and Wnt signaling during early inner ear development. BMC Dev. Biol 2015, 15, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sienknecht UJ; Fekete DM Mapping of Wnt, frizzled, and Wnt inhibitor gene expression domains in the avian otic primordium. J. Comp. Neurol 2009, 517, 751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tambalo M; Anwar M; Ahmed M; Streit A Enhancer activation by FGF signalling during otic induction. Dev. Biol 2020, 457, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fekete DM; Wu DK Revisiting cell fate specification in the inner ear. Curr. Opin. Neurobiol 2002, 12, 35–42. [DOI] [PubMed] [Google Scholar]
- 181.Groves AK; Fekete DM Shaping sound in space: The regulation of inner ear patterning. Development 2012, 139, 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kieman AE; Pelling AL; Leung KK; Tang AS; Bell DM; Tease C; Lovell-Badge R; Steel KP; Cheah KS Sox2 is required for sensory organ development in the mammalian inner ear. Nature 2005, 434, 1031–1035. [DOI] [PubMed] [Google Scholar]
- 183.Filova I; Dvorakova M; Bohuslavova R; Pavlinek A; Elliott KL; Vochyanova S; Fritzsch B; Pavlinkova G Combined Atoh1 and Neurod1 Deletion Reveals Autonomous Growth of Auditory Nerve Fibers. Mol. Neurobiol 2020, 57, 5307–5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hertzano R; Montcouquiol M; Rashi-Elkeles S; Elkon R; Yucel R; Frankel WN; Rechavi G; Moroy T; Friedman TB; Kelley MW; et al. Transcription profiling of inner ears from Pou4f3(dd1/dd1) identifies Gfi1 as a target of the Pou4f3 deafness gene. Hum. Mol. Genet 2004, 13, 2143–2153. [DOI] [PubMed] [Google Scholar]
- 185.Li J; Zhang T; Ramakrishnan A; Fritzsch B; Xu J; Wong EYM; Loh YE; Ding J; Shen L; Xu PX Dynamic changes in cis-regulatory occupancy by Six1 and its cooperative interactions with distinct cofactors drive lineage-specific gene expression programs during progressive differentiation of the auditory sensory epithelium. Nucleic Adds Res. 2020,48,2880–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wallis D; Hamblen M; Zhou Y; Venken KJ; Schumacher A; Grimes HL; Zoghbi HY; Orkin SH; Bellen HJ The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development 2003, 130, 221–232. [DOI] [PubMed] [Google Scholar]
- 187.Nakano Y; Jahan I; Bonde G; Sun X; Hildebrand MS; Engelhaidt JF; Smith RJ; Cornell RA; Fritzsch B; Bánfi B A mutation in the Srrm4 gene causes alternative splicing defects and deafness in the Bronx waltzer mouse. PLoS Genet. 2012, 8, e1002966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nakano Y; Wiechert S; Fritzsch B; Bánfi B Inhibition of a transcriptional repressor rescues hearing in a splicing factor-deficient mouse. Life Sci. Alliance 2020, 3, e202000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bulfone A; Menguzzato E; Broccoli V; Marchitiello A; Gattuso C; Mariani M; Consalez GG; Martinez S; Ballabio A; Banfi S Barhl1, a gene belonging to a new subfamily of mammalian homeobox genes, is expressed in migrating neurons of the CNS. Hum. Mol. Genet 2000, 9, 1443–1452. [DOI] [PubMed] [Google Scholar]
- 190.Chellappa R; Li S; Pauley S; Jahan I; Jin K; Xiang M Barhll regulatory sequences required for cell-specific gene expression and autoregulation in the inner ear and central nervous system. Mol. Cell. Biol 2008, 28, 1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lewis ER; Leverenz EL; Bialek WS The Vertebrate Inner Ear; CRC Press: Boca Raton, FL, USA, 1985; p. 248. [Google Scholar]
- 192.Lysakowski A Anatomy and microstrucctural organization of vestibular hair cells. In The Senses; Fritzsch B, Straka H, Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 6, pp. 173–184. [Google Scholar]
- 193.Fritzsch B; Elliott KL Evolution and Development of the Inner Ear Efferent System: Transforming a Motor Neuron Population to Connect to the Most Unusual Motor Protein via Ancient Nicotinic Receptors. Front. Cell Neurosci 2017, 11, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Eatock RA; Rusch A; Lysakowski A; Saeki M Hair cells in mammalian utricles. Otolaryngol. Head Neck Surg 1998, 119, 172–181. [DOI] [PubMed] [Google Scholar]
- 195.Platt C; Straka H Vestibular endorgans in vertebrates and adeqate sensory stimuli. In The Senses; Fritzsch B, Straka H, Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 6, pp. 108–128. [Google Scholar]
- 196.Curthoys IS The anatomical and physiological basis of clinical tests of otolith function. A tribute to Yoshio Uchino. Front. Neurol 2020, 11, 566895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Elliott KL; Fritzsch B; Duncan JS Evolutionary and Developmental Biology Provide Insights Into the Regeneration of Organ of Corti Hair Cells. Front. Cell. Neurosci 2018, 12, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hudspeth AJ How the ear’s works work. Nature 1989, 341, 397–404. [DOI] [PubMed] [Google Scholar]
- 199.Pan B; Géléoc GS; Asai Y; Horwitz GC; Kurima K; Ishikawa K; Kawashima Y; Griffith AJ; Holt JR TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 2013, 79, 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Duncan JS; Stoller ML; Francl AF; Tissir F; Devenport D; Deans MR Celsr1 coordinates the planar polarity of vestibular hair cells during inner ear development. Dev. Biol 2017, 423, 126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ratzan EM; Moon AM; Deans MR Fgf8 genetic labeling reveals the early specification of vestibular hair cell type in mouse utricle. Development 2020, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ketchum EM; Sheltz-Kempf SN; Duncan JS Molecular basis of vestibular organ formation during ontogeny. In The Senses; Fritzsch B, Straka H, Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 6, pp. 129–144. [Google Scholar]
- 203.Deans MR A balance of form and function: Planar polarity and development of the vestibular maculae. Semin. Cell Dev. Biol 2013, 24, 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Qno K; Keller J; Ramírez OL; Garrido AG; Zobeiri OA; Chang HHV; Vijayakumar S; Ayiotis A; Duester G; Della Santina CC Retinoic acid degradation shapes zonal development of vestibular organs and sensitivity to transient linear accelerations. Nat. Commun 2020, 11, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Balmer TS; Trussell LO Selective targeting of unipolar brush cell subtypes by cerebellar mossy fibers. eLife 2019, 8, e44964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bleckmann H Central lateral line pathways and central integration of lateral line information. In The Senses; Fritzsch B, Bleckmann H, Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 7, pp. 163–184. [Google Scholar]
- 207.Nicolson T The genetics of hair-cell function in zebrafish. J. Neurogenet 2017, 31, 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Krey JF; Chatterjee P; Dumont RA; O’Sullivan M; Choi D; Bird JE; Barr-Gillespie PG Mechanotransduction-dependent control of stereocilia dimensions and row identity in inner hair cells. Curr. Biol 2020, 30, 442–454.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Vischer HA The development of lateral-line receptors in Eigenmannia (Teleostei, Gymnotiformes). I. The mechanoreceptive lateral-line system (Part 1 of 2). Brain Behav. Evol 1989, 33, 205–213. [DOI] [PubMed] [Google Scholar]
- 210.Pujol-Martí J; Zecca A; Baudoin JP; Faucherre A; Asakawa K; Kawakami K; López-Schier H Neuronal birth order identifies a dimorphic sensorineural map. J. Neurosci. Off. J. Soc. Neurosci 2012, 32, 2976–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fattal D; Hansen M; Fritzsch B Aging-related balance impairment and hearing loss. In The Yiiley Handbook on the Aging Mind and Brain; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 315–336. [Google Scholar]
- 212.Engelmann J; Fritzsch B Lateral line input to ‘almost’ all vertebrates shares a common organization with different distinct connections. In The Evolution Of Sensory System Revealed: Gene Regulation, Cellular Networks And Processes; Fritzsch B, Elliott KL, Hall B, Moody S, Eds.; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- 213.Jacobo A; Dasgupta A; Erzberger A; Siletti K; Hudspeth AJ Notch-Mediated Determination of Hair-Bundle Polarity in Mechanosensory Hair Cells of the Zebrafish Lateral Line. Curr. Biol 2019, 29, 3579–3587.e7. [DOI] [PubMed] [Google Scholar]
- 214.Ji YR; Warrier S; Jiang T; Wu DK; Kindt KS Directional selectivity of afferent neurons in zebrafish neuromasts is regulated by Emx2 in presynaptic hair cells. eLife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ohta S; Ji YR; Martin D; Wu DK Emx2 regulates hair cell rearrangement but not positional identity within neuromasts. eLife 2020, 9, e60432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tarchini B; Jolicoeur C; Cayouette M A molecular blueprint at the apical surface establishes planar asymmetry in cochlear hair cells. Dev. Cell 2013, 27, 88–102. [DOI] [PubMed] [Google Scholar]
- 217.Kimura J; Suda Y; Kurokawa D; Hossain ZM; Nakamura M; Takahashi M; Hara A; Aizawa S Emx2 and Pax6 function in cooperation with Otx2 and Otx1 to develop caudal forebrain primordium that includes future archipallium. J. Neurosci. Off. J. Soc. Neurosci 2005, 25, 5097–5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Holley M; Rhodes C; Kneebone A; Herde MK; Fleming M; Steel KP Emx2 and early hair cell development in the mouse inner ear. Dev. Biol 2010, 340, 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bellono NW; Leitch DB; Julius D Molecular tuning of electroreception in sharks and skates. Nature 2018, 558, 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Simmons D; Duncan J; de Caprona DC; Fritzsch B Development of the inner ear efferent system. In Auditory and Vestibular Efferents; Springer: Berlin/Heidelberg, Germany, 2011; pp. 187–216. [Google Scholar]


