Abstract
Fluoroquinolone susceptibility heterogeneity between various Ehrlichia species has been previously demonstrated. In gram-negative bacteria, resistance to fluoroquinolones most often corresponds to specific amino acid variations in a portion of the protein sequence of the A subunit of DNA gyrase (GyrA), referred to as the quinolone resistance-determining region (QRDR). We suspected a similar mechanism to be responsible for natural resistance in some Ehrlichia species. To verify this hypothesis, we sequenced the entire gyrA gene of the quinolone-susceptible species Ehrlichia sennetsu and designed specific primers to amplify and sequence the QRDR of four other Ehrlichia species as well as the closely related species Cowdria ruminantium. We identified in the fluoroquinolone-resistant species Ehrlichia chaffeensis and Ehrlichia canis a specific GyrA QRDR amino acid sequence, also present in C. ruminantium (whose susceptibility to fluoroquinolones remains unknown). These three species belong to a single phylogenetic cluster referred to as the E. canis genogroup. A different GyrA QRDR pattern, shared by the Ehrlichia species representatives of the E. sennetsu and Ehrlichia phagocytophila genogroups, was identified. Three of the four species tested are known to be susceptible to fluoroquinolones. A serine residue in position 83 (Escherichia coli numbering) in the susceptible species is replaced by an alanine residue in fluoroquinolone-resistant species. These results are consistent with the current knowledge on fluoroquinolone resistance in other gram-negative bacteria. They are indicative of a natural gyrase-mediated resistance to fluoroquinolones in the E. canis genogroup.
Phylogenetic studies based on 16S rRNA gene sequence comparison have placed Ehrlichia species within the alpha group of the Proteobacteria, together with the genera Cowdria, Wolbachia, Neorickettsia, and Anaplasma (2, 14), and species belonging to these different genera have been placed into one of the four following phylogenetic groups (14). The Ehrlichia sennetsu genogroup contains the prototypic species E. sennetsu (the agent of human ehrlichiosis in the Far East and Southeast Asia), Ehrlichia risticii (the agent of Potomac horse fever), and the canine pathogen Neorickettsia helminthoeca. The Ehrlichia canis genogroup includes the prototypic species E. canis (the agent of canine monocytic ehrlichiosis), Ehrlichia chaffeensis (the agent of human monocytic ehrlichiosis), Ehrlichia ewingii (the agent of canine granulocytic ehrlichiosis), the murine pathogen Ehrlichia muris, the agent of ehrlichiosis in Venezuela, and the bovine pathogen Cowdria ruminantium. The Ehrlichia phagocytophila genogroup comprises the prototypic species E. phagocytophila, a European pathogen of ruminants, Ehrlichia equi (the agent of equine and canine granulocytic ehrlichiosis), Ehrlichia platys (the canine thrombocytic pathogen), the agent of human granulocytic ehrlichiosis (HGE), and species of the genus Anaplasma (which parasitizes bovine erythrocytes). The fourth genogroup includes only Wolbachia species (arthropod symbionts).
In vitro susceptibility to fluoroquinolones is dependent on the Ehrlichia species, with E. sennetsu and E. phagocytophila (including the HGE agent) being more susceptible (4, 20, 23) than E. chaffeensis and E. canis (5, 6). In gram-negative bacteria, acquired resistance to fluoroquinolones most often corresponds to mutations in the quinolone resistance-determining region (QRDR) of gyrA, which encodes the A subunit of DNA gyrase (22, 36, 37). Variations in the gyrA QRDR sequence are also found in species with natural resistance to these antibiotics (37). We suspected that a similar mechanism is involved in fluoroquinolone resistance in Ehrlichia species. Since the gyrA sequence of Ehrlichia spp. was not available, we first determined the entire gyrA sequence of the fluoroquinolone-susceptible species E. sennetsu. Then, the sequences of the gyrA QRDRs of four other Ehrlichia species and C. ruminantium were determined to assess the presence of specific variations in gyrA QRDR sequences which may potentially explain fluoroquinolone resistance.
MATERIALS AND METHODS
DNA preparation.
Ehrlichia species used and their respective sources were as follows: E. sennetsu Miyayama strain was obtained from G. A. Dasch (Naval Medical Research Institute, Bethesda, Md.); E. canis Oklahoma strain and E. chaffeensis Arkansas strain were from J. Dawson (Centers for Disease Control and Prevention, Atlanta, Ga.); E. phagocytophila (a sheep strain) was from A. Garcia Perez (SIMA, Derio, Spain); E. risticii HRC-IL was from the American Type Culture Collection (Rockville, Md.); HGE agent Webster strain was from J. S. Dumler (John Hopkins Hospital, Baltimore, Md.); and C. ruminantium was from C. E. Yunker (Onderstepoort Veterinary Institute, Onderstepoort, South Africa). Ehrlichia and Cowdria species were grown in culture systems, including the DH82 canine histiocytic cell line for E. sennetsu, E. chaffeensis, E. canis, and E. risticii, the human promyelocytic leukemia cell line HL60 (ATCC CCL-240) for E. phagocytophila and the HGE agent, and endothelial cells (E5 strain) for C. ruminantium (8). Infected cells were lysed by three freeze-thaw cycles (−80 and 37°C). Bacteria were purified on a sucrose gradient, using a previously described procedure (9). DNA was extracted from bacterial preparations using the QIAamp tissue kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions. These extracts were used as templates in different PCR assays. The amount of purified DNA was determined by measuring the absorbancy at 260 nm.
PCR assay with consensus degenerate primers.
The QRDR region of E. sennetsu was first amplified using consensus degenerate primers (here referred to as GYRAF and GYRAR) reported in the literature (21, 34). These primers have been previously determined by alignment of several gyrA sequences from various gram-positive and gram-negative bacterial species and correspond to positions 39 to 45 and 173 to 179 of the amino acid sequence of the E. coli gyrA product (21). The PCR conditions are given in Table 1 (experiment I). All PCR assays used in the present study started with a 3-min step at 95°C to allow separation of DNA strands, and all PCR cycles started with a 30-s step at 95°C and ended with a 5-min elongation step at 72°C. Elongase enzyme mix (Life Technologies, Gaithersburg, Md.) was used in all PCR assays. Amplified products were electrophoresed in 1% agar gel containing 0.5 μg of ethidium bromide (Sigma, St. Louis, Mo.) per ml and revealed the presence of four bands, including expected ∼450-bp fragments. A piece of agar gel containing the 450-bp fragment was cut out, and DNA fragments were extracted from the agar using the QIAquick gel extraction kit (Qiagen, Courtaboeuf, France) and following the manufacturer's instructions. DNA fragments obtained after gel purification were cloned as described below.
TABLE 1.
PCR assay conditions and primer sequencesa
| Expt | Primerb (amt [pmol]) | Sequence (5′→3′) | Temp and duration ofc:
|
|
|---|---|---|---|---|
| Annealing | Elongation | |||
| I | gyrAF (100) | GA(T/C)GGN(C/T)TNAA(G/A)CCNGTNCA | 48°C, 30 s | 72°C, 90 s |
| gyrAR (100) | GCCATNCCNACNGC(G/A/T)ATNCC | |||
| II | M13F (100) | GTAAAACGACGGCCAGT | 48°C, 30 s | 72°C, 90 s |
| M13R (100) | GGAAACAGCTATGACCATG | |||
| III | GF24 (50) | GACGGATTAAAACCTGTACA | 50°C, 30 s | 72°C, 90 s |
| GR22 (50) | GGCACATTCGTTGCCAT | |||
| GR23 (50) | TTAGTGGCCATACCAACTGC | |||
All PCRs used 5 μl of 2 mM deoxynucleoside triphosphates, 2 μl of buffer A, 8 μl of buffer B, and 1 μl of elongase.
F, forward; R, reverse.
Each PCR included 40 cycles.
Cloning reaction.
The purified 450-bp fragments of E. sennetsu obtained after amplification with primers gyrAF and gyrAR were cloned into Escherichia coli using the PCR-Script Amp cloning kit (Stratagene, Cambridge, United Kingdom), and following the manufacturer's instructions. Briefly, gyrAF-gyrAR PCR amplification products were cloned into the pPCR-Script Amp SK(+) cloning vector. Ligation of the PCR amplification products with the vector was obtained with T4 DNA ligase, and the ligation reaction mixture was used for transformation into Epicurian Coli XL1-Blue MRF′ Kan cells. After the transformation reaction mixture had been plated onto IPTG (isopropyl-β-d-thiogalactopyranoside)- and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)-supplemented Luria-Bertani agar plates containing 50 μg of ampicillin per ml, white colonies (i.e., Lac+, ampicillin-resistant colonies) were selected. Twenty of them were subcultured overnight at 37°C in ampicillin-containing Luria-Bertani broth, and bacterial growth from each culture was recovered by centrifugation (1,700 × g, 10 min). Plasmids from transformed E. coli were extracted using the QIAgen plasmid mini-kit (Qiagen, Courtaboeuf, France) following the manufacturer's recommendations. A DNA sequence was initially generated from each of the 20 clones using oligonucleotide primers (i.e., M13F and M13R) complementary to flanking sequences in the cloning vector (Table 1, experiment II). In half of them, an expected ∼650-bp fragment was revealed by electrophoresis. These fragments were sequenced using the procedure described below.
Genome walker assay.
The entire E. sennetsu gyrA sequence was determined using the genome walker procedure (33) (Universal Genome Walker kit; Clontech Laboratories, Palo Alto, Calif.), and following the manufacturer's instructions. We first determined the sequence of the E. sennetsu gyrA DNA adjacent to the DNA fragment amplified using GYRAF and GYRAR primers. Then, the newly defined sequence portion of the E. sennetsu gyrA served to define new primers allowing repeating the procedure until the entire gene sequence was determined. The entire gyrA sequence was then confirmed by further PCR-sequencing assays, using specific forward and reverse primers defined from the DNA sequence obtained using the genome walker procedure.
Sequencing procedure.
The cycle sequencing reactions were performed using the dRhodamine terminator cycle sequencing ready reaction kit with AmpliTaq DNA polymerase, FS (Perkin Elmer Applied Biosystems, Warrington, United Kingdom), according to the manufacturer's instructions. The 5′ ends of the amplified fragments obtained in the different PCR assays were sequenced after precipitation and purification with 70% ethanol and 0.5 mM MgCl2. Cycle sequencing reaction mixtures comprised 4 μl of ready reaction mix, 1 μl of forward primer (at 10 pmol/μl) for direct DNA strand sequencing or 1 μl of reverse primer (at 10 pmol/μl) for complementary DNA strand sequencing, and 2 μl (i.e., ∼200ng) of template DNA, brought to 30 μl with deionized water. Amplification was performed with 30 cycles of 95°C for 20 s, 50°C for 10 s, and 60°C for 4 min. Electrophoresis was performed with the ABI PRISM 310 genetic analyzer (Perkin Elmer).
QRDR amplification and sequencing for other Ehrlichia species and C. ruminantium.
Primers allowing amplification of the QRDR region of the remaining Ehrlichia species as well as for C. ruminantium were defined by alignment of the newly determined E. sennetsu gyrA sequence with the known Rickettsia prowazekii (39) and E. coli (41) gyrA sequences (GenBank accession numbers U02931 and X06744, respectively). These primers were referred to as GF24 for the forward primer and GR22 for the reverse primer for all species tested except for E. chaffeensis and C. ruminantium, for which the GR23 reverse primer was used (Table 1). PCR conditions were as described in Table 1 (experiment III).
Comparison of DNA and amino acid sequences.
E. sennetsu gyrA sequence and its putative protein counterpart were compared to known gyrA sequences deposited in GenBank, using BLAST software (1). DNA and amino acid sequences of the QRDRs of the six Ehrlichia species studied were aligned and compared using the CLUSTAL multialignment package (19).
Structural analysis.
The natural mutations in the gyrA QRDR sequence were analyzed in the context of the three-dimensional structure using the E. coli gyrA structure (protein database accession number 1AB4) (26). The alignment of the Ehrlichia species gyrA QRDR conserved sequences with that of E. coli were performed using the FASTA program (29), and TURBO software (38) was used to display the E. coli structure and localize the natural mutations at a structural level.
In vitro susceptibility of E. risticii to fluoroquinolones.
The susceptibility of E. risticii to fluoroquinolones was determined using an in vitro cell system. E. risticii was grown in DH82 cell cultures, incubated in minimum essential medium (Life Technologies, GIBCO BRL, Cergy Pontoise, France) supplemented with 12% fetal calf serum and 2 mM l-glutamine (Life Technologies, GIBCO BRL). E. chaffeensis Arkansas was grown under the same conditions and used as a fluoroquinolone-resistant control. Cell cultures were incubated at 37°C in a 5% CO2 atmosphere until 100% of cells were infected and cell lysis occurred due to intracellular bacterial multiplication. This cell suspension was recovered, centrifuged at 700 × g for 10 min to remove cell debris, and diluted 1:100 in fresh supplemented minimal essential medium. This inoculum was used to infect DH82 confluent cell monolayers grown in 24-well plates (1 ml per well) (D. Dutcher, Brumath, France). After a 1-h incubation of cultures at 37°C to allow entry of bacteria within cells, antibiotics were added to obtain twofold serial final concentrations ranging from 0.125 to 16 μg/ml. Ofloxacin and ciprofloxacin were used as the tested fluoroquinolone compounds, whereas amoxicillin and doxycycline were used as the negative and positive controls, respectively. Antibiotic-free wells served as growth controls. The percent infected cells was monitored every 3 days, using the Dif Quik technique (Biochemical Sciences, Paris, France) as previously described, until nearly 100% of cells were infected in antibiotic-free controls. At that time, the cell monolayer in each well was harvested by scraping, and cell smears were prepared by cytocentrifugation (5 min at 1,000 rpm using a cytospin; Shandon, Eragny, France) and stained with Dif Quik. Slides were examined under a light microscope (Leica Mikroscopie, Wetzlar, Germany) at a ×1,000 magnification. The MIC corresponded to the minimum antibiotic concentration allowing complete inhibition of morula formation.
RESULTS
E. sennetsu QRDR region.
PCR fragments amplified with GYRAF and GYRAR oligonucleotide primers were cloned, and several representative clones were sequenced. Two distinct sequences (∼450 bp) were found among the clones, which shared ∼70% identity at the nucleotide level. These specific DNA sequences were used as a template to design specific primers needed for the genome walker procedure. However, after about 1,000 bp was sequenced, only the sequence having the highest homology with other gyrA sequences, including E. coli and R. prowazekii gyrA sequences (39, 41), was considered specific for the E. sennetsu gyrA gene and was fully sequenced. This sequence showed 77.5 and 70% amino acid identity, respectively, with E. coli GyrA and ParC QRDR sequences.
E. sennetsu gyrA gene.
The entire E. sennetsu gyrA gene was sequenced by using the genome walker procedure. Within the 2,732 bp of E. sennetsu DNA sequenced, a 2,610-bp open reading frame (ORF), starting with an ATG at nucleotide 109 and extending to a stop codon, TAG, at nucleotide 2716, with a GC content of 41.9% was identified (Fig. 1). The ATG codon was designated as the start codon based on best-fit alignment with the E. coli and R. prowazekii gyrA sequences. However, a GTG codon preceding the ATG codon could also be considered based on alignment with the R. prowazekii gyrA for which a GTG codon is used at the beginning of the gyrA gene (39). The ORF is preceded by putative −10 and −35 promoter regions, separated by 20 bp (Fig. 1) and sharing a high degree of identity with the E. coli promoter (34, 41). The −10 region may correspond to the TATAAT DNA sequence, strictly conserved with the E. coli consensus sequence (34, 41), while the −35 region may correspond to the TCGCAA DNA sequence, comparable to the TTGACA E. coli consensus sequence (34, 41). Similarity with the E. coli ribosome binding site (i.e., AGGAGGT) sequence has also been identified three nucleotides upstream of the ATG codon (i.e., AGTAGTG). Translation of the ORF corresponded to a 869-amino-acid (aa)-long protein and a calculated molecular weight (Mr) of 97,365, comparable to the E. coli GyrA (i.e., 875 aa, Mr of 96,957) (34, 41) or the R. prowazekii GyrA (i.e., 905 aa, Mr of 101,048) (39). The BLAST program was used to compare the GyrA sequences of E. sennetsu and other species in order to determine sequence similarities and the presence of conserved residues (Fig. 2). The closest relative of the E. sennetsu GyrA sequence corresponds to the R. prowazekii sequence (39), with 47% identities at the nucleotide sequence level. The invariant active-site tyrosine residue, responsible for linking the enzyme to the cut DNA strands, was found at position 118, which corresponds to Y122 in E. coli GyrA (22). Other conserved regions included the HPHGD motif in the QRDR (37). As for other GyrA protein sequences, the most divergent part of the sequence corresponds to the C-terminal region (22).
FIG. 1.
DNA and amino acid sequences of E. sennetsu gyrA. The putative −10 and −35 promoter regions, the putative ribosome binding sites, the ATG start codon, the HPHGD conserved motif, and the active-site tyrosine residue (Y118) are underlined.
FIG. 2.
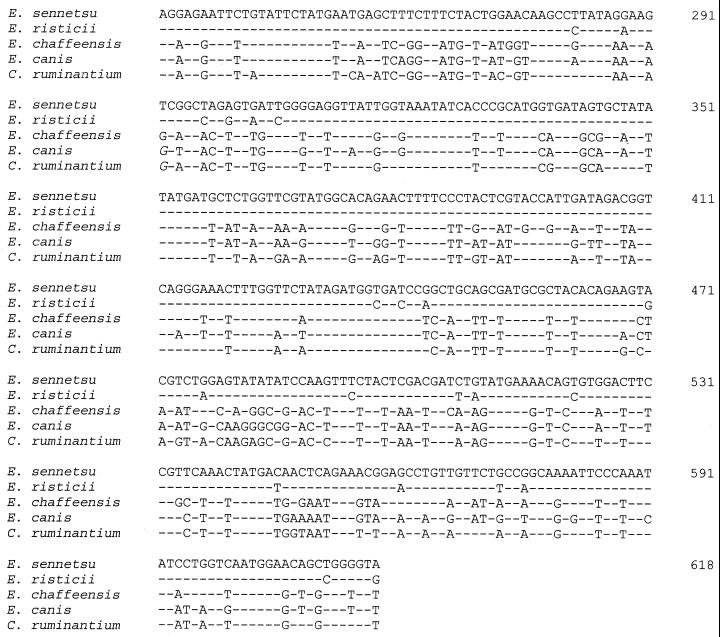
DNA sequence alignment of the QRDR of the six Ehrlichia species studied.
QRDR region for other Ehrlichia species and C. ruminantium.
Comparison of DNA sequences determined for the seven species studied revealed 100% homology in the QRDR region of E. sennetsu, E. phagocytophila, and the HGE agent, while silent mutations were found in the nucleotide sequence of E. risticii (Fig. 2), leading to a 100% amino acid identity in the QRDR for all four species. In contrast, a different consensus was found in QRDR sequences of E. chaffeensis, E. canis, and C. ruminantium (Fig. 2 and 3). The most striking differences in QRDR amino acid sequences between the different Ehrlichia species tested were found at positions 77 and 79 (corresponding to G81 and S83 in E. coli GyrA), with an alanine residue being found in species belonging to the E. canis genogroup and a glycine and a serine being found in the other species (Fig. 3).
FIG. 3.
Alignment of the putative amino acid sequences of the QRDR of the six Ehrlichia species studied, as well as E. coli GyrA and ParC and R. prowazekii GyrA.
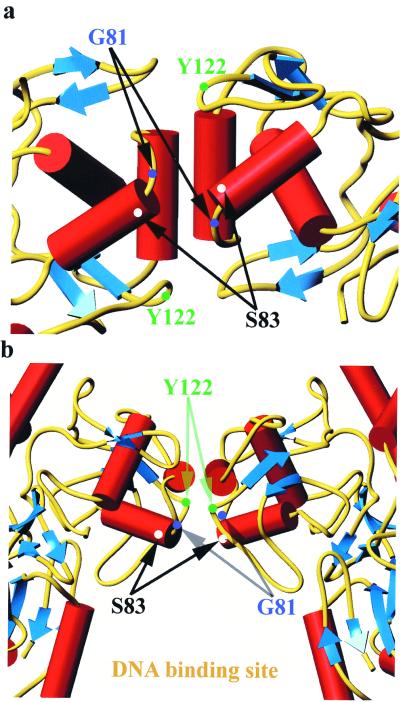
The E. sennetsu and E. coli QRDRs share 75.6% identity in a 41 aa overlap, which corresponds to a root mean square deviation of 1.09 Å in the positions of the main chain atoms (10) and a ternary structure comparable to the E. coli GyrA QRDR structure. Thus, we used the E. coli QRDR structure to highlight the specific amino acid positions (serine 83 and glycine 81 in E. coli numbering) (Fig. 4) and locate them at the structural level.
FIG. 4.
Stereoview of a ribbon diagram of the E. coli DNA gyrase A subunit (protein database accession code 1AB4). The blue arrows represent beta strands, the red ribbons represent alpha helices, and turns and loops are yellow. Residues involved in mutations associated with resistance phenotype (S83, A84, and D87) and the tyrosine residue (Y122) are marked on the picture. The figure was prepared using TURBO-FRODO (25).
In vitro susceptibility of E. risticii to fluoroquinolones.
Our model for Ehrlichia sp. antibiotic susceptibility testing allowed easy determination of MICs. In antibiotic-free controls, the percentage of infected cells, as determined by Dif Quik staining, increased progressively from 0% on the first day of experiment to 15% after 6 days of incubation, 35% after 9 days, 65% after 12 days, and near 100% after 15 days. At that time, almost every cell was heavily infected, with several morulae visible by Dif Quik staining. A similar result was found with amoxicillin at concentrations up to 16 μg/ml (i.e., MIC > 16 μg/ml). In contrast, complete inhibition of morula formation was obtained with doxycycline, ofloxacin, and ciprofloxacin at concentrations as low as 0.125 μg/ml (i.e., MIC < 0.125 μg/ml). MICs of ofloxacin and ciprofloxacin for E. chaffeensis Arkansas were ≥16 μg/ml.
DISCUSSION
DNA gyrase and topoisomerase IV are type II DNA topoisomerases catalyzing DNA topological changes necessary for DNA replication and transcription (13, 22). These two enzymes are natural targets of the fluoroquinolone antibiotics (13). Usually, the primary targets of fluoroquinolones are DNA gyrase in the gram-negative bacteria (e.g., E. coli and Neisseria gonorrhoeae) and topoisomerase IV in gram-positive bacteria (e.g., Streptococcus pneumoniae and Staphylococcus aureus) (13, 28). Both enzymes are composed of two subunits (in a tetrameric A2B2 structure), respectively encoded by gyrA and gyrB genes in the case of DNA gyrase and by parC and parE genes in the case of topoisomerase IV (37). Acquired resistance to fluoroquinolones in gram-negative bacteria most often corresponds to mutations in the gyrA DNA sequence (37), especially in a specific 41-aa region within the N-terminal portion of the GyrA protein corresponding to positions 67 to 106 of the E. coli GyrA protein sequence (22, 25, 37). This region is near the putative active site (i.e., Tyr122 in E. coli) and is supposed to be the interaction site between the A subunit of DNA gyrase and quinolones (40). It is referred to as the QRDR (40).
In the present study, we attempted to verify our hypothesis that the QRDR of GyrA may explain intrinsic quinolone resistance in some Ehrlichia species. We first determined the entire DNA sequence of gyrA of E. sennetsu, a fluoroquinolone-susceptible species. A PCR approach using universal degenerate primers was used to amplify a first portion of the E. sennetsu gyrA QRDR (22). These universal primers also amplify DNA from parC in about 40% of the organisms studied (22). Thus, using these degenerate primers, we amplified two different DNA sequences from E. sennetsu. A higher sequence homology is usually found between two gyrA genes from species belonging to the same phylogenetic lineage (i.e., usually near 50% identities between putative protein sequences) than between gyrA and parC genes from the same organism (22). Thus, the E. sennetsu DNA sequence sharing the highest homology with the E. coli gyrA QRDR sequence was considered to be the gyrA-specific gene. This sequence also displayed lower homology to the E. coli parC QRDR than to the E. coli gyrA QRDR. This sequence was then used to define specific primers to determine the entire gyrA sequence using the genome walker procedure. Not surprisingly, there is a high homology (i.e., 48% identity in a 900-residue overlap) between E. sennetsu and R. prowazekii DNA gyrase (alpha subunit) amino acid sequences, these genera being phylogenetically closely related (31, 32).
Tetracyclines are highly active in vitro against the human pathogens E. sennetsu, E. chaffeensis, and the HGE agent (4, 5, 23), and doxycycline is currently the first-line antibiotic for treating ehrlichial diseases (3, 14, 15, 17, 35). However, these antibiotics are relatively contraindicated in children less than 8 years old because of tooth discoloration and in pregnant women because of bone toxicity to the fetus, and they may induce gastric intolerance as a general side effect. Chloramphenicol has been shown to be inactive or poorly active against E. sennetsu, E. chaffeensis, and the HGE agent in vitro (4, 5, 23), and failures in patients with monocytic ehrlichiosis or human granulocytic ehrlichiosis treated with this drug have been reported (14, 16, 24). Rifampin is active in vitro against E. sennetsu and E. chaffeensis (4, 5), and it may represent an alternative to tetracyclines. Its clinical usefulness has been suggested for pregnant women with HGE (7). Fluoroquinolones have not been used extensively in ehrlichial diseases. In vitro, E. sennetsu and E. phagocytophila (including the HGE agent) are susceptible to fluoroquinolone compounds: the MIC of ciprofloxacin for E. sennetsu is ≤0.125 μg/ml (4), that of ofloxacin for E. phagocytophila is ≤2 μg/ml (20), and those of both compounds for the HGE agent are 2 μg/ml (23). Nalidixic acid, a narrow-spectrum quinolone, was not active against E. risticii (30), but we report here that for this species, ofloxacin and ciprofloxacin MICs are <0.125 μg/ml. In contrast, E. chaffeensis and E. canis appear to be more resistant to fluoroquinolones: MICs of ciprofloxacin for E. chaffeensis were 4 μg/ml (5) or even higher (i.e., >16 μg/ml) in the present study, and E. canis was able to grow in the presence of 2 μg of pefloxacin per ml (6). The antibiotic susceptibilities of C. ruminantium remain undetermined.
In gram-negative bacteria, fluoroquinolone resistance most often corresponds to the presence of specific amino acids at critical positions in the QRDR of GyrA, the A subunit of DNA gyrase (22, 36, 37). These key residues correspond to positions 83, 84, and 87 (E. coli numbering) of GyrA (37). As for acquired resistance to fluoroquinolones, mutations of S83 to A, W, and L in ciprofloxacin-resistant E. coli have been reported (11, 27, 41). Likewise, mutations of S83 (E. coli numbering) to T and V in Klebsiella pneumoniae, Pseudomonas aeruginosa, Rickettsia rickettsii, and Campylobacter jejuni increased by 1 log unit the quinolone MIC at which 90% of strains are inhibited (MIC90) (21). High ciprofloxacin resistance (MIC90 ranging from 1 to 8 μg/ml) has been found in Mycoplasma sp., Treponema sp., Borrelia sp., and Chlamydia sp. strains where S83 (E. coli numbering) is replaced by an amino acid other than S or T (21). Similar observations have been made with bacterial species bearing a natural resistance to fluoroquinolones (37). Interestingly, compared to the sequence in the quinolone-susceptible species E. sennetsu, amino acid sequence variations in the GyrA QRDR were found only in E. chaffeensis, E. canis, and C. ruminantium, two of these three species being known to be resistant to fluoroquinolones. The serine residue (corresponding to S83 in E. coli GyrA) is found at position 79 in the E. sennetsu GyrA QRDR as well as in all other fluoroquinolone-susceptible Ehrlichia species. This amino acid is replaced by an alanine in E. chaffeensis, E. canis, and C. ruminantium. Another substitution was observed in the QRDR of these species, with an alanine residue replacing the more classical glycine that is found in E. coli at position 81. This substitution corresponds to position 77 in all Ehrlichia species studied. Given the high level of conservation of the GyrA sequences we produced with the E. coli one, we can predict the two structures to be very similar, especially in the QRDR. Thus, the natural mutations observed in quinolone-resistant Ehrlichia species all appear to be located at the dimer interface in the DNA binding area of the GyrA structure. Altogether, our results suggest a gyrA-mediated natural resistance in the fluoroquinolone-resistant species E. chaffeensis and E. canis. Because only one strain for each of these species has been studied so far, determination of the gyrA QRDR in further strains is needed to confirm our hypothesis. To our knowledge, Mycobacterium is the only other genus for which heterogeneity in fluoroquinolone susceptibility between various species has been correlated with specific natural gyrA sequences (18).
E. chaffeensis, E. canis, and C. ruminantium belong to the same phylogenetic cluster, within the alpha group of the Proteobacteria (Fig. 5). In previous work from our team, among Rickettsia species, which also belong to the alpha group of the Proteobacteria, rpoB (the gene encoding the beta subunit of RNA polymerase)-mediated resistance to rifampin was found in specific species, including R. massiliae, R. montanensis, R. aeschlimannii, R. rhipicephali, and the tick isolate Bar 29 (12). All these species belong to a specific phylogenetic cluster referred to as the R. massiliae subgroup within the Rickettsia genus (12). Altogether, these results indicate that natural resistance to antibiotics whose targets are proteins (e.g., DNA gyrase and RNA polymerase for quinolones and rifamycins, respectively) or RNA (e.g., 16S rRNA or 23S rRNA for macrolides and chloramphenicol) encoded by highly conserved genes potentially represents a useful phylogenetic criterion that may help to better define phylogenetic clusters at a subgenus level.
FIG. 5.
Phylogenetic tree based on 16S rRNA gene sequences, and correlation between quinolone susceptibility and gyrA sequences in studied Ehrlichia species. S, susceptible (MIC ≤ 2 μg/ml); R, resistant (MIC > 2 μg/ml).
In conclusion, we have demonstrated that fluoroquinolone resistance in E. chaffeensis and E. canis, species belonging to the E. canis genogroup, is strongly correlated to the presence of a specific gyrA QRDR sequence and specifically to the presence of an alanine residue at positions 81 and 83 (E. coli numbering) at the dimer interface in the DNA binding area of the GyrA structure. These results are likely indicative of a natural gyrase-mediated resistance to fluoroquinolones in the E. canis genogroup. They also potentially represent useful data for ehrlichial phylogeny.
ACKNOWLEDGMENT
We thank J. S. Dumler for reviewing the manuscript.
REFERENCES
- 1.Altschul X, Stephen F, Thomas L, Madden X, Alejandro A, Schäffer X, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakken J S, Kreuth J, Wilson-Nordskog C, Tilden R L, Asanovitch K, Dumler J S. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 4.Brouqui P, Raoult D. In vitro susceptibility of Ehrlichia sennetsu to antibiotics. Antimicrob Agents Chemother. 1990;34:1593–1596. doi: 10.1128/aac.34.8.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brouqui P, Raoult D. In vitro antibiotic susceptibility of the newly recognized agent of human ehrlichiosis: Ehrlichia chaffeensis. Antimicrob Agents Chemother. 1992;36:2799–2803. doi: 10.1128/aac.36.12.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouqui P, Raoult D. Susceptibilities of Ehrlichiae to antibiotics. In: Raoult D, editor. Antimicrobial agents and intracellular pathogens. Boca Raton, Fla: CRC Press; 1993. pp. 181–199. [Google Scholar]
- 7.Buitrago M I, Ijdo J W, Rinaudo P, Simon H, Copel J, Gadbaw J, Heimer R, Fikrig E, Bia F J. Human granulocytic ehrlichiosis during pregnancy treated successfully with rifampin. Clin Infect Dis. 1998;27:213–215. doi: 10.1086/517678. [DOI] [PubMed] [Google Scholar]
- 8.Byrom B, Yunker C E, Donovan P L, Smith G E. In vitro isolation of Cowdria ruminantium from plasma of infected ruminants. Vet Microbiol. 1991;26:263–268. doi: 10.1016/0378-1135(91)90019-c. [DOI] [PubMed] [Google Scholar]
- 9.Chen S-M, Dumler J S, Feng H-M, Walker D H. Identification of the antigenic constituents of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1994;50:52–58. [PubMed] [Google Scholar]
- 10.Chotias C, Lesk A M. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986;5:823–826. doi: 10.1002/j.1460-2075.1986.tb04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cullen M, Wyke A, Kuroda R, Fisher L. Cloning and characterization of a DNA gyrase A gene from Escherichia coli that confers clinical resistance to 4-quinolones. Antimicrob Agents Chemother. 1989;33:886–894. doi: 10.1128/aac.33.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drancourt M, Raoult D. Characterization of mutations in the rpoB gene in naturally rifampin-resistant Rickettsia species. Antimicrob Agents Chemother. 1999;43:2400–2403. doi: 10.1128/aac.43.10.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumler J S, Bakken J S. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis. 1995;20:1102–1110. doi: 10.1093/clinids/20.5.1102. [DOI] [PubMed] [Google Scholar]
- 15.Dumler J S, Bakken J S. Human ehrlichioses: newly recognized infections transmitted by ticks. Annu Rev Med. 1998;49:201–213. doi: 10.1146/annurev.med.49.1.201. [DOI] [PubMed] [Google Scholar]
- 16.Dumler J S, Brouqui P, Aronson J, Taylor J P, Walker D H. Identification of ehrlichia in human tissue. N Engl J Med. 1991;325:1109–1110. doi: 10.1056/NEJM199110103251517. [DOI] [PubMed] [Google Scholar]
- 17.Fishbein D B, Dawson J E, Robinson L E. Human ehrlichiosis in the United States, 1985 to 1990. Ann Intern Med. 1994;120:736–743. doi: 10.7326/0003-4819-120-9-199405010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Guillemin I, Jarlier V, Cambau E. Correlation between quinolone susceptibility patterns and sequences in the A and B subunits of DNA gyrase in mycobacteria. Antimicrob Agents Chemother. 1998;42:2084–2088. doi: 10.1128/aac.42.8.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins D G, Sharp P M. Clustal: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 20.Horowitz H W, Hsieh T-C, Aguero-Rosenfeld M E, Kalantarpour F, Chowdhury I, Wormser G P, Wu J M. Antimicrobial susceptibility of Ehrlichia phagocytophila. Antimicrob Agents Chemother. 2001;45:786–788. doi: 10.1128/AAC.45.3.786-788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang W M. Multiple DNA gyrase-like genes in Eubacteria. In: Andoh T, Ideda H, Oguro M, editors. Molecular biology of DNA topoisomerases and its application to chemotherapy. Boca Raton, Fla: CRC Press; 1992. pp. 37–46. [Google Scholar]
- 22.Huang W M. Bacterial diversity based on type II DNA topoisomerase genes. Annu Rev Genet. 1996;30:79–107. doi: 10.1146/annurev.genet.30.1.79. [DOI] [PubMed] [Google Scholar]
- 23.Klein M B, Nelson C M, Goodman J L. Antibiotic susceptibility of the newly cultivated agent of human granulocytic ehrlichiosis: promising activity of quinolones and rifamycins. Antimicrob Agents Chemother. 1997;41:76–79. doi: 10.1128/aac.41.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeda K, Markowitz N, Hawley R C, Ristic M, Cox D, McDade J. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987;316:853–856. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell A. The molecular basis of quinolone action. J Antimicrob Chemother. 1992;30:409–414. doi: 10.1093/jac/30.4.409. [DOI] [PubMed] [Google Scholar]
- 26.Morais Cabral J H, Jackson A P, Smith C V, Shikotra N, Maxwell A, Liddington R. Crystal structure of the breakage-reunion domain of DNA gyrase. Nature. 1997;388:903–906. doi: 10.1038/42294. [DOI] [PubMed] [Google Scholar]
- 27.Oram M, Fisher L. 4-Quinolone resistance mutations in the DNA gyrase of Escherichia coli clinical isolates identified by using the polymerase chain reaction. Antimicrob Agents Chemother. 1991;35:387–389. doi: 10.1128/aac.35.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan X-S, Ambler J, Mehtar S, Fisher L M. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2321–2326. doi: 10.1128/aac.40.10.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1998;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rikihisa Y, Jiang B M. In vitro susceptibilities of Ehrlichia risticii to eight antibiotics. Antimicrob Agents Chemother. 1988;32:986–991. doi: 10.1128/aac.32.7.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roux V, Raoult D. Phylogenetic analysis of the genus Rickettsia by 16S rDNA sequencing. Res Microbiol. 1995;146:385–396. doi: 10.1016/0923-2508(96)80284-1. [DOI] [PubMed] [Google Scholar]
- 32.Roux V, Rydkina E, Eremeeva M, Raoult D. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int J Syst Bacteriol. 1997;47:252–261. doi: 10.1099/00207713-47-2-252. [DOI] [PubMed] [Google Scholar]
- 33.Siebert P D, Chenchik A, Kellogg D E, Lukyanov K A, Lukyanov S A. An improved PCR method for walking in uncloned genomic DNA. Nucleic Acids Res. 1995;23:1087–1088. doi: 10.1093/nar/23.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swanberg S L, Wang J C. Cloning and sequencing of the Escherichia coli gyrA gene coding for the A subunit of DNA gyrase. J Mol Biol. 1987;197:729–736. doi: 10.1016/0022-2836(87)90479-7. [DOI] [PubMed] [Google Scholar]
- 35.Walker D H, Dumler J S. Emergence of the ehrlichioses as human health problems. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J C. DNA topoisomerases. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 37.Waters B, Davies J. Amino acid variation in the GyrA subunit of bacteria potentially associated with natural resistance to fluoroquinolone antibiotics. Antimicrob Agents Chemother. 1997;41:2766–2769. doi: 10.1128/aac.41.12.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willmott C J R, Maxwell A A. A single point mutation in the DNA gyrase A protein greatly reduces the binding of fluoroquinolones to the gyrase-DNA complex. Antimicrob Agents Chemother. 1993;37:126–127. doi: 10.1128/aac.37.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wood D O, Waite R T. Sequence analysis of the Rickettsia prowazekii gyrA gene. Gene. 1994;151:191–196. doi: 10.1016/0378-1119(94)90655-6. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990;34:1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshida H, Kojima T, Yamagishi J-I, Nakamura S. Quinolone-resistant mutations of the gyrA gene of Escherichia coli. Mol Gen Genet. 1988;211:1–7. doi: 10.1007/BF00338386. [DOI] [PubMed] [Google Scholar]