The catecholamine neuromodulators dopamine and norepinephrine play key roles in cognition and the pathophysiology of psychiatric disorders. Much of the evidence for this comes from human studies using positron emission tomography (PET), which allows direct in vivo measurement of aspects of catecholamine function such as synthesis or transmission. However, the invasiveness and high degree of specialization associated with PET limit its applicability for clinical use and research, especially in pediatric populations and repeated longitudinal assessments of illness trajectory and treatment response. This underscores the need for developing biomarkers that, like PET-based measures, reflect neurobiological processes relevant to illnesses and their treatment, but which are more scalable and easily acquired.
Neuromelanin-sensitive MRI (NM-MRI)1 may fit these criteria. Neuromelanin (NM) is a dark pigment produced from catecholamine metabolism via iron-dependent oxidation. It accumulates gradually over the lifespan in granules stored in the body of catecholamine brainstem neurons. This creates a lasting record of the history of catecholamine turnover, as NM granules, which contain NM-iron complexes alongside proteins and lipids, cannot be removed from neurons—they are only cleared from tissue following cell death in neurodegenerative conditions like Parkinson’s disease. The paramagnetic properties of NM-iron complexes make it possible to image them non-invasively using MRI. NM-MRI localizes NM-rich nuclei like the dopaminergic substantia nigra (SN) and ventral tegmental area (VTA) and the noradrenergic locus coeruleus (LC) as hyperintense regions through a combination of T1 effects and direct or indirect magnetization transfer effects—in gradient-echo sequences with a magnetization-transfer pulse or turbo-spin-echo sequences, respectively2. Specifically, paramagnetic melanin-iron complexes in synthetic phantoms cause a T1 shortening that reduces magnetization-transfer effects and is thought to underlie the NM-MRI contrast3. The relative content of macromolecules versus water further influences magnetization transfer, and NM-MRI, in vivo: local NM-MRI contrast in SN-VTA and LC seems to be partly driven by the large size, and high water content, of catecholamine neurons relative to surrounding white-matter tissues rich in macromolecules4. Despite this non-specific and still-debated contrast mechanism, NM-MRI hyperintensities in SN-VTA and LC co-localize with and correlate with counts of NM-positive catecholamine neurons in postmortem histology2. Furthermore, cell loss in these regions is associated with decreases in NM-MRI signal such as those reliably observed in Parkinson’s disease5, which tend to parallel the loss of catecholamine neuron terminals in their target regions in studies imaging transporter density5. Until recently, it was however unclear whether in addition to its use as a marker of catecholamine cell loss in neurodegenerative conditions, NM-MRI could also be used as a marker of catecholamine function in non-neurodegenerative conditions such as those most relevant in psychiatry.
A recent postmortem study showed that NM-MRI is sensitive to variability in SN NM tissue concentration even in the absence of neurodegeneration6. Even more critically, it showed a correlation of SN NM-MRI signal with amphetamine-induced dopamine release in the dorsal striatum, a PET measure of dopamine function indexing storage and release capacity. This is consistent with the seminal preclinical finding that pharmacological induction of dopamine synthesis enhances NM accumulation2. NM-MRI signal also correlated with a functional MRI readout of local neural activity in SN6. Thus, beyond the structural information provided by NM-MRI and its ability to localize NM-rich structures, emerging evidence suggests that the intensity of the NM-MRI signal provides quantitative information about the molecular composition of the tissue, in particular NM concentration, which can serve as an indirect proxy for dopamine function.
Mounting evidence further suggests that NM-MRI may capture catecholamine dysfunction in psychiatric disorders without known catecholamine cell loss1,6–9. For example, consistent with the established finding of excess nigrostriatal-dopamine function in psychosis, increased NM-MRI signal was observed in medicated patients with schizophrenia1,8 and more recently in unmedicated patients and clinical high-risk individuals as a function of psychosis severity6. A more detailed topographical characterization of signal patterns across voxels—afforded by an anatomical resolution of NM-MRI up to an order of magnitude higher than PET—further revealed that SN alterations predominate in subregions projecting to the associative dorsal striatum. Other studies have reported alterations of SN and LC in major depressive disorder1,9 and cocaine addiction7.
These and other data show the potential of NM-MRI for developing psychiatric biomarkers. One desirable feature of NM-MRI in this regard is its stability. The slow accumulation of NM is likely to make NM-MRI stable over relatively long timescales and insensitive to short-term factors. NM-MRI indeed shows excellent test-retest reliability10, even for voxelwise analyses when implemented through standardized protocols6. Its stability and non-invasiveness situate NM-MRI well for potential development of risk or prognosis biomarkers, including for pediatric populations, and possibly for monitoring treatment response once the timescale of observable NM-MRI changes and their susceptibility to treatment are determined9. If proven to provide a longer-term readout of catecholamine function, NM-MRI could complement acute PET measures in the same way that, in diabetes mellitus, glycated hemoglobin (HbA1c) complements acute glycemic levels by indexing longer-term glycemic control.
For NM-MRI to fulfill its potential, however, important outstanding questions need to be addressed. First, further work should assess the specificity of NM-MRI to NM concentration versus other factors such as macromolecule content. Further refinement of NM-MRI sequences may maximize their specificity and resolution. Second, even in the unlikely case that NM-MRI signal corresponded perfectly to NM concentration, the latter will reflect a combination of processes affecting the cytosolic concentration of catecholamines, including synthesis and vesicular transport, and the various steps involved in NM synthesis and accumulation. Assessing the extent of their relative contributions to variability in NM-MRI signal in health and disease, perhaps through triangulation with PET measures, is thus critical. Third, further study is needed regarding the specific relationship of regional NM-MRI signals to the function of different catecholamine pathways, particularly those with distinct projections arising from small nuclei such as the VTA and LC. Furthermore, the ability of NM-MRI to measure NM concentration in LC still requires direct confirmation. Finally, development of harmonization protocols will facilitate multisite studies likely required for biomarker development.
We hope that the abovementioned work and proposed future directions will guide further development of NM-MRI as a valuable tool for elucidating disease mechanisms and ultimately informing clinical decisions in psychiatry.
Figure. Mechanisms and clinical applications of neuromelanin-sensitive MRI.
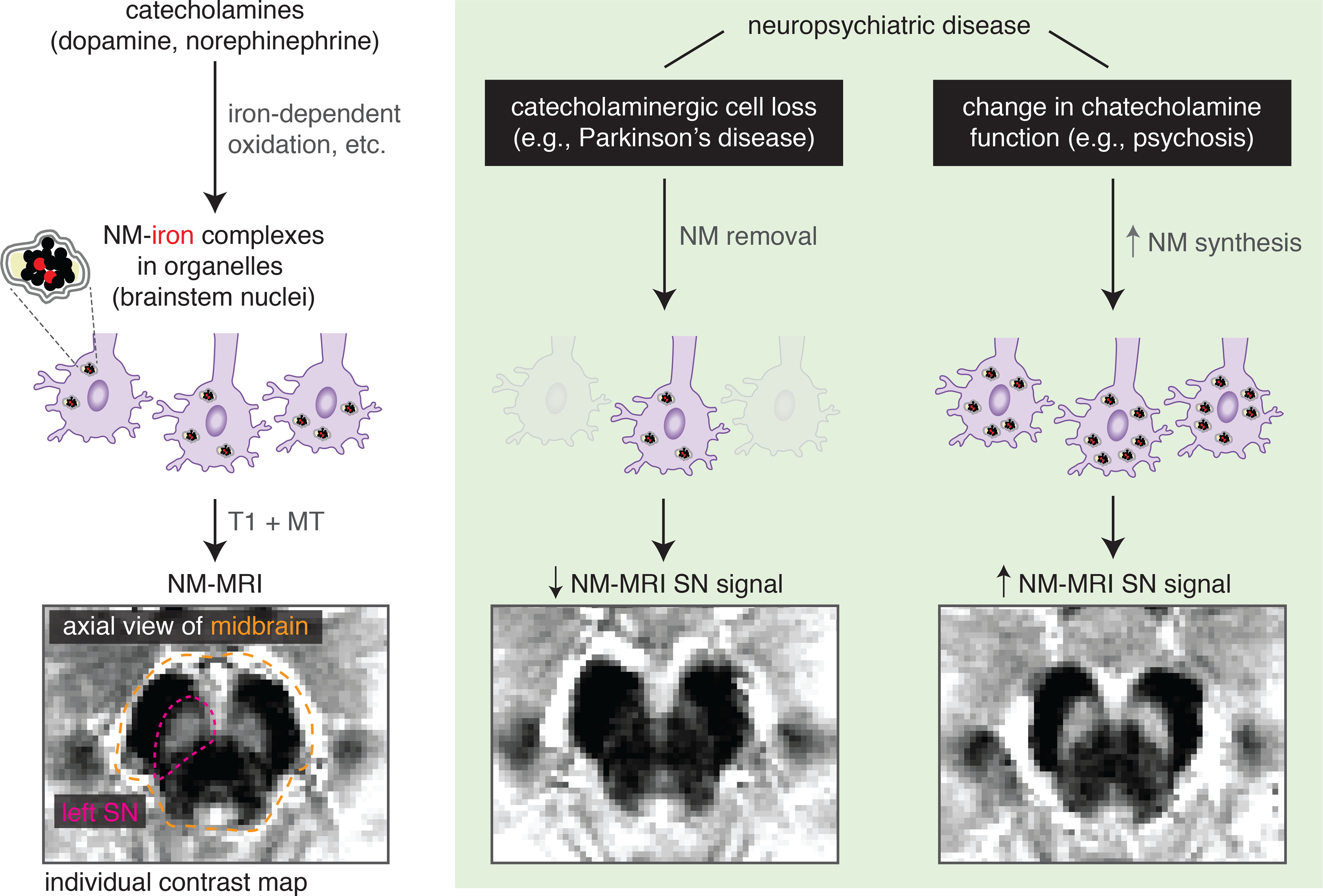
Left: neuromelanin granules are synthesized from the metabolism of catecholamines, accumulating in the body of catecholamine neurons located in brainstem nuclei (SN-VTA and LC). Through an MRI contrast mechanism involving T1 and magnetization-transfer (MT) effects, NM-MRI captures NM concentration in these nuclei. Right: in neuropsychiatric disease, NM-MRI can capture changes in NM concentrations. SN dopaminergic cell loss in Parkinson’s disease leads to decreased NM concentration and decreased NM-MRI signal in SN. In psychosis, in the absence of cell loss, increased synthesis of dopamine in SN is thought to drive increased NM concentration, resulting in the observed increases in NM-MRI signal in SN. Illustrative examples of individual contrast maps (contrast-ratio maps normalized to signal in a surrounding reference region of white matter) are shown for a healthy individual (left), one with Parkison’s disease and one with schizophrenia (right).
Acknowledgements.
The authors would like to thank Anissa Abi-Dargham, Luigi Zecca, and David Sulzer for invaluable discussions on the topics presented here, and to Lawrence Kegeles, Anna Konova, and Gaurav Patel for helpful feedback on the manuscript. This work was supported by NIMH grants R01 MH117323 and R01 MH114965 (to Dr. Horga).
Footnotes
Conflict statement. Drs. Horga, Wengler, and Cassidy are inventors on a patent describing a method for NM-MRI analysis but have received no licensing fees or royalties.
References
- 1.Sasaki M, Shibata E, Kudo K, Tohyama K. Neuromelanin-Sensitive MRI: Basics, Technique, and Clinical Applications. Clin Neuroradiol. 2008(18):147–153. [Google Scholar]
- 2.Sulzer D, Cassidy C, Horga G, et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease. NPJ Parkinsons Dis. 2018;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trujillo P, Summers PE, Ferrari E, et al. Contrast mechanisms associated with neuromelanin-MRI. Magn Reson Med. 2017;78(5):1790–1800. [DOI] [PubMed] [Google Scholar]
- 4.Priovoulos N, van Boxel SCJ, Jacobs HIL, et al. Unraveling the contributions to the neuromelanin-MRI contrast. Brain Struct Funct. 2020;225(9):2757–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter E, Roussakis AA, Lao-Kaim NP, Piccini P. Multimodal dopamine transporter (DAT) imaging and magnetic resonance imaging (MRI) to characterise early Parkinson’s disease. Parkinsonism Relat Disord. 2020;79:26–33. [DOI] [PubMed] [Google Scholar]
- 6.Cassidy CM, Zucca FA, Girgis RR, et al. Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc Natl Acad Sci U S A. 2019;116(11):5108–5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassidy CM, Carpenter KM, Konova AB, et al. Evidence for Dopamine Abnormalities in the Substantia Nigra in Cocaine Addiction Revealed by Neuromelanin-Sensitive MRI. Am J Psychiatry. 2020;177(11):1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe Y, Tanaka H, Tsukabe A, et al. Neuromelanin magnetic resonance imaging reveals increased dopaminergic neuron activity in the substantia nigra of patients with schizophrenia. PLoS One. 2014;9(8):e104619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wengler K, Ashinoff BK, Pueraro E, Cassidy CM, Horga G, Rutherford BR. Association between neuromelanin-sensitive MRI signal and psychomotor slowing in late-life depression. Neuropsychopharmacology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Langley J, Huddleston DE, Liu CJ, Hu X. Reproducibility of locus coeruleus and substantia nigra imaging with neuromelanin sensitive MRI. MAGMA. 2017;30(2):121–125. [DOI] [PubMed] [Google Scholar]


