Abstract
BACKGROUND & AIMS:
The aims of this study were to: (1) assess the performance of the Pancreatitis Activity Scoring System (PASS) in a large intercontinental cohort of patients with acute pancreatitis (AP); and (2) investigate whether a modified PASS (mPASS) yields a similar predictive accuracy and produces distinct early trajectories between severity subgroups.
METHODS:
Data was prospectively collected through the Acute Pancreatitis Patient Registry to Examine Novel Therapies In Clinical Experience (APPRENTICE) consortium (2015–2018) involving 22 centers from 4 continents. AP severity was categorized per the revised Atlanta classification. PASS trajectories were compared between the three severity groups using the generalized estimating equations model. Four mPASS models were generated by modifying the morphine equivalent dose (MED), and their trajectories were compared.
RESULT:
A total of 1393 subjects were enrolled (median age, 49 years; 51% males), The study cohort included 950 mild (68.2%), 315 (22.6%) moderately severe, and 128 (9.2%) severe AP. Mild cases had the lowest PASS at each study time point (all P < .001). A subset of patients with outlier admission PASS values was identified. In the outlier group, 70% of the PASS variation was attributed to the MED, and 66% of these patients were from the United States centers. Among the 4 modified models, the mPASS-1 (excluding MED from PASS) demonstrated high performance in predicting severe AP with an area under the receiver operating characteristic curve of 0.88 (vs area under the receiver operating characteristic of 0.83 in conventional PASS) and produced distinct trajectories with distinct slopes between severity subgroups (all P < .001).
CONCLUSION:
We propose a modified model by removing the MED component, which is easier to calculate, predicts accurately severe AP, and maintains significantly distinct early trajectories.
Keywords: Acute Pancreatitis, Disease Activity, PASS, Severity
Acute pancreatitis (AP) is a major cause of gastroenterology-related hospitalizations, with significant morbidity and mortality across the world.1,2 Pain control and successful resumption of an oral diet are the foremost aspects of management that impact the duration of hospitalization.3 AP may demonstrate highly variable clinical manifestations throughout its course, which impose a clinical challenge for the treating physicians.4 Even in the absence of local and systemic complications, hospitalizations can be protracted, with persistent symptoms such as abdominal pain and/or oral intolerance.5,6
An optimal disease activity index should capture the fluctuating clinical manifestations of AP and be reproducible across different practice patterns. We anticipate a well-designed AP activity metric to assist the treatment team with continuous monitoring of disease status and potentially improve the overall management. Furthermore, a valid and generalizable activity index can be utilized in clinical trials providing “real-time” quantification of disease status.
The Pancreatitis Activity Scoring System (PASS) is a dynamic index proposed to measure AP activity.7 Initial studies assessed the clinical application of PASS in patients from California, in the United States (U.S.).8 They reported that the admission PASS has a significant association with AP outcomes such as local complications and disease severity. The findings of a multicenter U.S. study from our group unraveled the importance of early PASS slopes and their association with the length of hospital stay.9
Our previous study from 8 U.S. centers revealed a subset of patients with mild AP with a markedly high admission PASS value. This was mainly driven by high doses of opioid analgesics, which resulted in a disproportionate contribution of the morphine equivalent dose (MED).9 This finding raised concerns about the generalizability of the PASS globally, especially in countries where opioids are infrequently administered in patients with AP.
To this end, we utilized the Acute Pancreatitis Patients Registry to Examine Novel Therapies in Clinical Experience (APPRENTICE), an international, multicenter collaboration with harmonized prospective recruitment of patients with AP.9–12 Such a diverse population provided a unique opportunity to assess the applicability of the PASS index.
The aims of this study were to: (1) assess the performance of PASS in a heterogeneous international AP cohort; and (2) investigate whether a modified PASS (mPASS) model yields an accurate prediction of severe AP while producing distinct early trajectories between severity subgroups.
Methods
The APPRENTICE study is an international prospective registry of patients with AP coordinated by the University of Pittsburgh Medical Center. Each participating site was required to obtain the approval of the respective institutional review board (IRB) and the University of Pittsburgh’s IRB acted as an umbrella IRB (PR015040389). The study was registered in clinicaltrials.gov (NCT03075618). The detailed methodology of APPRENTICE has been previously published.10
Data were collected from North America, Europe, South America, and Asia. The active enrollment was between August 2015 and January 2018. The diagnosis of AP was based on ≥2 of the following features: characteristic epigastric pain, serum lipase >3 times upper limit of normal, and/or characteristic radiologic findings of AP. Adult patients with AP identified within 7 days from pain onset were deemed eligible. Patients with chronic pancreatitis or pancreatic cancer were excluded.10 De-identified data was entered into the Research Electronic Data Capture tool.13
PASS consists of 5 separately weighted parameters:7 organ failure (×100 per organ), oral intolerance (×40), systemic inflammatory response syndrome (SIRS) (×25 per criterion), MED (×5), and pain score (×5). The score was measured at various time points. Data collected within the first 12 hours of hospitalization were assigned to the admission time point. Subsequently, study time points were assigned data from the preceding 12 hours in the following pattern: 24-hour (12- to 24-hour events), 48-hour (36- to 48-hour events), and 72-hour time points [60- to 72-hour events), and day 7 (156- to 168-hour events).
Organ failure was defined per modified Marshall score ≥2.14 Oral intolerance was defined as worsening abdominal pain and/or vomiting following the resumption of an oral feeding diet. SIRS score was defined as the sum of the following 4 variables: heart rate (>90 beats/minute), respiratory rate >20/minute or PCI2<32 mmHg, temperature (<36 or >38°C), and white blood cell count (<4000/mm3 or >12,000/mm3).15 The MED component was calculated by converting the dose of the opioid analgesics to the intravenous MED. Abdominal pain was based on the numeric visual analogue scale ranging from 0 (no pain) to 10 (worst pain).
In search for an optimal model to rectify MED score inside the PASS frame, we elected a categorical rather than continuous MED structure. Four mPASS models were proposed: (1) In the mPASS-1 model, the MED was completely removed; (2) In the mPASS-2 model, MED was dichotomized into 2 groups: subjects without opioids administration were assigned a value of 0, whereas subjects with any opioid administration received a score of 50; (3) In the mPASS-3 model, MED was stratified based on the third quartile of MED (Q3 = 17), and the following modified MED scores were assigned: no opioid, value of 0; low opioids (<17), 25; and high opioids (≥17), 50; (4) In the mPASS-4 model, we utilised MED quartiles again but with a higher assigned value for the “high opioid” group: no opioid, score of 0; low opioid (<17), 25; high opioid ≥17), 75.
The severity of AP was classified according to the Revised Atlanta Classification (RAC).16 AP subjects with persistent organ failure were considered as severe AP. Subjects with transient organ failure and/or local complications were categorized as moderately severe.
Statistical Methodology
Patients were considered to have missing SIRS score if ≥2 elements were missing. Patients missing all 3 of the SIRS, MED, and pain scores were excluded. For subjects with 3 SIRS components available and within the normal range, the SIRS score was considered to be “zero.” For those with 3 SIRS components available and at least another PASS point, pain and/or MED score data were imputed from patients with the same severity level.
Standard and modified PASS scores at each time point were calculated. Kruskal-Wallis tests were used for cross-sectional analyses comparing severity groups at each time point. The area under the receiver operating characteristic curve (AUC) was used to evaluate the predictive ability of the standard and modified PASS.
To examine differences in standard and modified PASS trajectories over time, a variable named delta PASS (ΔPASS) was generated, which reflected the change in the score compared with the baseline PASS at admission. Generalized Estimating Equations (GEE) models were then fit to compare PASS trajectories. When comparing the 3 RAC groups via the GEE model, mild strata served as the reference group.
We also identified a subgroup of subjects with an outlier high PASS level on admission. The 25th, 50th, and 75th percentile of admission PASS were determined. After calculation of median PASS and interquartile ranges (IQRs), the outlier PASS values were defined as those with score > [(1.5 × IQR) + 75th percentile].17 Raw and percent contribution to overall PASS score for each of the components, as well as patient characteristics, were compared between subjects with outlying PASS scores and those within the normal range using the Wilcoxon rank sum test for continuous variables and the χ2 test for categorical variables. Separately within the nonoutlier and the outlier groups, PASS was regressed on MED score, and the percent of the variation in PASS attributed to the MED score was calculated as model sums of squares divided by total sums of squares.
Data management was performed in R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria),18 and statistical analysis was performed in SAS version 9.4 (SAS Institute Inc, Cary, NC) by expert biostatisticians. All tests were 2-sided, and a P-value < .05 was considered significant.
Results
Baseline Characteristics
Among 1526 enrolled subjects, 133 cases were excluded due to missing PASS components or missing disease outcome data. A total of 1393 subjects from 10 countries with detailed data were included in this analysis. The baseline characteristics of the study population are tabulated in Table 1. The median age of subjects at the time of enrollment was 49 years (IQR, 34–63 years). The number of patients per continent were as follows: North America, 460; Latin America, 283; Europe, 347; and Asia, 303. Overall, 68.2% of the subjects had mild AP, whereas 22.6% experienced moderately severe and 9.2% severe AP per RAC.
Table 1.
The APPRENTICE Study Cohort Demographic and Clinical Characteristics
| Full cohort (N = 1393) | ||
|---|---|---|
| Age, y | 49.0 | 34.0–63.0 |
| BMI kg/m2 | 26.5 | 23.2–30.7 |
| Charlson Comorbidity Index | 1.0 | 0.0–2.0 |
| Male | 714 | 51.3 |
| Race | ||
| Asian Indian | 309 | 22.2 |
| Black or African American | 72 | 5.2 |
| Hispanic or Latino | 299 | 21.5 |
| White or Caucasian (non-Hispanic) | 699 | 50.2 |
| Other | 14 | 1.0 |
| Smoking | 315 | 22.8 |
| Alcohol | 532 | 38.5 |
| Etiology | ||
| Gallstones | 633 | 45.4 |
| Alcoholic | 291 | 20.9 |
| Idiopathic | 222 | 15.9 |
| HTG | 67 | 4.8 |
| Post-ERCP | 121 | 8.7 |
| Other | 59 | 4.2 |
| RAC | ||
| Mild | 950 | 68.20 |
| Moderate | 315 | 22.61 |
| Severe | 128 | 9.19 |
| Length of stay d | 8.0 | 5.0–13.0 |
| Length of Stay d | ||
| Short (0–2) | 98 | 7.04 |
| Intermediate (3–6) | 488 | 35.03 |
| Long (≥7) | 807 | 57.93 |
| Continent | ||
| Europe | 347 | 24.91 |
| Asiaa | 303 | 21.75 |
| Latin America | 283 | 20.32 |
| North America | 460 | 33.02 |
| PASS | ||
| Admission | 122.5 | 95.0–160.0 |
| 24 Hours | 101.7 | 65.0–155.0 |
| 48 Hours | 80.0 | 40.0–131.7 |
| 72 Hours | 60.0 | 20.0–120.0 |
| 7 Days | 30.0 | 0–80.0 |
Note: Data are presented as number (%) or median (IQR).
BMI, Body mass index; ERCP, endoscopic retrograde cholangiopancreatography; HTG, hypertriglyceridemia; IQR, interquartile range; PASS, Pancreatitis Activity Scoring System, RAC, Revised Atlanta Classification.
India was the contributing country from Asia.
Conventional PASS Trajectories
In the total cohort, the median admission PASS was 122.5 (IQR, 95–160). Subsequently, the activity showed a consistent decline: with the median score at 24 hours dropping to 101.7 (IQR, 65–155) and to 80.0 (IQR, 40.0–131.7) at the 48-hour timepoint. In the mild AP subset, the median PASS at admission was 115 (IQR, 90–142), which was significantly lower than in subjects with moderately severe AP (140 [IQR, 110–168]) and severe AP (172 [IQR, 140–263], P < .001). Similarly, at each subsequent timepoint, patients with mild AP continuously maintained a significantly lower activity level (Supplementary Table 1). Comparison of the PASS index among patients with mild AP revealed a significant difference at each study time point across the 4 continents (all P < .001). In comparison with the other 3 continents, patients with mild AP recruited at North American sites had the highest PASS on admission and at 24-hour time points (both P < .001) (Table 2).
Table 2.
PASS Values at Different Time Points by RAC Severity Groups Among 4 Continents
| PASS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Admission | 24 hours | 48 hours | 72 Hours | 7 Days | ||||||
| Mild | ||||||||||
| Europe | 105.0 | 80.0–130.0 | 65.0 | 45.0–95.0 | 45.0 | 15.0–75.0 | 25.0 | 0.0–40.0 | 0.0 | 0.0–25.0 |
| Asia | 115.0 | 90.0–140.0 | 100.0 | 75.0–125.0 | 80.0 | 50.0–110.0 | 60.0 | 25.0–85.0 | 25.0 | 0.0–55.0 |
| Latin America | 110.0 | 90.0–135.0 | 75.0 | 50.0–100.0 | 40.0 | 25.0–80.0 | 25.0 | 0.0–55.0 | 0.0 | 0.0–25.0 |
| North America | 132.7 | 97.9–185.0 | 111.7 | 75.0–168.6 | 78.8 | 35.0–140.0 | 73.3 | 25.0–126.7 | 56.3 | 15.0–100.8 |
| P-value | < .001 | < .001 | < .001 | < .001 | < .001 | |||||
| Moderate | ||||||||||
| Europe | 107.5 | 80.0–157.5 | 92.5 | 65.0–152.5 | 65.0 | 40.0–115.0 | 60.0 | 20.0–87.5 | 25.0 | 5.0–40.0 |
| Asia | 130.0 | 110.0–145.0 | 125.0 | 100.0–140.0 | 115.0 | 90.0–140.0 | 105.0 | 65.0–130.0 | 65.0 | 25.0–100.0 |
| Latin America | 140.0 | 115.0–165.0 | 127.5 | 85.0–165.0 | 90.0 | 55.0–140.0 | 57.5 | 35.0–105.0 | 25.0 | 0.0–65.0 |
| North America | 168.3 | 131.7–233.3 | 174.2 | 115.0–235.8 | 166.5 | 108.3–223.8 | 138.3 | 100.0–221.7 | 120.0 | 65.0–170.0 |
| P-value | < .001 | < .001 | < .001 | < .001 | < .001 | |||||
| Severe | ||||||||||
| Europe | 360.0 | 160.0–385.0 | 385.0 | 250.0–460.0 | 375.0 | 245.0–470.0 | 165.0 | 155.0–245.0 | 80.0 | 62.5–122.5 |
| Asia | 155.0 | 135.0–185.0 | 165.0 | 130.0–275.0 | 215.0 | 145.0–270.0 | 220.0 | 150.0–255.0 | 115.0 | 75.0–160.0 |
| Latin America | 165.0 | 140.0–215.0 | 230.0 | 215.0–255.0 | 195.0 | 155.0–240.0 | 120.0 | 70.0–200.0 | 70.0 | 25.0–90.0 |
| North America | 230.8 | 153.3–265.0 | 247.1 | 203.3–314.2 | 248.3 | 200.0–385.0 | 230.0 | 141.0–325.0 | 130.0 | 106.3–175.0 |
| P-value | < .001 | < .001 | < .001 | .023 | < .001 | |||||
Note: P-value testing for a significant difference between continents from Kruskal-Wallis tests.
Note: Data are presented as median (IQR).
Note: Boldface P values indicate statistical significance.
IQR, Interquartile range; PASS, Pancreatitis Activity Scoring System; RAC, Revised Atlanta Classification.
Conventional PASS: Outliers
We identified 100 subjects with markedly high PASS values on admission. In comparison with the normal-range subset, these outlier subjects were predominantly male (71% vs 50%; P = .004), of younger age (45 vs 49 years; P = .027), and more likely to be active drinkers (59% vs 37%; P < .001) and smokers (42% vs 21%; P < .001). Furthermore, the distribution of AP etiologies significantly differed between outliers and normal-range cases. The outlier group consisted of a higher rate of alcoholic etiology (43% vs 19%; P < .001) compared with prevalent biliary etiology in normal-range patients (47% vs 22%; P < .001 in outliers). The geographical distribution of outliers was also significantly different, with the majority of outlier cases (66% vs 30%; P < .001) registered from the North American centers (Supplementary Table 2).
Focusing on mild AP, 42 subjects had outlier scores on admission, and 908 had normal range scores. As summarized in Supplementary Table 3, all outliers with mild disease were enrolled from U.S. centers. Outlier subjects with mild AP were younger (39 vs. 50 years; P = .001), had lower Charlson comorbidity score (P = .002), a higher rate of smoking (42.9% vs 21.2%; P = .001), and shorter hospital stay (5 vs 6 days; P < .001).
Further analyses revealed that within the outlier subgroup, 69.7% of the variation in PASS score was attributed to the MED component, compared with only 29.5% of the variation in normal-range patients (Supplementary Figure 1). The median contribution of each of the PASS components are summarized in Supplementary Table 4.
The Variation in the MED Component
Overall, 913 patients (66%) did not receive any opioids, and their MED score on admission was 0. In those who received opioids, the mean MED score was 14.4 (±21.1), with the minimum recorded MED being 0.1 and the highest at 241.7.
Modified PASS (mPASS) Models
The mPASS models produced distinct trajectories between the 3 severity groups. Similar to the conventional PASS, subjects with severe AP had significantly higher mPASS levels at each of the study time points (P < .001 for all). Moreover, there was an increasing mPASS trend from admission to the 48-hour time point in severe AP, whereas mild disease exhibited a consistent mPASS decline throughout the first 72 hours of hospitalization. The detailed scores of all mPASS models are summarized in Supplementary Table 1.
With regard to the prediction of severe AP, the receiver operating characteristic curve for the conventional PASS revealed an AUC of 0.827 (95% confidence interval [CI], 0.788–0.865). The mPASS models showed similar high accuracies in predicting severe AP after adjusting for the continent. The AUC and 95% CI for 4 modified models after adjustment for continent were as follows: mPASS-1: 0.877 (95% CI, 0.846–0.907), mPASS-2: 0.870 (95% CI, 0.837–0.902), mPASS-3: 0.876 (95% CI, 0.845–0.907), mPASS-4: 0.874 (95% CI, 0.843–0.905). The mPASS-1 model had the simplest structure among the proposed models. The detailed mPASS-1 score across RAC subgroups are tabulated in Table 3, and its general trajectories are shown in Figure 1. Admission mPASS-1 demonstrated superior performance compared with SIRS (AUC = 0.68), hematocrit (AUC = 0.58), and blood urea nitrogen (AUC = 0.65) (Figure 2). The optimal cutoff score for the admission mPASS-1 in predicting severe AP was determined to be 118, with a sensitivity of 84.4%, specificity of 67.8%, and accuracy of 69.3%.
Table 3.
mPASS-1 Values at Different Time Points by RAC Severity Groups Among 4 Continents
| mPASS-1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Admission | 24 Hours | 48 hours | 72 Hours | 7 Days | ||||||
| Mild | ||||||||||
| Europe | 105.0 | 80.0–130.0 | 65.0 | 45.0–90.0 | 45.0 | 15.0–75.0 | 25.0 | 0.0–40.0 | 0.0 | 0.0–25.0 |
| Asia | 115.0 | 90.0–140.0 | 100.0 | 75.0–125.0 | 80.0 | 50.0–110.0 | 60.0 | 25.0–85.0 | 25.0 | 0.0–55.0 |
| Latin America | 105.0 | 90.0–125.0 | 70.0 | 50.0–90.0 | 40.0 | 25.0–65.0 | 25.0 | 0.0–50.0 | 0.0 | 0.0–25.0 |
| North America | 90.0 | 75.0–112.5 | 75.0 | 40.0–90.0 | 50.0 | 25.0–85.0 | 40.0 | 20.0–75.0 | 40.0 | 10.0–75.0 |
| P-value | < .001 | < .001 | < .001 | < .001 | < .001 | |||||
| Moderate | ||||||||||
| Europe | 107.5 | 80.0–145.0 | 92.5 | 65.0–150.0 | 65.0 | 40.0–115.0 | 60.0 | 20.0–87.5 | 25.0 | 5.0–40.0 |
| Asia | 130.0 | 110.0–145.0 | 125.0 | 100.0–140.0 | 115.0 | 90.0–140.0 | 105.0 | 65.0–130.0 | 65.0 | 25.0–100.0 |
| Latin America | 130.0 | 105.0–165.0 | 107.5 | 75.0–150.0 | 80.0 | 50.0–110.0 | 50.0 | 30.0–90.0 | 25.0 | 0.0–65.0 |
| North America | 122.5 | 90.0–140.0 | 120.0 | 90.0–145.0 | 105.0 | 85.0–140.0 | 100.0 | 65.0–130.0 | 70.0 | 45.0–115.0 |
| P-value | .121 | .083 | < .001 | < .001 | < .001 | |||||
| Severe | ||||||||||
| Europe | 360.0 | 160.0–380.0 | 365.0 | 250.0–460.0 | 375.0 | 245.0–470.0 | 165.0 | 155.0–245.0 | 80.0 | 62.5–122.5 |
| Asia | 155.0 | 135.0–185.0 | 165.0 | 130.0–275.0 | 215.0 | 145.0–270.0 | 220.0 | 150.0–255.0 | 115.0 | 75.0–160.0 |
| Latin America | 140.0 | 140.0–215.0 | 217.5 | 215.0–250.0 | 195.0 | 155.0–225.0 | 120.0 | 70.0–200.0 | 70.0 | 25.0–90.0 |
| North America | 140.0 | 120.0–190.0 | 170.0 | 130.0–250.0 | 202.5 | 130.0–285.0 | 180.0 | 120.0–240.0 | 100.0 | 75.0–115.0 |
| P-value | < .001 | < .001 | .001 | .033 | .019 | |||||
Note: P-value testing for a significant difference between continents from Kruskal-Wallis tests.
Note: Data are presented as median (IQR).
Note; Boldface P values indicate statistical significance.
IQR, Interquartile range; mPASS, modified Pancreatitis Activity Scoring System; RAC, Revised Atlanta Classification.
Figure 1.
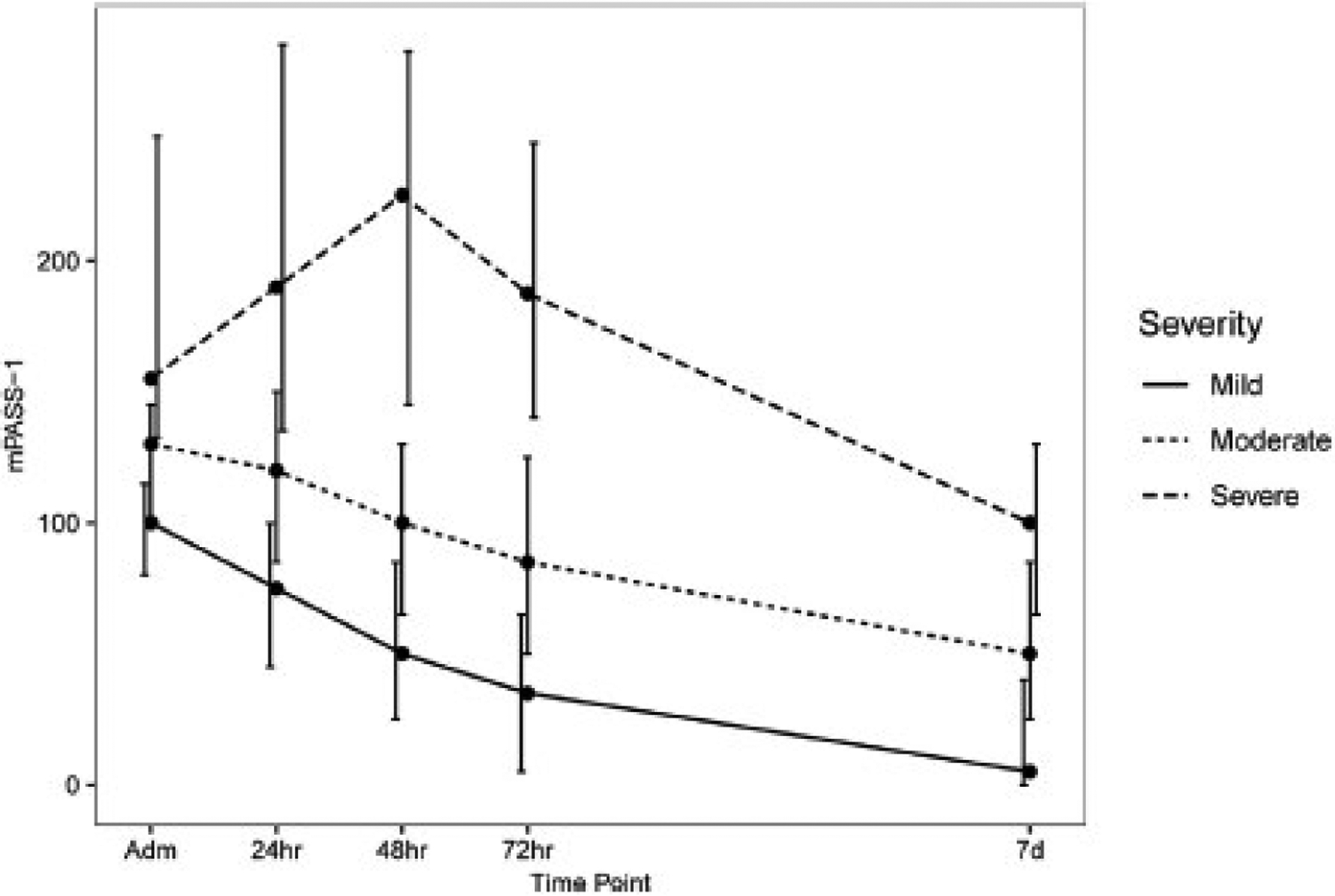
mPASS-1 trajectories at different time points across the 3 severity subsets.
Figure 2.
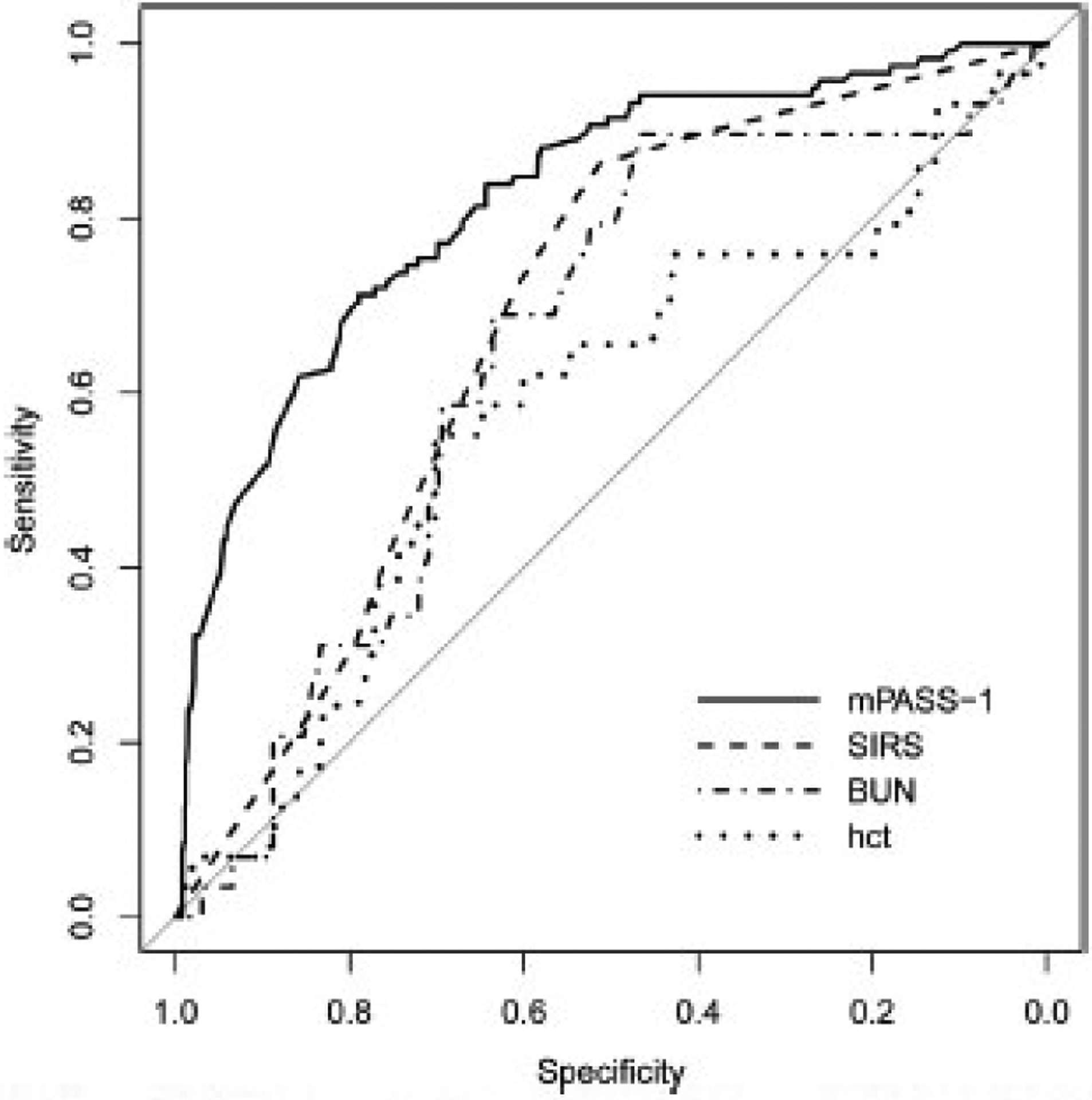
Receiver operating characteristic curves for the mPASS-1 model, SIRS, blood urea nitrogen (BUN), and hematocrit (hct) at admission after adjustment for continent.
The GEE analysis for ΔmPASS-1 showed a significant difference from baseline at 24, 48, and 72 hours between the 3 severity groups. As summarized in Table 4, patients with mild AP demonstrated the sharpest decline in activity score at 24-, 48-, and 72-hour time-points (All P < .001).
Table 4.
Generalized Estimating Equation Models Comparing mPASS-1 Trajectories Within RAC Severity Groups Adjusting for Continent
| Difference in mPASS-1 rate of change | Estimate | 95% CI | P-value |
|---|---|---|---|
| ΔPASS 24 Hours | |||
| Mild | Reference | ||
| Moderate | −15.90 | (−21.48 to −10.32) | < .001 |
| Severe | −70.04 | (−84.39 to −55.69) | < .001 |
| ΔPASS 48 Hours | |||
| Mild | Reference | ||
| Moderate | −16.46 | (−21.85 to −11.07) | < .001 |
| Severe | −57.29 | (−70.65 to −43.92) | < .001 |
| ΔPASS 72 Hours | |||
| Mild | Reference | ||
| Moderate | −17.01 | (−24.69 to −9.34) | < .001 |
| Severe | −44.54 | (−67.59 to −21.48) | < .001 |
Note: Boldface P values indicate statistical significance.
CI, Confidence interval; mPASS, modified Pancreatitis Activity Scoring System; RAC, Revised Atlanta Classification.
Discussion
In this international prospective study, we assessed the performance of the PASS index in a heterogeneous AP cohort across clinical settings located in 4 continents. Although the median value of the conventional PASS produced distinct trajectories in the 3 severity subgroups, we detected a subset of patients with outlier PASS values on admission, induced mainly by an inflated impact of the MED component on the total score. Of importance, MED is the only variable of the PASS that does not have a ceiling for an upper limit value. Aiming to contain the MED component, we developed and examined 4 mPASS indices. Our results showed the modified measures produced distinct trajectories between severity subgroups of AP. In addition, all 4 modified indices showed high accuracy in the prediction of severe disease. Among these scores, the mPASS-1 model, which excludes the MED component, demonstrated similar accuracy in predicting severe AP, while offering better pragmatic applicability due to removing the necessity of MED calculation.
There is an ongoing effort for new tools to facilitate research in AP, including disease activity scales like PASS, and patient-reported outcome measures addressing symptoms.19 The first 48 hours of inpatient care present the golden time of intervention in AP.20 Hence the interpretation of the AP clinical manifestations within this critical window is imperative for guiding management and ultimately resulting in a better outcome. Our previous study stressed the importance of early trends of the PASS index and the meaningful association with the length of hospitalization even in the absence of local or systemic complications.9 In the current study, we detected a distinct pattern in mPASS trajectories between the 4 RAC subgroups within the first 3 days of inpatient care. These distinguishable patterns further confirm the importance of repeated PASS measurements at the early stage of hospitalization.
In our global registry, we detected a subset of AP outliers with a very high admission PASS driven by the MED component Interestingly, a comparison of patients with mild AP with the outlier subset showed these patients to be younger, with a healthier comorbidity profile and requiring a shorter hospital stay. These findings show that in a subgroup of patients with AP, the inflated activity score does not reflect the clinical picture of disease but is mainly driven by chronic opioid use and increased tolerance. This finding was in line with our previous study, which was conducted in a small portion of the APPRENTICE cohort limited to the U.S., showing high variance in pain management, which resulted in an inflated PASS level among patients with mild AP requiring high doses of opioids.9 The outlier PASS group showed distinct composition, with the MED component driving around 70% of the score variance. Importantly, the geographical distribution of outliers showed the majority of outliers recorded from the U.S. sites. Comparison of the PASS index at each time point showed significant differences between the 4 continents even in the mild AP group, North American patients were found to have the highest PASS within the first 24 hours of inpatient care.
A previous study from our group revealed a very high rate of opioid administration throughout hospitalization among U.S. sites.21 This alarming pattern corresponds with previous reports on the increasing rate of opioid prescription in the U.S. medical setting.22,23 There is a high variability across practice patterns regarding the administration of opioid analgesics for pain control in patients with AP around the world.24 Furthermore, two-thirds of our study cohort did not receive any opioids. Thus, the disproportionate administration of opioid analgesics across different geographical areas and exaggerated MED values hamper the application of the conventional PASS as a global index.
To diminish the diverging impact of opioid administration, we limited the weight of MED in 4 mPASS models. Among the proposed models, the mPASS-1 model (mPASS-1), which is based on the complete removal of MED, demonstrated high performance. mPASS-1 at different study time points produced significantly different values between the 3 RAC subgroups with severe subjects having the highest scores. Comparison of PASS and mPASS-1 shows a pronounced decrease in modified activity scores in North American patients, whereas the changes in other continents were minimal, especially in the mild AP group. We also detected that the 3 RAC subgroups had distinguishable trajectories with significantly different mPASS-1 slopes within the first 3 days of hospital stay.
Overall, the mPASS-1 exhibited promising performance in capturing the dynamic picture of AP, as well as providing prognostic accuracy for the identification of patients at risk of persistent organ failure. The important advantages of mPASS-1 are its ease of use and clinical applicability, especially during hospital admission as a predictor of disease severity, but also as the disease progresses, and the elimination of the MED calculation at each 12-hour block makes it more reliable. The notion that MED is the key factor contributing to outlier PASS values on admission is a salient point for generalized PASS applicability. With the mPASS-1 model, the elimination of opioid dosage still maintained a promising predictive performance and, most importantly, demonstrated distinct early trajectories. Furthermore, our findings reiterated the clinical importance of repeated measurement of activity score, as mPASS slopes were significantly different across severity subgroups.
Strengths of our study include its prospective design, a systematic compilation of a large intercontinental dataset, a diverse patient population, use of RAC as the disease severity metric, and advanced analyses of repeated measurements via GEE models. Our study also has a few limitations. Most APPRENTICE sites were tertiary centers, which limits the generalizability of results to community settings. The PASS indices were calculated in certain time points (admission, 24, 48, and 72 hours, and 7 days) and not every 12 hours during hospitalization. This limitation may partly reflect the practicality of the conventional score, as it was difficult for clinicians to calculate all components every 12 hours. Furthermore, although mPASS yielded an acceptable predictive performance with each simplified model, the overall predictive value is similar to the previous prognostic tools. This underlines the main advantage of PASS is the real-time, dynamic monitoring of the clinical course of AP rather than predicting outcomes.
Conclusion
In conclusion, this large, international study highlights the challenges of the PASS as a global AP activity metric related to its MED component Because there is negligible utilization of opioids outside of the U.S., we evaluated an mPASS index by removing the MED component We propose this system as a metric for AP activity because the revised index is less complicated and maintains significantly distinct trajectories for the dynamic monitoring of AP activity over time. Prospective real-time application of the mPASS index is warranted for affirmative validation.
Supplementary Material
What You Need to Know.
Background
The Pancreatitis Activity Scoring System (PASS) is a dynamic index proposed to measure acute pancreatitis (AP) activity with high variability reported due to the morphine equivalent dose component.
Findings
The conventional PASS model was tested in an international, prospective cohort and an outlier subset was detected even within mild AP strata. Four modified PASS models were constructed. The modified PASS-1 model (excluding morphine equivalent dose from PASS) yielded similar performance on prediction of severe AP as well as generating distinct activity trajectories between severity subgroups
Implications for patient care
We propose a modified activity score for AP, the mPASS. This model provides a more applicable measure while maintaining high accuracy for predicting severe disease.
Funding
Pedram Paragomi was supported by National institutes of Health, United States T32CA186873 training grant in cancer epidemiology and prevention.
Abbreviations used in this paper:
- AP
acute pancreatitis
- APPRENTICE
Acute Pancreatitis Patient Registry to Examine Novel Therapies In Clinical Experience
- AUC
area under the receiver operating characteristic curve
- CI
confidence interval
- GEE
generalized estimating equation
- IQR
interquartile range
- IRB
institutional review board
- MED
morphine equivalent dose
- mPASS
modified Pancreatitis Activity Scoring System
- PASS
Pancreatitis Activity Scoring System
- RAC
Revised Atlanta Classification
- SIRS
systemic inflammatory response syndrome
- U.S.
United States
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjoumal.org, and at http://doi.org/10.1016/j.cgh.2021.09.014.
CRediT Authorship Statement
Pedram Paragomi (Conceptualization: Supposing; Data curation: Lead; Investigation: Lead; Methodology: Lead; Writing – original draft: Lead; Writing – review & editing: Equal)
Alice Hinton (Formal analysis: Lead; Methodology: Equal)
Ioannis Pothoulakis (Data curation: Supporting; Validation: Supporting)
Rupjyoti Talukdar (Investigation: Supporting; Writing – review & editing: Supporting)
Rakesh Kochhar (Writing – review & editing: Supporting)
Mahesh K. Goenka (Writing – review & editing: Supporting)
Aiste Gulla (Writing – review & editing: Supporting)
Jose A. Gonzalez (Writing – review & editing: Supporting)
Vikesh K. Singh (Methodology: Supporting; Writing – review & editing: Supporting)
Miguel Ferreira Bogado (Writing – review & editing: Supporting)
Tylar Stevens (Writing – review & editing: Supporting)
Sorin T. Barbu (Writing – review & editing: Supporting)
Haq Nawaz (Writing – review a editing: Supporting)
Silvia C. Gutierrez (Writing – review & editing: Supporting)
Narcis Zarnescu (Writing – review & editing: Supporting)
Livia Archibugi (Writing – review & editing: Supporting)
Jeffrey J. Easler (Writing – review – & editing: Supporting)
Konstantinos Triantafyllou (Writing – review & editing: Supporting)
Mario Peláez-Luna (Writing – review & editing: Supporting)
Shyam Thakkar (Writing – review & editing: Supporting)
Carlos Ocampo (Writing – review & editing: Supporting)
Enrique de-Madaria (Writing – review & editing: Supporting)
Gregory A. Cote (Writing – review & editing: Supporting)
Peter J. Lee (Writing – review & editing: Supporting)
Somashekar Krishna (Writing – review & editing: Supporting)
Luis F. Lara (Writing – review & editing: Supporting)
Samuel Han (Writing – review & editing: Supporting)
Bechien U. Wu (Conceptualization: Lead; Investigation: Equal; Writing – review & editing: Equal)
Georgios I. Papachristou (Conceptualization: Equal; Data curation: Equal; Investigation: Lead; Resources: Lead; Writing – original draft: Supporting; Writing – review & editing: Lead)
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144:1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg SK, Singh D, Sarvepalli S, et al. Incidence, admission rates, and economic burden of adult emergency visits for chronic pancreatitis: data from the National Emergency Department Sample, 2006 to 2012, J Clin Gastroenterol 2019; 53:e328–e333. [DOI] [PubMed] [Google Scholar]
- 3.Vaughn VM, Shuster D, Rogers MAM, et al. Early versus delayed feeding in patients with acute pancreatitis: a systematic review. Ann Intern Med 2017;166:883–892. [DOI] [PubMed] [Google Scholar]
- 4.Vega SS, DiMagno MJ, Forsmark CE, et al. Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute Technical Review. Gastroenterology 2018; 154:1103–1139. [DOI] [PubMed] [Google Scholar]
- 5.Singh H, Gougol A, Mounzer R, et al. Which patients with mild acute pancreatitis require prolonged hospitalization? Clin Transl Gastroenterol 2017;8:e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francisco M, Valentin F, Cubiella J, et al. Factors related to length of hospital admission in mild interstitial acute pancreatitis. Rev Esp Enferm Dig 2013;105:84–92. [DOI] [PubMed] [Google Scholar]
- 7.Wu BU, Batech M, Quezada M, et al. Dynamic measurement of disease activity in acute pancreatitis: the Pancreatitis Activity Scoring System. Am J Gastroenterol 2017; 112:1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buxbaum J, Quezada M, Chong B, et al. The Pancreatitis Activity Scoring System predicts clinical outcomes in acute pancreatitis: findings from a prospective cohort study. Am J Gastroenterol 2018;113:755–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paragomi P, Tuft M, Pothoulakis I, et al. Dynamic changes in the pancreatitis activity scoring system during hospital course in a multicenter, prospective cohort. J Gastroenterol Hepatoi 2021; 38:2416–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gl Papachristou, Machicado JD, Stevens T, et al. Acute pancreatitis patient registry to examine novel therapies in clinical experience (APPRENTICE): an international, multicenter consortium for the study of acute pancreatitis. Ann Gastroenterol 2017;30:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pothoulakis I, Paragomi P, Archibugi L, et al. Clinical features of hypertriglyceridemia-induced acute pancreatitis in an international, multicenter, prospective cohort (APPRENTICE consortium). Pancreatology 2020;20:325–330. [DOI] [PubMed] [Google Scholar]
- 12.Machicado JD, Gougol A, Tan X, et al. Mortality in acute pancreatitis with persistent organ failure is datermined by the number, type, and sequence of organ systems affected. United European Gastroenterol J 2021;9:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction scorn: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995:23:1638–1652. [DOI] [PubMed] [Google Scholar]
- 15.Singh VK, Wu BU, Bollen TL, et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol 2009;7:1247–1251. [DOI] [PubMed] [Google Scholar]
- 16.Banks PA, Bollen TL, Dervenis C, et al. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–111. [DOI] [PubMed] [Google Scholar]
- 17.Rosner B Fundamentals of biostatistics. 8th edition. Boston, MA: Cengage Learning, 2016. [Google Scholar]
- 18.R Core Team. R. A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing, 2017. [Google Scholar]
- 19.de-Madaria E, Sanchez-Marin C, Carrillo I, et al. Design and validation of a patient-reported outcome measure scale in acute pancreatitis: the PAN-PROMISE study. Gut 2021; 70:139–147. [DOI] [PubMed] [Google Scholar]
- 20.Fisher JM, Gardner TB. The “golden hours” of management in acute pancreatitis. Am J Gastroenterol 2012;107:1146–1150. [DOI] [PubMed] [Google Scholar]
- 21.Matta B, Gougol A, Gao X, et al. Worldwide variations in demographics, management, and outcomes of acute pancreatitis. Glin Gastroenterol Hepatol 2020;18:1567–1575.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herzig SJ, Rothberg MB, Cheung M, et al. , Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med 2014;9:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okie S A flood of opioids, a rising tide of deaths. N Engl J Med 2010;363:1981–1985. [DOI] [PubMed] [Google Scholar]
- 24.Wells N, Pasero C, McCaffery M. Improving the quality of care through pain assessment and management, in: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Advances in Patient Safety Rockville (MD): Agency for Healthcare Research and Quality (US), 2008. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


