Abstract
Desbutyl-benflumetol (DBB) is a novel antimalarial compound closely related to benflumetol (lumefantrine), of which it is a putative metabolite. The in vitro response of Plasmodium falciparum to DBB was studied in Mae Hong Son and Mae Sot, in northwest Thailand, in 1997 and 1998. In total, 155 fresh isolates were successfully tested using the World Health Organization standard in vitro microtest system (Mark II). The mean 50% effective concentration (EC50) and 90% effective concentration of DBB were 6.36 and 31.09 nmol/liter, respectively. The comparison of the activity of DBB and benflumetol yielded a highly significant potency ratio of 4.52, corresponding to a more than four times higher efficacy of DBB. A considerable potency difference was found between isolates from Mae Hong Son and those from Mae Sot, reflecting lesser sensitivity in the area with marked resistance to mefloquine and quinine. This observation is also supported by a highly significant activity correlation with benflumetol (P < 0.001) and to a similar degree with mefloquine (P < 0.001), reflecting a close relationship of DBB with the class II aryl amino alcohol blood schizontocides. A less distinct association was also found with artemisinin, which was significant only at the EC50 level, and there was no correlation at all with chloroquine. DBB is a promising antimalarial compound that merits further investigation in order to define its practical therapeutic potential.
At a time of increasing drug resistance, especially to mefloquine and quinine, the search for new antimalarial compounds has become a major concern, not only to countries where Plasmodium falciparum is endemic (9, 15). One of the new candidate compounds is desbutyl-benflumetol (DBB). It is a 2,3-benzindene (Fig. 1) and is closely related to benflumetol (lumefantrine), a compound already used as an antimalarial drug. Benflumetol shows marked blood schizontocidal activity in vitro and in vivo against mammalian plasmodia, including chloroquine-resistant P. falciparum (7, 12, 13, 18).
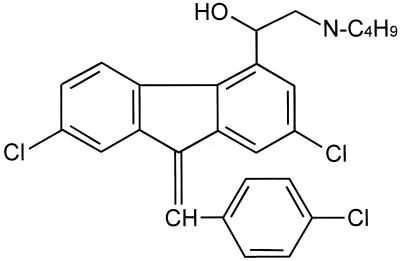
FIG. 1.
Chemical structure of DBB. DBB is a 2,3-benzindene and is closely related to benflumetol (lumefantrine).
Benflumetol carries a dibutylamino-ethanol side chain in the 4-position. In contrast, its putative metabolite DBB has a monobutylamino-ethanol group in the 4-position (Fig. 1). Under normal conditions in humans, probably only a small amount of benflumetol is metabolized to DBB, not enough to reach the threshold of detection. The structural and physicochemical relationship of DBB to the aryl amino alcohol antimalarials (class II blood schizontocides), including mefloquine, quinine, and halofantrine, could also indicate a similar mode of action. There is no information as yet on the pharmacokinetics and pharmacodynamics of DBB.
In association with β-artemether, a semisynthetic artemisinin derivative, benflumetol has been developed and registered for the oral treatment of uncomplicated falciparum malaria in areas afflicted by resistance to chloroquine and antifols (Riamet and Coartem; Novartis) after extensive clinical trials had shown this combination to be well tolerated and effective (4, 5, 10, 13, 14). Artemisinin is a sesquiterpene lactone that occurs naturally in the annual plant Artemisia annua Linné, from which it is obtained by extraction. Artemisinin bears a peroxide group and unlike most other antimalarials lacks a nitrogen-containing heterocyclic ring system. Its mode of action resides in the endoperoxide group and is thought to resemble that of disinfectants. It combines the advantages of rapid clinical-parasitological action with an exceptionally good tolerability profile.
The study was conducted with the primary objective of investigating the in vitro blood schizontocidal activity of DBB in a representative number of fresh isolates of P. falciparum, including chloroquine- and multidrug-resistant parasites, and to establish baseline sensitivity data for this compound. In addition, the activity of DBB was to be compared with that of benflumetol and to be investigated in relation to other presently used antimalarials.
MATERIALS AND METHODS
The studies were part of the program for the monitoring of the sensitivity of P. falciparum in Thailand and were conducted under the auspices of the Malaria Division, Ministry of Public Health of Thailand. In 1997 and 1998 in vitro sensitivity tests with DBB were carried out in parallel to sensitivity tests with benflumetol, mefloquine, quinine, chloroquine, and artemisinin in Mae Hong Son and Mae Sot, located in northwest Thailand in close proximity to the border to Myanmar.
Patients.
In 1997 blood samples originating from outpatients attending the malaria clinic (VBDC Unit 8) and the provincial hospital of Mae Hong Son yielded a total of 53 successful tests, and 38 successful tests were performed in 1998. In 1998 isolates were also obtained from 64 patients of the malaria clinic at Mae Sot.
The P. falciparum isolates came from patients who suffered from symptomatic, nonsevere monoinfections diagnosed by microscopic examination of Giemsa-stained blood films. The patients reported no intake of 4-aminochinolines within 14 days, of pyrimetamine and sulfonamides within 28 days, or of mefloquine within 63 days before blood sampling. The parasitemia of the isolates was between 1,000 and 80,000 asexual parasites per microliter of blood. Most of the patients had acquired their infections in malarious areas of Thailand or neighboring Myanmar.
Test procedure.
Drug sensitivity was tested using the standard World Health Organization (WHO) in vitro microtest Mark II (WHO document MAP/87.2, Rev. 1, 1990) that is based on assessing the inhibition of schizont maturation (11, 16). The predosed microtest plates (Falcon 3070) for the tests with chloroquine, quinine, and mefloquine were obtained from the WHO Test Kit Production Unit, Manila, Philippines. The test plates for artemisinin, benflumetol (batch 890901), and DBB (batch TA-2256) (benflumetol and DBB were provided by Novartis AG, Basel, Switzerland) were prepared at the Institute of Specific Prophylaxis and Tropical Medicine, University of Vienna, Austria. The individual wells were predosed with increasing concentrations at a range of 3 to 3,000 nmol of blood medium mixture (BMM) per liter. From each patient 300 μl of whole blood was collected by finger prick and added to 2.7 ml of RPMI 1640 medium with reduced contents of para-aminobenzoic acid and folic acid. At the same time, thick films were made to assess preculture parasitemia. Aliquots of 50 μl of BMM were added to each of the scheduled wells of the culture plates predosed with the appropriate drugs. The test plates were incubated in candle jars at 37.5°C for 25 h. Thick films were made from the cell sediment of each test well and were stained with Giemsa solution at pH 6.85. For the microscopic evaluation, the number of preschizonts and schizonts was counted against a total of 200 asexual parasites. Inhibition in the drug wells was expressed by relating the counts to the readings of the control wells. According to convention, tests were considered successful if ≥10% of the parasites in the control well developed into schizonts or preschizonts; i.e., parasites with three or more chromatins (16).
Statistical evaluation.
The statistical analysis was carried out using a computerized mathematical log-concentration–response-probit model (17) based on the method of Litchfield and Wilcoxon to fit a regression line to the data points which were directly derived from the counting results (6). This method is presently the most widely accepted analytic approach to dose-effect experiments showing a log-normal distribution of results. The 50 and 90% effective concentrations (EC50 and EC90, respectively), i.e., the drug concentrations at which 50 and 90% schizont maturation inhibition had occurred, were calculated from the mathematically derived regressions. Cutoff points, the lowest drug concentrations at which no schizont maturation was observed, were used as measures of full inhibition. Regressions were compared to assess the potency relations between individual drugs, performing tests for parallelism (slope ratio [SR]) and potency ratio (PR) (PR equals the EC50 of drug A divided by the EC50 of drug B). Pearson correlation analysis was used to calculate the activity correlations.
RESULTS
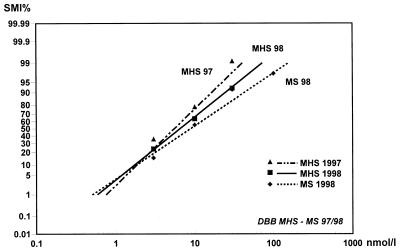
A total number of 155 isolates of P. falciparum were successfully tested for their susceptibility to DBB. The geometric mean of the schizont counts in the control wells was 99 (arithmetic mean, 113.7) per 200 asexual parasites. None of the isolates was fully inhibited at 3 nmol of BMM/liter, whereas all isolates were completely inhibited at 1,000 nmol of BMM/liter. The majority, however, had the cutoff point at 30 nmol/liter (50.3% of isolates) or 100 nmol/liter (31.6%), with a geometric mean cutoff point of 47.3 nmol/liter. The relative schizont maturation inhibition showed a steep increase above 3 nmol/liter, reaching 94.4% at 30 nmol/liter and finally 100% at 1,000 nmol/liter (Fig. 2).
FIG. 2.
Mean concentration response (SMI%) lines of DBB for the years 1997 and 1998 for P. falciparum from Mae Hong Son (MHS) and Mae Sot (MS), with data points. Higher EC values from Mae Sot are indicated by the flatter regression line. The mean EC50 from Mae Sot in 1998 (MS 98) was found to be significantly higher than the corresponding value from Mae Hong Son (MHS 98).
The overall mean EC50, i.e., the concentration at which schizont maturation showed 50% inhibition compared to control growth, was 6.36 nmol of BMM/liter, with lower and higher confidence limits (95%) of 5.24 and 7.72 nmol/liter. The mean EC90 value was 31.09 nmol/liter, with confidence intervals of 23.92 and 40.41 nmol/liter (Table 1). All χ2 values for heterogeneity were within permissible limits.
TABLE 1.
Effective concentrations, heterogeneity (χ2), and slopes of regression for DBB derived from log-probit regressionsa
| Data origin | n | Slope of regression | χ2 | EC01 | EC50 | EC90 | EC99 |
|---|---|---|---|---|---|---|---|
| MHS, 1997 | 53 | 2.5931 | 0.7592 | 0.4720 | 4.3849 | 14.9705 | 40.7325 |
| MHS, 1998 | 38 | 2.8027 | 0.2326 | 0.5872 | 6.5425 | 24.6901 | 72.8930 |
| MS, 1998 | 64 | 3.4183 | 7.2346 | 0.5043 | 8.9403 | 43.5785 | 158.5017 |
| Pooled data (1997 and 1998) | 155 | 3.4246 | 8.4774 | 0.3574 | 6.3637 | 31.0930 | 113.3093 |
Effective concentrations (in nmol/liter) are mean values obtained from all samples. Significantly higher EC values from Mae Sot (MS) reflect the lower DBB sensitivity in an area with a higher percentage of mefloquine and quinine resistance. MHS, Mae Hong Son.
The comparison of the 1998 data from Mae Hong Son and Mae Sot at the EC50 level yielded a significant potency difference (PR = 1.37). The regressions were parallel within experimental error. This phenomenon is mainly due to three isolates from Mae Sot which showed schizont maturation at a concentration of 300 nmol of BMM/liter (the same three isolates were also found to be highly resistant to mefloquine). The potency difference (PR = 1.49) between the EC50 values from 1997 and 1998 in Mae Hong Son was not found to be significant.
Only 1 of the 32 isolates tested for their in vitro response to chloroquine in parallel to DBB was found to be fully sensitive to chloroquine. The tests with quinine showed 15 out of 31 isolates to be resistant to this drug, whereas 18 out of 33 isolates (55%) were resistant to mefloquine, an observation that also reflects the clinical response to monotherapy with mefloquine in the study area (13).
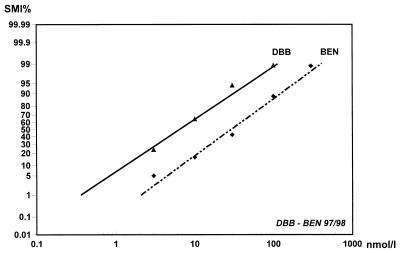
Simultaneous tests with DBB and benflumetol were conducted in 154 isolates, permitting the activity comparison between the two drugs. Both regression lines were parallel within experimental error (fSR > SR). The PR showed a highly significant value of 4.52, reflecting a more than fourfold higher efficacy of DBB compared to that of benflumetol. This fact is indicated by the relative position of the regression line as well as by the lower number of data points, illustrating earlier cutoff points (Fig. 3).
FIG. 3.
Mean concentration response (SMI%) lines for DBB and benflumetol (BEN) for P. falciparum, with 95% confidence intervals and data points. Parallel pooled data (n = 154) of 1997 and 1998 in Thailand are shown. The position of the regression line and the smaller number of data points reflect the higher activity of DBB.
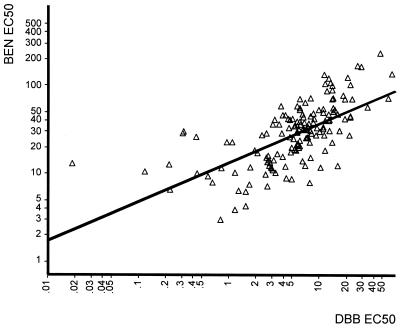
The same number of isolates was used to correlate the effective concentrations of DBB and benflumetol at the EC50 and EC90 levels using Pearson correlation analysis (Table 2). There was a highly significant positive correlation of responses between these two drugs at the EC50 (r = 0.687; P < 0.001) and the EC90 (r = 0.463; P < 0.001) (Fig. 4), obviously reflecting the close structural relationship. With values of r = 0.950 (EC50; P < 0.001) and r = 0.823 (EC90; P < 0.001), the activity correlation of DBB with mefloquine (n = 33) was even more distinct. The relationship with artemisinin (n = 33), on the other hand, was significant only at the EC50 level (r = 0.474; P < 0.01), whereas no correlation could be ascertained at the EC90 (r = 0.261 or P > 0.05). The results of correlation analysis with quinine (n = 31) and atovaquone (n = 25) were similar to those obtained with artemisinin. There was no indication of significant correlation between the activities of DBB and chloroquine (n = 32; at EC50 r = 0.064 and P > 0.05; at EC90 r = −0.018 and P > 0.05).
TABLE 2.
Pearson activity correlations at EC50 and EC90 levels between DBB and other antimalarialsa
| Drug correlated with DBB | n | Correlation at EC50
|
Correlation at EC90
|
||
|---|---|---|---|---|---|
| r | P | r | P | ||
| Benflumetol | 154 | 0.687 | <0.001 | 0.463 | <0.001 |
| Mefloquine | 33 | 0.950 | <0.001 | 0.823 | <0.001 |
| Chloroquine | 32 | 0.064 | >0.05 | −0.018 | >0.05 |
| Artemisinin | 33 | 0.474 | <0.01 | 0.261 | >0.05 |
| Quinine | 31 | 0.408 | <0.05 | 0.346 | >0.05 |
| Atovaquone | 25 | 0.641 | <0.001 | 0.381 | >0.05 |
Significant correlations suggest similarities in the mode of action and may reflect cross-sensitivities. P values in bold are significant.
FIG. 4.
Scatter diagram of individual EC50 values for DBB and benflumetol (BEN) for P. falciparum from Thailand, 1997 and 1998 (n = 154), showing distinct correlation and reflecting the close relationship between benflumetol and DBB. Concentrations are in nanomoles per liter.
DISCUSSION
Ever since the discovery of the first cases of chloroquine resistance along the Thai-Cambodian border in the early 1960s, the Indochina subcontinent has played an important role as a focus for the development of antimalarial drug resistance. The urgent need for new antimalarials in this region as well as the presence of multidrug-resistant P. falciparum make Thailand a key location for determining the activity of candidate drugs which are meant to replace antimalarials compromised by drug resistance.
The results of the in vitro tests with DBB, obtained with a large number of fresh parasite isolates, indicate that this novel antimalarial compound possesses high blood schizontocidal activity against P. falciparum, including multidrug-resistant strains prevalent in this part of Southeast Asia. Its in vitro activity is, on average, four to five times higher than that of the structurally related antimalarial benflumetol. The concentration-response regressions for both drugs are almost perfectly parallel. This may reflect a very similar mechanism of action. So far there are no clinical data on the pharmacokinetics and pharmacodynamics of DBB. Benflumetol is known to have low toxicity in animals (10) and to be very well tolerated at therapeutic doses (3, 5, 13, 14). This justifies hopes that DBB may have similar tolerability characteristics. However, the pharmacokinetics of DBB and particularly its absorption from the gastrointestinal tract require investigation, since absorption proved to be one of the constraints of benflumetol administration (2, 3). Although DBB is considered to be a putative metabolite of benflumetol, so far it has not been detected in patients or in healthy volunteers treated with benflumetol.
The absence of an activity correlation between DBB and chloroquine suggests that the activity of DBB is independent of the parasite's status of sensitivity to chloroquine and that no cross-resistance has to be anticipated. This may be an important detail for the eventual introduction of this drug in areas with a high percentage of chloroquine-resistant falciparum malaria, such as Southeast Asia or Africa.
The activity correlation between DBB and mefloquine and, at the EC50 level, also with quinine, identifies DBB as a class II aryl amino alcohol antimalarial. The somewhat poorer in vitro response found in Mae Sot, an area with a traditionally high degree of mefloquine and quinine resistance, may be a consequence of this close relationship, since benflumetol has never been used to a substantial extent in this area and DBB has not been used at all. Similarly there was also a moderate, though not significant, increase in the EC50 values in Mae Hong Son at the same site between 1997 and 1998. As evident with artemisinin and its derivatives, which also show an activity correlation with mefloquine (8, 19), this fact does not necessarily imply compromised activity in vivo. Moreover, the very high in vitro efficacy of DBB compared to that of benflumetol could eventually compensate for a certain degree of activity loss due to the activity correlation with other class II compounds.
In view of the absence of essential preclinical information it would seem to be too early to address the practical potential of DBB. Nevertheless, it may be considered a candidate for suppressive prophylaxis on its own and a candidate for therapeutic use, preferably in association with a fast-acting partner drug. A suitable artemisinin derivative may fulfill this purpose best, especially since benflumetol and artemether were shown to be synergistic (1), and the same might hold true for the combination of DBB with artemisinin-based compounds. The relatively weak activity correlation between DBB and artemisinin can be considered a point in favor of such a combination.
In conclusion, the results of the in vitro studies with DBB in the primary target parasite, multiresistant P. falciparum, indicate that DBB may be a highly promising candidate drug that merits further preclinical development with a view to determining its eventual suppressive and therapeutic potential.
ACKNOWLEDGMENTS
The support of the Ministry of Science and Communications of Austria to one of the authors (personal study grant, H.N.) is gratefully acknowledged.
We thank Novartis AG for providing DBB (batch TA-2256) and benflumetol (batch 890901). We thank the staff of the Office of Vector-Borne Disease Control Region 2 and the malaria clinics at Mae Hong Son and Mae Sot for efficient technical support.
REFERENCES
- 1.Alin M H, Björkman A, Wernsdorfer W H. Synergism of benflumetol and artemether in Plasmodium falciparum. Am J Trop Med Hyg. 1999;61:439–445. doi: 10.4269/ajtmh.1999.61.439. [DOI] [PubMed] [Google Scholar]
- 2.Ezzet F, Mull R, Karbwang J. Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients. Br J Clin Pharmacol. 1998;46:553–561. doi: 10.1046/j.1365-2125.1998.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ezzet F, van Vugt M, Nosten F, Looareesuwan S, White N J. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob Agents Chemother. 2000;44:697–704. doi: 10.1128/aac.44.3.697-704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatz C, Abdulla S, Mull R, Schellenberg D, Gathmann I, Kibatala P, Beck H-P, Tanner M, Royce C. Efficacy and safety of CGP 56697 (artemether and benflumetol) compared with chloroquine to treat acute falciparum malaria in Tanzanian children aged 1–5 years. Trop Med Int Health. 1998;3:498–504. doi: 10.1046/j.1365-3156.1998.00250.x. [DOI] [PubMed] [Google Scholar]
- 5.Jiao X, Liu G, Shan C O, Li X W, Gathmann I, Royce C. Phase II trial in China of a new, rapidly-acting and oral antimalarial, CGP 56697, for the treatment of Plasmodium falciparum malaria. Southeast Asian J Trop Med Public Health. 1997;28:476–481. [PubMed] [Google Scholar]
- 6.Litchfield J T, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharm Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 7.Noedl H, Prajakwong S, Wiedermann G, Kollaritsch H, Wernsdorfer G, Wernsdorfer W H. Benflumetol: in vitro sensitivity of Plasmodium falciparum in Thailand. Mitt Oesterr Ges Trop Parasitol. 1998;20:171–176. [Google Scholar]
- 8.Noedl, H., W. H. Wernsdorfer, S. Krudsood, P. Wilairatana, P. Viriyavejakul, H. Kollaritsch, G. Wiedermann, and S. Looareesuwan. An in vivo-in vitro model for the assessment of antimalarial cross-resistance. Am. J. Trop. Med. Hyg., in press. [DOI] [PubMed]
- 9.Nosten F, ter Kuile F, Chongsuphajaisiddhi T, Luxemburger C, Webster K H, Edstein M, Thew K L, White N J. Mefloquine resistant falciparum malaria on the Thai-Burmese border. Lancet. 1991;337:1140–1143. doi: 10.1016/0140-6736(91)92798-7. [DOI] [PubMed] [Google Scholar]
- 10.Novartis. Co-artemether: a novel therapy to meet the global malaria challenge, p. 5. Symposium of the Second European Congress of Tropical Medicine, Liverpool, England. Basel, Switzerland: Novartis Pharma AG; 1999. [Google Scholar]
- 11.Rieckmann K H, Campbell G H, Sax L J, Mrema J E. Drug sensitivity of Plasmodium falciparum. An in vitro micro technique. Lancet. 1978;i:22–23. doi: 10.1016/s0140-6736(78)90365-3. [DOI] [PubMed] [Google Scholar]
- 12.Tanarya P, Tippawangkoso P, Karbwang J, Na-Bangchang K, Wernsdorfer W H. In vitro sensitivity of Plasmodium falciparum and clinical response to lumefantrine (benflumetol) and artemether. Br J Clin Pharmacol. 2000;49:437–444. doi: 10.1046/j.1365-2125.2000.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Vugt M, Brockman A, Gemperli B, Luxemburger C, Gathmann I, Royce C, Slight T, Looareesuwan S, White N J, Nosten F. Randomized comparison of artemether-benflumetol and artesunate-mefloquine in treatment of multidrug-resistant falciparum malaria. Antimicrob Agents Chemother. 1998;42:135–139. doi: 10.1128/aac.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Seidlein L, Jaffar S, Pinder M, Haywood M, Snounou M, Gemperli B, Gathmann I, Royce C, Greenwood B. Treatment of African children with uncomplicated falciparum malaria with a new antimalarial drug, CGP 56697. J Infect Dis. 1997;176:1113–1116. doi: 10.1086/516524. [DOI] [PubMed] [Google Scholar]
- 15.Wernsdorfer W H. The development and spread of drug resistant malaria. Parasitol Today. 1991;7:297–303. doi: 10.1016/0169-4758(91)90262-m. [DOI] [PubMed] [Google Scholar]
- 16.Wernsdorfer W H, Payne D. Drug sensitivity tests in malaria parasites. In: Wernsdorfer W H, McGregor I A, editors. Malaria, principles and practice of malariology. Edinburgh, Scotland: Churchill Livingstone; 1988. pp. 1765–1800. [Google Scholar]
- 17.Wernsdorfer W H, Wernsdorfer M G. The evaluation of in vitro tests for the assessment of drug response in Plasmodium falciparum. Mitt Oesterr Ges Trop Parasitol. 1995;17:221–228. [Google Scholar]
- 18.Wernsdorfer W H, Landgraf B, Kilimali V A, Wernsdorfer G. Activity of benflumetol and its enantiomers in fresh isolates of Plasmodium falciparum from East Africa. Acta Tropica. 1998;70:9–15. doi: 10.1016/s0001-706x(97)00141-1. [DOI] [PubMed] [Google Scholar]
- 19.Wongsrichanalai C, Wimonwattrawatee T, Sookto P, Laoboonchai A, Heppner D G, Kyle D E, Wernsdorfer W H. In vitro sensitivity of Plasmodium falciparum to artesunate in Thailand. Bull WHO. 1999;77:392–398. [PMC free article] [PubMed] [Google Scholar]