Abstract
Introduction
With the advent of COVID-19 vaccines, hospitalization rates and progression to severe COVID-19 disease have reduced drastically. Most of the adverse events reported by the vaccine recipients were minor. However, autoimmune hematological complications such as vaccine-induced immune thrombotic thrombocytopenia (VITT), immune thrombocytopenic purpura (ITP) and TTP have also been reported post-COVID-19 vaccination. Given this, we sought to reflect on the existing cases of TTP, whether de novo or relapsing, reported after COVID-19 vaccination to further gain insight into any association, if present, and outcomes.
Methods
We searched PubMed, Embase, and Ebsco databases for published individual case reports on the occurrence or relapse of TTP after receiving any COVID-19 vaccine. A total of 23 articles (27 patients) were included in this qualitative analysis.
Results
The mean age for the patients who developed de novo TTP post-COVID-19 vaccination was 51.3 years. TTP episodes were seen mostly after BNT162b2 vaccine, followed by mRNA-1273 vaccine. All patients with immune TTP except one received plasma exchange (PLEX) and steroids. One patient passed away after two days of hospitalization, likely due to a sudden cardiovascular event.
Conclusion
Our review underscores the importance of in-depth anamnesis before vaccination and outlines characteristics of predisposed individuals. Evaluation of post-vaccine thrombocytopenia must include the possibility of TTP given the associated fatality with this condition.
Keywords: COVID-19 vaccine, BNT162b2 vaccine, mRNA-1273 vaccine, Ad26.COV2-S vaccine, ChAdOx1 nCoV-19 vaccine, TTP, Thrombotic thrombocytopenic purpura, Thrombocytopenia
1. Introduction
Thrombotic thrombocytopenic purpura (TTP) is an ultra-orphan hematological condition, affecting 3 to 10 adults per million population in a year and is defined by excessive deficiency (<10%) of ADAMTS13 (a disintegrin and metalloproteinase), a cleavage protein for von Willebrand factor (vWF) polymers [1]. In the absence of ADAMTS13, vWF polymers accumulate and cause severe platelet clumping with resultant microthrombi formation [1]. It can be acquired due to the formation of antibodies that promote neutralization or clearance of ADAMTS13 protein or congenital due to mutations in the ADAMTS13 gene [2]. Historically, TTP has been characterized by the classic pentad of microangiopathic hemolytic anemia, thrombocytopenia, changes in neurological status, fever, and renal dysfunction [2]. However, presentation with classical pentad is seen infrequently, only in 10% of the cases [3]. Therefore, guidelines recommend considering TTP diagnosis in patients who present with thrombotic microangiopathy after obvious causes have been ruled out [3]. Scores such as the PLASMIC score have been devised to help clinicians identify patients at high risk for having TTP [4]. Waiting for the confirmatory test (ADAMTS activity assay) can potentially delay treatment of this rapidly fatal illness [3]. Despite prompt treatment, TTP-associated mortality remains high at 10–20% [5].
With the emergence of COVID-19 infection came a dire need for vaccines to countermeasure this devastating viral illness. Various manufacturing companies used different techniques such as viral nucleic acids, inactivated virus, or viral vectors to create vaccines to alleviate this global pandemic [6]. Over 600 million COVID-19 vaccine doses have been administered in the United States as of March 2022 [7]. As per the vaccine adverse events reporting system (VAERS) data, there were around 300,000 reports of adverse events following mRNA vaccines (BNT162b2 and mRNA-1273) till June 2021, out of which 92% were non-serious events, such as local injection reaction, fatigue, headache, etc. [8]. Soon after their introduction, vaccine-induced immune thrombotic thrombocytopenia (VITT) was described in recipients of adenoviral-based vaccines (ChAdOx1 nCoV-19 and Ad26.COV2-S) who presented with thrombosis and thrombocytopenia [9]. Almost 100% of these patients were found to have elevated levels of antibody to platelet factor 4 heparin complex, a phenomenon analogous to heparin-induced thrombocytopenia [9]. Other hematological complications such as immune thrombocytopenic purpura (ITP) and TTP have also been reported post-COVID-19 vaccination [10]. Given this, we sought to reflect on the existing cases of TTP, whether de novo or relapsing, reported after COVID-19 vaccination to further gain insight into any association, if present, and outcomes.
1.1. Search strategies
Following preferred reporting items for systematic reviews and meta-analyses (PRISMA) recommendations, we searched PubMed, Embase, and Ebsco databases for published individual case reports on the occurrence or relapse of TTP after receiving any COVID-19 vaccine. There were no restrictions based on language, age, or country of origin. The first search was performed on February 20th, 2022, followed by an additional search on March 6th, 2022. Two authors independently screened all the search results from the three databases at the title and abstract levels, and conflicts, if any, were resolved either by discussion or adjudication by a third author. We retrieved all references in the included studies for additional sources. The following keywords were used for identifying reports with TTP and COVID-19 vaccine administration, respectively: “Purpura, Thrombotic Thrombocytopenic”[Mesh] OR “Thrombotic thrombocytopenic purpura, acquired” [Supplementary Concept] OR “platelet agglutinating protein, thrombotic thrombocytopenic purpura” [Supplementary Concept] OR “ADAMTS Proteins”[Mesh] OR “ADAMTS13 Protein”[Mesh] AND “COVID-19 Vaccines”[Mesh] OR “2019-nCoV Vaccine mRNA-1273”[Mesh] OR “BNT162 Vaccine”[Mesh] OR “ChAdOx1 nCoV-19”[Mesh] OR “Ad26COVS1”[Mesh].
1.2. Study selection
Thirty-one articles in PubMed, 26 articles in Embase, and 17 articles in Ebsco were retrieved from the aforementioned search keywords. After removal of duplicated articles, review papers, reports addressing VITT, ITP, or post-vaccine thrombocytopenia other than TTP, and articles reporting institution or registry data instead of individual patient data, a total of 23 articles (27 patients) were eligible for full-text screening and were included in this qualitative analysis. The following data was retrieved from each report: 1. First author's name and year of publication; 2. Age and sex of the patient; 3. Significant past medical history; 4. Type of vaccine received; 5. Clinical presentation on arrival to the hospital; 6. Day of symptom onset after receiving the vaccine; 7. Measured ADAMTS13 activity, antibody titer if present and reported; 8. Treatment received; and 9. Patient outcomes (Table 1 ).
Table 1.
Patients from respective case reports included in the analysis.
| Author, year | Age | Gender | Past medical history | Vaccine type | Clinical presentation | Onset of symptoms after vaccination | ADAMTS13 activity; autoantibody titers if reported; any other labs | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Yocum et al., 2021 [11] | 62 | F | HTN, HLD, hypothyroidism, GERD | Ad26.COV2-S | Acute onset of altered mental status | 37 days | <12%; no antibody documentation, however PF4 negative | PLEX and steroids | Unknown |
| Waqar et al., 2021 [6] | 69 | M | HTN, CKD, HIV (on HAART, with CD4 count 354), Hepatitis B, DVTs (on warfarin) | BNT162b2 (second dose) | Fatigue and dyspnea | 7 days | <2%; >90 IU/mL | PLEX, steroids, and rituximab | Discharged on outpatient rituximab |
| Sissa et al., 2021 [12] | 48 | F | Relapsing TTP | BNT162b2 (Second dose) | Ecchymoses | 6 days | <3%; 88 U/mL | PLEX and steroids | Discharged |
| Maayan et al., 2021 [13] | 40 | F | None | BNT162b2 (Second dose) | Somnolence, fever, hematuria, petechiae and ecchymoses | 8 days | 0%; 51 U/mL | PLEX, steroids and caplacizumab | Discharged with monitoring |
| Maayan et al., 2021 [13] | 28 | M | Morbid obesity | BNT162b2 (Second dose) | Dysarthria | 28 days | 0%; 113 U/mL | PLEX, steroids, caplacizumab and rituximab | Discharged with monitoring |
| Maayan et al., 2021 [13] | 31 | F | Recurrent TTP | BNT162b2 (First dose) | Vaginal bleeding and purpura | 13 days | 0%; 64 U/mL | PLEX, steroids, caplacizumab and rituximab | Discharged On caplacizumab |
| Maayan et al., 2021 [13] | 30 | M | Single episode of TTP 7 years prior | BNT162b2 (Second dose) | Purpura | 8 days | 0%; 21 U/mL | PLEX, steroids, caplacizumab and rituximab | Discharged with monitoring |
| Bruijn et al., 2021 [14] | 38 | F | None | BNT162b2 (first dose) | Petechiae, blurred vision due to central serous chorioretinopathy | 14 days | 0%; >1000 AU/mL; PF4 negative, high IgG antibodies against S1 receptor binding domain of SARS-CoV-2 (93.8 U/mL) | PLEX, steroids, low dose acetylsalicylic acid, caplacizumab and rituximab | Discharged with monitoring |
| Lee et al., 2021 [15] | 50 | F | HTN | ChAdOx1 nCoV-19 (first dose) | dysphasia, left upper extremity numbness and petechiae | 12 days | 0%; >94.93 U/mL, PF4 negative | PLEX, steroids, and rituximab | Discharged on tapering steroids |
| Guney et al., 2022 [16] | 48 | F | None | BNT162b2 (first dose) | Weakness, nausea, dizziness, and bruising | 3 days | <0.2%; >90 IU/mL | PLEX, steroids and rituximab | Unknown |
| Osmanodja et al., 2021 [17] | 25 | M | None | mRNA-1273 (first dose) | Malaise, fever, aphasia, vomiting, headache, petechiae and hematuria | 4 days | <1%; 72.2 IU/mL; PF-4 and enhanced platelet activation test negative | PLEX, steroids, caplacizumab and rituximab | Discharged on daily caplacizumab |
| Al-Ahmad et al., 2021 [18] | 37 | M | Heavy smoker complicated by secondary polycythemia and recent venesection (1 week) | ChAdOx1 nCoV-19 (first dose) | Dizziness, fatigue, headache, dyspnea hematuria, ecchymoses, and palpitations | 10–15 days | 2.6%; unknown titers but present | PLEX, steroids and rituximab | Discharged on rituximab and steroid taper |
| Yoshida et al., 2022 [19] | 57 | M | None | BNT162b2 (first dose) | Fatigue, jaundice, and appetite loss | 7 days | <0.5%, 1.9 BU/mL; PF-4 negative; high IgG antibodies (two types) against receptor binding domain of SARS-CoV-2 spike protein (23.5 AU/mL and 153 U/mL) | PLEX, steroids and rituximab | Discharged |
| Ruhe et al., 2022 [20] | 84 | F | None | BNT162b2 (first dose) | Petechiae, hypertension, and Partial hemiplegia due to subacute emboli | 16 days | 1.6%, 82.2 U/mL; PF-4 negative; HIPA and PIPA negative; high IgG antibodies against SARS-CoV-2 spike protein (28.6 AU/mL) | PLEX, steroids and rituximab | Improved |
| Giuffrida et al., 2021 [21] | 83 | F | Undifferentiated connective tissue disease (on low-dose steroids) and steroid induced DM | BNT162b2 (first dose) | Hematuria and petechiae | 14 days | <10%; 40 U/mL | PLEX, steroids and caplacizumab | Death after 2 days of treatment |
| Giuffrida et al., 2021 [21] | 30 | F | Beta-thalassemia carrier | BNT162b2 (first dose) | Headache, fatigue, and petechiae | 18 days | <10%; 77.6 U/mL | PLEX, steroids and caplacizumab | Discharged on caplacizumab |
| Kirpalani et al., 2022 [22] | 14 | F | Anxiety, iron deficiency, and postprandial abdominal pain with family history of maternal ITP | BNT162b2 (first dose) | Fatigue, headache, confusion, and petechiae | 14 days | < 1%; 72 μ/mL; boderline IgA and IgG antibodies against SARS-CoV-2 spike protein (checked after initiation of PLEX – unknow titers) | PLEX, steroids, caplacizumab and rituximab | Improved |
| Chamarti et al., 2021 [23] | 80 | M | HTN, DM (type II), HLD, Gout, iron deficiency anemia | BNT162b2 (second dose) | Malaise, weakness and petechiae | 12 days | <2%; 182% | PLEX, steroids, and rituximab | Discharged on rituximab |
| Innao et al., 2022 [24] | 33 | F | Lymphoma status post chemotherapy and allogenic HSCT (in remission for last 11 years), was on hormone therapy for unknown reasons | BNT162b2 (first dose) | Asthenia, headache, purpura, drowsiness, and nausea with abdominal pain | 9 days | 8%; defective sample – no comment on titers | PLEX, steroids and caplacizumab | Discharged on steroid taper and caplacizumab |
| Agbariah et al., 2021 [25] | 60 | M | Ischemic stroke one week after first dose of BNT162b2 | BNT162b2 (second dose) | Retrosternal pain and confusion | 10 days | <5%; negative; non-inhibitory ADAMTS13 IgG autoantibodies were weakly positive; PF-4 negative | PLEX and steroids | Improved |
| Pavenski et al., 2021 [26] | 84 | M | Immune TTP (diagnosed 14 months ago), Remote treated prostate cancer, gout, HLD, HTN, DM (Type II) | BNT162b2 (first dose) | Lethargy, myalgias and anorexia | 7 days | <1%; >15 IU/mL | PLEX, steroids, caplacizumab and rituximab | Discharged |
| Duecher et al., 2022 [27] | 28 | F | Immune TTP (diagnosed 30 months ago) | BNT162b2 (first dose) | Bruising and ataxia | 6 days | Undetectable; unknown | Prednisone, rituximab and caplacizumab (experience with use of Caplacizumab without PLEX) | Discharged on weekly rituximab |
| Wang et al., 2021 [29] | 75 | M | None | ChAdOx1 nCov-19 | Bleeding from tongue | 30 days | 0.8%; unknown; PF-4 complex antibodies negative | PLEX | Unknown |
| Karabulut et al., 2021 [30] | 48 | M | DM (Type II on insulin), TTP (8 years ago), ITP (5 years ago), COVID-19 pneumonia (2 months prior to presentation) | mRNA-1273 (First dose) | Paresthesia, Transient weakness, and dysarthria | 5 days | <3%; 6.6 BEU | PLEX, steroids and rituximab | Discharged on rituximab and steroid taper |
| Francisco et al., 2021 [31] | 57 | M | Immune TTP (5 years) | mRNA-1273 (second dose) | Petechiae | 49 days | <5%; titer 1.5 (normal <0.4) | PLEX, steroids, caplacizumab and rituximab | Discharged on rituximab and caplacizumab, however 3 weeks after completion of caplacizbumab (30 days duration), he had a relapse |
| Alislambouli et al., 2021 [32] | 61 | M | None | Pfizer (first dose) | Confusion, fever, headache, dark urine, and ecchymosis, with seizures on arrival (only patient with classic pentad) | 5 days | <3%; unknown | PLEX, steroids and rituximab | Discharged on rituximab and steroid taper |
| Dykes et al., 2022 [33] | 50 | F | Congenital TTP (maintained on plasma infusion every 4–6 weeks since 2016 - infusion got delayed by 2 weeks) | mRNA-1273 (second dose) | Malaise, neurologic deficits with seizures in ER | 7 days | <5%; no inhibitors as congenital, PF4 and SRA negative | Plasma infusions | Improved |
HTN: hypertension; HLD: hyperlipidemia; GERD: gastro-esophageal reflux disease; CKD: chronic kidney disease; HIV: human immunodeficiency virus; HAART: highly active anti-retroviral therapy; DVT: deep venous thrombosis; HIPA: heparin-induced platelet antibody; PIPA: platelet- iodinated protein A; PF4: platelet factor 4 heparin complex antibody; HSCT: hematopoietic stem cell transplant; PLEX: plasmapheresis; PF4: platelet factor 4.
2. Results
A total of 27 patients were included in this analysis (Table 1). The mean age for the patients who developed de novo TTP post-COVID-19 vaccination (n = 19) was 51.3 years. There was a nearly even distribution in terms of gender (14 females; 13 males). Eight patients had a prior history or an episode of TTP – seven had immune TTP, whereas one patient had congenital TTP. TTP episodes were seen mostly after BNT162b2 vaccine (after first dose, n = 12; after second dose, n = 7), followed by mRNA-1273 vaccine (after first dose, n = 2; after second dose, n = 4). Adenoviral vaccines were responsible for 4 cases (Ad26.COV2-S - 1, ChAdOx1 nCoV-19 - 3). The most common presenting symptom was mucocutaneous bleeding (n = 20), while 15 patients presented with neurological symptoms. The mean days of onset of symptoms post-vaccination were 13.4 days. ADAMTS13 activity ranged from 0% to <12%. All patients with immune TTP except one received plasma exchange (PLEX) and steroids. Twelve patients received caplacizumab, and 17 patients received rituximab, while the patient with congenital TTP received human plasma infusion. Only one patient had a relapse within 30 days after discharge from the hospital. One patient passed away after two days of hospitalization, likely due to a sudden cardiovascular event.
3. Discussion
The concept of TTP post-vaccination is not new. Rare instances of TTP have been reported after influenza, pneumococcal, H1N1, and rabies vaccines [34]. These were usually seen with vaccines against viral agents and within two weeks of vaccination [34]. Furthermore, there is robust evidence of the generation of autoantibodies by the vaccine itself or their adjuvant components resulting in cross-reactivity with or without aberrant stimulation of the immune system leading to the development of auto-immune diseases [21], [23]. Despite having an adequate insight into these immune mechanisms, it has been more intricate to monitor these with COVID-19 vaccines, given their emergent need that led to their expedited manufacturing [23]. Recognized autoimmune events post COVID-19 vaccines include VITT, Miller-Fisher syndrome, Guillain-Barre syndrome, and ITP [10]. TTP can camouflage like VITT (thrombocytopenia and thrombosis with neurological alterations) that can result in a delay in recognition and therapy. This becomes of particular concern in regard to PLEX initiation, which is considered as a second-line treatment for VITT, in contrast to TTP, where it's usually the first lifesaving modality offered [15]. Therefore, evaluation of post-vaccine thrombocytopenia poses a significant diagnostic dilemma, and clinicians must consider the possibility of TTP given the associated fatality with this condition and the need for expedient treatment as the mortality risk of TTP without early therapy remains high at 80–90% [4].
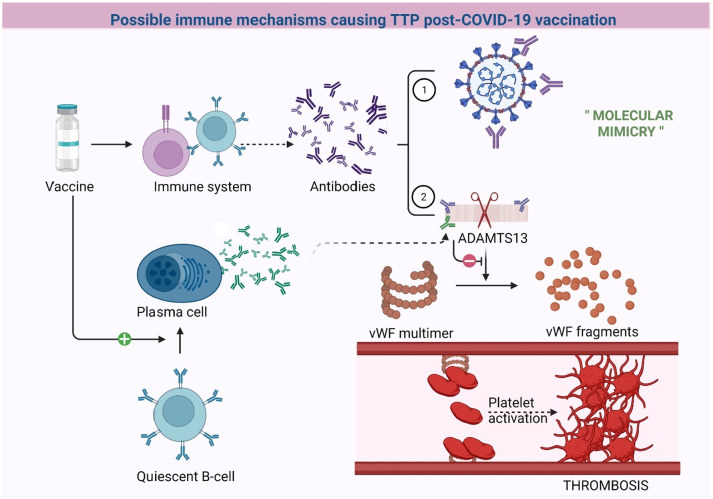
Pathophysiology of de-novo TTP after vaccination remains ambiguous with two schools of thought (Fig. 1 ). One concept outlines the notion of undiagnosed occult TTP manifesting as a full episode, after a trigger (vaccine), is exhibited by patients with symptom onset within a few days of vaccine receipt [17]. This concept is further supported by the expectation that immune cells usually demand more time to mount a response [17]. A second concept raises the possibility of autoantibodies formed against ADAMTS13 after an immunological trigger through mechanisms of molecular mimicry [20], [29] (Fig. A ). Furthermore, in patients with relapsing acquired or congenital TTP, there is data to support that ADAMTS13 deficiency alone is insufficient for a patient to develop an acute relapse [27]. There is a need for a “second hit” in the form of infection or inflammation to precipitate acute TTP, which in these cases was considered to be the COVID-19 vaccine [27].
Fig. 1.
PRISMA flow diagram for study selection.
Fig. A.
Graphical representation of the possible pathophysiology of TTP post-COVID-19 vaccination.
1 represents the normal response to COVID-19 vaccines, 2 represents formation of antibodies that cross-react with ADATMS13 (molecular mimicry). Quiescent B-cells represent the cells that have the ability to produce autoantibodies after an immunological trigger. Created with Biorender.com
Unanimously, all the authors mentioned above came to the suspicion of TTP in their respective patients given the chronology of events after receiving the COVID-19 vaccine and the absence of any other inciting factors. This was then confirmed by reduced ADAMTS13 activity and, in most reports, was supported by high titers of antibodies against ADAMTS13. In a few cases, an absence of PF-4 antibody further helped to refute the diagnosis of VITT. Moreover, the studies reporting high titers of SARS-CoV-2-IgG post-vaccination may also potentiate the hypothesis of vaccine-related TTP [22]. Notably, causality could not be assigned through this qualitative analysis, and whether these are just coincidental associations cannot be accurately judged.
Only in one study did the patient have a prior COVID-19 infection (asymptomatic) and received the mRNA vaccine (mRNA-1273) 2 months later [30]. The authors of this case hypothesized that an augmented immune response to the vaccine in context of a prior infection led to antibody production against ADAMTS-13 and hence, relapse of TTP. In one case, there was a genetic affliction to develop autoimmune disease (maternal ITP history), while in the other, the patient had connective tissue disease – both signifying an underlying vulnerability to autoimmunity that might have been unmasked by the vaccine [21], [22]. Additionally, there was one case of TTP in a patient with a history of malignancy [24]. However, the authors reported that the patient didn't show increased susceptibility to infections or autoimmunity in the prior years after receiving her chemotherapy or transplant, probably narrowing the diagnosis to vaccine associated TTP [24]. It is noteworthy to mention that a patient had documented evidence of ischemic stroke after the first dose of BNT162b2 vaccine due to unknown reasons, however, subsequently presented as de novo TTP after his second dose, subtly pointing towards a possible vaccine-associated unrecognized early hypercoagulable state [25].
Furthermore, all patients with acquired TTP except one received plasmapheresis and steroids to achieve remission. Deucher et al. reported abundant prior experience with the use of capalacizumab solely or in combination with other immunosuppressive agents to treat TTP, given their concerns about a potential increase in morbidity and mortality with PLEX [27]. Only one patient out of the 27 deceased after two days of inpatient treatment [21]. However, the patient refused treatment two weeks after her symptom onset and presented to the hospital one week later, emphasizing the role of timely medical management.
Most of the cases described above were advised against completing their vaccination series, either due to fear of another TTP relapse or due to concern for jeopardizing immune response post rituximab. Interestingly, the case reported by Deucher et al. safely received her second dose of BNT162b2 vaccine without any consequences, highlighting the importance of timing of vaccination in patients with a known history of TTP [27]. They proposed an ADATMS13 cutoff of 20% above which patients could be safely vaccinated. We also found a comment in literature from Doyle et al., who sought to correlate the TTP (de novo or relapse) occurrences with the vaccination program rollout in England [35]. They reported that there was no increase in the number of TTP presentations during peak vaccination times in a nationwide cohort, and that incidence of de novo cases was well within an expected range for their population [35]. On similar lines, in a small cohort of 12 patients with a known history of immune TTP, Schiepatti et al. observed no change in ADAMTS13 activity or inhibitor level on days 21 and 60 after the patients received their COVID-19 vaccine [36].
4. Conclusion
Our review underscores the importance of in-depth anamnesis before vaccination and provides a descriptive overview of the reported TTP cases post-COVID-19 immunization. In addition, further research is needed to determine epitope similarities, if any, between ADAMTS-13 and SARS-CoV-2 vaccine antigens, and to identify specific demographic or laboratory parameters that can predict TTP-occurrence and/or severity after COVID-19 vaccination. That said, comparing the number of TTP cases to the number of vaccine doses administered and outcomes of patients who develop TTP, we firmly believe that the benefits of receiving the COVID-19 vaccine outweigh risks, especially in immunocompromised patients, albeit with a need for closer surveillance.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
References
- 1.Blombery P., Scully M. Management of thrombotic thrombocytopenic purpura: current perspectives. J. Blood Med. 2014;5:15–23. doi: 10.2147/JBM.S46458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarode R., Bandarenko N., Brecher M.E., Kiss J.E., Marques M.B., Szczepiorkowski Z.M., et al. Thrombotic thrombocytopenic purpura: 2012 american Society for Apheresis (ASFA) consensus conference on classification, diagnosis, management, and future research. J. Clin. Apher. 2014;29(3):148–167. doi: 10.1002/jca.21302. [DOI] [PubMed] [Google Scholar]
- 3.Chiasakul T., Cuker A. Clinical and laboratory diagnosis of TTP: an integrated approach. Hematology. 2018;2018(1):530–538. doi: 10.1182/asheducation-2018.1.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanley M., Killeen R.B., Michalski J.M. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; Treasure Island (FL): 2022. Thrombotic Thrombocytopenic Purpura. StatPearls. [PubMed] [Google Scholar]
- 5.Rock G.A., Shumak K.H., Buskard N.A., Blanchette V.S., Kelton J.G., Nair R.C., et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N. Engl. J. Med. 1991;325(6):393–397. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 6.Waqar S.H.B., Khan A.A., Memon S. Thrombotic thrombocytopenic purpura: a new menace after COVID bnt162b2 vaccine. Int. J. Hematol. 2021;114(5):626–629. doi: 10.1007/s12185-021-03190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention . CDC; 2022. COVID data tracker. Atlanta GUDoHaHS.https://covid.cdc.gov/covid-data-tracker March 10. . [Available from: [Google Scholar]
- 8.HG Rosenblum J Gee R Liu PL Marquez B Zhang P Strid et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect. Dis. [DOI] [PMC free article] [PubMed]
- 9.Sharifian-Dorche M., Bahmanyar M., Sharifian-Dorche A., Mohammadi P., Nomovi M., Mowla A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J. Neurol. Sci. 2021;428 doi: 10.1016/j.jns.2021.117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Y Chen Z Xu P Wang X-M Li Z-W Shuai D-Q Ye , et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology.n/a(n/a). [DOI] [PubMed]
- 11.Yocum A., Simon E.L. Thrombotic thrombocytopenic purpura after Ad26.COV2-S vaccination. Am. J. Emerg. Med. 2021;49 doi: 10.1016/j.ajem.2021.05.001. 441.e3-.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sissa C., Al-Khaffaf A., Frattini F., Gaiardoni R., Mimiola E., Montorsi P., et al. Relapse of thrombotic thrombocytopenic purpura after COVID-19 vaccine. Transfus. Apher. Sci. 2021;60(4) doi: 10.1016/j.transci.2021.103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maayan H., Kirgner I., Gutwein O., Herzog-Tzarfati K., Rahimi-Levene N., Koren-Michowitz M., et al. Acquired thrombotic thrombocytopenic purpura: a rare disease associated with BNT162b2 vaccine. J. Thromb. Haemost. 2021;19(9):2314–2317. doi: 10.1111/jth.15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Bruijn S., Maes M.B., De Waele L., Vanhoorelbeke K., Gadisseur A. First report of a de novo iTTP episode associated with an mRNA-based anti-COVID-19 vaccination. J. Thromb. Haemost. 2021;19(8):2014–2018. doi: 10.1111/jth.15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H.P., Selvaratnam V., Rajasuriar J.S. Thrombotic thrombocytopenic purpura after ChAdOx1 nCoV-19 vaccine. BMJ Case Rep. 2021;14(10) doi: 10.1136/bcr-2021-246049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Güney T., Can F., Akıncı S., Soyer Kösemehmetoğlu Ö., Dilek İ. Immune-mediated thrombotic thrombocytopenic purpura after BNT162b2 vaccine. Turk. J. Haematol. 2022;39(1):74–75. doi: 10.4274/tjh.galenos.2021.2021.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osmanodja B., Schreiber A., Schrezenmeier E., Seelow E. First diagnosis of thrombotic thrombocytopenic purpura after SARS-CoV-2 vaccine - case report. BMC Nephrol. 2021;22(1):411. doi: 10.1186/s12882-021-02616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Ahmad M., Al-Rasheed M., Shalaby N.A.B. Acquired thrombotic thrombocytopenic purpura with possible association with AstraZeneca-Oxford COVID-19 vaccine. EJHaem. 2021;2(3):534–536. doi: 10.1002/jha2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida K., Sakaki A., Matsuyama Y., Mushino T., Matsumoto M., Sonoki T., et al. Acquired thrombotic thrombocytopenic purpura following BNT162b2 mRNA coronavirus disease vaccination in a Japanese patient. Intern. Med. 2022;61(3):407–412. doi: 10.2169/internalmedicine.8568-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruhe J., Schnetzke U., Kentouche K., Prims F., Baier M., Herfurth K., et al. Acquired thrombotic thrombocytopenic purpura after first vaccination dose of BNT162b2 mRNA COVID-19 vaccine. Ann. Hematol. 2022;101(3):717–719. doi: 10.1007/s00277-021-04584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giuffrida G., Condorelli A., Di Giorgio M.A., Markovic U., Sciortino R., Nicolosi D., et al. Immune-mediated thrombotic thrombocytopenic purpura following Pfizer-BioNTech COVID-19 vaccine. Haematologica. 2021;107(4) doi: 10.3324/haematol.2021.279535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirpalani A., Garabon J., Amos K., Patel S., Sharma A.P., Ganesan S.L., et al. Thrombotic thrombocytopenic purpura temporally associated with BNT162b2 vaccination in an adolescent successfully treated with caplacizumab. Br. J. Haematol. 2021;196(1):e11–e14. doi: 10.1111/bjh.17782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chamarti K., Dar K., Reddy A., Gundlapalli A., Mourning D., Bajaj K. Thrombotic thrombocytopenic purpura presentation in an elderly gentleman following COVID vaccine circumstances. Cureus. 2021;13(7) doi: 10.7759/cureus.16619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Innao V., Urso S., Insalaco M., Borraccino A., Consoli U. Immune thrombotic thrombocytopenic purpura following Pfizer-BioNTech anti-COVID-19 vaccination in a patient healed from lymphoma after allogeneic hematopoietic stem cell transplantation. Thromb. Res. 2022;210:91–93. doi: 10.1016/j.thromres.2021.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agbariah N., Bütler V.A., Wieland A., Andina N., Hammann F., Kremer Hovinga J.A. Acquired immune-mediated thrombotic thrombocytopenic pur-pura (iTTP) following mRNA-based COVID-19 vaccination (BNT162b2) Swiss Med. Wkly. 2021;151(SUPPL 255):20S. doi: 10.3389/fmed.2022.890661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavenski K. Relapse of immune thrombotic thrombocytopenic purpura following vaccination with covid19 mRNA vaccine. TH Open. 2021;5(3) doi: 10.1055/s-0041-1732342. E335-E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deucher W., Sukumar S., Cataland S.R. Clinical relapse of immune-mediated thrombotic thrombocytopenic purpura following COVID-19 vaccination. Res. Pract. Thromb. Haemost. 2022;6(1) doi: 10.1002/rth2.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y.C., Chen T.C., Teng C.L.J., Wu C.H. ChAdOx1 nCov-19 vaccine-induced thrombotic thrombocytopenic purpura successfully treated with plasmapheresis. Ann. Hematol. 2021;2021 doi: 10.1007/s00277-021-04701-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karabulut K., Andronikashvili A., Kapici A.H. Recurrence of thrombotic thrombocytopenic Purpura after mRNA-1273 COVID-19 vaccine administered shortly after COVID-19. Case Rep. Hematol. 2021;2021 doi: 10.1155/2021/4130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francisco M.T., Kaufman A.E., Northfelt D., Padrnos L., Rosenthal A.C., Andres J., et al. Relapsed refractory acquired thrombotic thrombocytopenic purpura (aTTP) following COVID-19 vaccination. Blood. 2021;138:4218. [Google Scholar]
- 32.Alislambouli M., Veras Victoria A., Matta J., Yin F. Acquired thrombotic thrombocytopenic purpura following Pfizer COVID-19 vaccination. eJHaem. 2022;3(1):207–210. doi: 10.1002/jha2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dykes K.C., Kessler C.M. First report of COVID-19 vaccine induced flare of compensated congenital thrombotic thrombocytopenic purpura. Blood Coagul. Fibrinolysis. 2022;33(1):71–73. doi: 10.1097/MBC.0000000000001097. [DOI] [PubMed] [Google Scholar]
- 34.Yavaşoğlu İ. Vaccination and thrombotic thrombocytopenic purpura. Turk. J. Haematol. 2020;37(3):218–219. doi: 10.4274/tjh.galenos.2020.2020.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doyle A.J., Springell D., Dutt T., Kenworthy J., Ling G., Desborough M. Acquired thrombotic thrombocytopenic purpura: a rare disease associated with BNT162b2 vaccine: comment from Doyle et al. J. Thromb. Haemost. 2022;20(3) doi: 10.1111/jth.15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schieppati F., Russo L., Marchetti M., Galimberti E., Palladino A.M., Gamba S., et al. Hemostatic markers, Adamts-13 profile and anti-sars-Cov-2 antibody levels in patients with immune thrombotic thrombocytopenic purpura receiving BNT162b2 vaccination. Blood. 2021;138:1022. [Google Scholar]