Abstract
Importance:
Long-term sleep disturbances in menopausal women are closely related to cardiovascular disorders, metabolic disorders, and cognitive impairment. At present, hormone therapy (HT) is a standard treatment for menopausal symptoms. However, it remains unclear whether HT can improve sleep quality.
Objective:
We did a systematic review and meta-analysis to assess the effects of different HT regimens on menopausal sleep quality.
Evidence Review:
We systematically searched MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, PsycINFO, CINAHL, and Web of Science for randomized controlled trials of menopausal HT on sleep disturbances up to June 14,2021. Information about ongoing and unpublished trials was collected by searching WHOICTRP and ClinicalTrials.gov. Our primary outcome was sleep quality with objective measurements. We estimated the standardized mean difference (SMD) using random-effects models.
Findings:
We identified a total of 3,059 studies and finally included 15 studies in the meta-analysis. Compared with placebo, HT improved self-reported sleep outcomes (SMD = –0.13; 95% CI, –0.18 to -0.08, P < 0.00001 and I2 = 41%), but not sleep parameters measured by polysomnography. Subgroup analyses according to the regimen of HT showed that 17β-estradiol (17β-E2) (SMD = –0.34; 95% CI, –0.51 to -0.17, P < 0.0001, and I2 = 0%) and conjugated equine estrogens (SMD = –0.10; 95% CI, −0.12 to −0.07, P < 0.00001, and I2 = 0%) improved sleep quality. Moreover, transdermal administration (SMD = −0.35; 95% CI, −0.64 to −0.06, and P = 0.02) was more beneficial than oral (SMD = −0.10; 95% CI, −0.14 to −0.07, and P < 0.00001). In addition, the combination of estrogen and progesterone had a positive effect on sleep disturbance (SMD = −0.10; 95% CI, −0.13 to −0.07, P < 0.00001, and I2 = 0%), while estrogen monotherapy did not. The results showed that estrogen/micronized progesterone (SMD = −0.22; 95% CI, −0.37 to −0.06, P = 0.007, and I2 = 0%) and estrogen/medroxyprogesterone acetate (SMD = −0.10; 95% CI, −0.13 to −0.07, P < 0.00001, and I2 = 0%) could alleviate sleep disturbance.
Conclusions and Relevance:
HT has a beneficial effect on sleep disturbance to some extent, and the formulations and routes of administration of hormonal agents influence the effect size.
Keywords: Estrogen, Menopause, Progesterone, Sleep
Key Points
Question/Objective: What are the effects of different regimens of hormone therapy (HT) on menopausal sleep quality?
Findings: Fifteen randomized controlled trials (n = 27,715) evaluating the effect of different regimens of HT on menopausal sleep quality were included. The present study demonstrated that HT improved subjective sleep quality in 12 randomized controlled trials. More specifically, 17β-estradiol (17β-E2) and conjugated equine estrogens improved sleep quality, whereas estradiol valerate did not. In addition, transdermal regimens were more beneficial than oral.
Meaning: Based on the meta-analysis, HT could improve sleep quality, and the formulations and routes of administration of hormonal agents influence the effect size.
Sleep disturbance is one of the distinctive features of menopausal symptoms and a well-recognized global health problem.1,2 Compared to young women and their male counterparts, premenopausal women have major sleep disturbances.3,4 Self-reported sleep problems were reported to increase by 2 to 3.5 times in women during the menopausal transition.5 Forty to sixty percent of women report problems sleeping during perimenopause and postmenopause.6 Menopausal sleep disturbances are significantly associated with vasomotor symptoms, such as hot flashes and night sweats,7 and are two to three times more likely to increase the risk of depression.8 Furthermore, long-term sleep disturbances are closely related to cardiovascular and metabolic disorders, cognitive impairment, deficits in immune function, and even malignant tumors.9,10 Therefore, sleep disturbance is an important issue for menopausal women.
McEwen and Alves11 reported that estrogen acted on the areas of sleep regulation in the brain, and the change of estrogen was the primary factor for sleep disturbance. Progesterone also decreased during menopause, and accumulating clinical data demonstrated its actions on the central nervous system.12,13 The close relationship between sleep problems and decreased levels of reproductive hormones in menopausal women has led to consideration of hormone therapy (HT) for their relief.14 However, there is a lack of effective treatment at present.
Montplaisir et al15 observed improved sleep efficiency in the oral conjugated equine estrogens (o-CEE) plus micronized progesterone group, but not in the medroxyprogesterone acetate (MPA) group. Cintron et al16 reported alleviated sleep disturbances in the transdermal 17β-estradiol (t-17β-E2) group and the o-CEE group. However, Heinrich and Wolf17 did not find beneficial effects of estradiol valerate (EV) or estradiol plus micronized progesterone on sleep quality. Due to the heterogeneity of the participants, different formulations and doses of HT, and different measurements for sleep quality in previous studies,18 there is no consistent evidence that HT could improve sleep quality. It is also unclear which is the most effective therapeutic regimen for improving sleep quality. Hence, synthesized evidence is needed to help women and clinicians to choose the most appropriate treatment for menopausal women with sleep disorders.
A previous meta-analysis found that HT improved sleep quality in postmenopausal women with vasomotor symptoms, nevertheless, it failed to compare the effects of different formulations of HT on sleep disorders with each other owing to the limited number of original studies. Besides, the study was short of the measurement and analysis of objective indicators for sleep disturbances.19 Therefore, with several newly-published clinical studies, we updated and optimized the previous meta-analysis, and also did a systematic review of randomized controlled trials (RCTs) to evaluate the effects of different formulations and routes of administration of HT on sleep disturbance, aiming to probe into the association between HT therapy and sleep disturbance in menopausal women and consequently take effective measures to promote the physical and mental health of menopausal women.
METHODS
This systematic review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and was registered with the International Prospective Register of Systematic Reviews (PROSPERO), number CRD42021256551.
Search strategy and eligibility criteria
We selected relevant studies in the following databases: MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, PsycINFO, CINAHL, and Web of Science. We developed a search strategy from text and MeSH terms related to “hormone replacement therapy,” “estrogen,” “progesterone,” “menopause,” “insomnia,” and “sleep” up to June 14, 2021. Information about ongoing and unpublished trials was obtained by searching WHOICTRP and ClinicalTrials.gov.
We included studies that considered HT administration as either the treatment or the control group. We identified eligible studies according to the following criteria: participants had to be women during menopause, including perimenopause and postmenopause; minimal intervention length was 4 weeks; the outcomes were related to sleep quality measured with polysomnography or self-reported questionnaires; study design must be double-blind RCTs. Studies with HT at any dose, formulations, and routes of administration, such as oral, subdermal, transdermal, or intravenous, were eligible for inclusion. Exclusion criteria were as follows: observation and retrospective studies; studies in which HT was combined with compounds other than progesterone derived or selective estrogen receptor modulators; studies not published in English. For the overlapped sample sources, we included the report with more information and larger sample sizes.
Two independent investigators reviewed study titles and abstracts, and studies that satisfied the inclusion criteria were retrieved for full-text assessment. The agreement of both investigators determined final eligibility. Disagreements were referred to a third reviewer to reach a consensus.
Data extraction and assessment of risk bias
Two reviewers independently extracted the following data from each included study using a specifically designed form: study design, sample sizes, participant characteristics, details of HT, duration, and sleep outcomes. We extracted the mean and standard deviation for the result. Disagreements were referred to a third reviewer to reach a consensus.
Two independent reviewers assessed the included studies for risk of bias using the Cochrane risk of bias tool, containing seven specific domains. We resolved any disagreements by consensus or by a discussion with a third author. Each domain was assigned a judgment relating to the risk of bias for that study classified as low, high, or unclear risk.
Statistical analysis
Since the included studies used different scales to evaluate sleep quality, we calculated pooled estimates of the standardized mean differences (SMDs) and 95% confidence intervals (CIs) with a random-effects model. To overcome a unit-of-analysis error for multiarms studies, we combined groups to create a single pair-wise comparison. For most studies, lower scores indicated better sleep quality except five studies,20-24 in which we reversed the direction to keep consistent with the others. We assessed statistical heterogeneity using the I2 statistic, with values greater than 50% regarded as moderate-to-high heterogeneity. We performed prespecified subgroup analyses according to the following parameters: formulation of HT; routes of administration of HT; andperimenopause or postmenopause. We did Egger tests to assess funnel plot asymmetry, and defined significant publication bias with the P value lower than 0.1. We conducted sensitivity analyses to determine whether the conclusions were robust. We presented the overall quality of the evidence for each outcome using the GRADE criteria. We conducted the statistical analyses with the Review Manager (Revman5.3.3, Cochrane Collaboration, Copenhagen, Denmark, 2014) and Stata software (version 14.0, StataCorp LP, TX, 1985-2015).
RESULTS
Search results
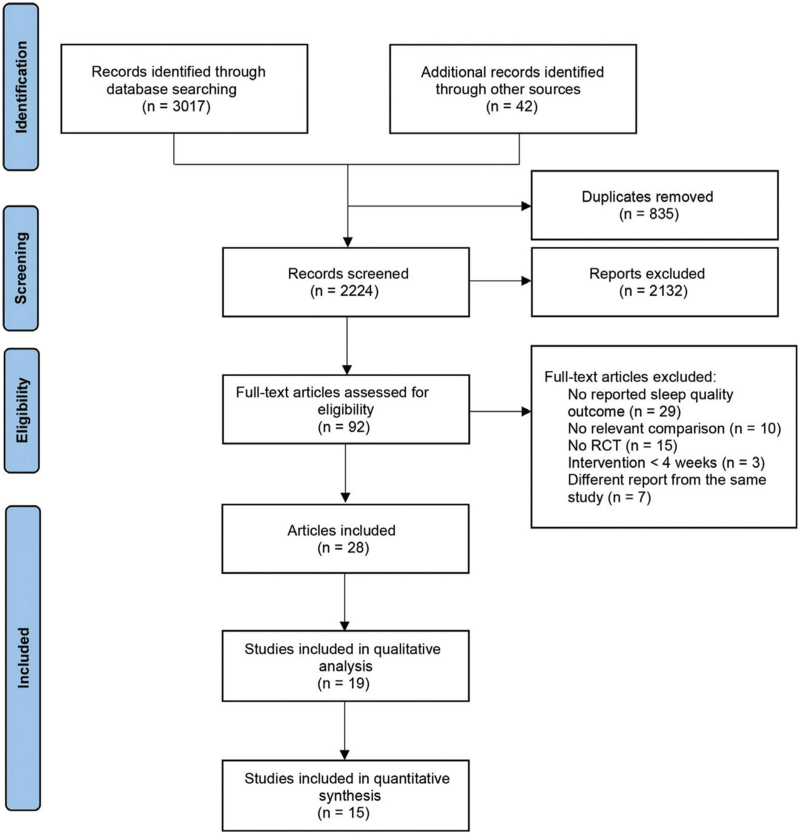
In total, we identified 3,059 studies, and 2,224 studies remained after we removed duplicates. Subsequently, we excluded 2,132 studies after reviewing the titles and abstracts. Of the remaining 92 articles assessed for eligibility, 28 studies met the inclusion criteria. Nineteen studies reported sufficient data,16,17,20-36 whereas 15 with placebo arm as comparator were included in the quantitative analysis16,17,20-26,28,29,31,33,35,36 (Fig. 1).
FIG. 1.
Flow diagram for study selection.
Description of included studies
The meta-analysis contained 27,715 participants: 14,058 women in the intervention group and 13,657 in the control group. Mean age ranged from 49.7 ± 4.4 to 64.1 ± 0.6 years old. Polysomnography was used in five trials,22,26,28,33,35 and subjective sleep questionnaires were used in 12 trials to evaluate sleep disorders.16,17,20-25,28,29,31,36 Six studies included women with vasomotor symptoms.16,20,21,23,25,35 (Table 1).
TABLE 1.
Characteristics of included trials
| Y | Study design | Sample sizes | Participant characteristics | Details of HT (/d) | Control | Duration | Sleep outcomes | |
| Brunner et al20 | 2005 | RCT | 10,739 | postmenopausal women with hysterectomy, mean age 63.6 y | o-CEE 0.625 mg | placebo | 12 mo | WHIIRS |
| Cintron et al16 | 2018 | RCT | 727 | postmenopausal women, mean age 52.6 y | o-CEE 0.45 mg + micronized progesterone 200 mg, t-17β-E2 50 μg + micronized progesterone 200 mg | placebo | 48 mo | PSQI |
| Ensrud et al25 | 2015 | RCT | 339 | menopause, postmenopausal or undergone hysterectomy women, mean age 54.6 y | o-17β-E2 0.5 mg | placebo | 8 wk | PSQI |
| Hachul et al26 | 2008 | RCT | 33 | postmenopausal women, mean age 55.9 ± 4.5 y | o-CEE 0.625 mg | placebo | 24 wk | polysomnography |
| Hays et al21 | 2003 | RCT | 8,506 | postmenopausal women, mean age 63.2 y | o-CEE 0.625 mg + MPA 2.5 mg | placebo | 12 mo | WHIIRS |
| Heinrich and Wolf17 | 2005 | RCT | 51 | postmenopausal women with hysterectomy, mean age 64.1 ± 0.6 y | o-EV 2 mg, o-EV 2 mg + micro-micronized progesterone 200 mg | placebo | 24 wk | Sleep item from ADSK and from Menopausal Index |
| Kagan et al27 | 2018 | RCT | 1,835 | postmenopausal women, mean age 55 y | oral TX-001HR (1 mg E2/ 100 mg P4, 0.5 mg E2/ 100 mg P4, 0.5 mg E2/50 mg P4, 0.25 mg E2/50 mg P4) | placebo | 12 mo | MOS-sleep questionnaire |
| Kalleinen et al28 | 2008 | RCT | 18 | postmenopausal women, mean age 62.9 y | o-EV 2 mg + norethisterone 0.7 mg | placebo | 6 mo | BNSQ and polysomno-graphy |
| LeBlanc et al29 | 2007 | RCT | 37 | late menopausal or early postmenopause women, mean age 52.3 y | o-E2 2 mg | placebo | 8 wk | OHSU SL sleep dairy |
| Leeangkoonsathian et al30 | 2017 | RCT | 100 | perimenopause, early menopause or late menopause women, mean age 52.1 ± 4.1 y | o-EV 1 mg + micronized progesterone 10 mg, o-EV 1mg + dydrogesteron 100 mg | none | 3 mo | PSQI |
| Meeuwsen et al31 | 2002 | RCT | 85 | postmenopausal women, mean age 54.2 ± 4.7 y | o-tibolone 2.5 mg | placebo | 12 mo | NHP questionnaire |
| Polisseni et al32 | 2013 | RCT | 130 | postmenopausal women, mean age 52.6 ± 3.6 y | tibolone 2.5 mg, estradiol 1mg + norethindrone acetate 0.5 mg | calcium carbonate 50 mg + VitD3 200IU | 12 wk | WHQ sleep item |
| Polo-Kantola et al33 | 1999 | RCT | 71 | postmenopausal women, mean age 56.4 ± 4.4 y | estrogel 2.5 g, estrogen patch 50 ug | placebo | 3 mo | polysomnography |
| Saletu-Zyhlarz et al22 | 2003 | RCT | 55 | insomniac postmenopausal women, mean age 58 ± 5y | o-EV 2 mg + dienogest 3 mg, o-EV 2 mg | placebo | 8 wk | PSQI and polysomnography |
| Savolainen-Peltonen et al23 | 2014 | RCT | 150 | postmenopausal women, mean age 53.2 y | t-E2 1 mg, o-EV 2 mg, o-EV 2mg + MPA 5 mg | placebo | 6 mo | WHQ sleep item |
| Shulman et al34 | 2002 | RCT | 845 | postmenopausal women≥45 y | t-17β-E2/LNG (0.045 mg + 0.015 mg, 0.045 mg + 0.030 mg, 0.045 mg + 0.040 mg), t-17β-E2 | none | 1y | WHQ sleep item |
| Silva et al35 | 2011 | RCT | 12 | postmenopausal women, mean age 49.7 ± 4.4 y | 0.045 mg o-E2 1mg + trimegestone .125 mg | placebo | 4 wk | polysomnography |
| Tansupswatdikil et al24 | 2015 | RCT | 40 | insomniac postmenopausal women, mean age 54.4 y | t-17β-E2 50 μg | placebo | 2 mo | ISI |
| Welton et al36 | 2008 | RCT | 3,721 | postmenopausal women with or without subtotal hysterectomy, mean age 63.8 y | o-CEE 0.625 mg + MPA2.5 mg, o-CEE 0.625 mg + MPA 5mg | placebo | 12 mo | WHQ sleep item |
17β-E2,17β-estradiol; ADSK, German short version of the Center for Epidemiological Studies Depression Scale; BNSQ, Basic Nordic Sleep Questionnaire; CEE, conjugated equine estrogens; E2, estradiol; EV, estradiol valerate; HT, hormone therapy; ISI, Insomnia Severity Index; LNG, levonorgestrel; MOS, Medical Outcomes Study; MPA, medroxyprogesterone acetate; NHP, Nottingham Health Profile; o-, oral; o-CEE, oral conjugated equine estrogen; OHSU SL, Oregon Health and Science University Sleep Laboratory; P4, progesterone; PSQI, Pittsburgh Sleep Quality Index; RCT, randomized controlled trials; t-, transdermal; TX-001HR, a single, oral softgel capsule that contains hormones that are biologically identical to endogenous 17β-estradiol and progesterone; VitD3, vitamin D3; WHIIRS, Women's Health Initiative Insomnia Rating Scale; WHQ, Women's Health Questionnaire.
Only three studies reported adverse effects. Tansupswatdikul et al24 demonstrated that the t-17β-E2 group experienced more breast pain and vaginal discharge than the placebo group. Welton et al36 reported 37% of women with vaginal bleeding in the o-CEE plus MPA group and 4% in the placebo group. Meeuwsen et al31 reported eight women with bleeding and eight with spotting in the tibolone group, and three women with bleeding and one with spotting in the placebo group.
Four RCTs could not be included in the quantitative analysis due to the absence of outcomes in the placebo group. Leeangkoonsathia et al30 reported that both EV combined with micronized progesterone and dydrogesterone improved sleep quality, whereas no significant difference between the two groups. Shulman et al34 demonstrated that both t-17β-E2 monotherapy and three doses of combined 17β-E2/levonor-gestrel transdermal system improved sleep quality, whereas no significant difference among those groups. Kagan et al27 observed the beneficial effects of TX-001HR, an oral softgel capsule containing hormones that were biologically identical to endogenous 17β-E2 and progesterone ranging from 0.25 mg E2/50 mg P4 to 1 mg E2/100 mg P4, on MOS-sleep scores. Polissen et al32 reported alleviated sleep disturbances in the tibolone and the estradiol plus norethindrone acetate groups.
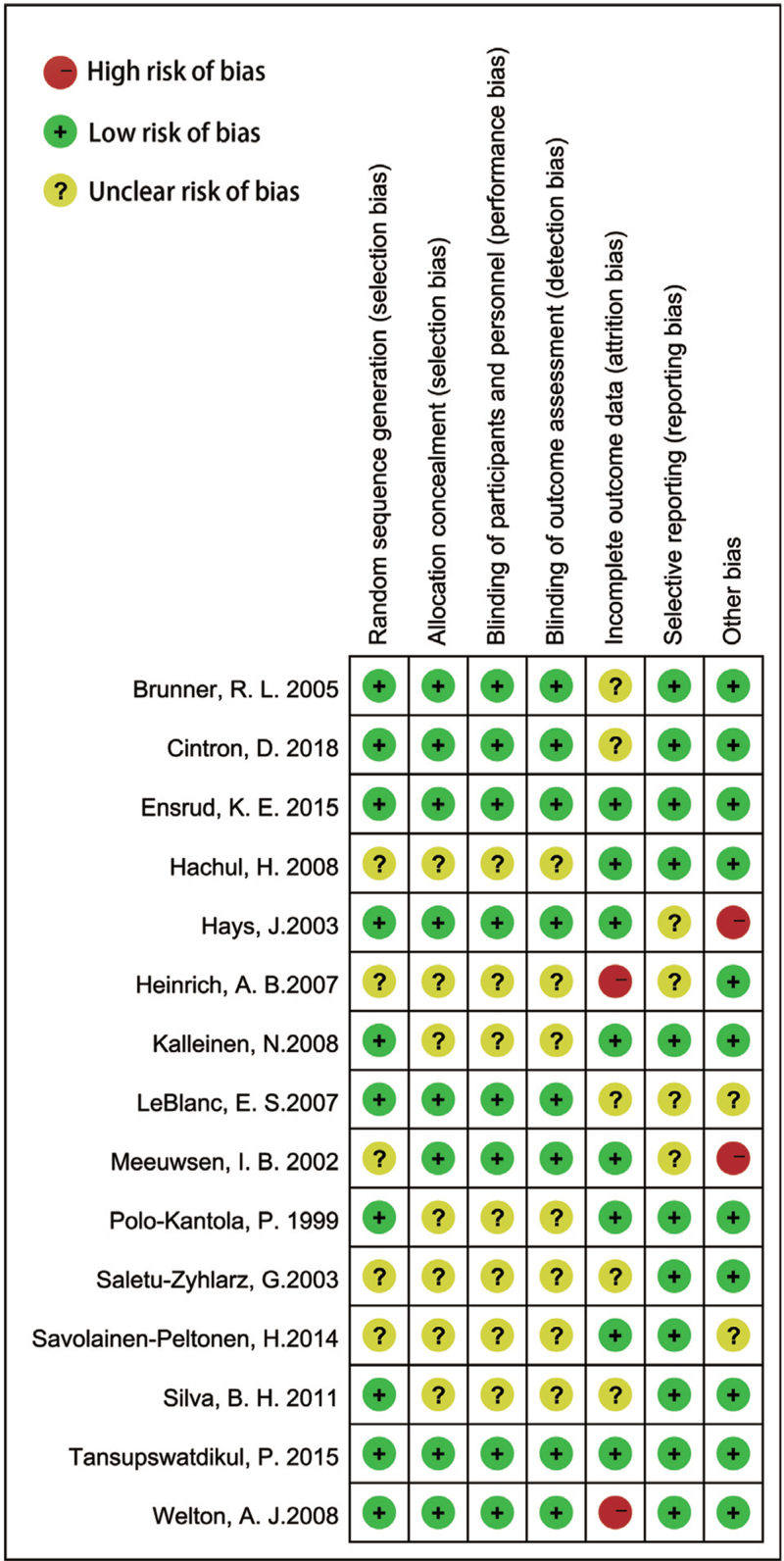
Risk of bias in included studies
We assessed the risk of bias in all included studies, as demonstrated in Figure 2. Ten studies reported adequate methods for random sequence generation.16,20,21,24,25,28,29,33,35,36 Seven studies did not specify whether data collectors and outcome assessors were masked to treatment allocation.17,22,23,26,28,33,35 Two studies rated at low risk of bias,16,35 and four were judged to be at high risk because of incomplete outcome data or funded by industry.17,21,31,36
FIG. 2.
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
Primary outcomes
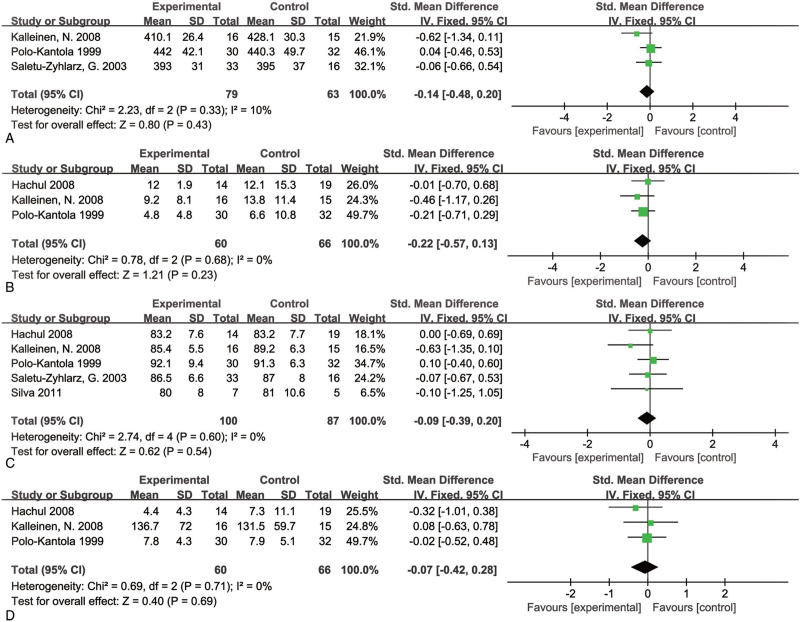
Meta-analysis of polysomnography studies showed no significant improvement in sleep parameters in the HT group, including total sleep time (SMD = −0.14; 95% CI, −0.48 to 0.20, P = 0.43, and I2 = 10%), sleep latency (SMD = −0.22; 95% CI, −0.57 to 0.13, P = 0.23, and I2 = 0%), sleep efficiency (SMD = −0.09; 95% CI, −0.39 to 0.20, P = 0.54, and I2 = 0%), and arousals number (SMD = −0.07; 95% CI, −0.42 to 0.28, P = 0.69, and I2 = 0%) (Fig. 3). These studies did not show any heterogeneity. However, we downgraded the evidence to low quality using GRADE criteria due to the few participants of the included studies (Supplemental Digital Content 1).
FIG. 3.
Forest plot showing individual and combined effect size estimates and 95% confidence intervals (CIs) in studies that evaluated the effect of hormone therapy using polysomnography. Horizontal lines indicate 95% CIs, boxes show the study-specific weight, diamond represents combined effect size. (A) Total sleep time; (B) sleep latency; (C) sleep efficiency; and (D) arousals number.
Pooled analysis of self-reported sleep outcomes of the 12 included studies showed the significant improvement of sleep quality in the HT group (SMD = −0.13; 95% CI, −0.18 to −0.08, and P < 0.00001)] (Fig. 4), with moderate between-study heterogeneity (I2 = 41%). We downgraded the evidence to moderate quality because of some included studies’ unclear risk for allocation concealment (Supplemental Digital Content 2).
FIG. 4.
Self-reported scores of subjective sleep questionnaire and subgroup analysis. (A) Self-reported scores of subjective sleep questionnaire among participants receiving hormone therapy versus placebo; (B) postmenopause subgroup analysis; (C) sample size subgroup analysis; (D) duration subgroup analysis; (E) different estrogen regimens subgroup analysis; (F) oral and transdermal subgroup analysis; (G) ET and EPT subgroup analysis; and (H) estrogen combined with different progesterone subgroup analysis. 17β-E2,17β-estradiol; CEE, conjugated equine estrogens; E, estrogen; EPT, estrogen plus progestogen therapy; ET, estrogen therapy; EV, estradiol valerate; MP, micronized progesterone; MPA, medroxyprogesterone acetate;P, progesterone.
Subgroup analysis
In the postmenopausal subgroup, HT improved self-reported sleep quality (SMD = −0.10; 95% CI, −0.13 to −0.08, and P < 0.00001) (Fig. 4), and there was no significant heterogeneity between studies (I2 = 20%). Subgroup analysis on the basis of sample size suggested a favorable sleep quality improvement of HT in the large sample subgroup (SMD = −0.12; 95% CI, −0.17 to −0.08, P < 0.00001, and I2 = 46%). We also conducted subgroup analysis based on the duration of studies and found that more than 6 months of HT improved sleep quality (SMD = -0.10; 95% CI, −0.13 to -0.08, P < 0.00001, and I2 = 6%). Subgroup analyses by different regimens of HT showed that 17β-E2 (SMD = −0.34; 95% CI, −0.51 to −0.17, P < 0.0001, and I2 = 0%) and CEE (SMD = −0.10; 95% CI, −0.12 to −0.07, P < 0.00001, and I2 = 0%) improved sleep quality, but EV had no positive effect (Fig. 4). Both oral and transdermal subgroup showed beneficial effects on sleep disturbances (oral: SMD = −0.10; 95% CI, −0.14 to −0.07, P < 0.00001, and I2 = 17%; transdermal: SMD = −0.35; 95% CI, −0.64 to −0.06, P = 0.02, and I2 = 18%), and the transdermal subgroup had a larger effect (Fig. 4). In addition, estrogen plus progesterone was an adequate intervention (SMD = −0.10; 95% CI, −0.13 to −0.07, P < 0.00001, and I2 = 0%), whereas estrogen monotherapy was not (SMD = −0.14; 95% CI, −0.40 to 0.12, P = 0.29, and I2 = 57%) (Fig. 4). Furthermore, subgroup analysis of estrogen combined with different progesterone showed that both estrogen plus micronized progesterone (SMD = −0.22; 95% CI, −0.37 to −0.06, P = 0.007, and I2 = 0%) and estrogen plus MPA (SMD = −0.10; 95% CI, -0.13 to −0.07, P < 0.00001, and I2 = 0%) positively associated with sleep disturbances, whereas other progesterone did not (Fig. 4).
Sensitivity analysis
Tansupswatdikul et al24 measured subjective sleep quality with Insomnia Severity Index, which is quite different from other sleep questionnaires. We conducted a sensitivity analysis to assess the contribution of this study to the synthesized outcome. We generated similar pooled SMD and 95% CI with the removal of this study (SMD = −0.12; 95% CI, −0.17 to −0.08, P < 0.00001, and I2 = 36%), thus indicating no significant influence of the trial on the overall estimation of self-reported sleep quality.
Publication bias
The funnel plots for self-reported sleep quality models and regression analyses of Egger's test suggested publication bias in this analysis (Supplemental Digital Content 3; Supplemental Digital Content 4).
DISCUSSION
We performed the systematic review and meta-analysis to comprehensively analyze the effect of HT on sleep disturbance with both subjective and objective sleep outcomes. We included 15 RCTs with similar interventions and sufficient quantitative data for statistical pooling. The pooled effects of subjective sleep quality showed a significant improvement in the HT group. Our pooled results were stable, and the heterogeneity among studies was moderate.
We did subgroup analyses to eliminate heterogeneity and evaluate the effect of different regimens of HT on sleep quality. The results showed that both oral and transdermal regimens positively impacted sleep disturbance, and transdermal administration was more helpful. Both 17β-E2 and CEE improved sleep quality, whereas EV did not. Furthermore, we found more favorable sleep quality improvement in the 17β-E2 subgroup. Considering the 17β-E2 subgroup contained three RCTs and two of them compared transdermal 17β-E2 to placebo, we speculated that transdermal 17β-E2 administration led to better effects. The underlying mechanism may be that transdermal estrogen delivery avoids the first-pass effect, resulting in more stable serum estradiol level and higher bioavailability comparing with oral administration.
In the subgroup analysis, the large sample subgroup showed improved sleep quality. Since large sample studies were more likely to avoid sampling error and better represent the actual effect, this result revealed a beneficial effect of HT on sleep disturbance. Furthermore, in the subgroup analysis of the duration of studies, we found that more than 6 months of HT improved sleep quality. Our results provide evidence for the clinical application of HT on menopausal sleep disturbances.
A previous study has proved the sedative and hypnotic effects of progesterone.37 Lancel et al38 demonstrated that progesterone metabolites could produce similar changes to sleep architecture as benzodiazepines. We conducted subgroup analyses to estimate the effect of progesterone on sleep quality. Estrogen plus progesterone alleviated sleep disturbance, but estrogen monotherapy did not. Furthermore, both the estrogen plus micronized progesterone group and the estrogen plus MPA group improved sleep quality, and the former showed a better effect. However, formulations of estrogen may be a confounding factor. Consistent with the study, Leeangkoonsathia et al30 reported improved sleep quality in the EV plus micronized progesterone group and the EV plus dydrogesterone group. Montplaisir et al observed improved sleep efficiency in the micronized progesterone group. In postmenopausal women taking estrogen, they also observed increased subjective sleep quality in the micronized progesterone and the MPA group.15 Together with our results, the evidence indicated the critical role of progesterone on sleep.
A previous meta-analysis favored oral micronized progesterone for sleep onset latency but not total sleep time or sleep efficiency.39 We also analyzed the effects of HT on different sleep parameters of polysomnography. Because previous studies showed significant improvements in other menopausal symptoms in women who received estrogen therapy for four weeks,40,41 we excluded the studies with HT less than four weeks. Our pooled results indicated that HT had no beneficial effects on sleep parameters in postmenopausal women, including total sleep time, sleep latency, sleep efficiency, and arousals.
In addition, the pooled-analysis outcomes of polysomnography were inconsistent with that of subjective sleep scores. The possible reasons may be as follows: first, the studies measured with polysomnography were conducted in small samples on one or few nights, the polysomnography without long-term monitoring might not always correlate with perceived sleep quality.42,43 Second, objective sleep outcomes were assessed only in a few studies. Therefore, the pooled analysis results may be dubious. Mansikkamaki et al44 demonstrated that subjective symptoms were vital to assess sleep quality. A previous systematic review also showed that patients reported measurements were highly predictive of sleep quality.45 A clinical guideline-recommended subjective sleep measurements as instruments to diagnose and evaluate chronic insomnia.46 However, self-reported sleep quality items have some defects, such as a lack of standardized assessment tools. It is of importance for standardizing sleep assessments tools in further study.
Limitations
There are several limitations of this systematic review and meta-analysis. First, the studies included in our meta-analysis may have publication bias. One of the underlying reasons may be that some studies meeting the eligibility criteria did not report sufficient data for quantitative analysis. The attempts to communicate with authors to obtain missing data were unsuccessful. Second, some of the evidence in this review cannot discern the magnitude of effect on sleep quality through indirect reduction of vasomotor symptoms, which was known to affect sleep quality. Third, since lacking original studies, our meta-analysis could not directly estimate the effects of different routes of administration and formulations of HT on sleep quality. Fourth, we found that more than 6 months of HT improved sleep quality, but the ideal timing for the duration of HT on sleep disturbance remains vague. Finally, several studies carried potential risks of bias, such as attrition bias and pharmaceutical industry funding. Despite this, our study provides a comprehensive review of the current literature guided by a prospectively registered protocol. Overall, the conclusions drawn from this review represent a current collation of best evidence.
CONCLUSIONS
In conclusion, HT has a beneficial effect on sleep disturbance to some extent, and the formulations and routes of administration of hormonal agents influence the effect size. Our findings from indirect evidence support the use of transdermal 17β-estradiol combined with micronized progesterone for at least 6 months in menopausal women with sleep disturbance. However, further head-to-head RCTs with multicenter and larger sample sizes are needed to evaluate the effects of different routes of administration and formulations of HT on sleep quality to provide direct evidence.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
We are particularly grateful to Deying Kang for his revision in the methodological section.
Footnotes
Funding/support: None reported.
Financial disclosure/conflicts of interest: None reported.
Z. P., S. W., and L. X. conceived and designed the study; M. Y. and X. S. performed the literature search and collected the data; and Z. P. and S. W. contributed the data analysis and wrote the manuscript. X. Q. and L. X. revised the manuscript. All authors reviewed and approved the manuscript before submission.
Supplemental digital content is available for this article.
REFERENCES
- 1.Tom SE, Kuh D, Guralnik JM, Mishra GD. Self-reported sleep difficulty during the menopausal transition: results from a prospective cohort study. Menopause 2010; 17:1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocha FL, Guerra HL, Lima-Costa MF. Prevalence of insomnia and associated socio-demographic factors in a Brazilian community: the Bambuí study. Sleep Med 2002; 3:121–126. [DOI] [PubMed] [Google Scholar]
- 3.Leger D, Guilleminault C, Dreyfus JP, Delahaye C, Paillard M. Prevalence of insomnia in a survey of 12 778 adults in France. J Sleep Res 2000; 9:35–42. [DOI] [PubMed] [Google Scholar]
- 4.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause 2003; 10:19–28. [DOI] [PubMed] [Google Scholar]
- 5.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep 2008; 31:979–990. [PMC free article] [PubMed] [Google Scholar]
- 6.Moline ML, Broch L, Zak R, Gross V. Sleep in women across the life cycle from adulthood through menopause. Sleep Med Rev 2003; 7:155–177. [DOI] [PubMed] [Google Scholar]
- 7.Kim MJ, Yim G, Park HY. Vasomotor and physical menopausal symptoms are associated with sleep quality. PloS One 2018; 13:e0192934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caruso D, Masci I, Cipollone G, Palagini L. Insomnia and depressive symptoms during the menopausal transition: theoretical and therapeutic implications of a self-reinforcing feedback loop. Maturitas 2019; 123:78–81. [DOI] [PubMed] [Google Scholar]
- 9.Pines A. Sleep duration and midlife women's health. Climacteric 2017; 20:528–530. [DOI] [PubMed] [Google Scholar]
- 10.Luyster FS, Strollo PJ, Jr, Zee PC, Walsh JK. Sleep: a health imperative. Sleep 2012; 35:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev 1999; 20:279–307. [DOI] [PubMed] [Google Scholar]
- 12.Bernardi F, Pluchino N, Pieri M, et al. Progesterone and medroxypro-gesterone acetate effects on central and peripheral allopregnanolone and beta-endorphin levels. Neuroendocrinology 2006; 83:348–359. [DOI] [PubMed] [Google Scholar]
- 13.Gruber CJ, Huber JC. Differential effects of progestins on the brain. Maturitas 2003; 46: (Suppl 1): S71–S75. [DOI] [PubMed] [Google Scholar]
- 14.Sarti CD, Chiantera A, Graziottin A, et al. Hormone therapy and sleep quality in women around menopause. Menopause 2005; 12:545–551. [DOI] [PubMed] [Google Scholar]
- 15.Montplaisir J, Lorrain J, Denesle R, Petit D. Sleep in menopause: differential effects of two forms of hormone replacement therapy. Menopause 2001; 8:10–16. [DOI] [PubMed] [Google Scholar]
- 16.Cintron D, Lahr BD, Bailey KR, et al. Effects of oral versus transdermal menopausal hormone treatments on self-reported sleep domains and their association with vasomotor symptoms in recently menopausal women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). Menopause 2018; 25:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinrich AB, Wolf OT. Investigating the effects of estradiol or estradiol/progesterone treatment on mood, depressive symptoms, menopausal symptoms and subjective sleep quality in older healthy hysterectomized women: a questionnaire study. Neuropsychobiology 2005; 52:17–23. [DOI] [PubMed] [Google Scholar]
- 18.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep 2014; 37:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cintron D, Lipford M, Larrea-Mantilla L, et al. Efficacy of menopausal hormone therapy on sleep quality: systematic review and meta-analysis. Endocrine 2017; 55:702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunner RL, Gass M, Aragaki A, et al. Effects of conjugated equine estrogen on health-related quality of life in postmenopausal women with hysterectomy: results from the Women's Health Initiative randomized clinical trial. Arch Intern Med 2005; 165:1976–1986. [DOI] [PubMed] [Google Scholar]
- 21.Hays J, Ockene JK, Brunner RL, et al. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med 2003; 348:1839–1854. [DOI] [PubMed] [Google Scholar]
- 22.Saletu-Zyhlarz G, Anderer P, Gruber G, et al. Insomnia related to postmenopausal syndrome and hormone replacement therapy: sleep laboratory studies on baseline differences between patients and controls and double-blind, placebo-controlled investigations on the effects of a novel estrogen-progestogen combination (Climodien, Lafamme) versus estrogen alone. J Sleep Res 2003; 12:239–254. [DOI] [PubMed] [Google Scholar]
- 23.Savolainen-Peltonen H, Hautamäki H, Tuomikoski P, Ylikorkala O, Mikkola TS. Health-related quality of life in women with or without hot flashes: a randomized placebo-controlled trial with hormone therapy. Menopause 2014; 21:732–739. [DOI] [PubMed] [Google Scholar]
- 24.Tansupswatdikul P, Chaikittisilpa S, Jaimchariyatam N, Panyakhamlerd K, Jaisamrarn U, Taechakraichana N. Effects of estrogen therapy on postmenopausal sleep quality regardless of vasomotor symptoms: a randomized trial. Climacteric 2015; 18:198–204. [DOI] [PubMed] [Google Scholar]
- 25.Ensrud KE, Guthrie KA, Hohensee C, et al. Effects of estradiol and venlafaxine on insomnia symptoms and sleep quality in women with hot flashes. Sleep 2015; 38:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hachul H, Bittencourt LR, Andersen ML, Haidar MA, Baracat EC, Tufik S. Effects of hormone therapy with estrogen and/or progesterone on sleep pattern in postmenopausal women. Int J Gynaecol Obstet 2008; 103:207–212. [DOI] [PubMed] [Google Scholar]
- 27.Kagan R, Constantine G, Kaunitz AM, Bernick B, Mirkin S. Improvement in sleep outcomes with a 17β-estradiol-progesterone oral capsule (TX-001HR) for postmenopausal women. Menopause 2018; 26:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalleinen N, Polo O, Himanen SL, Joutsen A, Polo-Kantola P. The effect of estrogen plus progestin treatment on sleep: a randomized, placebo-controlled, double-blind trial in premenopausal and late postmenopausal women. Climacteric 2008; 11:233–243. [DOI] [PubMed] [Google Scholar]
- 29.LeBlanc ES, Neiss MB, Carello PE, Samuels MH, Janowsky JS. Hot flashes and estrogen therapy do not influence cognition in early menopausal women. Menopause 2007; 14:191–202. [DOI] [PubMed] [Google Scholar]
- 30.Leeang koonsathian E, Pantasri T, Chaovisitseree S, Morakot N. The effect of different progestogens on sleep in postmenopausal women: a randomized trial. Gynecol Endocrinol 2017; 33:933–936. [DOI] [PubMed] [Google Scholar]
- 31.Meeuwsen IB, Samson MM, Duursma SA, Verhaar HJ. The influence of tibolone on quality of life in postmenopausal women. Maturitas 2002; 41:35–43. [DOI] [PubMed] [Google Scholar]
- 32.Polisseni AF, Andrade AT, Ribeiro LC, et al. Effects of a continuous-combined regimen of low-dose hormone therapy (oestradiol and norethindrone acetate) and tibolone on the quality of life in symptomatic postmenopausal women: a double-blind, randomised study. Maturitas 2013; 74:172–178. [DOI] [PubMed] [Google Scholar]
- 33.Polo-Kantola P, Erkkola R, Irjala K, Pullinen S, Virtanen I, Polo O. Effect of short-term transdermal estrogen replacement therapy on sleep: a randomized, double-blind crossover trial in postmenopausal women. Fertil Steril 1999; 71:873–880. [DOI] [PubMed] [Google Scholar]
- 34.Shulman LP, Yankov V, Uhl K. Safety and efficacy of a continuous once-a-week 17beta-estradiol/levonorgestrel transdermal system and its effects on vasomotor symptoms and endometrial safety in postmenopausal women: the results of two multicenter, double-blind, randomized, controlled trials. Menopause 2002; 9:195–207. [DOI] [PubMed] [Google Scholar]
- 35.Silva BH, Martinez D, Wender MC. A randomized, controlled pilot trial of hormone therapy for menopausal insomnia. Arch Womens Ment Health 2011; 14:505–508. [DOI] [PubMed] [Google Scholar]
- 36.Welton AJ, Vickers MR, Kim J, et al. Health related quality of life after combined hormone replacement therapy: randomised controlled trial. BMJ 2008; 337:a1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arafat ES, Hargrove JT, Maxson WS, Desiderio DM, Wentz AC, Andersen RN. Sedative and hypnotic effects of oral administration of micronized progesterone may be mediated through its metabolites. Am J Obstet Gynecol 1988; 159:1203–1209. [DOI] [PubMed] [Google Scholar]
- 38.Lancel M, Faulhaber J, Holsboer F, Rupprecht R. Progesterone induces changes in sleep comparable to those of agonistic GABAA receptor modulators. Am J Physiol 1996; 271:E763–E772. [DOI] [PubMed] [Google Scholar]
- 39.Nolan BJ, Liang B, Cheung AS. Efficacy of micronized progesterone for sleep: a systematic review and meta-analysis of randomized controlled trial data. J Clin Endocrinol Metab 2021; 106:942–951. [DOI] [PubMed] [Google Scholar]
- 40.Notelovitz M, Lenihan JP, McDermott M, Kerber IJ, Nanavati N, Arce J. Initial 17beta-estradiol dose for treating vasomotor symptoms. Obstet Gynecol 2000; 95:726–731. [DOI] [PubMed] [Google Scholar]
- 41.Haas S, Walsh B, Evans S, Krache M, Ravnikar V, Schiff I. The effect of transdermal estradiol on hormone and metabolic dynamics over a six-week period. Obstet Gynecol 1988; 71:671–676. [PubMed] [Google Scholar]
- 42.Ameratunga D, Goldin J, Hickey M. Sleep disturbance in menopause. Intern Med J 2012; 42:742–747. [DOI] [PubMed] [Google Scholar]
- 43.Kay DB, Buysse DJ, Germain A, Hall M, Monk TH. Subjective-objective sleep discrepancy among older adults: associations with insomnia diagnosis and insomnia treatment. J Sleep Res 2015; 24:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mansikkamaki K, Raitanen J, Nygård CH, et al. Sleep quality and aerobic training among menopausal women–a randomized controlled trial. Maturitas 2012; 72:339–345. [DOI] [PubMed] [Google Scholar]
- 45.Devine EB, Hakim Z, Green J. A systematic review of patient-reported outcome instruments measuring sleep dysfunction in adults. Pharmacoe-conomics 2005; 23:889–912. [DOI] [PubMed] [Google Scholar]
- 46.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 2008; 4:487–504. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.