Abstract
The safety and pharmacokinetics of a once-daily high intravenous dose of levofloxacin (750 mg) in 18 healthy volunteers were studied in a double-blind, randomized, placebo-controlled, single-center parallel group study. Levofloxacin was well tolerated, and higher maximum concentration of drug in serum and area under the concentration-time curve values were achieved. For difficult-to-treat infections, high daily doses of levofloxacin may be beneficial, and intravenous administration may be preferred in certain clinical settings, such as when treating patients in intensive care units, warranting further evaluation.
A levofloxacin regimen of 500 mg administered once daily has been efficacious in the treatment of respiratory and uncomplicated skin infections (6, 7, 10–12). However, infections that are more difficult to treat (i.e., complicated skin and skin structure infections, bacterial endocarditis, and nosocomial pneumonia) may necessitate higher daily doses of levofloxacin. The higher dose provides greater confidence in treating infections due to organisms for which drug MICs are high or patients with compromised vasculature that limits perfusion of the infection site. Having established the safety and pharmacokinetics of a 750-mg oral dose of levofloxacin (3), we conducted a pilot investigation to evaluate the safety and pharmacokinetics of 750 mg of intravenous (i.v.) levofloxacin administered as a single dose and then once daily for 7 days.
(This study was presented, in part, at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, 28 September to 1 October 1997, Toronto, Canada.)
Eighteen healthy male and female volunteers, ages 26 to 54, participated in the study after granting written, informed consent as approved by the local institutional review board. Subjects were judged healthy on the basis of normal findings on medical history, physical examination, clinical laboratory tests, and electrocardiography (ECG). Eligibility for study participation also included no relevant history of chronic illness and no acute illness 7 days prior to the study's commencement. In addition, subjects were not to have ingested alcoholic beverages or caffeine- or methylxanthine-containing substances 48 h prior to or during the study.
The study was conducted as a single-center, double-blind, randomized, placebo-controlled, parallel group study. Subjects were randomized in a 2:1 ratio to receive either levofloxacin (n = 12) or a placebo (n = 6) by i.v. infusion over 1.5 h. On study day 1, a single i.v. infusion was administered, followed by a washout phase (days 2 and 3). From study day 4 through study day 10, 7 once-daily i.v. infusions were administered.
The safety evaluation included the following: determinations of vital signs and clinical laboratory tests on days 1 (including prior to drug administration), 2, 4, 10, and 13; a physical examination on day 13; 12-lead ECG on days 10 and 13; and 24-h Holter monitoring on day 10. Each subject was observed after every dose of the study drug and throughout the postdosing period for possible adverse events (AEs).
Blood samples of 5 ml were obtained from the arm contralateral to the infusion site at 0 (immediately prior to study drug administration), 0.5, 1, 1.5, 2, 2.5, 3, 4, 8, 12, 24, 36, 48, 60, and 72 h after the start of study drug administration on days 1 and 10. In addition, blood samples were obtained immediately prior to dosing on days 5 through 9. Blood samples were collected in heparinized tubes and centrifuged; the plasma was separated and frozen at −20°C until it was assayed.
Concentrations of levofloxacin in plasma were assayed using validated high-performance liquid chromatography methodology (14) at PPD Development Inc., Middleton, Wisc. The assay was validated over the concentration ranges of 0.125 to 13.75 μg/ml (plasma), and the intraday precision values (as expressed by percent coefficient of variation) were <12%, while the corresponding interday precision values were <9%.
Pharmacokinetic analysis was performed as described previously (3, 9). Compartmental analysis utilized a linear dispositional model with first-order elimination from the central compartment (Win Nonlin, version 1.1; Scientific Consulting, Inc., Apex, N. C.). Estimated parameters included peak drug concentration in plasma (Cmax), trough drug concentration in plasma (Cmin), area under the concentration-versus-time curve (AUC), terminal disposition half-life (t1/2), total body clearance (CL), and steady-state volume of distribution (Vss). Data sets analyzed included data collected on days 1 through 3, 10 through 13, and 1 through 13. Model selection was based on the Akaike Information Criterion (1), and the sum of squared residuals was minimized using the Gauss-Newton algorithm with Levenberg and Hartley modification (5). Accumulation of levofloxacin following multiple dosing was estimated as the ratio of Cmax at steady state (day 10) to Cmax following the single dose (day 1) and as the ratio of AUC0–24 on day 10 to AUC0–24 on day 1.
Levofloxacin is eliminated primarily through the kidneys; therefore, the renal function of each subject was estimated. Creatinine clearance (CLcr) was calculated by the Cockcroft and Gault method (4) using the baseline serum creatinine value of the subject and was employed as an index of each subject's renal function.
Page's test was used to test for attainment of steady-state conditions following once-daily dosing, using Cmin data from days 5 through 10 and the 24-h plasma concentration value after day 10 (multiple dosing) (13).
Equal percentages of men and women were enrolled in the study. The majority of subjects (83%) randomized to receive a placebo were Hispanic; subjects randomized to receive levofloxacin were Caucasian (42%) and Hispanic (50%). Subjects who received a placebo were younger (mean age, 35.7 years) than subjects who received levofloxacin (mean age, 41.2 years).
All 18 subjects completed study participation. Four (33%) of the 12 levofloxacin recipients and 3 (50%) of the 6 placebo recipients reported one or more treatment-emergent AEs. In the levofloxacin group, this included two cases each of peripheral edema at the infusion site and erythematous rash at the infusion site and one case each of dizziness, headache, pruritus at the infusion site, infusion site reaction, and euphoria. In the placebo group, the AEs included two cases of headache and one case each of peripheral edema at the infusion site, dizziness, infusion site edema, and purpura. All of these events were mild in nature and transient. No clinically significant alterations in clinical laboratory evaluations, vital sign measurements, physical-examination findings, 12-lead ECG, or 24-h Holter monitor findings were noted over the course of the study.
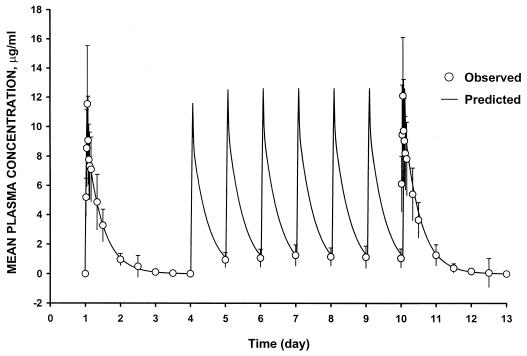
The mean plasma concentration-versus-time curve for the levofloxacin recipients is illustrated in Fig. 1. Based on Akaike Information Criterion values, a two-compartment model was selected to characterize the levofloxacin plasma concentration-versus-time data. For the 12 volunteers randomized to receive levofloxacin, a mean (± standard deviation [SD]) peak concentration of levofloxacin in plasma of 11.3 ± 3.6 and 12.4 ± 3.9 μg/ml and an AUC value of 110 ± 40 and 108 ± 34 μg/ml · h were measured on days 1 and 10, respectively.
FIG. 1.
Mean (±SD) plasma levofloxacin concentration-versus-time profile for 12 healthy subjects following both single and multiple 750-mg once-daily i.v. doses.
Results of Page's test revealed that steady state was achieved 24 h from the start of multiple dosing (i.e., on study day 5). Plasma levofloxacin concentration-versus-time profiles following single (day 1) and multiple (day 10) doses were similar. As reflected by the Cmax and AUC0–24 ratios (Table 1), drug accumulation following multiple dosing was minimal. Parameter values following single (day 1) and multiple (day 10) doses were also similar. Correlation between the observed and predicted concentration-versus-time profiles for levofloxacin in plasma exceeded 0.91 in all cases.
TABLE 1.
Summary of levofloxacin pharmacokinetic parameters for all volunteers, including subjects with differing renal function, using a two-compartment modela
| Subject groupb and dosage | Cmax (μg/ml) | Cmin (μg/ml) | AUC0–24 (μg · h/ml) | AUC0–∞ (μg · h/ml) | t1/2β (h) | CL (ml/min) | Vss (liters) | Cmax ratio | AUC0–24 ratio |
|---|---|---|---|---|---|---|---|---|---|
| CLCR > 80 ml/min (n = 4) | 6.91 ± 0.83 | 186 ± 5 | 106 ± 12 | 1.07 ± 0.03 | 1.10 ± 0.03 | ||||
| Single dose (day 1) | 8.12 ± 0.99 | 0.62 ± 0.11 | 61.1 ± 1.3 | 67.4 ± 1.75 | |||||
| Steady state (day 10) | 8.71 ± 0.90 | 0.68 ± 0.15 | 67.4 ± 1.75 | 74.3 ± 3.9 | |||||
| CLCR ≤ 80 ml/min (n = 8) | 7.82 ± 1.76 | 107 ± 21 | 69.0 ± 16.7 | 1.11 ± 0.05 | 1.14 ± 0.08 | ||||
| Single dose (day 1) | 12.9 ± 3.3 | 1.28 ± 0.47 | 106 ± 23 | 121 ± 28 | |||||
| Steady state (day 10) | 14.2 ± 3.4 | 1.48 ± 0.61 | 121 ± 28 | 139 ± 35 | |||||
| All (n = 12) | 7.51 ± 1.54 | 133 ± 42 | 81.2 ± 23.3 | 1.10 ± 0.05 | 1.13 ± 0.07 | ||||
| Single dose | 11.3 ± 3.6 | 1.06 ± 0.50 | 90.9 ± 28.9 | 103 ± 35 | |||||
| Steady state (day 10) | 12.4 ± 3.9 | 1.21 ± 0.63 | 103 ± 35 | 117 ± 42 |
Data are presented as means ± SD. Cmax ratio, ratio of Cmax at steady state to Cmax following the single dose; AUC0–24 ratio, ratio of AUC0–24 on day 10 to AUC0–24 on day 1.
n, number of subjects.
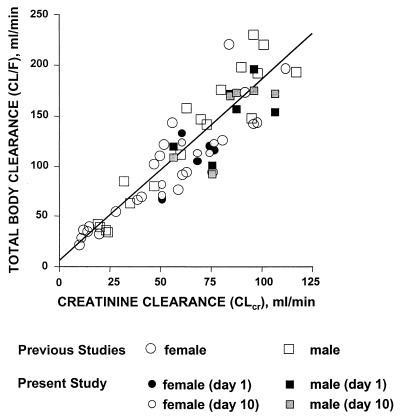
Intersubject variability in levofloxacin pharmacokinetic parameters was normalized to variations in body weight and renal function. Levofloxacin Vss values and body weight were highly correlated (r = 0.91), and mean ± SD of this steady-state volume distribution normalized for body weight was 1.13 ± 0.17 liters/kg. Baseline estimated CLcr values ranged from 51 to 107 ml/min (mean ± SD = 74 ± 18 ml/min), and CL and CLcr values were highly correlated (r = 0.84) (Fig. 2). When data were analyzed as two subgroups of CLcr values of <80 ml/min (n = 8) and >80 ml/min (n = 4), linear and predictable pharmacokinetics and minimal drug accumulation were consistently observed in both subgroups (Table 1).
FIG. 2.
Relationship between creatinine clearance and apparent total body clearance of levofloxacin following a single 500-mg oral dose (previous studies) or 750-mg single and multiple once-daily i.v. doses of levofloxacin (present study) in subjects with various degrees of renal function (the line indicates the expected relationship between apparent total body clearance and estimated creatinine clearance from linear regression analysis) (2, 8).
This is the first report to evaluate the safety and pharmacokinetics of levofloxacin with volunteers receiving a 750-mg, once-daily dose of levofloxacin after a single i.v. infusion followed by i.v. administration on seven consecutive days. At high (750-mg)-multiple-dose i.v. administration of levofloxacin, steady state was achieved by day 2. The Cmax and the AUC at steady state were predictable based on 500-mg i.v. dosing (2). The pharmacokinetic profiles for oral versus parenteral administration of 750-mg doses were similar. The 100% bioavailability of oral levofloxacin allows for convenient conversion from i.v. dosing to oral dosing.
For patients with reduced renal function, levofloxacin Cmax, AUC, and t1/2β values are known to increase (8). Since more than 80% of levofloxacin is eliminated unchanged in the urine, this observation is not surprising and allows for reduced dosing in patients with renal impairment. In the present study, the variation in pharmacokinetic parameters appeared to be related to renal function and body weight. Subjects having reduced renal function (CLcr of ≤80 ml/min) have higher Cmax, AUC, and t1/2β values than subjects with CLcr values of >80 ml/min. No increase in drug retention was observed, and pharmacokinetics remained linear.
The higher Cmax and AUC/MIC ratios achieved with the higher dosing allow greater confidence in treating patients who may be infected with organisms for which levofloxacin MICs are high. In addition, patients with limited tissue perfusion secondary to compromised vascularity would benefit from receiving 750 mg of levofloxacin to treat infections such as complicated skin and skin structure infections, bacterial endocarditis, and nosocomial pneumonia.
Parenteral levofloxacin at the 750-mg dose level was well tolerated by the healthy volunteers, with mild and transient i.v.-site reactions predominating among the treatment-emergent AEs. These findings are consistent with the excellent safety profile of levofloxacin. Further studies are needed, however, to confirm these results with seriously ill patients receiving the higher (750-mg) dose. The results of this study support the clinical evaluation of a high-dose i.v. regimen to treat serious infection.
REFERENCES
- 1.Akaike H. An information criterion (AIC) Math Sci. 1976;14:5–9. [Google Scholar]
- 2.Chien S-C, Rogge M C, Gisclon L G, Curtin C, Wong F, Natarajan J, Williams R R, Fowler C L, Cheung W K, Chow A T. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob Agents Chemother. 1997;41:2256–2260. doi: 10.1128/aac.41.10.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chien S-C, Wong F A, Fowler C L, Callery-D'Amico SV, Williams R R, Nayak R, Chow A T. Double-blind evaluation of the safety and pharmacokinetics of multiple oral once-daily 750-milligram and 1-gram doses of levofloxacin in healthy volunteers. Antimicrob Agents Chemother. 1998;42:885–888. doi: 10.1128/aac.42.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cockcroft D W, Gault M H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 5.Davis M, Whiting I J. Numerical methods for non-linear optimization. New York, N.Y: Academic Press; 1972. A modified form of Levenberg's correction. [Google Scholar]
- 6.DeAbate C A, Russell M, McElvaine P, Faris H, Upchurch J, Fowler C L, Polak E M, Morgan N S. Safety and efficacy of oral levofloxacin versus cefuroxime axetil in acute bacterial exacerbation of bronchitis. Respir Care. 1997;42:206–213. [Google Scholar]
- 7.File T M, Jr, Segreti J, Dunbar L, Player R, Kohler R, Williams R R, Kojak C, Rubin A. A multicenter, randomized study comparing the efficacy and safety of intravenous and/or oral levofloxacin versus ceftriaxone and/or cefuroxime axetil in treatment of adults with community-acquired pneumonia. Antimicrob Agents Chemother. 1997;41:1965–1972. doi: 10.1128/aac.41.9.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fish D N, Chow A T. The clinical pharmacokinetics of levofloxacin. Clin Pharmacokinet. 1997;32:101–119. doi: 10.2165/00003088-199732020-00002. [DOI] [PubMed] [Google Scholar]
- 9.Gibaldi M, Perrier D. Pharmacokinetics. New York, N.Y: Marcel Dekker, Inc; 1982. [Google Scholar]
- 10.Habib M P, Gentry L O, Rodriguez-Gomez G, Morowitz W, Polak E, Rae J K, Morgan N S, Williams R R. Multicenter, randomized study comparing efficacy and safety of oral levofloxacin and cefaclor in treatment of acute bacterial exacerbations of chronic bronchitis. Infect Dis Clin Pract. 1998;7:101–109. [Google Scholar]
- 11.Klimberg I W, Cox II C E, Fowler C L, King W, Kim W K, Callery-D'Amico S. A controlled trial of levofloxacin and lomefloxacin in the treatment of complicated urinary tract infection. Urology. 1998;51:610–615. doi: 10.1016/s0090-4295(97)00708-5. [DOI] [PubMed] [Google Scholar]
- 12.Nichols R L, Smith J W, Gentry L O, Gezon J, Campbell T, Sokol P, Williams R R. Multicenter, randomized study comparing levofloxacin and ciprofloxacin for uncomplicated skin and skin structure infections. South Med J. 1997;90:1193–1200. doi: 10.1097/00007611-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Smith I L, Schentag J J. Noncompartmental determination of the steady-state volume of distribution during multiple dosing. J Pharm Sci. 1984;73:281–282. doi: 10.1002/jps.2600730239. [DOI] [PubMed] [Google Scholar]
- 14.Wong F A, Juzwin S J, Flor S C. Rapid stereospecific high-performance liquid chromatographic determination of levofloxacin in human plasma and urine. J Pharm Biomed Anal. 1997;15:765–771. doi: 10.1016/s0731-7085(96)01890-0. [DOI] [PubMed] [Google Scholar]