Abstract
COVID-19 caused by SARS-CoV-2 coronavirus has been associated with severe illness in pregnant women. Furthermore, COVID-19 during pregnancy is associated with adverse fetal outcomes including preterm labor. Pregnant women were largely excluded from initial clinical trials investigating the safety and efficacy of COVID-19 vaccines; however, they have since been included as part of the routine roll-out of these vaccines. This narrative review synthesizes the evidence on the safety, immunogenicity, and effectiveness predominantly of the mRNA COVID-19 vaccines which have been most widely used in pregnant women.
Keywords: safety, immunogenicity, transplacental transfer, antibodies
Implications of COVID-19 during pregnancy and vaccine development
Since the beginning of the COVID-19 pandemic, pregnant women were identified as a high-risk group for severe complications. Observational studies revealed that SARS-CoV-2 infection in pregnant women is associated with an increased risk of hospital admission, admission to an intensive care unit, and invasive ventilation relative to non-pregnant women [1., 2., 3.]. Pregnant women with COVID-19, compared with contemporaneous or historical groups of pregnant women without SARS-CoV-2 infection, had higher rates of pre-eclampsia (see Glossary), eclampsia, stillbirth, and preterm birth (birth before completing 37 weeks of gestation) which were attributed to COVID-19-associated proinflammatory mechanisms [3., 4., 5., 6.]. Furthermore, 1–5% of newborns to women infected with SARS-CoV-2 at the time of delivery were subsequently identified to be infected by the virus [7., 8., 9., 10.]. In neonates, SARS-CoV-2 infection generally manifests as mild illness or is asymptomatic (20%); however, there are reports of young infants being more susceptible to severe COVID-19 than older children [11,12].
COVID-19 vaccines were expeditiously developed and deployed to protect against disease, including vaccines developed using less conventional platforms such as non-replicating vector based-vaccines and mRNA vaccines [13]. COVID-19 vaccines were initially tested with large numbers of subjects during Phase III randomized controlled trials and were shown to be efficacious at preventing infection and disease caused by wild-type SARS-CoV-2 and the alpha variant in non-pregnant adults [14., 15., 16., 17., 18., 19., 20.]. The initial pivotal COVID-19 vaccine efficacy trials excluded pregnant women, leading to uncertainty regarding its safety and efficacy in this population [21]. Nevertheless, by late 2020 and early 2021 the American College of Obstetricians and Gynecologists (ACOG), the US Centers for Disease Control (CDC), the World Health Organization (WHO), and other medical organizations and committees endorsed the routine use of COVID-19 vaccines in pregnant women based on risk–benefit analysis [22., 23., 24., 25., 26.]. Since these recommendations, pregnant women opting to be vaccinated were included in observational studies and vaccine safety monitoring registers, and have provided crucial information on the immunogenicity and safety of the available vaccines in this population [27]. Inadvertently, safety data on COVID-19 vaccines were also collected in some of the initial clinical trials when incidental pregnancies occurred during the studies [28].
Vaccination of pregnant women against other pathogens such as influenza virus, Bordetella pertussis, and tetanus is routine in many countries [29]. The use of COVID-19 vaccines employing the newer non-replicating vector and mRNA technologies merits ongoing monitoring, notably because future vaccination of pregnant women will involve an increasing proportion of individuals with underlying immunity from past infection or vaccine receipt before becoming pregnant. In addition to vaccination of pregnant women to protect them against COVID-19, vaccination could also protect against adverse COVID-19-associated pregnancy outcomes. Furthermore, COVID-19 vaccination during pregnancy could confer transient protection to their offspring over the first few months of life through transplacental transfer of antibodies or the acquisition of breastmilk antibody by the young infants. In a narrative review, we synthesize recent developments on COVID-19 vaccines in pregnant women, including published data on the safety, immunogenicity, and effectiveness of vaccination against SARS-CoV-2 infection.
COVID-19 vaccines in pregnant women
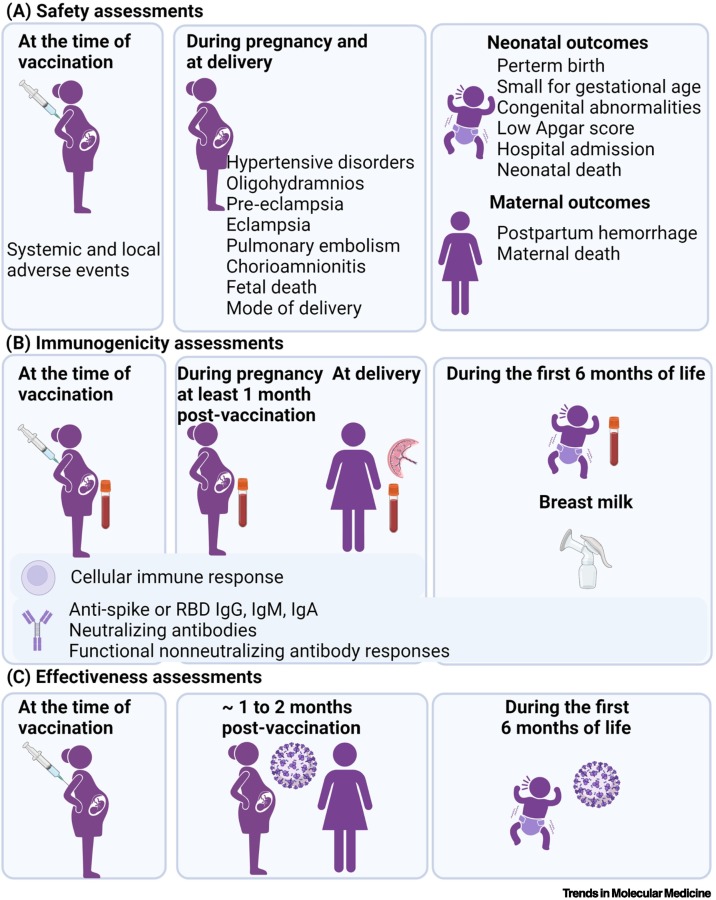
As of the end of February 2022, the majority of reports on COVID-19 vaccines in pregnant women reported on the BNT162b2 (Pfizer/BioNTech™) and mRNA-1273 (Moderna™) mRNA vaccines, and less information has emerged on the use of the non-replicating adenovirus vector-based vaccines ChAdOx1 (Oxford/AstraZeneca™) and Ad.26.COV2.S (Janssen, Johnson & Johnson™) [30., 31., 32., 33., 34., 35., 36., 37., 38., 39., 40., 41., 42., 43., 44., 45., 46., 47., 48., 49., 50., 51., 52., 53., 54., 55., 56., 57., 58., 59.]. Most studies have been performed in high-income countries where mRNA vaccines were used for pregnant populations. The most common reported findings from mainly observational studies have been on safety and antibody responses, including antibody concentrations in the women and cord blood at the time of delivery, following a single or two doses of vaccine (Figure 1 ).
Figure 1.
Different approaches used to determine COVID-19 vaccine safety, immunogenicity, and effectiveness in pregnant women.
(A) The safety of COVID-19 vaccines in pregnant women has been described for systemic and local adverse events, and for pregnancy, maternal, and neonatal outcomes. (B) Anti-SARS-CoV-2 binding (IgG, IgM) and neutralizing antibodies have been quantified in maternal and cord blood samples. Functional non-neutralizing antibody responses were assessed by systems serology approaches in maternal and cord blood samples. IgG, IgM, IgA have also been measured in breastmilk and IgG in infants up to 6 months of age. Cellular immune responses have been described in pregnant women. (C) The effectiveness of maternal COVID-19 vaccination against documented infection of the women during the 2 months after vaccination, and in their infants <6 months of age against COVID-19-associated hospitalization. Figure created with biorender.com.
Safety of COVD-19 vaccination during pregnancy
The safety of COVID-19 vaccines in pregnant women is described in terms of systemic and local adverse events, as well as pregnancy, maternal, and neonatal outcomes (Figure 1).
Systemic and local adverse events following COVID-19 vaccination during pregnancy
Comprehensive reporting of vaccine-related adverse events has been presented from Israel for BNT162b2, from Romania for BNT162b2 and Ad26.COV2.S (results presented combined for both vaccines), and four studies from the USA which evaluated BNT162b2 and mRNA-1273 vaccines. The study methods included case–control studies among 390 pregnant and 260 age-matched non-pregnant BNT162.b2 vaccinated women who received a digital questionnaire after the second dose in Israel, and 173 non-pregnant and 173 pregnant women vaccinated in their third pregnancy trimester in Romania. The studies from the USA entailed (i) analyses of national vaccine safety surveillance systems among pregnant and non-pregnant women; (ii) an online prospective cohort study including 7809 pregnant women, 6815 lactating women, and 2901 participants who were neither pregnant nor lactating but planned on falling pregnant at the time of the receipt of their first vaccine dose; (iii) a prospective cohort of 84 pregnant and 16 non-pregnant vaccinated women; and (iv) a cross-sectional study of 38 pregnant and 991 non-pregnant women who were vaccinated with BNT162b2 or mRNA-1273 [31,36,37,48,58,59].
The most common reported systemic adverse symptoms among pregnant women were tiredness (14–72%), headache (5–55%), myalgia (2–54%), chills (1–47%), nausea (5–29%) and fever (2–45%). In general, the rate of systemic adverse events was higher following the second vaccine dose for both mRNA vaccines [31,36,48], as was also evident in non-pregnant individuals [14,16,58]. Furthermore, a higher frequency of adverse events was reported after the higher-concentration mRNA-1273 vaccine (100 μg) than for the BNT162b2 vaccine (30 μg) [48]. Among pregnant women, injection-site pain (57–97%) was the most common local adverse event for both mRNA vaccines.
In general, the frequency of local and systemic events and the increased rate of reactogenicity after the second dose were similar among non-pregnant and pregnant women irrespective of the gestational age at time of vaccination. The exception was in Israel, where myalgia (24.1% vs. 49.2%), arthralgia (4.1% vs. 21.5%), headache (10.3% vs. 48.8%), injection-site pain (91.8% vs. 96.2%), and lymphadenopathy (2.1% vs. 9.6%) were less common among pregnant than non-pregnant BNT162b2 vaccinated women, whereas paresthesia (4.6% vs. 1.2%) was more frequent in pregnant women [31]. Similarly, the study from Romania also reported a lower frequency of myalgia (6.6% vs. 12.7%), lymphadenopathy (4.8% vs. 10.5%), and fever (10.1% vs. 16.7%) in pregnant than in non-pregnant women, although fatigue (82.8% vs. 67.8%) was more frequently reported in the pregnant women. Furthermore, the study from Romania also reported that pregnant women who had a previous SARS-CoV-2 infection compared to those without past infection had a higher frequency of fever (25.9% vs. 10.9%) and lymphadenopathy (14.8% vs. 4.6%) after completing the full vaccination scheme [59].
Pregnancy, maternal, and neonatal outcomes following antenatal COVID-19 vaccination
The first preliminary findings on pregnancy outcomes among women who received mRNA vaccines were reported using data from three USA vaccine safety monitoring systems – the 'V-Safe after Vaccination Health Checker' surveillance system, the V-Safe Pregnancy Registry, and the Vaccine Adverse Event Reporting System (VAERS) [60., 61., 62.]. The pregnancy registry enrolled 3958 women, 2.3% of whom were reportedly vaccinated during the pre-conception period, and 28.6%, 43.3%, and 25.7% in the first, second, and third trimesters of pregnancy, respectively. At the time of the analysis, there were 712 live births including 9.4% preterm births, 3.2% newborns small for gestational age (SGA), and 2.2% with congenital anomalies; the rate of stillbirths was 0.1%. The profile of pregnancy outcomes in the V-Safe Pregnancy Registry was similar to the national rate of the respective pregnancy outcomes in the pre-COVID-19 pandemic era. None of the women whose children had congenital anomalies were vaccinated during the first trimester or pre-conception period. In addition, no neonatal deaths were reported in babies born to the vaccinated women [48].
Fetal death was the pregnancy outcome most often investigated in the studies conducted to date. Two studies in the USA using the CDC Vaccine Safety Databases and one case–control study from Norwegian registries investigated whether vaccination during pregnancy increased the risk of early fetal death [39,41,56]. In a large case–control surveillance of 105 446 pregnancies there was no association between receipt of a COVID-19 mRNA vaccine within 28 days before spontaneous abortion (8.6%) compared to ongoing pregnancies [8.0%; adjusted odds ratio (OR) 1.02; 95%CI 0.96–1.08] [39]. In another US study evaluating mRNA COVID-19 vaccination during pregnancy or in the pre-conception period, the cumulative risk of spontaneous abortion (14.1%; 95%CI 12.1–16.1) was within the risk range expected from two historical cohorts from the USA (13–21%) [56,63,64]. In Norway, where mRNA and the ChAdOx1 COVID-19 vaccines were used, the vaccination rate was 5.1% among 4521 women with a miscarriage before 14 weeks of gestation compared to 5.5% in 13 956 women with pregnancies that advanced beyond 14 weeks gestational age. In this study, vaccination in the previous 3 weeks (OR 0.91; 95%CI 0.75–1.10) or 5 weeks (OR 0.81; 95%CI 0.69–0.95) was not associated with a higher odds for miscarriage compared to no vaccination [41].
Although informative, the initial studies using the US registries were based on passive surveillance and were limited to self-reporting of vaccine reactions and pregnancy events, and may thus be subject to selection bias [48,56]. Health administration databases were used to investigate the association between maternal COVID-19 vaccination and pregnancy outcomes in Israel, the USA, Scandinavia, and Canada [34,35,50,53,54]. In Israel, no differences in pregnancy outcomes between BNT162b2-vaccinated and unvaccinated women were observed in either of the two population-based cohort studies using electronic registries [34,35], nor in a retrospective cohort study which included women vaccinated in the second or third trimesters [54]. The assessed outcomes in the Israeli studies included pregnancy-related hypertensive disorders, oligohydramnios, pre-eclampsia, maternal death and obstetric pulmonary embolism, mode of delivery, abortion, stillbirth, gestational age at delivery, frequency of SGA, newborn respiratory complications, congenital malformations, all-cause hospitalizations, and infant death.
In the USA, a retrospective cohort study of 46 079 live births from eight Vaccine Safety Datalink healthcare organizations compared the risk of preterm birth and SGA among vaccinated (10 064, 21.8%) and unvaccinated pregnant women, accounting for time-dependent vaccine exposures and propensity to be vaccinated [65]. Of the vaccinated women, 98% received their first dose during the second or third trimesters (4.2% received Ad.26.COV2.S and 85.8% an mRNA vaccine). Vaccination during pregnancy was not associated with an increased risk for preterm birth overall [adjusted hazard ratio (aHR) 0.91; 95%CI 0.82–1.01)] or SGA (0.95; 95%CI 0.87–1.03), or when evaluating whether one or two doses of mRNA vaccines were received or the trimester of vaccination during pregnancy, compared to the risk in unvaccinated pregnant women [65]. Another US study used a comprehensive vaccine registry combined with a delivery database for an integrated healthcare system to create a delivery cohort [50]. This study mainly reported on outcomes after third-trimester vaccination among 140 women (one received Ad.26.COV2.S, 12 received mRNA-1273, and 127 received BNT162b2), and the frequency of a composite measure of maternal and neonatal pregnancy complications was similar among the vaccinated (5.0%, 7/140) and unvaccinated (4.9%, 91/1862) women. No maternal or early neonatal deaths occurred in the cohort [50]. Also in the USA, 424 pregnant women who received a mRNA vaccine in the first (29%), second (46%), or third (25%) trimesters showed no difference in pregnancy outcomes relative to national or their institution rates [53].
Reassuring safety findings were also reported from two large population-based observational retrospective studies that addressed potential sources of bias in observational studies of vaccination during pregnancy, including healthy vaccinee bias and confounding by indication, immortal time bias, or cohort truncation [66,67]. The report from Canada linked the Ontario Birth Registry with the provincial vaccination system and identified 97 590 pregnant women who were eligible for COVID-19 vaccination during the study period, of whom 23% had been vaccinated with at least one dose (<1% vaccinated in the first trimester; >99% received mRNA vaccine). The women who received a COVID-19 vaccine compared to unvaccinated pregnant women had a similar frequency of post-partum hemorrhage, chorioamnionitis, cesarean delivery; and no difference in low Apgar score or neonatal care admission in their newborn [66]. Similarly, data from the Swedish Pregnancy Register and the Norwegian Birth Registry were linked with the vaccine registries in the respective countries. Together, the databases from Sweden and Norway included 157 521 pregnant women, 18.1% who received a COVID-19 vaccine (3.9% vaccinated in the first trimester; >98% received an mRNA vaccine). There was no association between receipt of COVID-19 vaccine and risk of preterm birth, stillbirth, SGA, low newborn Apgar score, or neonatal care admission [67].
The impact of ChAdOx1 vaccination on fertility rates and birth outcomes was assessed among pregnancies that occurred during the conduct of Phase I–III clinical trials in three countries (UK, Brazil, and South Africa) [28]. Although pregnancy was an exclusion criterion for all the trials, pregnancies transpired during the follow-up phase of the study after women had been vaccinated pre-conception. Overall, 121 (1%) pregnancies were reported among the 9755 women ≤49 years of age who participated in the studies. Fertility rates were similar among women who received ChAdOx1 (1.02%) compared to those in the control groups (0.89%, P = 0.53). Although 52% of the pregnancies were still ongoing at the time of analysis (July 2021), the rate of miscarriage was similar in pregnancies among the ChAdOx1 (14%) and the control groups (21%, P = 0.51). An analysis restricted to the 15 live births identified three preterm births in the ChAdOx1 group. No stillbirths or neonatal deaths were reported in either group [28].
mRNA vaccines induce an immune response through activation of Toll-like receptors (TLRs) including TLR3. Activation of TLR3 by other routes has been linked to adverse placenta-associated pregnancy outcomes in rodent models, including growth restriction, preterm delivery, and fetal death [68., 69., 70.]. Furthermore, SARS-CoV-2 infection during pregnancy has been associated with placental anomalies such as decidual arteriopathy, fetal vascular malperfusion, and chronic histiocytic intervillositis [71,72]. Hence, it is important to evaluate the frequency of possible placental pathology in pregnant women vaccinated with mRNA vaccines. One report detected no difference in the frequency of placental histopathological lesions in 84 pregnant women vaccinated during pregnancy (vaccine brand not stated) compared to 116 unvaccinated women. Furthermore, high-grade chronic villitis was more common in the unvaccinated (14%) versus vaccinated (5%, P = 0.04) groups [47].
The current data suggest that vaccination of pregnant women with COVID-19 vaccines is not associated with an increased risk of adverse pregnancy, maternal, and neonatal outcomes. However, most of the vaccinations of the pregnant women involved mRNA vaccines administered during the second and third trimesters of pregnancy, and this should be considered when interpreting the findings.
Immunogenicity of COVD-19 vaccination during pregnancy
Anti-SARS-CoV-2 antibody responses in pregnant women after vaccination
Although 17 observational studies analyzed the immunogenicity of COVID-19 vaccines in pregnant women up until the end of February 2022, the majority enrolled <100 participants (Table 1). Furthermore, the studies varied in the immunological assays used to analyze antibody responses following vaccination, preventing comparisons between studies or different vaccines. Most of the published studies were conducted in the USA (38%) or Israel (50%) [30., 31., 32.,36,38,40,42., 43., 44., 45., 46.,49,51,55].
Table 1.
Immunogenicity measures in maternal blood or cord blood samples after COVID-19 vaccination during pregnancya
| Location and study dates | Vaccines during pregnancy | Number of pregnant women vaccinated | Timing of vaccination during pregnancy | Comparator groups | Immune response measures | Responses in womenb | Responses in cord blood | Placental antibody transfer | Other results | Assays used for antibody quantification | Refs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Israel January–March 2021 |
At least one dose BNT162b2 |
86 | First dose: 34.5 (±7.5) weeks GA | 65 Women who had COVID-19 during pregnancy at 28.1 (±8.3) weeks GA 62 Unvaccinated uninfected pregnant women |
Anti-RBD IgG and IgM; anti-S IgG and IgM Reported as net fluorescence intensity |
Robust IgG response by day 15 after the first dose, further increase in IgG responses after the second dose At delivery, higher anti-S and anti-RBD IgG in vaccinated women than in infected women |
IgG responses by day 15 after the first dose, further increase in IgG responses after second dose Anti-S and anti-RBD IgG no different between the vaccinated and infected groups IgM response in five samples from the infected group |
Similar transfer profiles in the vaccinated and infected groups | Association between maternal and cord blood IgG responses | Milliplex MAP SARS-CoV-2 Antigen Panel, MerckMillipore | [30] |
| Israel January–February 2021 |
Two doses BNT162b2 |
96 Tested 2 months following the second dose |
2–40 weeks GA | 96 Non-pregnant vaccinated women | Anti-RBD IgG Reported as signal to cutoff ratios |
At 2 weeks to 2 months following the second dose Overall: 100% positive Pregnant: 27.03 (±10.72) Non-pregnant: 34.35 (±10.25) |
Not shown | Not shown | Anti-RBD IgG responses did not differ according to trimester of vaccination | Access SARS-CoV-2 IgG assay, Beckman Coulter | [31] |
| USA December 2020–March 2021 |
Two doses BNT162b2: 37% mRNA-1273: 63% |
30 | Vaccinated: 16 lactating women; 57 non-pregnant nonlactating women Previously infected: 6 non-pregnant women and 22 pregnant women |
Anti-RBD IgG Neutralization responses Functional antibody responses T cell responses |
Binding, neutralizing, and functional non-neutralizing antibody responses, and CD4 and CD8 T cell responses were present in pregnant, lactating, and non-pregnant women following vaccination | Binding and neutralizing antibodies were detected in cord blood | Vaccinated group: anti-RBD IgG cord blood 19 873 vs. mother 14 953 Neutralizing antibody in cord blood 324 vs. mother 1016 Infected group: anti-RBD IgG cord blood 635 vs. mother 1342 Neutralizing antibody in cord blood 164 vs. mother 151 |
Descriptive statistics only | In-house enzyme-linked immunosorbent assay | [32] | |
| USA December 2020–March 2021 |
At least one dose BNT162b2: 49% mRNA-1273: 51% |
84 Tested at baseline (v0), at the second dose (v1), at 2–6 weeks after the second dose (v2), and at delivery (v3) |
First dose: 23.2 (16.3–32.1) weeks GA; 13% during the first trimester, 46% during the second trimester, 40% during the third trimester From second dose to delivery 14 (IQR: 11–16) days |
Vaccinated: 31 lactating women 16 non-pregnant women 37 Women who had COVID-19 during pregnancy at 4–12 weeks before delivery |
Anti-S IgM, IgG, and IgA Anti-RBD IgM, IgG, and IgA Neutralization responses at v3 Reported as mean fluorescence intensity |
Antibody responses were similar in pregnant/lactating women and nonpregnant women Increase for all isotypes across all antigens from v0 to v1, further increase in IgG responses from v1 to v2 Spike responses increased quicker than RBD at v1 and v2 IgM and IgA responses were induced after first dose but not after second dose Neutralizing antibody: 104.7 (IQR: 61.2–188.2) |
100% positive anti-S and anti-RBD IgG Neutralizing antibody: 52.3 (IQR: 11.7–69.6) |
Not shown | Placental transfer ratio of anti-S IgG (but not anti-RBD) positively correlated with time from second dose to delivery No association between maternal responses and time of vaccination during pregnancy Higher responses is vaccinated women compared to pregnant women with natural infection |
Multiplexed Luminex assay, ThermoFisher | [36] |
| Israel February–March 2021 |
Two doses BNT162b2 |
29 | Third trimester | 29 Women who had COVID-19 during pregnancy 21 Pregnant women not infected, not vaccinated |
Anti-RBD IgG Reported as U/ml |
Not shown | Vaccinated group: 100% positive; 224.7 U/ml (±64.3) Infected group: 100% positive; 83.7 U/ml (±91.6) |
Not shown | Association between maternal and cord blood responses | Elecsys Anti-SARS-CoV-2 S immunoassay, Roche Diagnostics | [38] |
| Israel May–July 2021 |
Two doses, ≥7 days before delivery BNT162b2 |
129 | Second trimester First dose: 21.9 (±3.3) weeks GA; second dose: 24.9 (±3.3) weeks GA From second dose to delivery 14.4 (±3.0) weeks |
None | Anti-RBD IgG Reported as AU/ml |
Overall: 100% positive 1185.2 AU/ml (range: 146.6–32415.1) |
Overall: 100% positive; 3315.7 AU/ml (range: 350.1–17 643.5) | Approximately 2.6 | Association between maternal and cord blood responses Maternal and cord blood responses correlated positively with GA at second dose Maternal and cord blood responses correlated inversely with the time from second dose to delivery and with maternal age |
SARS-CoV-2 IgG II Quant, Abbott | [40] |
| USA January–March 2021 |
At least one dose BNT162b2: 64% mRNA-1273: 18% Unknown vaccine: 14% Two doses: 74% |
27 | Third trimester First dose: 33 (±2) weeks GA |
None | Anti-RBD IgG and IgM Reported as AU/ml |
96% positive anti-RBD IgG 56% positive anti-RBD IgM |
89% positive anti-RBD IgG (negative cord bloods from two women who received their first dose <3 weeks before delivery); 0 positive anti-RBD IgM. | 1.0 ± 0.6 | Cord blood and placental transfer ratio of IgG associated positively with time from vaccination Cord blood IgG response associated positively with having received second dose. |
DXI Platform, Beckman Coulter | [42] |
| Israel February–March 2021 |
Two doses, ≥14 days before delivery BNT162b2 |
64 | Third trimesterS econd dose 33.5 (±3.2) weeks GA From second dose to delivery 21.7 (±11.0) days |
11 Women who had COVID-19 during pregnancy at 92.5 (±75.8) days before delivery | Anti-RBD IgG Reported as signal to cutoff ratio |
Vaccinated group: 100% positive; 26.1 (IQR: 22.0–39.7) Infected group: 2.6 (IQR: 0.9–3.5). |
Vaccinated group: 98.3% positive; 20.2 (IQR: 12.7–29.0) Infected group cord blood: 3.3 (IQR: 0.5–4.6) |
0.77 | Association between maternal and cord blood responses Maternal responses associated negatively with time from second dose to delivery (but no correlation with cord blood IgG levels) |
Access SARS-CoV-2 IgG assay, Beckman Coulter | [43] |
| USA January–March 2021 |
At least one dose BNT162b2: 70% mRNA-1273: 30% Two doses: 55% |
122 | Not shown | None | Anti-RBD IgG and IgM | Overall: 71% IgG-positive, 16% IgG- plus IgM-positive, 13% no detectable response (all women delivered within 4 weeks of first dose) By 4 weeks after first dose: 100% IgG-positive |
Women who received 1 dose: 44% IgG-positive Women who received 2 doses: 99% IgG-positive |
Not shown | Association between maternal and cord blood IgG responses Placental transfer ratio correlated positively with the number of weeks from second dose to delivery Maternal IgG responses increased week on week, from 2 weeks after first dose |
Pylon 3D platform, ET HealthCare | [44] |
| Israel February 2021 |
Two doses BNT162b2 |
20 | Third trimester From first dose to delivery 33 (IQR: 30–37) days From second dose to delivery 11 (IQR: 9–15) days |
None | Anti-RBD IgG and IgM, and anti-S IgG Reported as AU/ml |
100% positive anti-S and anti-RBD IgG; 30% positive anti-RBD IgM Anti-S IgG: 319 AU/ml (IQR: 211–1033) Anti-RBD IgG 11 150 (IQR: 6154–17 575) |
100% positive anti-S and anti-RBD IgG 0% positive anti-RBD IgM Anti-S IgG: 193 AU/ml (IQR: 111–260) Anti-RBD IgG 3494 (IQR: 1817–6163) |
Anti-S IgG: 0.44 (IQR: 0.25–0.61) Anti-RBD IgG: 0.34 (IQR: 0.27–0.56) |
Association between maternal and cord blood anti-S and anti-RBD IgG responses Cord blood anti-S and anti-RBD IgG were associated positively with time from first dose to delivery |
Anti-spike IgG and anti-RBD IgM: Liaison, DiaSorin, Saluggia, Italy Anti-RBD IgG: SARSCoV-2 IgG II Quant, Abbott |
[46] |
| Israel February–April 2021 |
Two doses BNT162b2 |
171 | Third trimester Early third trimester (27–31 weeks GA): 49% From first dose to delivery 71 (IQR: 63–79) days Late third trimester (32–36 weeks GA) From first dose to delivery 41 (IQR 34–50) days |
None | Anti-RBD IgG and IgM Anti-S IgG Neutralization responses reported as AU/ml |
Overall: 100% positive anti-S and anti-RBD IgG Early third trimester – anti-S IgG: 200 AU/ml (IQR: 143–296), anti-RBD IgG: 3980 AU/ml (IQR: 2414–7022), anti-RBD IgM: 0% Late third trimester – anti-S IgG: 292 AU/ml (IQR: 209–963), anti-RBD IgG: 8506 AU/ml (IQR: 4601–15 094), anti-RBD IgM: 18% |
Overall: 100% positive anti-S and anti-RBD IgG 0% positive anti-RBD IgM Early third trimester: anti-RBD IgG: 9620 AU/ml (IQR: 5131–15 332) Late third trimester: anti-RBD IgG: 6697 AU/ml (IQR: 3157–14 731) |
Early third trimester – anti-S IgG: 1.3 (IQR: 1.1–1.6), anti-RBD IgG: 2.3 (IQR: 1.7–3.0), neutralization: 1.9 (IQR: 1.7–2.5) Late third trimester – anti-S IgG: 0.9 (IQR: 0.6–1.1), anti-RBD IgG: 0.7 (IQR: 0.5–1.2), neutralization: 0.8 (IQR: 0.5–1.1) |
Association between maternal and cord blood anti-S, anti-RBD IgG responses and neutralizing activity Maternal anti-S and anti-RBD IgG responses associated negatively with time from first dose to delivery Cord blood anti-RBD IgG and placental transfer ratio of anti-S, anti-RBD IgG and neutralizing activity higher after early vs. late third trimester vaccination, and were associated positively with time from first dose to delivery |
Anti-spike IgG and anti-RBD IgM: Liaison, DiaSorin, Saluggia, Italy Anti-RBD IgG: SARSCoV-2 IgG II Quant, Abbott |
[45] |
| USA July–October 2021 |
BNT162b2: 68% mRNA-1273: 32% |
77 | First dose: 20–32 weeks GA From first dose to delivery 85 (±46) days |
12 Women who had COVID-19 during pregnancy at 20–32 weeks GA | Anti-S IgG Reported as optical density |
Vaccinated group: 2.03 (±0.47) Infected group: 0.65 (±0.76) |
Vaccinated group: cord blood 2.17 (±0.50); at 2 months of age 1.29 (±0.53), 98% positive; at 6 months of age 0.33 (±0.46), 57% positive Infected group: cord blood 1.00 (±0.83); at 6 months of age 0 (±0.01), 8% positive |
Not shown | Responses at 2 months of age correlated positively with maternal and cord blood responses | In-house enzyme-linked immunosorbent assay | [49] |
| Israel May–July 2021 |
Two doses, ≥1 week before delivery BNT162b2 |
28 | Second trimester Second dose: 26 (IQR: 14–34) weeks GA From second dose to delivery 11.1 (IQR: 9.3–15) weeks |
12 Women who had COVID-19 during pregnancy at 20.6 (IQR: 17.6–36.9) weeks before delivery | Anti-S IgG Reported as AU/ml |
Vaccinated group: 145 AU/ml (IQR: 113–202) Infected group: 41 AU/ml (IQR: 19–95) |
Vaccinated group: 216 AU/ml (IQR: 155–316) Infected group: 64 AU/ml (IQR: 23–219) |
Vaccinated group: 1.48 (IQR: 1.18–1.82) Infected group: 1.35 (IQR: 1.19–1.84) |
Association between maternal and cord blood responses No association between maternal responses and placental transfer ratio No association between maternal responses and interval from vaccination to delivery Placental transfer ratio correlated positively with birthweight |
Anti-spike IgG, Liaison, DiaSorin, Saluggia, Italy | [51] |
| Germany Start date not reported–June 2021 |
At least 1 dose BNT162b2: 72% mRNA-1273: 28% Two doses: 97% |
36 | First dose 6% during the first trimester, 83% during the second trimester, 11% during the third trimester From second dose to delivery 13 (range: 5.9–24.9) weeks |
None | Anti-S IgG Reported as U/ml |
100% positive | 100% positive | Not shown | Elecsys anti-SARS-CoV-2 assay, Roche Diagnostics | [52] | |
| USA March–October 2021 |
At least one dose BNT162b2: 75% mRNA-1273: 22% Ad26.COV2.S: 2% Two doses: 89% Booster dose: 1% |
1359 12.8% had history of SARS-CoV-2 infection |
First dose: 3% pre-pregnancy, 14% during the first trimester, 51% during the second trimester, 32% during the third trimester | None | Anti-S IgG Reported as relative index value |
Anti-S IgG levels in fully vaccinated women by trimester of vaccination: 4.1 (pre-pregnancy), 4.2 (first trimester), 4.9 (second trimester), 6.2 (third trimester) | Anti-S IgG levels in cord blood from fully vaccinated women by trimester of vaccination: 4.5 (pre-pregnancy); 4.7 (first trimester); 5.5 (second trimester); 6.3 (third trimester) | Not shown | Third trimester vaccination associated with highest responses in maternal and cord blood Ad26.COV2.S no difference in responses by trimester of vaccination and always lower than the mRNA vaccines In women with history of SARS-CoV-2 infection, maternal and cord blood responses after vaccination in early pregnancy were comparable to third trimester vaccination in pregnant women without history of infection |
Pylon 3D platform, ET HealthCare | [55] |
| Poland Dates not reported |
Two doses BNT162b2 |
16 | First dose: 31.8 (±2.1) weeks GA Second dose 35.1 (±2.1) weeks GA From second dose to delivery 5.5 (±2.1) weeks |
None | Anti-RBD IgG Reported as U/ml |
100% positive 984.37 U/ml (±689.4) |
100% positive 1026.51 U/ml (±769.25) |
1.28 (±0.80) | Cord blood and placental transfer ratio associated positively with time from first dose and second dose to delivery | Elecsys Anti-SARS-CoV-2-S-RBD assay, Roche Diagnostics | [57] |
| Romania May–December 2021 |
BNT162b2 Ad26.CO |
227 | Third trimester | 54 Pregnant women who had COVID-19 529 Non-pregnant vaccinated women, uninfected |
Anti-RBD IgG Reported as U/ml |
Vaccinated group not previously infected – before vaccination: 0.41 (0.31–0.45); 1 month post-fully vaccinated: 2433 (1752–3094); 2 months post-fully vaccinated: 1697 (1393–2115); 3 months post-fully vaccinated: 1314 (1095–1762); 4 months post-fully vaccinated: 1083 (896–1468) Vaccinated group previously infected – before vaccination: 145 (98.2–208.1); 1 month post-fully vaccinated: 15 360 (13 551–16 318); 2 months post-fully vaccinated: 14 571 (12 628–15 337); 3 months post-fully vaccinated: 12 870 (10 644–14 349); 4 months post-fully vaccinated: 10 759 (9043–12 571) Non-pregnant group – before vaccination: 0.40 (0.32–0.47); 1 month post-fully vaccinated: 2461 (1719–3176); 2 months post-fully vaccinated: 1705 (1452–2179); 3 months post-fully vaccinated: 1377 (1147–1822); 4 months post-fully vaccinated: 1114 (909–1483) |
Not shown | Not shown | Elecsys Anti-SARS-CoV-2-S-RBD assay, Roche Diagnostics | [59] |
Abbreviations: AU/ml, arbitrary units per milliliter; GA, gestational age; IQR, interquartile range; RBD, receptor-binding domain; S, spike protein; U/ml, units per milliliter.
Blood samples collected at delivery if not stated otherwise.
Serum immunoglobulin G (IgG) responses against the receptor-binding domain (RBD) of the SARS-CoV-2 spike (S) protein or against S1 and S2 were measured in the women across all studies [30., 31., 32.,36,38,40,42., 43., 44., 45., 46.,49,51,52,55,57,59], IgM concentrations were reported in six studies [30,36,42,44., 45., 46.] and IgA concentrations in one [36]. Because breastmilk antibodies could confer protection to infants against illness, IgA, IgG, or IgM in breastmilk were measured in three studies in women who had been vaccinated during pregnancy [32,36,43]. Neutralizing antibody activity against SARS-CoV-2, which has been established as a surrogate for protection against infection and mild-to-moderate COVID-19 caused by the original SARS-CoV virus, was also reported in three studies [32,36,45,73]. Figure 1 summarizes the different immunological responses measured.
Overall, pregnant women mounted robust humoral immune responses to vaccination, and IgG seropositivity was 100% following the second dose of an mRNA vaccine [40,43., 44., 45., 46.,52,57]. Unsurprisingly, women who received two vaccine doses had higher serum IgG concentrations than those receiving a single dose [30,36,44]. The anti-RBD IgG, anti-S IgG, and neutralization antibody activity in pregnant women following one or two doses of an mRNA vaccine were, in general, similar to those in non-pregnant women [32,36,59]. A case–control study in Israel, however, detected a significantly lower serum anti-RBD IgG concentration 2 weeks to 2 months after the second dose in 96 pregnant women vaccinated at 2–40 weeks of gestation (signal to cutoff median, 27.03 ± 10.72) compared to 96 control non-pregnant women (34.35 ± 10.25; P < 0.001) [31]. Immune responses to RBD and spike have also been compared after vaccination with mRNA vaccines in pregnant women relative to convalescent serum following natural SARS-CoV-2 infection during pregnancy [30,32,36,38,43,49,51]. Although the timing of the events (vaccination and infection) during pregnancy was not always matched, vaccine-induced antibody concentrations were always higher than those induced by infection [32,36,43,49,51]. One study, in which pregnant women vaccinated in their third trimester with BNT162b2 or Ad26.COV2.S were stratified by SARS-CoV-2 seropositivity before vaccination (i.e., a surrogate for previous infection status), showed that anti-RBD IgG levels increased in both groups from pre-vaccination to after vaccination for a 4 month period, and were significantly higher in the baseline seropositive group, indicating an anamnestic response via priming induced by previous SARS-CoV-2 infection [59].
The proportion of women with serum IgM to RBD and spike assessed after vaccination was consistently lower than for IgG responses [30,36,42,44., 45., 46.]. Unsurprisingly, one study reported that – in contrast to IgG responses to spike – IgM responses increased after the first dose but not after the second mRNA vaccine dose [36]. Although vaccine-induced anti-RBD and anti-S IgG antibodies were detected in breastmilk samples of all women, the concentrations were lower than serum IgG concentrations [32,36,43].
Two studies from the USA described humoral responses to COVID-19 mRNA vaccines in pregnant compared to non-pregnant women, including serum IgG, and used systems serology to investigate antibody effector functions [32,74]. Both studies reported similar anti-S specific antibody-dependent neutrophil phagocytosis, antibody-dependent complement deposition, and antibody-dependent cellular phagocytosis in pregnant women compared to non-pregnant women. One study found, however, that antibody effector functions and Fc receptor (FcR) binding were lower in pregnant compared to non-pregnant counterparts after the first vaccine dose, but were similar after the second vaccine dose [74]. In addition, despite overall lower IgG concentrations against multiple SARS-CoV-2 IgG epitopes in cord blood compared to maternal blood, mRNA COVID-19 vaccination during the third pregnancy trimester was associated with enrichment of functional RBD-specific FcγR-binding antibodies in cord blood, as was also observed following SARS-CoV-2 infection in pregnant women [75]. The other study also assessed cellular immune responses to mRNA vaccination by quantifying S-specific CD4 and CD8 T cells and central memory T cells, and reported a similar frequency of S-specific IFN-γ-secreting cells in pregnant and non-pregnant women measured by enzyme-linked immunospot assay [32].
Transplacental transfer of anti-SARS-CoV-2 antibodies to the newborn after maternal vaccination
It is established that binding and neutralizing antibodies induced by vaccines undergo maternal to fetus transplacental transfer [31,32,36,40,42,43,45,46,49,51,55,57]. All studies, independently of which mRNA vaccine and when vaccination occurred during pregnancy, reported a positive correlation between maternal and cord blood IgG concentrations. The cord-to-maternal blood IgG ratios were generally ~1.0, with the highest ratio of 2.6 for anti-RBD IgG among 114 term newborns whose mothers had received BNT162b2 in the second trimester of pregnancy – indicating active transplacental transfer of the anti-protein antibodies [40]. Lower ratios of cord-to-maternal blood anti-S (0.4–0.9) and anti-RBD (0.34–0.7) IgG were reported in term neonates whose mothers received two doses of BNT162b2 from late third trimester [45,46]. These data suggest that earlier timing of COVID-19 vaccination is associated with a higher cord-to-maternal IgG ratio; however, this could be offset by waning antibody concentrations in women who were vaccinated earlier in pregnancy relative to the time of delivery. This was corroborated in a study that also reported a higher cord-to-maternal ratio (1.3 for anti-S IgG and 2.3 for anti-RBD IgG) if women were vaccinated earlier in the third trimester at 27–31 weeks gestational age compared to later vaccination [45]. Overall, IgM was not identified in cord blood following maternal vaccination even in women vaccinated toward the late stages of pregnancy, suggesting no primary stimulation of the humoral immune system of the fetus [30,42,45,46].
Factors affecting anti-SARS-CoV-2 antibody responses in pregnant women and newborns
Although vaccination as early as possible during pregnancy is ideal to optimize the duration of maternal protection against infection, direct protection of the young infant would depend on the efficiency and concentration of transplacental acquired antibodies. Most studies in which pregnant women were vaccinated in the third trimester found that IgG levels in cord blood and placental transfer ratio correlated positively with increasing time between vaccination and delivery [36,42,44., 45., 46.,57]. Early third trimester vaccination at 27–31 gestational weeks also resulted in higher cord blood neutralizing antibody levels compared to later vaccination [45]. In a cohort study of 1359 pregnant women (2.4% received Ad.26.COV2.S and 97.6% an mRNA vaccine), although early third trimester vaccination was associated with the highest anti-S IgG concentration in maternal and cord blood, newborns to fully vaccinated women (even if vaccinated early in the first trimester) had similar or higher IgG concentration than those born to women who were partially vaccinated before delivery [55]. Moreover, anti-S IgG concentrations after mRNA COVID-19 vaccination in pregnant women with SARS-CoV-2 infection before vaccination were comparable to IgG concentrations in women without a history of SARS-CoV-2 infection who were vaccinated in the third trimester of pregnancy [55]. However, an inverse correlation was observed between both maternal and cord blood anti-RBD IgG responses at delivery and the time interval between receipt of the second dose of BNT162b2 (≥7 days before delivery, at 21.9 weeks gestation) and delivery [40]. In addition to the time from vaccination to delivery, birthweight was also found to be associated positively with transplacental anti-S IgG transfer [51], and maternal age was inversely correlated with maternal and neonatal IgG concentrations at delivery [40]. It will be important to understand what factors affect the immune response to vaccines and transplacental transfer of antibodies to delineate the preferred time for vaccination of pregnant women to optimize protection against COVID-19 in the women and their newborns.
Persistence of maternally derived IgG in young infants
The potential direct protection of the infant against SARS-CoV-2 infections via transplacental acquisition of maternal IgG is dependent on the duration over which the antibody remains above the threshold associated with risk reduction of being infected or developing COVID-19. It is unclear whether the putative thresholds of anti-S IgG or neutralizing antibody against wild-type virus would also apply to young infants; and more so in the context of relatively antibody-evasive variants of concern for which no correlates are established even in adults. When the persistence of maternally derived anti-S IgG seropositivity was evaluated in young infants born to women vaccinated between 20 and 32 weeks of gestation, seropositivity was 98% (48 out of 49) at 2 months of age and 57% (16 out of 28) by 6 months of age, compared to 8% (1 of 12) in infants born to unvaccinated women who had SARS-CoV-2 infection during pregnancy at similar gestational ages as vaccination occurred in the vaccinated group [49]. These data, unsurprisingly, indicate waning of anti-SARS-CoV-2 IgG over time.
Protection against SARS-CoV-2 infection by maternal vaccination
Three observational studies reported on SARS-CoV-2 infection among mRNA vaccinated and unvaccinated pregnant women [33,34,50]. An initial retrospective cohort study from Israel evaluated the protection of BNT162b2 vaccination during pregnancy against PCR-confirmed SARS-CoV-2 infection at least 28 days after the first vaccine dose [34]. The cohort included 7530 vaccinated (first dose administered from December 2020 to February 2021) and 7530 matched unvaccinated women, followed-up for a median of 37 days. Overall, 118 and 202 SARS-CoV-2 infections occurred in BNT162b2-vaccinated and unvaccinated women, respectively. During the 28–70 days follow-up period, there were 10 infections in the vaccinated group and 46 in the unvaccinated group (aHR 0.22; 95%CI 0.11–0.43) [34]. In the second study from Israel, 10 861 pregnant women who received BNT162b (26%, 48%, and 26% in the first, second, and third trimesters, respectively) between December 2020 and June 2021 were matched with the same number of unvaccinated women [33]. During a median follow-up of 77 days, 131 and 235 infections were documented among the vaccinated and unvaccinated groups, respectively. The estimated vaccine effectiveness from 7 days through to 56 days after the second vaccination was 96% (95%CI 89–100%) for any documented infection, 97% (95% CI 91–100%) for any symptomatic COVID-19, and 89% (95% CI 43–100%) for COVID-19-related hospitalization [33]. A USA study evaluating the rate of SARS-CoV-2 infection after vaccination, regardless of temporal relationship, also reported a lower frequency of infection before delivery among vaccinated women (2/140, 1.4%) compared to unvaccinated women (210/1861; 11.3%) between December 2020 and April 2021, which presumably mainly involved infections from the wild-type virus or the alpha variant of concern [50].
The effectiveness of mRNA vaccines during pregnancy against COVID-19-associated hospitalization in infants <6 months age was evaluated in 17 US states from July 2021 to January 2022 (during which time the delta and omicron variants of concern circulated) using a test-negative, case–control study design [76,77]. Included in the analysis were 379 hospitalized infants (176 cases and 203 controls); maternal vaccination coverage was 16% among cases and 32% among controls, yielding a vaccine effectiveness of 61% (95% CI 31–78%). Vaccine effectiveness of two doses given early in pregnancy (the first 20 weeks of gestation) against COVID-19-associated hospitalization of infants was 32% (95% CI –43% to 68%) and 80% (95% CI 55–91%) when administered from 21 weeks gestation through to 14 days before delivery [76].
In response to the emergence of new SARS-CoV-2 variants of concern and to address potential waning of immunity over time, many countries now recommend at least a three-dose schedule of mRNA vaccines, as well as a mRNA vaccine in those who previously received two doses of ChAdOx1 or a single Ad.26.COV2.S (Box 1 ). Data on the effect of such additional doses of mRNA vaccines, either in homologous or heterologous schedules, are sparse in pregnant women.
Box 1. COVID-19 vaccine booster doses.
Despite initial reports of high vaccine efficacy against symptomatic COVID-19 caused by the wild-type virus or the alpha variant of concern, a decline in vaccine effectiveness has been reported after 4–5 months following the second vaccine dose, albeit persistently high protection against severe COVID-19. Furthermore, the emergence of other variants of concern such as beta and omicron has resulted in further decline in vaccine effectiveness against COVID-19 [85]. Studies conducted during the time of circulation of the delta variant demonstrated that a third BNT162b2 dose administered at least 5 months after the second dose significantly reduced the rate of symptomatic COVID-19 and severe COVID-19 compared to receipt of only two doses [86,87]. A two-dose mRNA COVID-19 vaccine schedule provides limited protection against symptomatic COVID-19 caused by the omicron variant, and also lesser protection against severe COVID-19 compared to the effectiveness against wild-type, alpha, beta, or delta variants of concern. In several observational studies a third dose of mRNA was associated with increased effectiveness against severe COVID-19 caused by omicron, and vaccine effectiveness approached that observed with two doses against the wild-type virus and earlier variants of concern [88., 89., 90.].
Regarding pregnant women, the CDC, the ACOG, and the UK Health Security Agency, among others, recommend that pregnant women stay up to date with their COVID-19 vaccines, including receiving a third mRNA dose [22., 23., 24.]. In addition, pregnant women may receive any vaccine product available for their third dose, although mRNA vaccines are recommended.
Three studies published to date suggest that an mRNA vaccine administered as a third dose is in principle safe and induces a robust IgG response in the mother that is transferred to the newborn [55,91,92]. In Israel, 294 pregnant women who received three doses of mRNA vaccines had similar rates of preterm and SGA births compared to women who were unvaccinated (N = 3368) or had only received two doses (N = 2854). There was, however, a higher rate of post-partum hemorrhage in those who received three doses (9.5%) than in the unvaccinated (3.2%) or double-vaccinated (3.5%) groups [91]. Although the underlying potential mechanism for this difference is unknown, the safety of additional vaccine doses beyond the initial two-dose schedule warrants further study.
In Germany, three pregnant women who received ChAdOx1 as primary vaccination at gestational ages between 21 and 28 weeks, and had a third dose 12 weeks thereafter with either BNT162b2 or mRNA-1273, had a measurable boost in their anti-S IgG responses [92]. Similarly, an mRNA booster dose in the third trimester given to 20 women (previously vaccinated with an mRNA vaccine) was also associated with the highest maternal and cord blood anti-S IgG responses [55].
Alt-text: Box 1
Concluding remarks
Vaccination during pregnancy with the current mRNA vaccines demonstrated no major adverse events and similar reactogenicity profiles between pregnant women and the general population (see Clinician’s corner). Safety information was mostly collected following mRNA vaccine administration, and it will be important to collect more data for other COVID-19 vaccines such as inactivated virus vaccines as well as vector-based and protein-based vaccines. In addition, more safety data on vaccination during the first trimester are needed, and investigations are warranted on whether COVID-19 vaccination reduces COVID-19-related pregnancy complications.
Clinician’s corner.
Pregnant women are at risk of more severe disease following SARS-CoV-2 infection than non-pregnant individuals, and COVID-19 in pregnant women is associated with greater risk of preterm labor.
Although excluded from initial Phase I–III vaccine clinical trials, COVID-19 vaccines are now widely recommended for use in pregnant women by the World Health Organization and other bodies.
Post-introduction surveillance has not identified any major safety concerns on the use of mainly the mRNA COVID-19 vaccines in pregnant women in relation to the wellbeing of the women, pregnancy outcome, or the wellbeing of the offspring.
The reactogenicity of mRNA COVID-19 vaccines in pregnant women was mainly mild, local and/or systemic, and more common after the second dose, as is the case for the general population, and the frequency was similar or lower than for non-pregnant women.
Vaccination of pregnant women with mRNA COVID-19 vaccines has been shown to be effective in protecting the women against disease.
The safety of vaccine by trimester of immunization remains to be determined; however, current evidence does not indicate any serious side effects related to vaccination, including with regard to congenital abnormalities.
Alt-text: Clinician’s corner
The coverage of COVID-19 vaccination among pregnant women is increasing and we should use the opportunity to address remaining issues (see Outstanding questions). Different trials are ongoing and will soon provide important information on the use of COVID-19 vaccines in pregnant women. These include a Phase II, randomized, placebo-controlled, observer-blind trial evaluating the safety, tolerability, and immunogenicity of two doses of BNT162b2 given 21 days apart, in ~700 (randomized 1:1) healthy pregnant women vaccinated at 24–34 gestational weeks, started in February 2021, and has an estimated completion date in mid-2022 (NCT04754594i). Another Phase II, randomized, single-blind, platform trial in pregnant women is ongoing to assess the safety, reactogenicity, and immunogenicity of COVID-19 vaccines available in the UK [78]. Also ongoing is an open-label, Phase II trial to evaluate the safety, reactogenicity, and immunogenicity of Ad26.COV2.S in 400 healthy pregnant women at 16–38 gestational weeks as a one-dose schedule at the standard dose level, or two doses at a lower dose level (NCT04765384ii). In addition, at least 14 observational studies and vaccine registries are currently listed on clinicaltrials.gov that are evaluating the safety and/or immunogenicity of COVD-19 vaccination during pregnancy.
Outstanding questions.
What is the safety of COVID-19 vaccines for birth outcomes, including non-replicating vector-based vaccines, inactivated vaccines, and protein-based vaccines?
What are the immune responses following vaccination with non-replicating vector-based vaccines, inactivated vaccines, and protein-based vaccines?
What is the effectiveness of COVID-19 vaccines against adverse pregnancy outcomes such as preterm labor and stillbirth?
Does COVID-19 vaccination during pregnancy protect the neonates against COVID-19, either via transplacental transfer of vaccine-induced neutralizing antibody or by the acquisition of secretory IgA from breastmilk?
What is the optimal timing of COVID-19 vaccination during pregnancy to maximize protection against COVID-19 in the women and the fetus, as well as to minimize any adverse pregnancy outcomes?
What will be the benefit of COVID-19 vaccination in pregnant women once there is widespread vaccine- or natural infection-induced immunity, and when SARS-CoV-2 circulation is established to be endemic?
What is the need for COVID-19 vaccination in subsequent pregnancies regarding benefits to the women, fetus, and infant triad?
What will be the safety and immunogenicity profiles when COVID-19 vaccines are coadministered with other routine vaccines during pregnancy?
Alt-text: Outstanding questions
It remains important to include pregnant women in future COVID-19 vaccine research and in response to efforts to prevent emerging and re-emerging infectious diseases. New approaches to public health preparedness will be necessary to guarantee that the needs of pregnant women and their offspring are fairly addressed in ethically and socially respectful ways [79]. Moreover, for the successful introduction of new vaccines for use in pregnancy, there needs to be close integration of vaccination programs with antenatal, maternal, and child healthcare services. In addition, surveillance systems for pregnancy that can be used by large numbers of women need to be supported, particularly in many low- and middle-income countries where only limited or no surveillance capacity currently exists [80].
Long-term vaccine safety data in the offspring are also warranted, as well as the durability of the maternal SARS-CoV-2 antibodies in the young infants, and whether these antibodies might influence the immunogenicity of other childhood vaccines [81].
Two studies have suggested differences in the humoral immune response to the two current mRNA vaccines, including higher antibody responses and more robust functional antibodies induced by mRNA-1273 compared to the BNT162b2 vaccine [36,74]. Whether these differences between the two vaccines have any clinical implications on mother–newborn dyads is unclear, but the robust immune responses to both mRNA vaccines suggests that they are likely to be as efficacious in pregnant women as in non-pregnant adults.
For Tdap (tetanus-toxoid, reduced diphtheria-toxoid, pertussis) and influenza vaccines it has been shown that the timing of vaccine administration during pregnancy impacts on the magnitude of transplacental IgG transfer to the fetus [82,83]. Nonetheless, for infections that cause substantial morbidity in the pregnant women, such as influenza and now COVID-19, vaccine administration should be timed to optimize maternal immunity relative to viral circulation. Of note, it is likely that immune responses to COVID-19 vaccines to date in high-income settings have predominantly been primary immune responses rather than anamnestic responses following infection-induced immunity, but this is now changing with the evolving pandemic.
The only vaccine effectiveness analysis assessing protection of maternal COVID-19 vaccination in young infants indicates protection against COVID-19 hospitalization during early infancy [76], and this requires further study.
To date, COVID-19 vaccine studies in pregnant women have mainly been undertaken in the USA or Israel. No studies have been reported involving women from ethnic minorities or from low- and middle-income countries, and these are warranted because the immune responses and vaccine efficacies could differ in these populations because of host genetic factors, differences in the prevalence of comorbidities, and/or differences in the efficiency of transplacental antibody transfer to the fetus and newborn [84].
Acknowledgments
Acknowledgments
This work was supported in part by funding from the Department of Science and Technology: South African Research Chair Initiative in Vaccine Preventable Diseases, and by the South African Medical Research Council.
Declaration of interests
M.C.N. reports grants to her institution from the Bill & Melinda Gates Foundation (BMFG), the European and Developing Countries Clinical Trials Partnership (EDCTP), Pfizer, AstraZeneca, and Sanofi-Pasteur, as well as personal honoraria from Pfizer and Sanofi-Pasteur unrelated to the manuscript. S.A.M. reports grants to his institution related to COVID-19 epidemiology and vaccine studies from BMFG, the South African Medical Research Council, Novavax, Pfizer, Gritstone (PATH), Providence, Johnson & Johnson, AstraZeneca, and the EDCTP. Additional non-COVID-19 grants to the institution have also been received from GSK and Minervax. S.A.M. also declares personal honoraria from BMGF unrelated to the manuscript.
Glossary
- Anamnestic response
a memory response in which there is renewed rapid production of an antibody on the second (or subsequent) encounter with the same antigen.
- Antibody-dependent cellular phagocytosis
a functional assay that detects monocyte phagocytosis (clearance) of antibody–antigen immune complexes.
- Antibody-dependent complement deposition
a functional assay that detects antibody-driven specific complement activation.
- Antibody-dependent neutrophil phagocytosis
a functional assay that detects neutrophil phagocytosis (clearance) of antibody–antigen immune complexes.
- Arthralgia
joint stiffness or pain.
- Chorioamnionitis
bacterial infection of the chorion and amnion (the membranes that surround the fetus) and the amniotic fluid (in which the fetus floats) that occurs before or during labor.
- Chronic histiocytic intervillositis
a disorder of pregnancy characterized by infiltration of maternal macrophages into the intervillous space of the human placenta, often with accompanying perivillous fibrin deposition.
- Decidual arteriopathy
an abnormality of maternal vascular malperfusion involving injury to the maternal decidual vessels and altered uterine and intervillous blood flow.
- Eclampsia
a severe complication of pre-eclampsia. It is a rare but serious condition in which high blood pressure results in seizures during pregnancy.
- Fetal vascular malperfusion
a group of placental lesions indicating reduced or absent perfusion of the villous parenchyma by the fetus.
- Lymphadenopathy
lymph nodes that are abnormal in size (swollen lymph nodes) or consistency.
- Myalgia
soreness and achiness in the muscles that can range from mild to severe.
- Oligohydramnios
low amniotic fluid during pregnancy.
- Paresthesia
burning or prickling sensation that is usually felt in the hands, arms, legs, or feet but can also occur in other parts of the body.
- Pre-eclampsia
a condition in which pregnant women have high blood pressure and protein in their urine; there may also be swelling in the legs, feet, and hands. It can range from mild to severe. It usually happens late in pregnancy, although it can arise earlier or immediately after delivery.
- Pulmonary embolism
a condition in which one or more arteries in the lungs become blocked by a blood clot.
- Small for gestational age (SGA)
newborns who are smaller than expected from the number of weeks of pregnancy, and have a birth weight below the 10th percentile.
- Transplacental transfer
active transport across the placenta from the maternal side to the fetus.
Resources
ihttps://clinicaltrials.gov/ct2/show/NCT04754594iihttps://clinicaltrials.gov/ct2/show/NCT04765384References
- 1.Zambrano L.D., et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status – United States, January 22–October 3, 2020. Morb. Mortal. Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciapponi A., et al. COVID-19 and pregnancy: an umbrella review of clinical presentation, vertical transmission, and maternal and perinatal outcomes. PLoS One. 2021;16 doi: 10.1371/journal.pone.0253974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allotey J., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villar J., et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021;175:817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinn J., et al. Characteristics and outcomes of women with COVID-19 giving birth at US academic centers during the COVID-19 pandemic. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei S.Q., et al. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193:E540–E548. doi: 10.1503/cmaj.202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight M., et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adhikari E.H., et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.29256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelidou A., et al. Association of maternal perinatal SARS-CoV-2 infection with neonatal outcomes during the COVID-19 pandemic in Massachusetts. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodworth K.R., et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy – SET-NET, 16 Jurisdictions, March 29–October 14, 2020. Morb. Mortal. Wkly Rep. 2020;69:1635–1640. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellos I., et al. Maternal and perinatal outcomes in pregnant women infected by SARS-CoV-2: a meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021;256:194–204. doi: 10.1016/j.ejogrb.2020.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui X., et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19) J. Med. Virol. 2021;93:1057–1069. doi: 10.1002/jmv.26398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 14.Polack F.P., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voysey M., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baden L.R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logunov D.Y., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime–boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadoff J., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinde V., et al. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N. Engl. J. Med. 2021;384:1899–1909. doi: 10.1056/NEJMoa2103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanriover M.D., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adhikari E.H., Spong C.Y. COVID-19 vaccination in pregnant and lactating women. JAMA. 2021;325:1039–1040. doi: 10.1001/jama.2021.1658. [DOI] [PubMed] [Google Scholar]
- 22.American College of Obstetricians and Gynecologists ACOG; 2020. COVID-19 Vaccination Considerations for Obstetric–Gynecologic Care. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care?utm_source=redirect&utm_medium=web&utm_campaign=int# Published online December 2020.
- 23.Centers for Disease Control and Prevention CDC; 2022. COVID-19 Vaccines while Pregnant or Breastfeeding. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html Published online March 3, 2022.
- 24.UK Health Security Agency UK Government; 2022. COVID-19 Vaccination: A Guide on Pregnancy and Breastfeeding. https://www.gov.uk/government/publications/covid-19-vaccination-women-of-childbearing-age-currently-pregnant-planning-a-pregnancy-or-breastfeeding/covid-19-vaccination-a-guide-for-women-of-childbearing-age-pregnant-planning-a-pregnancy-or-breastfeeding Published online April 11, 2022.
- 25.World Health Organization WHO; 2022. The Moderna COVI-19 (mRNA-1273) Vaccine: What You Need To Know. https://www.who.int/news-room/feature-stories/detail/the-moderna-covid-19-mrna-1273-vaccine-what-you-need-to-know Published online February 23, 2022.
- 26.World Health Organization WHO; 2022. The Pfizer BioNTech (BNT162b2) COVID-19 Vaccine: What You Need To Know. https://www.who.int/news-room/feature-stories/detail/who-can-take-the-pfizer-biontech-covid-19--vaccine-what-you-need-to-know Published online January 21, 2022.
- 27.Pratama N.R., et al. mRNA Covid-19 vaccines in pregnancy: a systematic review. PLoS One. 2022;17 doi: 10.1371/journal.pone.0261350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hillson K., et al. Fertility rates and birth outcomes after ChAdOx1 nCoV-19 (AZD1222) vaccination. Lancet. 2021;398:1683–1684. doi: 10.1016/S0140-6736(21)02282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loubet P., et al. Immunization during pregnancy. Expert Rev. Vaccines. 2018;17:383–393. doi: 10.1080/14760584.2018.1471988. [DOI] [PubMed] [Google Scholar]
- 30.Beharier O., et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Invest. 2021;131 doi: 10.1172/JCI154834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bookstein Peretz S., et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet. Gynecol. 2021;58:450–456. doi: 10.1002/uog.23729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collier A.Y., et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325:2370–2380. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dagan N., et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat. Med. 2021;27:1693–1695. doi: 10.1038/s41591-021-01490-8. [DOI] [PubMed] [Google Scholar]
- 34.Goldshtein I., et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326:728–735. doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldshtein I., et al. Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes. JAMA Pediatr. 2022 doi: 10.1001/jamapediatrics.2022.0001. Published online February 10, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gray K.J., et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am. J. Obstet. Gynecol. 2021;225:303.e1–303.e17. doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadali R.A.K., et al. Adverse effects of COVID-19 messenger RNA vaccines among pregnant women: a cross-sectional study on healthcare workers with detailed self-reported symptoms. Am. J. Obstet. Gynecol. 2021;225:458–460. doi: 10.1016/j.ajog.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kashani-Ligumsky L., et al. Titers of SARS CoV-2 antibodies in cord blood of neonates whose mothers contracted SARS CoV-2 (COVID-19) during pregnancy and in those whose mothers were vaccinated with mRNA to SARS CoV-2 during pregnancy. J. Perinatol. 2021;41:2621–2624. doi: 10.1038/s41372-021-01216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kharbanda E.O., et al. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA. 2021;326:1629–1631. doi: 10.1001/jama.2021.15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kugelman N., et al. Maternal and neonatal SARS-CoV-2 immunoglobulin G antibody levels at delivery after receipt of the BNT162b2 messenger RNA COVID-19 vaccine during the second trimester of pregnancy. JAMA Pediatr. 2022;176:290–295. doi: 10.1001/jamapediatrics.2021.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magnus M.C., et al. Covid-19 vaccination during pregnancy and first-trimester miscarriage. N. Engl. J. Med. 2021;385:2008–2010. doi: 10.1056/NEJMc2114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mithal L.B., et al. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am. J. Obstet. Gynecol. 2021;225:192–194. doi: 10.1016/j.ajog.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nir O., et al. Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy. Am. J. Obstet. Gynecol. MFM. 2022;4 doi: 10.1016/j.ajogmf.2021.100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prabhu M., et al. Antibody response to coronavirus disease 2019 (COVID-19) messenger RNA vaccination in pregnant women and transplacental passage into cord blood. Obstet. Gynecol. 2021;138:278–280. doi: 10.1097/AOG.0000000000004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rottenstreich A., et al. Timing of SARS-CoV-2 vaccination during the third trimester of pregnancy and transplacental antibody transfer: a prospective cohort study. Clin. Microbiol. Infect. 2022;28:419–425. doi: 10.1016/j.cmi.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rottenstreich A., et al. Efficient maternofetal transplacental transfer of anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike antibodies after antenatal SARS-CoV-2 BNT162b2 messenger RNA vaccination. Clin. Infect. Dis. 2021;73:1909–1912. doi: 10.1093/cid/ciab266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shanes E.D., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: measures of immunity and placental histopathology. Obstet. Gynecol. 2021;138:281–283. doi: 10.1097/AOG.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimabukuro T.T., et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N. Engl. J. Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shook L.L., et al. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA. 2022;327:1087–1089. doi: 10.1001/jama.2022.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Theiler R.N., et al. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am. J. Obstet. Gynecol. MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Treger S., et al. Transplacental transfer of SARS-CoV-2 antibodies in recovered and BNT162b2-vaccinated patients. Am. J. Obstet. Gynecol. 2022;226:587–589. doi: 10.1016/j.ajog.2021.11.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trostle M.E., et al. High antibody levels in cord blood from pregnant women vaccinated against COVID-19. Am. J. Obstet. Gynecol. MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trostle M.E., et al. COVID-19 vaccination in pregnancy: early experience from a single institution. Am. J. Obstet. Gynecol. MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wainstock T., et al. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine. 2021;39:6037–6040. doi: 10.1016/j.vaccine.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y.J., et al. Association of gestational age at coronavirus disease 2019 (COVID-19) vaccination, history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and a vaccine booster dose with maternal and umbilical cord antibody levels at delivery. Obstet. Gynecol. 2022;139:373–380. doi: 10.1097/AOG.0000000000004693. [DOI] [PubMed] [Google Scholar]
- 56.Zauche L.H., et al. Receipt of mRNA Covid-19 vaccines and risk of spontaneous abortion. N. Engl. J. Med. 2021;385:1533–1535. doi: 10.1056/NEJMc2113891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zdanowski W., Wasniewski T. Evaluation of SARS-CoV-2 spike protein antibody titers in cord blood after COVID-19 vaccination during pregnancy in Polish healthcare workers: preliminary results. Vaccines (Basel) 2021;9:675. doi: 10.3390/vaccines9060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kachikis A., et al. Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Citu I.M., et al. Immunogenicity following administration of BNT162b2 and Ad26.COV2.S COVID-19 vaccines in the pregnant population during the third trimester. Viruses. 2022;14:307. doi: 10.3390/v14020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention CDC; 2022. V-Safe After Vaccination Health Checker. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html#print Published online January 20, 2022.
- 61.Investigators C.D.C., Collaborators CDC; 2020. V-Safe Pregnancy Surveillance (Amendment) https://www.cdc.gov/vaccinesafety/pdf/vsafe-pregnancy-surveillance-protocol-508.pdf Published online December 29, 2020.
- 62.Shimabukuro T.T., et al. Safety monitoring in the vaccine adverse event reporting system (VAERS) Vaccine. 2015;33:4398–4405. doi: 10.1016/j.vaccine.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mukherjee S., et al. Risk of miscarriage among black women and white women in a U.S. Prospective Cohort Study. Am. J. Epidemiol. 2013;177:1271–1278. doi: 10.1093/aje/kws393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldhaber M.K., Fireman B.H. The fetal life table revisited: spontaneous abortion rates in three Kaiser Permanente cohorts. Epidemiology. 1991;2:33–39. [PubMed] [Google Scholar]
- 65.Lipkind H.S., et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth – eight integrated health care organizations, United States, December 15, 2020–July 22, 2021. MMWR Morb. Mortal. Wkly Rep. 2022;71:26–30. doi: 10.15585/mmwr.mm7101e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fell D.B., et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA. 2022;327:1478–1487. doi: 10.1001/jama.2022.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Magnus M.C., et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA. 2022;327:1469–1477. doi: 10.1001/jama.2022.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koga K., et al. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am. J. Reprod. Immunol. 2009;61:196–212. doi: 10.1111/j.1600-0897.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pardi N., et al. mRNA vaccines – a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J., et al. Toll-like receptor 3 agonist induces impairment of uterine vascular remodeling and fetal losses in CBA × DBA/2 mice. J. Reprod. Immunol. 2007;74:61–67. doi: 10.1016/j.jri.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 71.Shanes E.D., et al. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Resta L., et al. SARS-CoV-2 and placenta: new insights and perspectives. Viruses. 2021;13:723. doi: 10.3390/v13050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khoury D.S., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 74.Atyeo C., et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abi8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Atyeo C., et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell. 2021;184:628–642. doi: 10.1016/j.cell.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Halasa N.B., et al. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 months – 17 states, July 2021–January 2022. MMWR Morb. Mortal. Wkly Rep. 2022;71:264–270. doi: 10.15585/mmwr.mm7107e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Centers for Disease Control and Prevention CDC; 2022. COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#variant-proportions Updated online April 26, 2022.
- 78.St George's Vaccine Institute University of London; 2022. Preg-CoV Trial. https://vaccine.ac.uk/research/preg-cov-trial/
- 79.Krubiner C.B., et al. Pregnant women & vaccines against emerging epidemic threats: ethics guidance for preparedness, research, and response. Vaccine. 2021;39:85–120. doi: 10.1016/j.vaccine.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kochhar S., et al. Introduction of new vaccines for immunization in pregnancy - Programmatic, regulatory, safety and ethical considerations. Vaccine. 2019;37:3267–3277. doi: 10.1016/j.vaccine.2019.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maertens K., et al. The effect of maternal pertussis immunization on infant vaccine responses to a booster pertussis-containing vaccine in Vietnam. Clin. Infect. Dis. 2016;63:S197–S204. doi: 10.1093/cid/ciw551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cuningham W., et al. Optimal timing of influenza vaccine during pregnancy: a systematic review and meta-analysis. Influenza Other Respir. Viruses. 2019;13:438–452. doi: 10.1111/irv.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eberhardt C.S., et al. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin. Infect. Dis. 2016;62:829–836. doi: 10.1093/cid/ciw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chu H.Y., Englund J.A. Maternal immunization. Clin. Infect. Dis. 2014;59:560–568. doi: 10.1093/cid/ciu327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feikin D.R., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barda N., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bar-On Y.M., et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N. Engl. J. Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andrews N., et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N. Engl. J. Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lauring A.S., et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376 doi: 10.1136/bmj-2021-069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chenchula S., et al. Current evidence on efficacy of COVID-19 booster dose vaccination against the omicron variant: a systematic review. J. Med. Virol. 2022 doi: 10.1002/jmv.27697. Published online March 4, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dick A., et al. Safety of 3rd SARS-CoV-2 vaccine (booster dose) during pregnancy. Am. J. Obstet. Gynecol. MFM. 2022 doi: 10.1016/j.ajogmf.2022.100637. Published online April 7, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gloeckner S., et al. Newborns' passive humoral SARS-CoV-2 immunity following heterologous vaccination of the mother during pregnancy. Am. J. Obstet. Gynecol. 2022;226:261–262. doi: 10.1016/j.ajog.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]