Abstract
PURPOSE
Women have more adverse events (AEs) from chemotherapy than men, but few studies have investigated sex differences in immune or targeted therapies. We examined AEs by sex across different treatment domains.
METHODS
We analyzed treatment-related AEs by sex in SWOG phase II and III clinical trials conducted between 1980 and 2019, excluding sex-specific cancers. AE codes and grade were categorized using the Common Terminology Criteria for Adverse Events. Symptomatic AEs were defined as those aligned with the National Cancer Institute's Patient-Reported Outcome–Common Terminology Criteria for Adverse Events; laboratory-based or observable/measurable AEs were designated as objective (hematologic v nonhematologic). Multivariable logistic regression was used, adjusting for age, race, and disease prognosis. Thirteen symptomatic and 14 objective AE categories were examined.
RESULTS
In total, N = 23,296 patients (women, 8,838 [37.9%]; men, 14,458 [62.1%]) from 202 trials experiencing 274,688 AEs were analyzed; 17,417 received chemotherapy, 2,319 received immunotherapy, and 3,560 received targeted therapy. Overall, 64.6% (n = 15,051) experienced one or more severe (grade ≥ 3) AEs. Women had a 34% increased risk of severe AEs compared with men (odds ratio [OR] = 1.34; 95% CI, 1.27 to 1.42; P < .001), including a 49% increased risk among those receiving immunotherapy (OR = 1.49; 95% CI, 1.24 to 1.78; P < .001). Women experienced an increased risk of severe symptomatic AEs among all treatments, especially immunotherapy (OR = 1.66; 95% CI, 1.37 to 2.01; P < .001). Women receiving chemotherapy or immunotherapy experienced increased severe hematologic AE. No statistically significant sex differences in risk of nonhematologic AEs were found.
CONCLUSION
The greater severity of both symptomatic AEs and hematologic AEs in women across multiple treatment modalities indicates that broad-based sex differences exist. This could be due to differences in AE reported, pharmacogenomics of drug metabolism/disposition, total dose received, and/or adherence to therapy. Particularly large sex differences were observed for patients receiving immunotherapy, suggesting that studying AEs from these agents is a priority.
INTRODUCTION
Sex differences in response to treatment have been observed in multiple disease settings.1,2 Yet despite growing evidence identifying patient sex as a predictor of disease sequelae, sex is rarely included in the evaluation of risk.2,3 This is surprising given the increased individualization of treatments in an era of precision medicine.
CONTEXT
Key Objective
Women have more adverse events (AEs) from chemotherapy than men, but almost no research has examined the experience of women compared with men receiving immune or targeted therapies. We examined AEs by sex across different treatment domains using combined data from 23,296 patients enrolled in 202 clinical trials from 1989 to 2019.
Knowledge Generated
Women had a 34% increased risk of severe AEs compared with men (odds ratio = 1.34; 95% CI, 1.27 to 1.42; P < .001), including a 49% increased risk among those receiving immunotherapy (odds ratio = 1.49; 95% CI, 1.24 to 1.78; P < .001).
Relevance
The greater severity of AEs in women across multiple treatment modalities indicates that broad-based sex differences exist. Particularly large sex differences were observed for patients receiving immunotherapy. These findings support the idea that sex may independently modulate drug toxicity, including for novel treatments.
For patients with cancer, female sex has been associated with an increased risk of adverse events (AEs) from cytotoxic therapy.4-6 However, almost no research has examined the experience of women and men receiving immune and targeted therapies. Differences in the toxic effects and outcomes from treatment may be due to multiple factors, such as subjective differences in reporting, or differences in pharmacokinetics, pharmacodynamics, and pharmacogenomics, or differences in drug therapy received.7-10 Indeed, sex-based differences in the experience of disease have recently been highlighted by worse symptoms and mortality observed for men with COVID-19 infection.11,12
In this study, we systematically examined the role of patient sex in the experience of both symptomatic and objective AEs across multiple cancer treatment paradigms including cytotoxic, immune, and targeted therapies. To improve power to detect possible sex differences in AEs, we combined data from several decades of therapeutic clinical trials. Patients receiving care under study are uniformly followed for acute AEs while on treatment; thus, analyses from a large-scale, well-characterized clinical trials database provides a unique opportunity to explore this issue.
METHODS
Data
Data were obtained from the SWOG Cancer Research Network. We included data on eligible patients evaluable for AE assessment from phase II and III clinical treatment trials over 30 years from July 1, 1989 to June 30, 2019; data were collected through January 1, 2020, thereby allowing at least 6-months for data collection. More recent follow-up was not included to limit the potential for confounding of the patient treatment experience by the COVID-19 pandemic.11,12 Trials in sex-specific or sex-dominant cancers (eg, prostate and breast) were excluded. The focus was on systemic therapies only. Study arms that included observation, autologous/allogeneic transplant, or surgery were excluded. Only the initial on-protocol treatment was evaluated. For patients enrolled in multiple studies, only data from the first study were included. Studies with fewer than 10 patients were excluded to limit study-level heterogeneity.
All study protocols included in this analysis were approved by local Institutional Review Boards, and all patients gave written informed consent.
AE Coding and Definitions
To establish a common reference, all AE codes and grades were mapped to Version 4 of the Common Terminology Criteria for Adverse Events (CTCAE).13 Given evidence that clinician reports of subjective AEs may be less reliable and under-reported, the NCI developed a set of patient-reported toxicity criteria for symptomatic AEs, termed Patient-Reported Outcome (PRO)-CTCAE.14 Symptomatic AEs were defined as those aligned with PRO-CTCAE using the PRO-CTCAE item library (Data Supplement, online only).15 Each AE in this item library was matched with the corresponding CTCAE v4.0 terminology.16 To ensure that the CTCAE codes and terms were used in a consistent manner over time, corresponding terms and grading from CTCAE v3.0 and v2.0 for each of the CTCAE v4.0 terms were identified.13 Borrowing from prior conceptualizations, laboratory-based or objective/measurable AEs were termed objective AEs.14,17 Some AE categories included both symptomatic and objective AEs (cardiovascular, skin, gastrointestinal, neurologic, respiratory, and visual); within these categories, individual AE types were categorized into symptomatic or objective domains on the basis of the NCI specifications.15,16 On the basis of observed patterns, objective AEs were further categorized as hematologic (blood/bone marrow) versus nonhematologic.
The CTCAE are categorized according to grade, ranging from 0 to 5, where 0 indicates no toxicity; 1, mild; 2, moderate; 3, severe; 4, life-threatening; and 5, death.13 Only the highest degree for each category of AE experienced during treatment was recorded. Unknown AEs and AEs with unknown grade were excluded. Also, AEs that were uniformly rare (< 5% incidence) for both women and men across all diseases (clotting, endocrine, lymphatics, musculoskeletal, and syndromes) were excluded, as were sex-specific AEs (gynecologic and male and female sexual and sex-specific urinary function).
Data Collection
Institutional reports of AEs were collected with study-specific case report forms. Historically, AE data were derived from study flow sheets. Beginning in 2002, toxicities were reported according to electronic case report forms. All data were reviewed and subject to confirmation by the study principal investigator.
Treatment Types
Patients were identified as having received cytotoxic therapy (with or without radiation therapy), immunotherapy, or targeted therapy (eg, kinase inhibitors) on the basis of published definitions and therapy names (Data Supplement).18-20 Specific categories within immunotherapy (immune checkpoint inhibitors and immune system modulators [cytokines and biologic response modifiers]) were examined separately. Vaccine-based interventions, a category of immunotherapy, were excluded. Also, patients receiving combinations of different domains of systemic therapies (eg, cytotoxic therapy plus targeted therapy) were excluded since attribution to a treatment domain could not be clearly ascertained.
Statistical Methods
The primary end point was the occurrence of one or more treatment-related severe or greater (grade ≥ 3) AEs. This level of AE severity was chosen because hospitalization is commonly required. We analyzed severe AEs by self-reported sex within treatment domains using multivariable logistic regression. The number of individual severe AE categories (≥ 5 v < 5, the cut point best approximating the overall median) was also analyzed by sex using a similar logistic regression approach. Thirteen symptomatic and 14 objective AE categories were examined. Regression models incorporated demographic factors such as age (< 65 v ≥ 65 years), race (Black v Others; White v Others), and prestudy obesity status (body mass index ≥ 30 [obese] v < 30). Each analysis was stratified by study-level prognosis (advanced, poor prognosis (2-year overall survival < 25%) versus advanced, intermediate (2-year overall survival 25%-75%) versus adjuvant; denoted as cancer stratum) to reflect disease severity and treatment intensity. Finally, we adjusted for decade of trial registration (< 2000 v 2000-2009 v 2010+ using indicator variables).
We also examined associations of sex with AE categories at each grade-specific cut point, with results depicted using heat maps. To test a global association within AE domains (symptomatic v objective) and overall, for each AE category (eg, sleep) for a given AE domain (eg, symptomatic), we calculated the mean of the z-scores from the individual, grade-specific logistic regression models. A one-sample t-test was used to determine whether this sample of z-scores statistically significantly differed from zero. Differences in clinical characteristics between groups were tested using chi-square tests for categorical data and t-statistics from quantile regression for medians.21
Analyses were conducted using SAS, version 9.4 (SAS Institute Inc) and R, version 4.0.2 (R Foundation for Statistical Computing). Two-sided P values are reported.
Sensitivity Analyses
Our base-case model adjusted for overall prognosis to account for disease severity and treatment intensity. Alternative modeling approaches were also used that accounted for potential trial effects, including adjusting for study as a conditioning variable in logistic regression and by treating study as a clustering variable using generalized estimating equations. Also, AE domains (eg, symptomatic) were examined within levels of adjuvant versus advanced disease for each treatment. Finally, we evaluated potential differences in the relationship between sex and AEs across treatment domains using interaction tests, rather than in subsets of treatments.
RESULTS
Cohort and Patient Characteristics
In total, N = 23,296 unique patients (women, 8,838 [37.9%]; men, 14,458 [62.1%]) from 202 trials experiencing 274,688 of the specified 27 AE categories were analyzed. Cohort sample sizes by treatment domain included 17,417 for chemotherapy, 2,319 for immunotherapy, and 3,560 for targeted therapy.
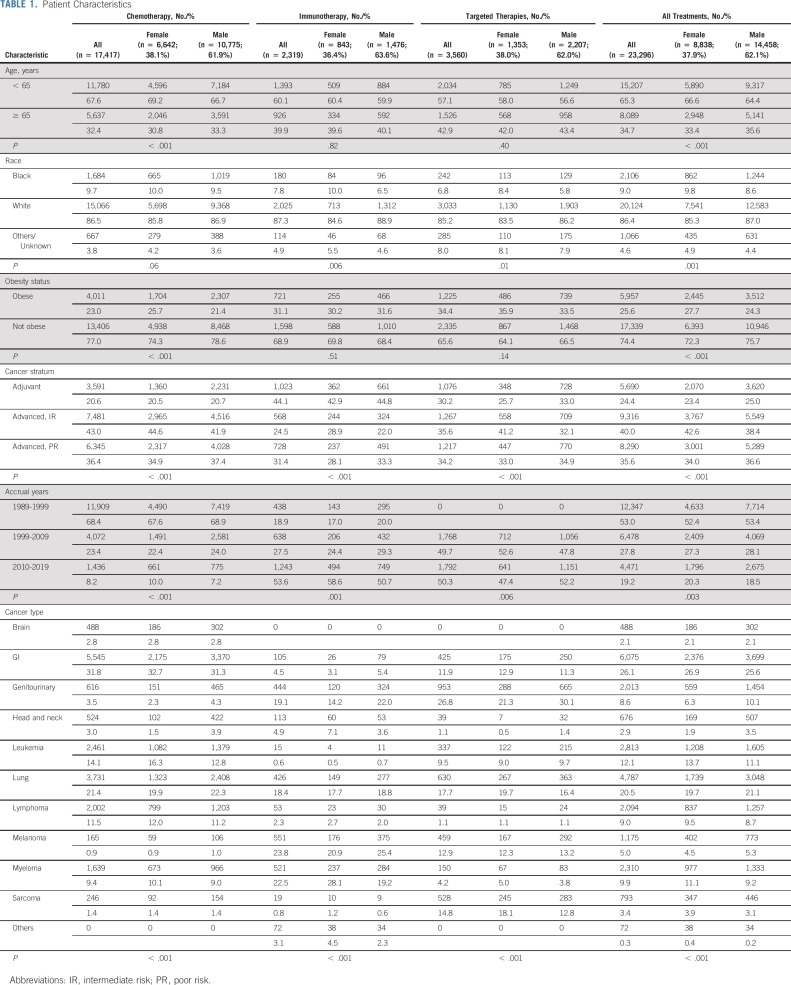
The most common cancers were gastrointestinal (26.1%), lung (20.5%), and leukemia (12.1%; Table 1). Overall, 34.7% of patients were 65 years or older, 9.0% were Black, and 25.6% were obese. Female patients were more likely to be < 65 years (66.6% v 64.4%, P < .001), Black (9.8% v 8.6%, P = .001), and obese (27.7% v 24.3%, P < .001). One quarter (24.4%) of patients received adjuvant treatment. Chemotherapy was particularly common in trials from 1989 to 1999 (68.4%), whereas immunotherapy (53.6%) and targeted therapies (50.3%) were more common from 2010 to 2019.
TABLE 1.
Patient Characteristics
The median treatment time was 88 (interquartile range, 34-170) days for women and 84 (interquartile range, 37-167) days for men (P = .16).
Severe AEs
Among all patients, 64.6% (n = 15,051) experienced one or more severe AEs. Women had a 34% increased risk of severe toxicity compared with men (68.6% v 62.2%, odds ratio [OR] = 1.34; 95% CI, 1.27 to 1.42; P < .001; Fig 1). An increased risk of severe toxicity for women versus men was consistently observed for each treatment domain, with the greatest increased risk for immunotherapy (OR = 1.49; 95% CI, 1.24 to 1.78; P < .001).
FIG 1.
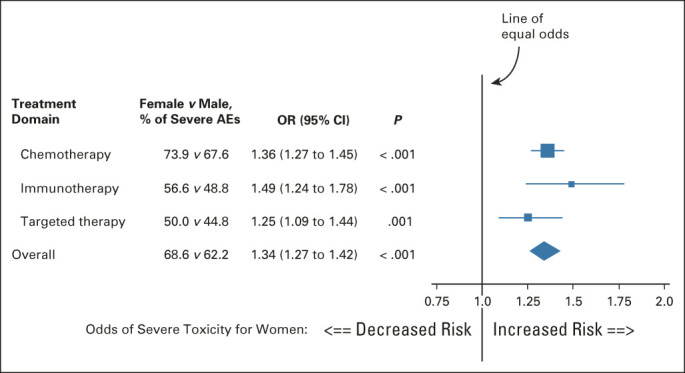
Forest plot of the association of patient sex with the risk of severe AEs. The boxes indicate the OR, and the horizontal lines indicate the 95% CIs. Boxes to the right of the vertical line (the line of equal odds) indicate increased risk of severe AEs for females, and to the left, for males. For each treatment domain, women had an increased risk of severe AEs of any kind. AE, adverse event; OR, odds ratio.
Number of Toxicity Categories
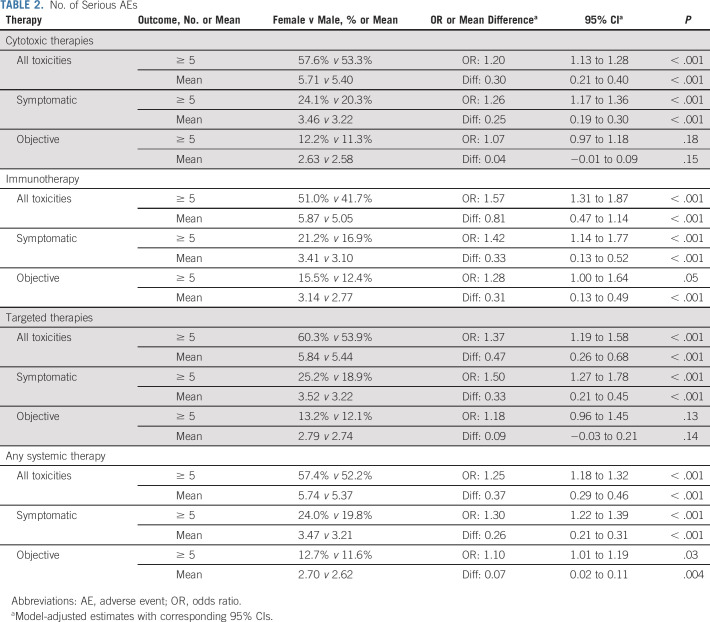
Among all treatments, women had a 25% higher risk of experiencing ≥ 5 severe AEs (females, 57.4% v males, 52.2%, OR = 1.25; 95% CI, 1.18 to 1.32; P < .001; Table 2). The increased risk of ≥ 5 severe AEs was greater for symptomatic AEs versus objective AEs. This pattern was generally consistent across treatment domains. The association was strongest for women receiving immunotherapy (OR = 1.42; 95% CI, 1.14 to 1.77; P < .001) or targeted therapy (OR = 1.50; 95% CI, 1.27 to 1.78; P < .001).
TABLE 2.
No. of Serious AEs
Symptomatic and Objective AEs by Sex
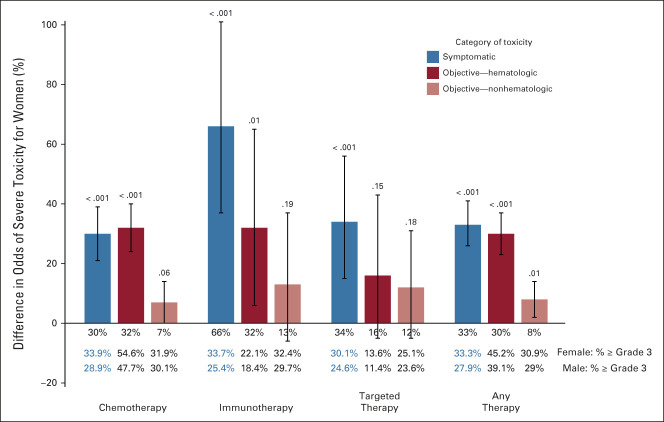
Women were at 30% or higher risk of experiencing symptomatic (female, 33.3% v male, 27.9%; OR = 1.33; 95% CI, 1.26 to 1.41; P < .001) and hematologic (female, 45.2% v male, 39.1%; OR = 1.30; 95% CI, 1.23 to 1.37; P < .001) AEs (Fig 2). The risk of objective, nonhematologic AEs was also statistically significantly greater in women (female, 30.9% v male, 29.0%; OR = 1.08; 95% CI, 1.02 to 1.14; P = .01). Similar patterns of notably higher increased risk of symptomatic and hematologic AEs for females versus males were greatest among patients treated with immunotherapy (female, 33.7% v male, 25.4%; OR = 1.66; 95% CI, 1.37 to 2.01; P < .001). The risk of objective, nonhematologic AEs was similar for females compared with males across the treatment domains.
FIG 2.
Difference in the odds of severe AEs by category of adverse events. AEs were categorized as symptomatic versus objective and hematologic versus objective and nonhematologic. The vertical bars indicate the percentage increased odds, and the vertical lines show the 95% CIs. The observed percentage of patients experiencing severe (grade ≥ 3) AEs for a given category are also shown. AE, adverse event.
Association of Sex and AE Outcomes in Subgroups of Immunotherapy Treatment
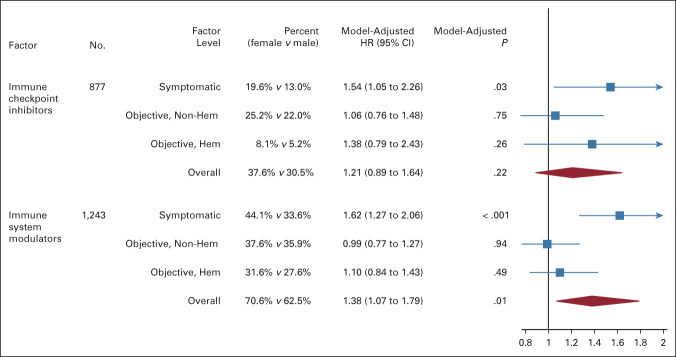
Within subsets of patients receiving immunotherapy, the risk of symptomatic AEs was greater for females receiving immune checkpoint inhibitors (n = 877; female, 19.6% v male, 13.0%; OR = 1.54; 95% CI, 1.05 to 2.26; P = .03) and immune system modulators (n = 1,243; female, 44.1% v male, 33.6%; OR = 1.62; 95% CI, 1.27 to 2.06; P < .001; Fig 3). This association was not observed for nonsymptomatic AEs.
FIG 3.
Forest plot of the association of patient sex with the risk of severe AEs in subgroups of immunotherapy treatment. The boxes indicate the OR, and the horizontal lines indicate the 95% CIs. Boxes to the right of the vertical line (the line of equal odds) indicate increased risk of severe AEs for females, and to the left, for males. AE, adverse event; HR, hazard ratio.
Individual Categories of Symptomatic and Objective AEs by Sex
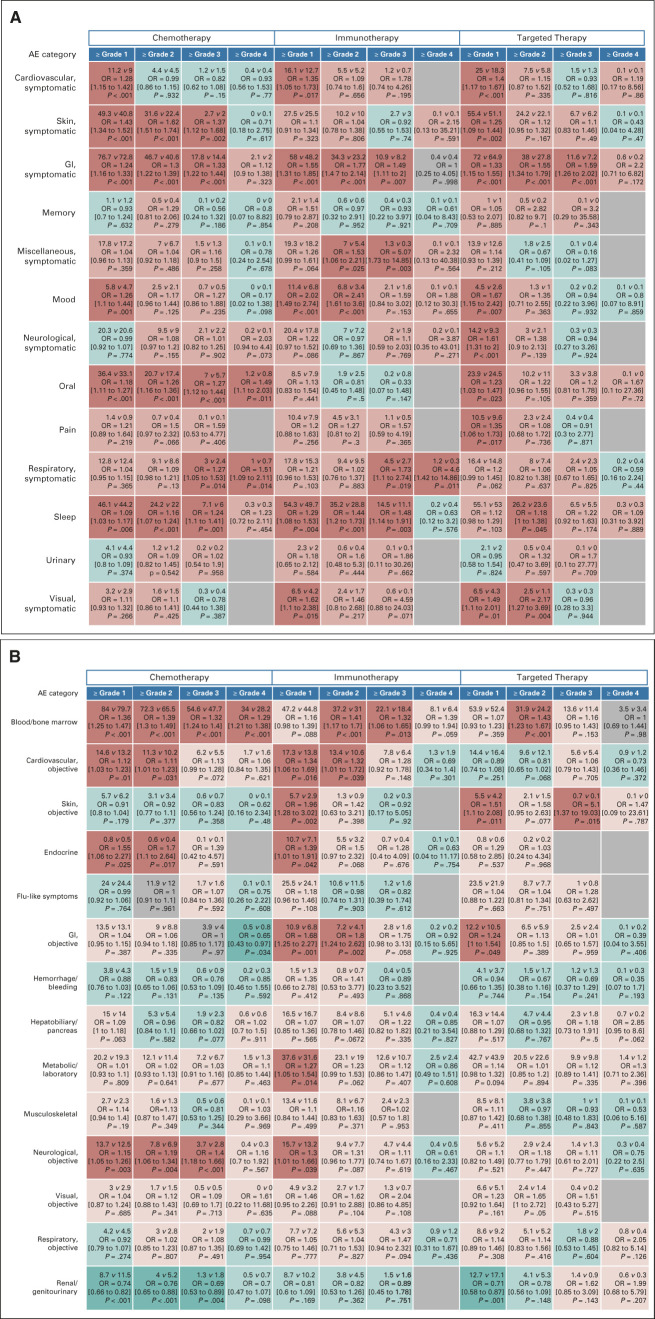
Figures 4A and 4B provide a heat map representation of the individual categories of symptomatic and objective AEs by sex, for different cut point levels (grades 1-4) of severity. Among symptomatic AEs, women receiving chemotherapy had statistically significantly increased risk of symptomatic skin AEs (grades ≥ 1, ≥ 2, and ≥ 3) and oral AEs (grades ≥ 1 through ≥ 4; Fig 4A). For each treatment domain, women were also at statistically significantly increased risk of symptomatic gastrointestinal AEs at grades ≥ 1 through ≥ 3. Among those receiving chemotherapy and immunotherapy, women were at increased risk of sleep-related AEs. Among 141 evaluable comparisons, females had a statistically significantly increased risk of a specific AE category at a given grade cut point 43 times, compared with 0 for males (30.5% v 0.0%, P < .001).
FIG 4.
Risk of AEs by sex for individual AE categories by the treatment domain and grade cut point. For each AE, the proportion of patients with grade ≥ 1 through ≥ 4 AEs was analyzed in a logistic regression model, adjusted for covariates. (A) Symptomatic AEs and (B) objective AEs. Within each cell, the observed percentage of women versus men experiencing at least the indicated grade level of AE, with the corresponding multivariable logistic regression model ORs, 95% CIs, and P-values, are shown. OR > 1.0 indicates greater risk of a given AE at the cut point level within the specific treatment domain for females and < 1.0 for males. Instances where women had greater odds of AEs than men are coded red, with blue indicating the reverse and gray indicating no difference or missing because of lack of events. The color intensity corresponds to greater divergence by sex in a given direction; thus, the darker red or darker blue indicates statistically significant (P < .05) findings and lighter red and blue indicate nonstatistically significant trends in favor of greater risk for females and males, respectively. The depiction thus represents a heat map of toxicity outcomes. The predominance of red over blue coloring indicates a generalized pattern of worse AE outcomes for women than men. AE, adverse event; OR, odds ratio.
Among objective AEs, women were at increased risk of hematologic AEs among those receiving chemotherapy (grades ≥ 1 through ≥ 4), immunotherapy (grades ≥ 2 and ≥ 3), and targeted therapy (grade ≥ 2). Among those receiving chemotherapy or immunotherapy, women had statistically significantly increased risk of objective cardiovascular AEs (grades ≥ 1 and ≥ 2). Among 154 evaluable comparisons, females had a statistically significantly increased risk of a specific AE category at a given grade cut point 25 times, compared with five for males (16.2% v 3.2%, P = .009).
Among all 27 symptomatic and objective AE categories, among 295 evaluable comparisons, there were 68 instances where females had a statistically significantly increased risk of a specific AE category at a given grade cut point and five instances for males (23.1% v 1.7%, P < .001).
Sensitivity Analyses
Nearly all estimates using alternative analytic approaches corresponded closely to the base-case analysis, suggesting that the primary analysis results are robust to the modeling approach (Data Supplement).
When results were stratified by adjuvant versus advanced disease, the increased risk of severe symptomatic and hematologic AEs for women was greater among patients on adjuvant trials, particularly those using chemotherapy (interaction P value ≤ .01). These patterns were consistent but less extreme in patients receiving immune or targeted agents (Data Supplement). Importantly, there remained a strong, statistically significantly increased risk of severe symptomatic and hematologic toxicities in women versus men in advanced disease trials.
Finally, estimates of the increased risk of AEs for women versus men were similar in an aggregate model (Data Supplement).
DISCUSSION
Our study showed that women are at substantially greater risk of severe symptomatic AEs across multiple treatment domains, including patients receiving immune checkpoint inhibitor therapy and targeted therapies with kinase inhibitors. In fact, women receiving immunotherapy had a 66% increased risk of symptomatic AEs compared with men. Moreover, women were more likely to experience severe hematologic AEs among those receiving chemotherapy and immunotherapy. These results are robust because of the breadth of the data, the large sample size, and the quality of the prospective, clinical trial–based data.
Major research advisory and regulatory agencies of the federal government, including the National Institutes of Health and US Food and Drug Administration, have issued mandates and guidelines to better understand the possible disease outcome differences between men and women.22-25 Identification of possible sex-related differences might lead to potential sex-specific interventions.2 There are several possible explanations for sex differences in AEs. For instance, given average body type differences, women may receive greater relative dose, although importantly we included covariate adjustment for obesity status to account for body type.26-28 It has been suggested that medication adherence for oral therapies may differ by sex29,30 although this may not apply as much in the trial setting. Finally, biases may exist in the reporting or interpretation of AEs, or men and women may differentially report AEs because of potential sex-related differences in symptom perception.31,32 However, in our study, objectively assessed hematologic AEs were also more commonly experienced in women.
Pharmacokinetics and pharmacodynamics could play a role. For the systemic therapies examined in this study, the literature is mixed. For instance, studies have found that women have lower capacity to clear fluorouracil than men.33,34 By contrast, no differences between men and women in drug clearance were found in a study of patients receiving imatinib.35 Evidence is conflicting regarding differential clearance rates of doxorubicin by sex.36,37 Pharmacogenetics (ie, how drug metabolism and elimination of genetic polymorphisms modulate drug responses) may also vary by sex. For instance, survival in female patients with metastatic colon cancer treated with fluorouracil has been found to differ according to methylenetetrahydrofolate reductase gene polymorphism, but not in male patients.38 The protein ABCG2/BCRP/MXR/ABCP, an ATP-binding cassette transporter, influences the absorption, distribution, and excretion of drugs and may be regulated by sex hormones, with potential differential effects on drug toxicity for agents that interact with high affinity with ABCG2.39-41 In 2016, Yuan et al42 conducted a comprehensive analysis of sex differences in molecular profiles for 13 cancers, identifying two distinct groups of genes that predicted distinct incidence and mortality profiles. The gut microbiome may also be implicated given its role in regulating metabolic and immune inflammatory pathways.43
Findings regarding individual AE categories may serve as hypotheses for future research. The occurrence of sleep-related AEs among women receiving chemotherapy and immunotherapy is intriguing and could be a function of hormonal effects interacting with cancer treatment.44 Disrupted sleep could contribute to an increased risk of poor cardiovascular outcomes.45,46 The more common experience of severe hematologic AEs in female patients has been observed in patients receiving adjuvant treatment for colon cancer.4,47 Female sex has been routinely identified as a risk factor for anthracycline-induced cardiotoxicity for patients with pediatric cancer.48 Our findings indicate that among nonsex-specific cancers, the risk of cardiotoxicity may be higher for women than men, especially among those treated with chemotherapy or immunotherapy. Although the observation of increased risk of severe symptomatic gastrointestinal AEs is consistent with previous findings among those receiving chemotherapy, we have now shown that sex-based differences in gastrointestinal AEs in immunotherapy and targeted therapy exist as well.49-51
Our study has limitations. First, clinical trial patients tend to be younger and healthier than nontrial patients on average, and therefore, toxicities may be greater in nontrial patients.52,53 Also, because only the worst toxicity grade in each category was recorded, our data do not allow for observation of toxicity patterns over time. Furthermore, toxicity must be considered in the context of survival; indeed, increased toxicity for women compared with men has been shown to occur in conjunction with improved survival.49,54 However, the causal relationship between AEs and survival may be challenging to interpret since improved survival may allow more time/exposure to develop AEs or alternatively, increased AEs may represent, on average, increased delivery/efficacy of the anticancer agents, which could result in improved survival. In addition, reporting of AE data may be subject to misclassification, especially when CTCAE criteria are unable to depict subtle symptoms. For this reason, our primary analysis was based on severe AEs that are more clearly recognizable and commonly require hospitalization. Some conditions might have existed before study although all toxicities that we analyzed were deemed possibly, probably, or definitely related to treatment. For instance, some patients might have had existing sarcopenia, which might influence toxicity patterns and could not be fully accounted for by adjusting for obesity status. Also, although we were able to define symptomatic AEs, these symptomatic AEs were not actually reported by patients themselves. In this context, the incorporation of patient-reported symptomatic AEs into routine monitoring could shed further light on potential sex-related differences. Finally, although pooling across clinical trial databases is necessary to enhance statistical power to identify trends in AEs by sex,2 pooling may also mask potentially meaningful associations.
Ideally, the goal in cancer therapy is to maximize treatment efficacy while limiting toxicity. Increasingly, treatment will be individualized on the basis of patient and tumor characteristics to optimize this relationship. In this context, our findings are supportive of the argument advanced by Özdemir et al, who stated that “sex as an independent modulator of drug efficacy and toxicity merits consideration for further individualization of treatments.”2 Indeed, if confirmed, our findings suggest that underlying mechanisms may result in generalized worse toxicity outcomes for women, with or without corresponding survival improvements or detriments. Therefore, more awareness of symptom differences or reporting differences in women versus men is needed. A better understanding of the nature of the underlying mechanisms could potentially lead to interventions or delivery modifications to reduce toxicity in women (in particular). In such cases, cancer treatment may then be able to be simultaneously modified or augmented, with the ultimate goal of extending therapeutic benefits.
Kathy S. Albain
Honoraria: Encore Medical Education
Research Funding: Seattle Genetics (Inst), Quantum Leap Healthcare Collaborative (Inst)
Other Relationship: Seattle Genetics
Michael LeBlanc
Consulting or Advisory Role: Agios
Carolyn C. Gotay
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
N. Lynn Henry
Research Funding: Blue Note Therapeutics (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/27894/summary
Michael J. Fisch
Employment: AIM Specialty Health
Stock and Other Ownership Interests: Anthem
Patents, Royalties, Other Intellectual Property: Healthcore, Inc, A subsidiary of Anthem, Inc
Open Payments Link: https://openpaymentsdata.cms.gov/physician/767578
Shing M. Lee
Consulting or Advisory Role: PTC Therapeutics
Research Funding: Merck, Genentech/Roche
Dawn L. Hershman
Consulting or Advisory Role: AIM Specialty Health
No other potential conflicts of interest were reported.
SUPPORT
Supported by National Institutes of Health, National Cancer Institute, National Cancer Trials Network grants U10CA180888 and U10CA180819, and Community Oncology Research Program (NCORP) grant UG1CA189974 and by The Hope Foundation for Cancer Research (SWOG Impact Award grant [S.M.L./J.M.U.] and Secondary Data Analysis grant [J.M.U.]).
AUTHOR CONTRIBUTIONS
Conception and design: Joseph M. Unger, Kathy S. Albain, Lori M. Minasian, Carolyn C. Gotay, Shing M. Lee, Dawn L. Hershman
Financial support: Joseph M. Unger
Administrative support: Charles D. Blanke
Collection and assembly of data: Joseph M. Unger, Riha Vaidya
Provision of study material or patients: Kathy S. Albain
Data analysis and interpretation: Joseph M. Unger, Riha Vaidya, Michael LeBlanc, Carolyn C. Gotay, N. Lynn Henry, Michael J. Fisch, Shing M. Lee, Charles D. Blanke, Dawn L. Hershman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Sex Differences in Risk of Severe Adverse Events in Patients Receiving Immunotherapy, Targeted Therapy, or Chemotherapy in Cancer Clinical Trials
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kathy S. Albain
Honoraria: Encore Medical Education
Research Funding: Seattle Genetics (Inst), Quantum Leap Healthcare Collaborative (Inst)
Other Relationship: Seattle Genetics
Michael LeBlanc
Consulting or Advisory Role: Agios
Carolyn C. Gotay
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
N. Lynn Henry
Research Funding: Blue Note Therapeutics (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/27894/summary
Michael J. Fisch
Employment: AIM Specialty Health
Stock and Other Ownership Interests: Anthem
Patents, Royalties, Other Intellectual Property: Healthcore, Inc, A subsidiary of Anthem, Inc
Open Payments Link: https://openpaymentsdata.cms.gov/physician/767578
Shing M. Lee
Consulting or Advisory Role: PTC Therapeutics
Research Funding: Merck, Genentech/Roche
Dawn L. Hershman
Consulting or Advisory Role: AIM Specialty Health
No other potential conflicts of interest were reported.
REFERENCES
- 1.Legato MJ, Johnson PA, Manson JE: Consideration of sex differences in medicine to improve Health care and patient outcomes. JAMA 316:1865-1866, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Özdemir BC, Csajka C, Dotto GP, et al. : Sex differences in efficacy and toxicity of systemic treatments: An undervalued issue in the era of precision Oncology. J Clin Oncol 36:2680-2683, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Garcia M, Mulvagh SL, Merz CN, et al. : Cardiovascular disease in women: Clinical perspectives. Circ Res 118:1273-1293, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chansky K, Benedetti J, Macdonald JS: Differences in toxicity between men and women treated with 5-fluorouracil therapy for colorectal carcinoma. Cancer 103:1165-1171, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Gotay CC, Phillips PH, Cheson BD: Male-female differences in the impact of cancer therapy. Oncology (Williston Park) 7:67-74, 1993. discussion 74, 77 [PubMed] [Google Scholar]
- 6.Sloan JA, Goldberg RM, Sargent DJ, et al. : Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. J Clin Oncol 20:1491-1498, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Anderson GD: Gender differences in pharmacological response. Int Rev Neurobiol 83:1-10, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Domecq C, Naranjo CA, Ruiz I, et al. : Sex-related variations in the frequency and characteristics of adverse drug reactions. Int J Clin Pharmacol Ther Toxicol 18:362-366, 1980 [PubMed] [Google Scholar]
- 9.Soldin OP, Mattison DR: Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet 48:143-157, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Huang Y: Pharmacogenomics of sex difference in chemotherapeutic toxicity. Curr Drug Discov Technol 4:59-68, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Scully EP, Haverfield J, Ursin RL, et al. : Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol 20:442-447, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sha J, Qie G, Yao Q, et al. : Sex differences on clinical characteristics, severity, and mortality in adult patients with COVID-19: A multicentre retrospective study. Front Med (Lausanne) 8:607059, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Division of Cancer Treatment & Diagnosis : Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40 [Google Scholar]
- 14.Basch E, Reeve BB, Mitchell SA, et al. : Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 106:dju244, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health, National Cancer Institute : Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE™). https://healthcaredelivery.cancer.gov/pro-ctcae/ [Google Scholar]
- 16.National Cancer Institute. Division of Cancer Control & Population Sciences : Correspondence Between PRO-CTCAE and CTCAE Terminologies. https://healthcaredelivery.cancer.gov/pro-ctcae/terms.html [Google Scholar]
- 17.Bentzen SM, Dörr W, Anscher MS, et al. : Normal tissue effects: Reporting and analysis. Semin Radiat Oncol 13:189-202, 2003 [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute : Targeted cancer therapies. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet#q4
- 19.National Cancer Institute : Immunotherapy to treat cancer. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy
- 20.National Cancer Institue : Chemotherapy to Treat Cancer.https://www.cancer.gov/about-cancer/treatment/types/chemotherapy [Google Scholar]
- 21.Koenker R, Bassett GW: Regression quantiles. Econometrica 46:33-50, 1978 [Google Scholar]
- 22.National Institutes of Health : NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm [Google Scholar]
- 23.Institute of Medicine : Exploring the Biological Contributions to Human Health: Does Sex Matter? Washington, DC, The National Academies Press, 2001. 10.17226/10028 [DOI] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration : FDA Research, Policy, and Workshops on Women in Clinical Trials. https://www.fda.gov/science-research/womens-health-research/fda-research-policy-and-workshops-women-clinical-trials [Google Scholar]
- 25.National Institutes of Health, Office of Research on Women's Health : NIH Policy on Sex as a Biological Variable. https://orwh.od.nih.gov/sex-gender/nih-policy-sex-biological-variable [Google Scholar]
- 26.Singh S, Parulekar W, Murray N, et al. : Influence of sex on toxicity and treatment outcome in small-cell lung cancer. J Clin Oncol 23:850-856, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Griggs JJ, Mangu PB, Temin S, et al. : Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. JCO Oncol Pract 8:e59-e61, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HI, Lim H, Moon A: Sex differences in cancer: Epidemiology, genetics and therapy. Biomol Ther (Seoul) 26:335-342, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein SL, Morgan R: The impact of sex and gender on immunotherapy outcomes. Biol Sex Differ 11:24, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rolnick SJ, Pawloski PA, Hedblom BD, et al. : Patient characteristics associated with medication adherence. Clin Med Res 11:54-65, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Wijk CM, Kolk AM: Sex differences in physical symptoms: The contribution of symptom perception theory. Soc Sci Med 45:231-246, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Palma-Gudiel H, Peralta V, Deuschle M, et al. : Epigenetics-by-sex interaction for somatization conferred by methylation at the promoter region of SLC6A4 gene. Prog Neuropsychopharmacol Biol Psychiatry 89:125-131, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Milano G, Etienne MC, Cassuto-Viguier E, et al. : Influence of sex and age on fluorouracil clearance. J Clin Oncol 10:1171-1175, 1992 [DOI] [PubMed] [Google Scholar]
- 34.Port RE, Daniel B, Ding RW, et al. : Relative importance of dose, body surface area, sex, and age for 5-fluorouracil clearance. Oncology 48:277-281, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Delbaldo C, Chatelut E, Ré M, et al. : Pharmacokinetic-pharmacodynamic relationships of imatinib and its main metabolite in patients with advanced gastrointestinal stromal tumors. Clin Cancer Res 12:6073-6078, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Dobbs NA, Twelves CJ, Gillies H, et al. : Gender affects doxorubicin pharmacokinetics in patients with normal liver biochemistry. Cancer Chemother Pharmacol 36:473-476, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Hu OY, Chang SP, Jame JM, et al. : Pharmacokinetic and pharmacodynamic studies with 4'-epi-doxorubicin in nasopharyngeal carcinoma patients. Cancer Chemother Pharmacol 24:332-337, 1989 [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Press OA, Haiman CA, et al. : Association of methylenetetrahydrofolate reductase gene polymorphisms and sex-specific survival in patients with metastatic colon cancer. J Clin Oncol 25:3726-3731, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Allikmets R, Schriml LM, Hutchinson A, et al. : A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res 58:5337-5339, 1998 [PubMed] [Google Scholar]
- 40.Krishnamurthy P, Schuetz JD: Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol 46:381-410, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Merino G, van Herwaarden AE, Wagenaar E, et al. : Sex-dependent expression and activity of the ATP-binding cassette transporter breast cancer resistance protein (BCRP/ABCG2) in liver. Mol Pharmacol 67:1765-1771, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Yuan Y, Liu L, Chen H, et al. : Comprehensive characterization of molecular differences in cancer between male and female patients. Cancer Cell 29:711-722, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pala L, Nezi L, De Pas T, et al. : Sex differences in efficacy and toxicity of systemic cancer treatments: Role of the microbiome. J Clin Oncol 37:439, 2019 [DOI] [PubMed] [Google Scholar]
- 44.Han MK, Arteaga-Solis E, Blenis J, et al. : Female sex and gender in lung/sleep health and disease. Increased understanding of basic biological, pathophysiological, and behavioral mechanisms leading to better health for female patients with lung disease. Am J Respir Crit Care Med 198:850-858, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang T, Mariani S, Redline S: Sleep irregularity and risk of cardiovascular events: The multi-ethnic study of atherosclerosis. J Am Coll Cardiol 75:991-999, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Javaheri S, Redline S: Insomnia and risk of cardiovascular disease. Chest 152:435-444, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cristina V, Mahachie J, Mauer M, et al. : Association of patient sex with chemotherapy-related toxic effects: A retrospective analysis of the PETACC-3 trial conducted by the EORTC Gastrointestinal Group. JAMA Oncol 4:1003-1006, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meiners B, Shenoy C, Zordoky BN: Clinical and preclinical evidence of sex-related differences in anthracycline-induced cardiotoxicity. Biol Sex Differ 9:38, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Athauda A, Nankivell M, Langley RE, et al. : Impact of sex and age on chemotherapy efficacy, toxicity and survival in localised oesophagogastric cancer: A pooled analysis of 3265 individual patient data from four large randomised trials (OE02, OE05, MAGIC and ST03). Eur J Cancer 137:45-56, 2020 [DOI] [PubMed] [Google Scholar]
- 50.Davidson M, Wagner AD, Kouvelakis K, et al. : Influence of sex on chemotherapy efficacy and toxicity in oesophagogastric cancer: A pooled analysis of four randomised trials. Eur J Cancer 121:40-47, 2019 [DOI] [PubMed] [Google Scholar]
- 51.Sharma R, Cunningham D, Smith P, et al. : Inflammatory (B) symptoms are independent predictors of myelosuppression from chemotherapy in Non-Hodgkin Lymphoma (NHL) patients—Analysis of data from a British National Lymphoma Investigation phase III trial comparing CHOP to PMitCEBO. BMC Cancer 9:153, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hutchins LF, Unger JM, Crowley JJ, et al. : Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 341:2061-2067, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Unger JM, Hershman DL, Fleury ME, et al. : Association of patient comorbid conditions with cancer clinical trial participation. JAMA Oncol 5:326-333, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wakelee HA, Wang W, Schiller JH, et al. : Survival differences by sex for patients with advanced non-small cell lung cancer on Eastern Cooperative Oncology Group trial 1594. J Thorac Oncol 1:441-446, 2006 [PubMed] [Google Scholar]