Abstract
PURPOSE
Preclinical data suggest the combination of an anti–programmed death receptor 1 antibody plus dabrafenib and trametinib to have superior antitumor activity compared with dabrafenib plus trametinib alone. These observations are supported by translational evidence suggesting that immune checkpoint inhibitors plus targeted therapy may improve treatment outcomes in patients with BRAF V600–mutant metastatic melanoma. COMBI-i is a phase III trial evaluating spartalizumab, an anti–programmed death receptor 1 antibody, in combination with dabrafenib and trametinib (sparta-DabTram), versus placebo plus dabrafenib and trametinib (placebo-DabTram) in patients with BRAF V600–mutant unresectable or metastatic melanoma.
METHODS
Patients received spartalizumab 400 mg intravenously every 4 weeks plus dabrafenib 150 mg orally twice daily and trametinib 2 mg orally once daily or placebo-DabTram. Participants were age ≥ 18 years with unresectable or metastatic BRAF V600–mutant melanoma. The primary end point was investigator-assessed progression-free survival. Overall survival was a key secondary end point (ClinicalTrials.gov identifier: NCT02967692).
RESULTS
At data cutoff (July 1, 2020), the median progression-free survival was 16.2 months (95% CI, 12.7 to 23.9 months) in the sparta-DabTram arm versus 12.0 months (95% CI, 10.2 to 15.4 months) in the placebo-DabTram arm (hazard ratio, 0.82 [95% CI, 0.66 to 1.03]; P = .042 [one-sided; nonsignificant]). The objective response rates were 69% (183 of 267 patients) versus 64% (170 of 265 patients), respectively. Grade ≥ 3 treatment-related adverse events occurred in 55% (146 of 267) of patients in the sparta-DabTram arm and 33% (88 of 264) in the placebo-DabTram arm.
CONCLUSION
The study did not meet its primary end point; broad first-line use of sparta-DabTram is not supported by these results. Further biomarker-driven investigation may identify patient subpopulations who could benefit from checkpoint inhibitor plus targeted therapy combinations.
INTRODUCTION
Systemic therapies have revolutionized the management of advanced melanoma, with significant improvements in overall survival (OS) observed in patients treated with both BRAF- and MEK-targeted therapies as well as immune checkpoint inhibitors.1-5 Despite these advancements, nearly half of the patients with unresectable or metastatic melanoma die within 5 years of starting treatment. In patients with BRAF V600–mutant disease, accounting for approximately 40% of all melanoma cases, current guidelines recommend sequential use of these two treatment strategies.6-8 However, translational evidence has suggested that combining checkpoint inhibition with BRAF and MEK inhibition may help improve treatment outcomes in patients with BRAF V600–mutant melanoma.9-11
CONTEXT
Key Objective
It was hypothesized that upfront combination of immune checkpoint inhibitors and BRAF plus MEK inhibitor targeted therapies might yield durable responses in more patients with BRAF V600–mutant metastatic melanoma. Thus, we conducted this randomized, double-blind, placebo-controlled, phase III trial (COMBI-i; ClinicalTrials.gov identifier: NCT02967692) to evaluate spartalizumab plus dabrafenib and trametinib (sparta-DabTram) versus placebo plus dabrafenib and trametinib (placebo-DabTram) in this patient population.
Knowledge Generated
The trial did not meet its primary end point of improved progression-free survival with sparta-DabTram versus placebo-DabTram. Combination of dabrafenib plus trametinib with spartalizumab was associated with higher rates of adverse events and dose modifications than dabrafenib plus trametinib alone.
Relevance
Sparta-DabTram exhibited modest efficacy and increased toxicity over placebo-DabTram. These results do not support broad use of first-line immunotherapy plus targeted therapy combination, but they provide additional data toward understanding the optimal application of these therapeutic classes in patients with BRAF V600–mutant metastatic melanoma.
Although part 3 of the phase II KEYNOTE-022 study (ClinicalTrials.gov identifier: NCT02130466) did not meet the primary end point of investigator-assessed progression-free survival (PFS) at initial analysis, a second analysis, with an extended median follow-up of 36.6 months, demonstrated a numerically higher PFS rate in patients treated with the anti–programmed death receptor 1 (PD-1) antibody pembrolizumab in combination with dabrafenib and trametinib than in patients treated with dabrafenib plus trametinib alone (hazard ratio [HR], 0.53 [95% CI, 0.34 to 0.83]).12 In addition, the anti–programmed death ligand 1 (PD-L1) antibody atezolizumab in combination with vemurafenib plus cobimetinib has also been evaluated in the phase III IMspire150 study (ClinicalTrials.gov identifier: NCT02908672).13 The triple combination demonstrated a significantly improved median PFS of 15.1 months compared with 10.6 months with vemurafenib plus cobimetinib alone (HR, 0.78 [95% CI, 0.63 to 0.97]; P = .0249 [two-sided]). These placebo-controlled studies provide clinical evidence of first-line treatment efficacy with checkpoint inhibitor plus targeted therapy combination.
COMBI-i (ClinicalTrials.gov identifier: NCT02967692) is a global, randomized, phase III trial evaluating the safety and efficacy of the anti–PD-1 antibody spartalizumab in combination with dabrafenib and trametinib (sparta-DabTram).14,15 Findings from the open-label parts 1 (safety run-in; n = 9) and 2 (biomarker cohort; n = 27) showed the treatment regimen to have an acceptable safety profile and promising efficacy. The objective response rate (ORR) was 78% (28 of 36 patients), including a complete response rate of 44% (16 of 36 patients).15 Here, we report the primary analysis of the randomized, double-blind, placebo-controlled part 3, comparing sparta-DabTram with placebo plus dabrafenib and trametinib (placebo-DabTram) in patients with BRAF V600–mutant unresectable or metastatic melanoma.
METHODS
Study Design and Participants
COMBI-i (ClinicalTrials.gov identifier: NCT02967692) is a global phase III study consisting of three parts: a safety run-in (part 1); a biomarker cohort (part 2); and the randomized, double-blind, placebo-controlled part 3, which was conducted at 179 centers in 29 countries worldwide. The trial was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. The study Protocol (online only) was approved by the institutional review board or human research ethics committee at each site. All patients provided written informed consent.
Part 3 enrolled patients age ≥ 18 years with histologically confirmed unresectable or metastatic (according to the American Joint Committee on Cancer's Cancer Staging Manual, 7th edition) BRAF V600–mutant cutaneous melanoma. Additional eligibility criteria included no clinically active brain metastases, Eastern Cooperative Oncology Group performance status ≤ 2, and no prior systemic anticancer treatment for unresectable or metastatic melanoma. Prior locoregional, neoadjuvant, and/or adjuvant therapy was acceptable as long as it did not occur within 6 months of the start of study treatment. Full inclusion and exclusion criteria are provided in the study Protocol.
Random Assignment and Blinding
Patients were randomly assigned (1:1) to receive sparta-DabTram (treatment arm) or placebo-DabTram (control arm). Dabrafenib plus trametinib is an internationally approved treatment for patients with BRAF V600–mutant unresectable or metastatic melanoma and was considered an accepted comparator for this patient population at the time of study initiation.16,17 Random assignment by investigators or study site staff followed a random permuted block scheme and was conducted using interactive response technology. Treatment identity was blinded from the time of random assignment until the primary analysis database lock. Patients could be unblinded to manage medical emergencies, for regulatory reporting purposes or, if required, to determine subsequent therapy following progressive disease (PD); unblinded patients discontinued study treatment but remained in follow-up. Patients were stratified by Eastern Cooperative Oncology Group performance status (0 v 1 v 2) and lactate dehydrogenase levels (< 1 × upper limit of normal [ULN] v ≥ 1 to < 2 × ULN v ≥ 2 × ULN).
Procedures
COMBI-i part 1 determined the recommended phase III regimen of intravenous spartalizumab 400 mg every 4 weeks in combination with the approved full doses of oral dabrafenib 150 mg twice daily and oral trametinib 2 mg once daily.15 Treatment began on day 1 of cycle 1 and continued until PD or unacceptable toxicity; cycles were defined as 28 days. Treatment beyond PD per RECIST version 1.1 was allowed if Protocol-specific criteria were met.
Outcomes
The primary end point was investigator-assessed PFS (per RECIST 1.1), defined as the time from random assignment to first documented disease progression or death because of any cause. OS was a key secondary end point. Additional secondary end points included ORR, duration of response (DOR), disease control rate, safety and tolerability, patient-reported outcomes, pharmacokinetics, prevalence and incidence of antidrug antibodies, and outcomes on the basis of PD-L1 expression. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 and reviewed by an independent data monitoring committee. Biomarker analyses are described in the Data Supplement (online only).
Statistical Analysis
The primary analysis was planned after the target number of 352 events was observed or when all patients had at least 24 months of follow-up, whichever came first. Rationale and further details are provided in the Data Supplement. Efficacy analyses were performed using the full analysis set of all patients randomly assigned to receive study treatment; safety analyses included all patients who received at least one dose of spartalizumab or placebo, dabrafenib, or trametinib. Kaplan-Meier analysis was used to estimate PFS and OS distributions. HRs were calculated from a stratified Cox model on the basis of the random assignment stratification factors. Significance was determined by a stratified log-rank test at an overall one-sided 2.5% level.
RESULTS
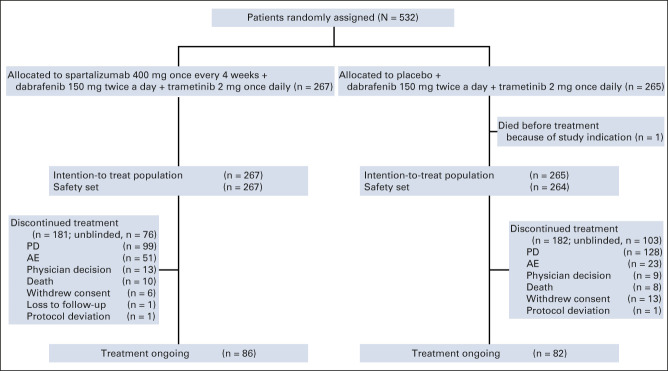
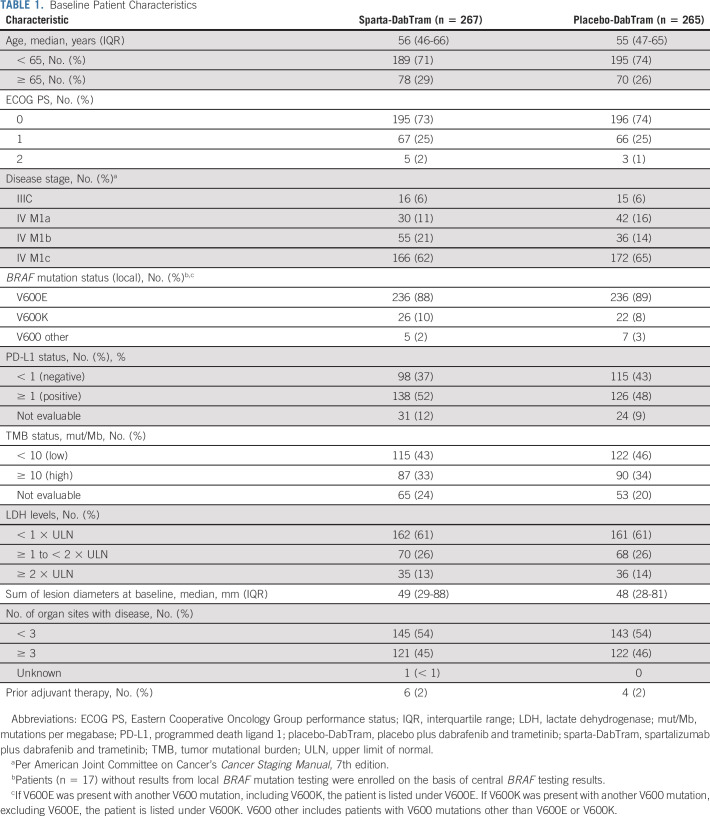
Between September 13, 2017, and July 4, 2018, 532 patients were randomly assigned to receive sparta-DabTram (n = 267) or placebo-DabTram (n = 265; Fig 1). Baseline characteristics were well balanced between treatment arms (Table 1). At the data cutoff (July 1, 2020), corresponding to a median follow-up of 27.2 months (interquartile range [IQR], 25.4-29.0 months), 86 of 267 patients (32%) remained on treatment in the sparta-DabTram arm and 82 of 265 (31%) remained on treatment in the placebo-DabTram arm; 181 of 267 (68%) and 182 of 265 (69%) had discontinued treatment, respectively. PD was the most common reason for discontinuation, occurring in 99 of 267 patients (37%) in the sparta-DabTram arm and 128 of 265 (48%) in the placebo-DabTram arm (Fig 1; Data Supplement); discontinuation because of AEs occurred in 51 of 267 (19%) and 23 of 265 patients (9%), respectively.
FIG 1.
COMBI-i part 3 CONSORT diagram. AE, adverse event; PD, progressive disease.
TABLE 1.
Baseline Patient Characteristics
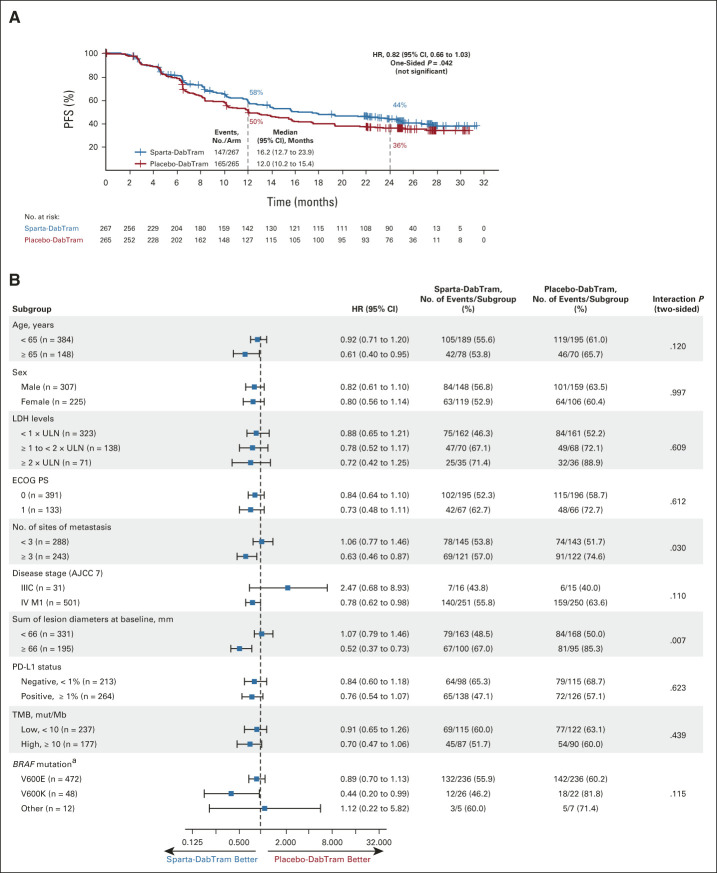
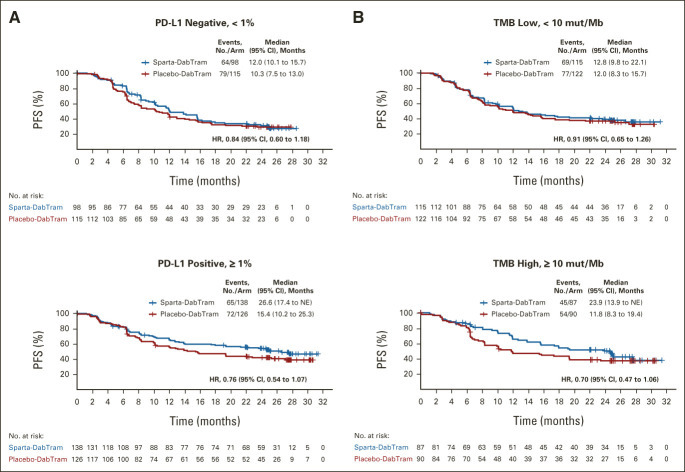
At the data cutoff, 147 of 267 patients (55%) in the sparta-DabTram arm had a PFS event versus 165 of 265 patients (62%) in the placebo-DabTram arm (HR, 0.82 [95% CI, 0.66 to 1.03]; P = .042 [one-sided; nonsignificant]), with a median PFS of 16.2 months (95% CI, 12.7 to 23.9 months) versus 12.0 months (95% CI, 10.2 to 15.4 months). Estimated 24-month PFS rates were 44% (95% CI, 37 to 50) with sparta-DabTram and 36% (95% CI, 30 to 42) with placebo-DabTram (Fig 2A). In prespecified exploratory subgroup analyses (Fig 2B), two-sided interaction tests were significant for baseline sum of lesion diameters (P = .007) and number of sites of metastasis (P = .030). PD-L1 status appeared to be prognostic in both treatment arms, with longer median PFS observed in patients with PD-L1–positive (≥ 1%) tumors than in patients with PD-L1–negative (< 1%) tumors (Fig 3A). However, although HRs were numerically lower in subgroups defined by PD-L1–positive versus –negative tumors (0.76 v 0.84) or high tumor mutational burden (TMB; ≥ 10 mutations per megabase) versus low TMB (0.70 v 0.91), there was not a significant PFS benefit with sparta-DabTram versus placebo-DabTram in any of these subgroups (Fig 3B).
FIG 2.
(A) Kaplan-Meier estimates of investigator-assessed PFS in the intention-to-treat population and (B) analysis of PFS in predetermined prognostic subgroups. (A) The log-rank P value of .042 is one-sided and thus not significant. (B) P values are two-sided for treatment by subgroup interaction. aBRAF V600 mutation as determined by local testing. AJCC 7, American Joint Committee on Cancer's Cancer Staging Manual, 7th edition; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; LDH, lactate dehydrogenase; mut/Mb, mutations per megabase; PD-L1, programmed death ligand 1; PFS, progression-free survival; placebo-DabTram, placebo plus dabrafenib and trametinib; sparta-DabTram, spartalizumab plus dabrafenib and trametinib; TMB, tumor mutational burden; ULN, upper limit of normal.
FIG 3.
Kaplan-Meier estimates of investigator-assessed PFS in predefined (A) PD-L1 (< 1% [negative] or ≥ 1% [positive]) and (B) TMB (< 10 mut/Mb [low] or ≥ 10 mut/Mb [high]) subgroups. HRs are based on stratified analyses. HR, hazard ratio; mut/Mb, mutations per megabase; NE, not estimable; PD-L1, programmed death ligand 1; PFS, progression-free survival; placebo-DabTram, placebo plus dabrafenib and trametinib; sparta-DabTram, spartalizumab plus dabrafenib and trametinib; TMB, tumor mutational burden.
A total of 90 of 267 patients (34%) treated with sparta-DabTram and 103 of 265 patients (39%) treated with placebo-DabTram had died as of the data cutoff (HR, 0.79 [95% CI, 0.59 to 1.05]). Although OS cannot be formally tested because the primary end point was not met, interim analysis found that medians were not reached in either arm (Data Supplement). Estimated 24-month OS rates were 68% (95% CI, 61 to 73) with sparta-DabTram and 62% (95% CI, 55 to 67) with placebo-DabTram. Exploratory subgroup analyses did not yield any significant two-sided interaction tests (Data Supplement). Among patients who discontinued study treatment, 101 of 267 (38%) in the sparta-DabTram arm and 106 of 265 (40%) in the placebo-DabTram arm received subsequent anticancer therapy, most commonly checkpoint inhibitors. More patients received anti–PD-1 monotherapy in the placebo-DabTram arm (57 of 265 [22%]) than in the sparta-DabTram arm (28 of 267 [11%]; Data Supplement).
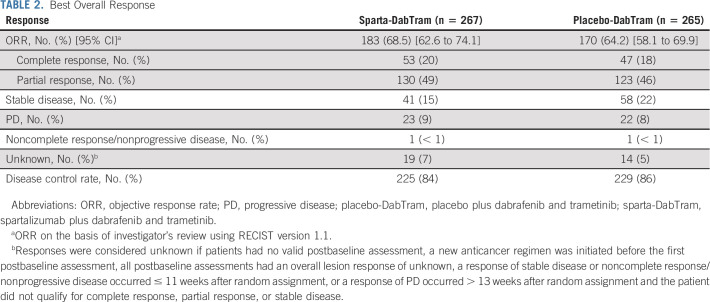
In patients treated with sparta-DabTram, the ORR was 69% (183 of 267; 95% CI, 62.6 to 74.1), with 53 of 267 patients (20%; 95% CI, 15.2 to 25.1) achieving a complete response. In comparison, the ORR was 64% (170 of 265; 95% CI, 58.1 to 69.9) in patients treated with placebo-DabTram, with 47 of 265 patients (18%; 95% CI, 13.3 to 22.9) achieving a complete response (Table 2). The median DOR was not reached (95% CI, 18.6 months to not estimable) in the sparta-DabTram arm versus 20.7 months (95% CI, 13.0 months to not estimable) in the placebo-DabTram arm. Estimated 24-month DOR rates were 55% (95% CI, 47 to 62) in the sparta-DabTram arm and 48% (95% CI, 39 to 56) in the placebo-DabTram arm (Fig 4).
TABLE 2.
Best Overall Response
FIG 4.
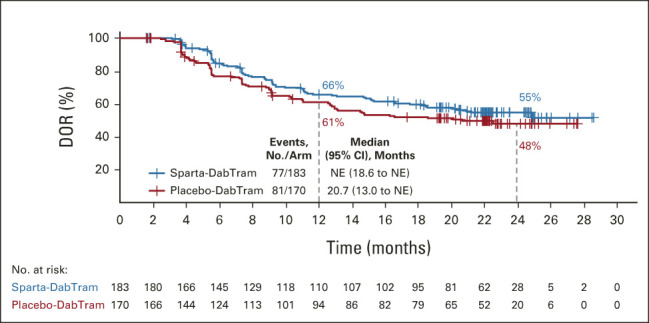
Kaplan-Meier estimates of DOR in the intention-to-treat population. DOR, duration of response; NE, not estimable; placebo-DabTram, placebo plus dabrafenib and trametinib; sparta-DabTram, spartalizumab plus dabrafenib and trametinib.
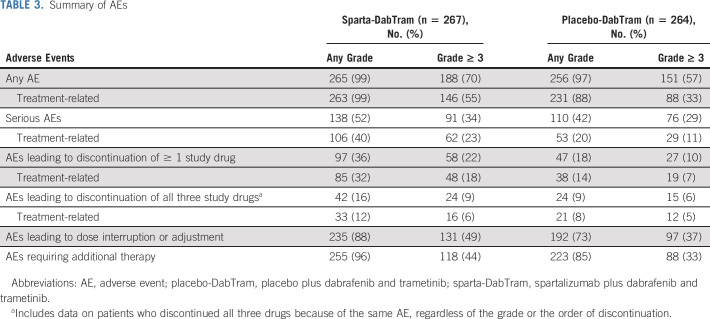
The median duration of exposure was 13.2 months (IQR, 6.4-25.2 months) in the sparta-DabTram arm and 11.8 months (IQR, 6.4-24.9 months) in the placebo-DabTram arm. AEs independent of treatment relationship were observed in the majority of patients (≥ 97%) across treatment arms, with 70% (188 of 267) of patients treated with sparta-DabTram and 57% (151 of 264) treated with placebo-DabTram experiencing at least one grade ≥ 3 AE (Table 3). Increases in blood creatine phosphokinase (21 of 267 [8%]), pyrexia (14 of 267 [5%]), and increases in aspartate aminotransferase (10 of 267 [4%]) were the most common grade ≥ 3 AEs in patients treated with sparta-DabTram (Data Supplement). Treatment-related AEs of any grade occurred in 99% (263 of 267) of patients in the sparta-DabTram arm and 88% (231 of 264) in the placebo-DabTram arm; grade ≥ 3 treatment-related AEs occurred in 55% (146 of 267) and 33% (88 of 264), respectively (Table 3). Pyrexia (177 of 267 [66%]), chills (78 of 267 [29%]), diarrhea (65 of 267 [24%]), and nausea (65 of 267 [24%]) were the most common treatment-related AEs in patients treated with sparta-DabTram and occurred at a higher rate than in patients who received placebo-DabTram (Data Supplement). In particular, grade ≥ 3 treatment-related pyrexia occurred in 5% (14 of 267) of patients in the sparta-DabTram arm and 3% (7 of 264) in the placebo-DabTram arm. There were no treatment-related deaths in the sparta-DabTram arm, whereas two patients in the placebo-DabTram arm died because of AEs deemed by investigators to be related to treatment (pancreatitis and cerebrovascular accident, n = 1 each).
TABLE 3.
Summary of AEs
In the sparta-DabTram arm, 235 of 267 patients (88%) experienced AEs leading to dose modifications versus 192 of 264 (73%) in the placebo-DabTram arm (Table 3). Among patients treated with sparta-DabTram, pyrexia (170 of 267 [64%]), chills (51 of 267 [19.1%]), and diarrhea (28 of 267 [10.5%]) were the most common AEs leading to dose modifications of any drug. A higher frequency of dose interruptions and reductions was observed in patients treated with sparta-DabTram, contributing to a lower relative dose intensity than that observed in patients treated with placebo-DabTram (87% v 98% for both dabrafenib and trametinib; Data Supplement). In the sparta-DabTram arm, 182 and 120 of 267 patients (68% and 45%) experienced at least one dose reduction of dabrafenib and trametinib, respectively, because of AEs; full doses of dabrafenib and trametinib were received by only a respective 32% (85 of 267) and 55% (147 of 267) of patients. By contrast, full doses of dabrafenib and trametinib were received by more patients in the placebo-DabTram arm, a respective 54% (142 of 264) and 74% (196 of 264) of patients (Data Supplement). Permanent discontinuation of all three study drugs because of treatment-related AEs occurred in 12% (33 of 267) of patients in the sparta-DabTram arm versus 8% (21 of 264) in the placebo-DabTram arm (Table 3).
DISCUSSION
COMBI-i did not show a statistically significant difference in investigator-assessed PFS in the broad population of patients with BRAF V600–mutant metastatic melanoma treated with sparta-DabTram versus placebo-DabTram. Increased efficacy of placebo-DabTram compared with Protocol assumptions and lower relative dose intensities of dabrafenib and trametinib in the sparta-DabTram arm because of increased toxicity may have contributed to this result. Although OS cannot be formally tested because the primary end point was not met, patients remain in follow-up, and future exploratory OS analyses are planned. However, the results of this primary analysis do not support routine first-line use of sparta-DabTram in patients with BRAF V600–mutant metastatic melanoma.
In addition to COMBI-i, the phase II KEYNOTE-022 and phase III IMspire150 trials also investigated first-line checkpoint inhibitor plus targeted therapy combinations.13 Although IMspire150 was the only one of these trials to show a statistically significant difference in investigator-assessed PFS, the performances of the regimens evaluated in all three trials appear similar, although the caveats of cross-trial comparison preclude definitive conclusions.13,18 Differences in study design (including a targeted therapy run-in in IMspire150), patient populations, and statistical parameters may have contributed to their differing outcomes, but collectively, these studies suggest only a modest efficacy benefit with checkpoint inhibitor plus targeted therapy combination compared with targeted therapy alone.12,13,18 Moreover, although additional follow-up was associated with increased efficacy in KEYNOTE-022, this observation is unlikely to translate to COMBI-i.12,18 The primary COMBI-i analysis was conducted on the basis of a minimum follow-up of 24 months at 312 events (per protocol), which was below the target of 352 events, at which time the trend was for a weakening in the overall treatment effect estimate. The low number of events per month observed at this time and the performance of the placebo-DabTram arm suggest there is little chance of a significant outcome within an additional 2 years.
Notable in COMBI-i was the improved performance of the comparator arm versus that observed in historical data with dabrafenib plus trametinib from the phase III COMBI-d/v studies.2,19,20 This may be reflective of increased clinician experience with dabrafenib plus trametinib, as this combination has been available since 2015 and continues to be a robust targeted therapy option for patients with BRAF V600–mutant melanoma.21,22 Differences in the per-protocol management of pyrexia, the most common AE observed with dabrafenib plus trametinib, also could have contributed to improved outcomes in patients treated with placebo-DabTram in this study. In previous studies, pyrexia was managed through interruption of dabrafenib alone.22 In COMBI-i, an adapted algorithm mandated interruption of both dabrafenib and trametinib at the first signs of pyrexia or its prodrome.23 Post hoc analysis suggested that this algorithm was associated with improvements in severe pyrexia-related outcomes in the placebo-DabTram arm compared with historical data from COMBI-d/v.24
Treatment-related AEs occurred at a higher frequency in patients receiving sparta-DabTram than in patients receiving placebo-DabTram. Toxicity appeared to be a barrier to patients receiving the full dose, as there were more dose modifications in the sparta-DabTram arm than in the placebo-DabTram arm. Management of certain AEs such as pyrexia or abnormalities in liver function may have led to extended time off treatment as health care providers evaluated possible relationship to treatment (eg, potential immune-related hepatitis v liver abnormalities related to dabrafenib and trametinib). Taken together, the modest efficacy and increased toxicity of sparta-DabTram over placebo-DabTram suggest that the risk-benefit profile of upfront immunotherapy plus targeted therapy combination is not favorable for most patients.
Although COMBI-i did not reach the primary end point, the results provide further insight into the optimal use of checkpoint inhibitors and targeted therapies in the treatment of BRAF V600–mutant metastatic melanoma. Treatment with both a checkpoint inhibitor and targeted therapy in the first-line setting would leave few options for subsequent therapeutic lines in patients who experience PD. Instead, because upfront combination of a checkpoint inhibitor plus targeted therapy appears not to be an ideal therapeutic strategy for most patients, continued evaluation of sequencing approaches may be warranted. Targeted therapy and immunotherapy sequencing is under evaluation in several clinical trials (eg, DREAMseq, ClinicalTrials.gov identifier: NCT02224781; ImmunoCobiVem, ClinicalTrials.gov identifier: NCT02902029; EBIN, ClinicalTrials.gov identifier: NCT03235245; SECOMBIT, ClinicalTrials.gov identifier: NCT02631447); however, as of this writing, no definitive conclusions have been reached. Preliminary results from the phase II SECOMBIT trial evaluating a targeted therapy–to-immunotherapy switch at PD, an immunotherapy-to-targeted therapy switch at PD, or an 8-week targeted therapy sandwich before immunotherapy suggest that the latter two approaches may be associated with higher 3-year total PFS and OS rates, although these results were not statistically significant.25 As additional data from sequencing studies become available, they will join the results of COMBI-i, IMspire150, and KEYNOTE-022 in furthering the understanding of how to apply checkpoint inhibitors and targeted therapies most effectively in BRAF V600–mutant metastatic melanoma in the first-line setting and beyond.
In COMBI-i, preplanned exploratory subgroup analyses suggested a greater PFS benefit with sparta-DabTram in patients with features indicative of higher disease burden, such as ≥ 3 sites of metastasis or a median sum of lesion diameters ≥ 66 mm, which may warrant further risk-benefit analyses. Numerically lower PFS HRs were also observed in patients with PD-L1–positive tumors or high TMB, features that have previously been associated with improved clinical outcomes with first-line anti–PD-1 monotherapy.26,27 Thus, although the results of COMBI-i do not support broad first-line use of sparta-DabTram in patients with BRAF V600–mutant melanoma, further biomarker-driven analyses may help to determine whether there are subpopulations that could benefit from upfront immunotherapy plus targeted therapy combination.
ACKNOWLEDGMENT
The authors thank the patients and their families for their participation, as well as study site staff and additional investigators. They also thank Maurizio Voi (Novartis Pharmaceuticals Corporation) for guidance and critical review of the report. Medical writing assistance was provided by Amy Ghiretti, PhD, and Allison Lytle, PhD (ArticulateScience LLC), funded by Novartis.
Reinhard Dummer
Honoraria: Roche, Novartis, Bristol Myers Squibb, MSD, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome, Regeneron, Alligator Bioscience, MaxiVax, touchIME, T3 Pharmaceuticals, Pfizer
Consulting or Advisory Role: Roche, Bristol Myers Squibb, MSD, Novartis, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome, Alligator Bioscience, touchIME, MaxiVax, Regeneron, Pfizer, T3 Pharmaceuticals
Research Funding: Roche (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), MSD (Inst), Amgen (Inst)
Georgina V. Long
Honoraria: BMS, Pierre Fabre
Consulting or Advisory Role: Aduro Biotech, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Hexal, Highlight Therapeutics, Merck Sharpe & Dohme, Novartis, OncoSec, Pierre Fabre, QBiotics, Regeneron, SkylineDx, Specialised Therapeutics, Array BioPharma, Evaxion Biotech A/S
Caroline Robert
Consulting or Advisory Role: Bristol Myers Squibb, Roche, Novartis, Pierre Fabre, MSD, Sanofi, AstraZeneca, Pfizer
Hussein A. Tawbi
Consulting or Advisory Role: Novartis, Bristol Myers Squibb, Genetech/Roche, Merck, Array BioPharma, Eisai, Iovance Biotherapeutics, Karyopharm Therapeutics, Boxer Capital
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Merck (Inst), GlaxoSmithKline (Inst), Genentech/Roche (Inst), Celgene (Inst)
Keith T. Flaherty
Stock and Other Ownership Interests: Clovis Oncology, Loxo, X4 Pharma, Strata Oncology, PIC Therapeutics, Shattuck Labs, Apricity Health, Oncoceutics, FOGPharma, Tvardi Therapeutics, Checkmate Pharmaceuticals, Kinnate Biopharma, Scorpion Therapeutics, ALX Oncology, xCures, Monopteros Therapeutics, Vibliome Therapeutics, Transcode Therapeutics, Soley Therapeutics,
Consulting or Advisory Role: Novartis, Lilly, Oncoceutics, Tvardi Therapeutics, Takeda, Boston Biomedical, Debiopharm Group, FOGPharma
Paolo A. Ascierto
Stock and Other Ownership Interests: PrimeVax
Consulting or Advisory Role: Bristol Myers Squibb, Roche/Genentech, Merck Sharp & Dohme, Novartis, Array BioPharma, Merck Serono, Pierre Fabre, Incyte, MedImmune, AstraZeneca, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore, 4SC, Alkermes, Italfarmaco, Nektar, Boehringer Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, OncoSec, Nouscom, Takis Biotech, Lunaphore Technologies, Seattle Genetics, ITeos Therapeutics
Research Funding: Bristol Myers Squibb (Inst), Roche/Genentech (Inst), Array BioPharma (Inst), Sanofi (Inst)
Travel Accommodations, Expenses: Merck Sharp & Dohme
Paul D. Nathan
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, MSD, Immunocore, Pfizer, Pierre Fabre, Novartis, GlaxoSmithKline, Ipsen, 4SC, Merck
Speaker's Bureau: Bristol Myers Squibb, Novartis, MSD, Merck,
Travel Accommodations, Expenses: Bristol Myers Squibb, MSD
Piotr Rutkowski
Honoraria: Bristol Myers Squibb, MSD, Novartis, Roche, Pfizer, Pierre Fabre, Sanofi, Merck
Consulting or Advisory Role: Novartis, Blueprint Medicines, Bristol Myers Squibb, Pierre Fabre, MSD, Amgen
Speaker's Bureau: Pfizer, Novartis, Pierre Fabre
Research Funding: Novartis (Inst), Roche (Inst), Bristol Myers Squibb (Inst)
Travel Accommodations, Expenses: Orphan Europe, Pierre Fabre
Caroline Dutriaux
Honoraria: Bristol Myers Squibb, MSD, Novartis, Pierre Fabre
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Novartis, Pierre Fabre
Travel Accommodations, Expenses: Bristol Myers Squibb, MSD, Novartis, Pierre Fabre
Mario Mandalà
Honoraria: MSD Oncology, Novartis, Pierre Fabre
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Novartis, Pierre Fabre
Research Funding: Novartis (Inst)
Paul Lorigan
Honoraria: Novartis, Pierre Fabre, Merck, BMS, MSD, NeraCare GmbH, Amgen, Roche, Oncology Education, Nektar
Consulting or Advisory Role: Merck Sharp & Dohme, Bristol Myers Squibb, Amgen, Pierre Fabre, Novartis, Nektar
Speaker's Bureau: Merck Sharp & Dohme, Novartis, Bristol Myers Squibb, Pierre Fabre, BMS
Travel Accommodations, Expenses: Merck Sharp & Dohme, Bristol Myers Squibb
Pier Francesco Ferrucci
Consulting or Advisory Role: Bristol Myers Squibb, Roche, Novartis, MSD Oncology, Pierre Fabre
Research Funding: BMS (Inst)
Travel Accommodations, Expenses: MSD Oncology, Bristol Myers Squibb
Jean Jacques Grob
Consulting or Advisory Role: BMS, MSD Oncology, Roche/Genentech, Novartis, Amgen, Pierre Fabre, Sun Pharma, Merck KGaA, Sanofi, Pfizer, Roche
Speaker's Bureau: Novartis
Travel Accommodations, Expenses: BMS, MSD Oncology, Novartis, Pierre Fabre
Nicolas Meyer
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Roche/Genentech, Novartis, Pierre Fabre, AbbVie, Sun Pharma, Merckle GmbH
Research Funding: Bristol Myers Squibb (Inst), MSD Oncology (Inst)
Helen Gogas
Honoraria: Bristol Myers Squibb, MSD Oncology, Pierre Fabre, Sanofi/Regeneron
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Amgen, Pierre Fabre, Sanofi/Regeneron
Research Funding: Bristol Myers Squibb, Roche, MSD Oncology, Amgen (Inst), Novartis (Inst)
Travel Accommodations, Expenses: Bristol Myers Squibb, MSD, Amgen, Pfizer
Daniil Stroyakovskiy
Speaker's Bureau: Roche/Genentech, Bristol Myers Squibb, BioCad, AstraZeneca
Travel Accommodations, Expenses: AstraZeneca, Novartis, Roche
Ana Arance
Consulting or Advisory Role: BMS, Roche, Novartis, Pierre Fabre, MSD, Merck, Sanofi
Speaker's Bureau: Pierre Fabre, Novartis, MSD, BMS, Roche, Merck, Sanofi
Research Funding: Pierre Fabre, Novartis, Roche, BMS, MSD, Merck, Sanofi, Amgen
Travel Accommodations, Expenses: BMS, MSD, Novartis, Pierre Fabre, Roche, Merck, Sanofi
Jan C. Brase
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Patents, Royalties, Other Intellectual Property: Coinventor on patent application
Steven Green
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Tomas Haas
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Aisha Masood
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Other Relationship: Novartis
Eduard Gasal
Employment: Novartis, Innovent Biologics
Leadership: Innovent Biologics
Stock and Other Ownership Interests: Novartis, Innovent Biologics, Revolution Medicines, Amgen, Natera
Travel Accommodations, Expenses: Novartis, Innovent Biologics
Antoni Ribas
Leadership: PACT Pharma, Arcus Biosciences, Lutris
Stock and Other Ownership Interests: Compugen, CytomX Therapeutics, Advaxis, Tango Therapeutics, PACT Pharma, Merus, ImaginAb, Lutris, Highlight Therapeutics, MapKure, 4c Biomed, Kite/Gilead, Isoplexis, Appia, Synthekine, Pluto, Inspirna, RAPT Therapeutics, ImmPACT-Bio
Honoraria: Merck Sharp & Dohme, Novartis, Amgen, Chugai/Roche, Genentech/Roche, Sanofi, Vedanta Biosciences, AstraZeneca
Consulting or Advisory Role: Merck, Amgen, Novartis, Chugai Pharma, Sanofi
Research Funding: Agilent (Inst), Bristol Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: Nonviral gene editing to Arsenal Bio
Dirk Schadendorf
Honoraria: Roche/Genentech, Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Immunocore, Merck Serono, Array BioPharna, Pfizer, Pierre Fabre, Philogen, Regeneron, 4SC, Sanofi/Regeneron, NeraCare GmbH, Sun Pharma, InflarxGmbH, Ultimovacs, Sandoz
Consulting or Advisory Role: Roche/Genentech, Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, 4SC, Pierre Fabre, Sanofi/Regeneron, Nektar
Speaker's Bureau: Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre, Sanofi/Regeneron, Merck KGaA
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Roche (Inst), MSD Oncology (Inst), Array BioPharma/Pfizer (Inst)
Travel Accommodations, Expenses: Roche/Genentech, Bristol Myers Squibb, Merck Serono, Novartis, Merck Sharp & Dohme, Pierre Fabre, Sanofi/Regeneron
No other potential conflicts of interest were reported.
See accompanying editorial on page 1393
PRIOR PRESENTATION
Presented in part at the ESMO Virtual Congress 2020, September 19-21, 2020 (abstract LBA43).
SUPPORT
Supported by Novartis Pharmaceuticals Corporation.
CLINICAL TRIAL INFORMATION
R.D. and G.V.L. contributed equally to this work and share the position of first author. A.R. and D. Schadendorf contributed equally to this work and share the position of senior author.
DATA SHARING STATEMENT
Novartis is committed to sharing, with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. Requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on ClinicalStudyDataRequest.com.
AUTHOR CONTRIBUTIONS
Conception and design: Reinhard Dummer, Georgina V. Long, Caroline Robert, Hussein A. Tawbi, Keith T. Flaherty, Paolo A. Ascierto, Paul D. Nathan, Jan C. Brase, Steven Green, Tomas Haas, Aisha Masood, Eduard Gasal, Antoni Ribas
Administrative support: Dirk Schadendorf
Provision of study materials or patients: Reinhard Dummer, Caroline Robert, Hussein A. Tawbi, Paul D. Nathan, Piotr Rutkowski, Caroline Dutriaux, Mario Mandalà, Paul Lorigan, Nicolas Meyer, Helen Gogas, Daniil Stroyakovskiy, Antoni Ribas, Dirk Schadendorf
Collection and assembly of data: Reinhard Dummer, Georgina V. Long, Caroline Robert, Hussein A. Tawbi, Paul D. Nathan, Piotr Rutkowski, Oleg Leonov, Caroline Dutriaux, Paul Lorigan, Jean Jacques Grob, Nicolas Meyer, Helen Gogas, Jan C. Brase, Steven Green, Aisha Masood, Antoni Ribas, Dirk Schadendorf
Data analysis and interpretation: Reinhard Dummer, Caroline Robert, Hussein A. Tawbi, Paolo A. Ascierto, Paul D. Nathan, Piotr Rutkowski, Mario Mandalà, Paul Lorigan, Pier Francesco Ferrucci, Jean Jacques Grob, Daniil Stroyakovskiy, Ana Arance, Jan C. Brase, Steven Green, Tomas Haas, Aisha Masood, Eduard Gasal, Antoni Ribas, Dirk Schadendorf
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase III Trial Evaluating Spartalizumab Plus Dabrafenib and Trametinib for BRAF V600–Mutant Unresectable or Metastatic Melanoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Reinhard Dummer
Honoraria: Roche, Novartis, Bristol Myers Squibb, MSD, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome, Regeneron, Alligator Bioscience, MaxiVax, touchIME, T3 Pharmaceuticals, Pfizer
Consulting or Advisory Role: Roche, Bristol Myers Squibb, MSD, Novartis, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome, Alligator Bioscience, touchIME, MaxiVax, Regeneron, Pfizer, T3 Pharmaceuticals
Research Funding: Roche (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), MSD (Inst), Amgen (Inst)
Georgina V. Long
Honoraria: BMS, Pierre Fabre
Consulting or Advisory Role: Aduro Biotech, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Hexal, Highlight Therapeutics, Merck Sharpe & Dohme, Novartis, OncoSec, Pierre Fabre, QBiotics, Regeneron, SkylineDx, Specialised Therapeutics, Array BioPharma, Evaxion Biotech A/S
Caroline Robert
Consulting or Advisory Role: Bristol Myers Squibb, Roche, Novartis, Pierre Fabre, MSD, Sanofi, AstraZeneca, Pfizer
Hussein A. Tawbi
Consulting or Advisory Role: Novartis, Bristol Myers Squibb, Genetech/Roche, Merck, Array BioPharma, Eisai, Iovance Biotherapeutics, Karyopharm Therapeutics, Boxer Capital
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Merck (Inst), GlaxoSmithKline (Inst), Genentech/Roche (Inst), Celgene (Inst)
Keith T. Flaherty
Stock and Other Ownership Interests: Clovis Oncology, Loxo, X4 Pharma, Strata Oncology, PIC Therapeutics, Shattuck Labs, Apricity Health, Oncoceutics, FOGPharma, Tvardi Therapeutics, Checkmate Pharmaceuticals, Kinnate Biopharma, Scorpion Therapeutics, ALX Oncology, xCures, Monopteros Therapeutics, Vibliome Therapeutics, Transcode Therapeutics, Soley Therapeutics,
Consulting or Advisory Role: Novartis, Lilly, Oncoceutics, Tvardi Therapeutics, Takeda, Boston Biomedical, Debiopharm Group, FOGPharma
Paolo A. Ascierto
Stock and Other Ownership Interests: PrimeVax
Consulting or Advisory Role: Bristol Myers Squibb, Roche/Genentech, Merck Sharp & Dohme, Novartis, Array BioPharma, Merck Serono, Pierre Fabre, Incyte, MedImmune, AstraZeneca, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore, 4SC, Alkermes, Italfarmaco, Nektar, Boehringer Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, OncoSec, Nouscom, Takis Biotech, Lunaphore Technologies, Seattle Genetics, ITeos Therapeutics
Research Funding: Bristol Myers Squibb (Inst), Roche/Genentech (Inst), Array BioPharma (Inst), Sanofi (Inst)
Travel Accommodations, Expenses: Merck Sharp & Dohme
Paul D. Nathan
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, MSD, Immunocore, Pfizer, Pierre Fabre, Novartis, GlaxoSmithKline, Ipsen, 4SC, Merck
Speaker's Bureau: Bristol Myers Squibb, Novartis, MSD, Merck,
Travel Accommodations, Expenses: Bristol Myers Squibb, MSD
Piotr Rutkowski
Honoraria: Bristol Myers Squibb, MSD, Novartis, Roche, Pfizer, Pierre Fabre, Sanofi, Merck
Consulting or Advisory Role: Novartis, Blueprint Medicines, Bristol Myers Squibb, Pierre Fabre, MSD, Amgen
Speaker's Bureau: Pfizer, Novartis, Pierre Fabre
Research Funding: Novartis (Inst), Roche (Inst), Bristol Myers Squibb (Inst)
Travel Accommodations, Expenses: Orphan Europe, Pierre Fabre
Caroline Dutriaux
Honoraria: Bristol Myers Squibb, MSD, Novartis, Pierre Fabre
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Novartis, Pierre Fabre
Travel Accommodations, Expenses: Bristol Myers Squibb, MSD, Novartis, Pierre Fabre
Mario Mandalà
Honoraria: MSD Oncology, Novartis, Pierre Fabre
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Novartis, Pierre Fabre
Research Funding: Novartis (Inst)
Paul Lorigan
Honoraria: Novartis, Pierre Fabre, Merck, BMS, MSD, NeraCare GmbH, Amgen, Roche, Oncology Education, Nektar
Consulting or Advisory Role: Merck Sharp & Dohme, Bristol Myers Squibb, Amgen, Pierre Fabre, Novartis, Nektar
Speaker's Bureau: Merck Sharp & Dohme, Novartis, Bristol Myers Squibb, Pierre Fabre, BMS
Travel Accommodations, Expenses: Merck Sharp & Dohme, Bristol Myers Squibb
Pier Francesco Ferrucci
Consulting or Advisory Role: Bristol Myers Squibb, Roche, Novartis, MSD Oncology, Pierre Fabre
Research Funding: BMS (Inst)
Travel Accommodations, Expenses: MSD Oncology, Bristol Myers Squibb
Jean Jacques Grob
Consulting or Advisory Role: BMS, MSD Oncology, Roche/Genentech, Novartis, Amgen, Pierre Fabre, Sun Pharma, Merck KGaA, Sanofi, Pfizer, Roche
Speaker's Bureau: Novartis
Travel Accommodations, Expenses: BMS, MSD Oncology, Novartis, Pierre Fabre
Nicolas Meyer
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Roche/Genentech, Novartis, Pierre Fabre, AbbVie, Sun Pharma, Merckle GmbH
Research Funding: Bristol Myers Squibb (Inst), MSD Oncology (Inst)
Helen Gogas
Honoraria: Bristol Myers Squibb, MSD Oncology, Pierre Fabre, Sanofi/Regeneron
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Amgen, Pierre Fabre, Sanofi/Regeneron
Research Funding: Bristol Myers Squibb, Roche, MSD Oncology, Amgen (Inst), Novartis (Inst)
Travel Accommodations, Expenses: Bristol Myers Squibb, MSD, Amgen, Pfizer
Daniil Stroyakovskiy
Speaker's Bureau: Roche/Genentech, Bristol Myers Squibb, BioCad, AstraZeneca
Travel Accommodations, Expenses: AstraZeneca, Novartis, Roche
Ana Arance
Consulting or Advisory Role: BMS, Roche, Novartis, Pierre Fabre, MSD, Merck, Sanofi
Speaker's Bureau: Pierre Fabre, Novartis, MSD, BMS, Roche, Merck, Sanofi
Research Funding: Pierre Fabre, Novartis, Roche, BMS, MSD, Merck, Sanofi, Amgen
Travel Accommodations, Expenses: BMS, MSD, Novartis, Pierre Fabre, Roche, Merck, Sanofi
Jan C. Brase
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Patents, Royalties, Other Intellectual Property: Coinventor on patent application
Steven Green
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Tomas Haas
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Aisha Masood
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Other Relationship: Novartis
Eduard Gasal
Employment: Novartis, Innovent Biologics
Leadership: Innovent Biologics
Stock and Other Ownership Interests: Novartis, Innovent Biologics, Revolution Medicines, Amgen, Natera
Travel Accommodations, Expenses: Novartis, Innovent Biologics
Antoni Ribas
Leadership: PACT Pharma, Arcus Biosciences, Lutris
Stock and Other Ownership Interests: Compugen, CytomX Therapeutics, Advaxis, Tango Therapeutics, PACT Pharma, Merus, ImaginAb, Lutris, Highlight Therapeutics, MapKure, 4c Biomed, Kite/Gilead, Isoplexis, Appia, Synthekine, Pluto, Inspirna, RAPT Therapeutics, ImmPACT-Bio
Honoraria: Merck Sharp & Dohme, Novartis, Amgen, Chugai/Roche, Genentech/Roche, Sanofi, Vedanta Biosciences, AstraZeneca
Consulting or Advisory Role: Merck, Amgen, Novartis, Chugai Pharma, Sanofi
Research Funding: Agilent (Inst), Bristol Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: Nonviral gene editing to Arsenal Bio
Dirk Schadendorf
Honoraria: Roche/Genentech, Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Immunocore, Merck Serono, Array BioPharna, Pfizer, Pierre Fabre, Philogen, Regeneron, 4SC, Sanofi/Regeneron, NeraCare GmbH, Sun Pharma, InflarxGmbH, Ultimovacs, Sandoz
Consulting or Advisory Role: Roche/Genentech, Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, 4SC, Pierre Fabre, Sanofi/Regeneron, Nektar
Speaker's Bureau: Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre, Sanofi/Regeneron, Merck KGaA
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Roche (Inst), MSD Oncology (Inst), Array BioPharma/Pfizer (Inst)
Travel Accommodations, Expenses: Roche/Genentech, Bristol Myers Squibb, Merck Serono, Novartis, Merck Sharp & Dohme, Pierre Fabre, Sanofi/Regeneron
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ascierto PA, Dréno B, Larkin J, et al. : 5-year outcomes with cobimetinib plus vemurafenib in BRAFV600-mutation-positive advanced melanoma: Extended follow-up of the coBRIM study. Clin Cancer Res 27:5225-5235, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Grob JJ, Stroyakovskiy D, et al. : Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 381:626-636, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Ascierto PA, Dummer R, Gogas HJ, et al. : Update on tolerability and overall survival in COLUMBUS: Landmark analysis of a randomised phase 3 trial of encorafenib plus binimetinib vs vemurafenib or encorafenib in patients with BRAF V600-mutant melanoma. Eur J Cancer 126:33-44, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Ribas A, Schachter J, et al. : Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 20:1239-1251, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535-1546, 2019 [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology. Melanoma: Cutaneous. V1.2022. 2021. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
- 7.Michielin O, van Akkooi ACJ, Ascierto PA, et al. : Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:1884-1901, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Cancer Council Australia Melanoma Guidelines Working Party : Clinical practice guidelines for the diagnosis and management of melanoma. 2020. https://wiki.cancer.org.au/australiawiki/index.php?oldid=213448
- 9.Wilmott JS, Haydu LE, Menzies AM, et al. : Dynamics of chemokine, cytokine, and growth factor serum levels in BRAF-mutant melanoma patients during BRAF inhibitor treatment. J Immunol 192:2505-2513, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Wilmott JS, Long GV, Howle JR, et al. : Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res 18:1386-1394, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Dummer R, Ascierto PA, Nathan P, et al. : Rationale for immune checkpoint inhibitors plus targeted therapy in metastatic melanoma: A review. JAMA Oncol 6:1957-1966, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. : KEYNOTE-022 part 3: A randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer 8:e001806, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutzmer R, Stroyakovskiy D, Gogas H, et al. : Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 395:1835-1844, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Nathan P, Dummer R, Long GV, et al. : Spartalizumab plus dabrafenib and trametinib (Sparta-DabTram) in patients (pts) with previously untreated BRAF V600–mutant unresectable or metastatic melanoma: Results from the randomized part 3 of the phase III COMBI-i trial. Ann Oncol 31:S1172, 2020. (suppl 4; abstr LBA43) [Google Scholar]
- 15.Dummer R, Lebbé C, Atkinson V, et al. : Combined PD-1, BRAF and MEK inhibition in advanced BRAF-mutant melanoma: Safety run-in and biomarker cohorts of COMBI-i. Nat Med 26:1557-1563, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Dummer R, Hauschild A, Lindenblatt N, et al. ; ESMO Guidelines Committee: Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v126-v132, 2015. (suppl 5) [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology. Cutaneous Melanoma. V1.2017, 2017
- 18.Ascierto PA, Ferrucci PF, Fisher R, et al. : Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med 25:941-946, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Long GV, Flaherty KT, Stroyakovskiy D, et al. : Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K–mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann Oncol 28:1631-1639, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert C, Karaszewska B, Schachter J, et al. : Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 372:30-39, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Tafinlar (Dabrafenib) [Summary of Product Characteristics]. Dublin, Ireland, Novartis Europharm Limited, 2018. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002604/WC500149671.pdf [Google Scholar]
- 22.Tafinlar (Dabrafenib) [Package Insert]. East Hanover, NJ, Novartis Pharmaceuticals Corporation, 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202806s010lbl.pdf [Google Scholar]
- 23.Atkinson V, Long GV, Menzies AM, et al. : Optimizing combination dabrafenib and trametinib therapy in BRAF mutation-positive advanced melanoma patients: Guidelines from Australian melanoma medical oncologists. Asia Pac J Clin Oncol 12:5-12, 2016. (suppl 7) [DOI] [PubMed] [Google Scholar]
- 24.Ascierto PA, Robert C, Nathan PD, et al. : Pyrexia-related outcomes upon application of an adapted pyrexia management algorithm in patients with BRAF V600–mutant unresectable or metastatic melanoma treated with dabrafenib plus trametinib in the COMBI-i trial. Presented at the 2021 American Society of Clinical Oncology Annual Meeting, June 4-8, 2021 (abstr 9560)
- 25.Ascierto PA, Mandala M, Ferrucci PF, et al. : SECOMBIT: The best sequential approach with combo immunotherapy [ipilimumab (I)/nivolumab (N)] and combo target therapy [encorafenib (E)/binimetinib (B)] in patients with BRAF mutated metastatic melanoma. A phase II randomized study. Presented at the European Society for Medical Oncology Congress 2021, September 16-21, 2021 (abstr LBA40)
- 26.Hodi SF, Wolchok JD, Schadendorf D, et al. : Genomic analyses and immunotherapy in advanced melanoma. Cancer Res 79, 2019. (13 suppl; abstr CT037) [Google Scholar]
- 27.Ribas A, Robert C, Schachter J, et al. : Tumor mutational burden (TMB), T cell-inflamed gene expression profile (GEP) and PD-L1 are independently associated with response to pembrolizumab (pembro) in patients with advanced melanoma in the KEYNOTE (KN)-006 study. Cancer Res 79, 2019. (13 suppl; abstr 4217) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Novartis is committed to sharing, with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. Requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on ClinicalStudyDataRequest.com.