Abstract
OBJECTIVES:
The Sequential Organ Failure Assessment (SOFA) score is a predictor of mortality in ICU patients. Although it is widely used and has been validated as a reliable and independent predictor of mortality and morbidity in cardiac ICU, few studies correlate early postoperative SOFA with long-term survival.
DESIGN:
Retrospective observational cohort study.
SETTING:
Tertiary academic cardiac surgery ICU.
PATIENTS:
One-thousand three-hundred seventy-nine patients submitted to cardiac surgery.
INTERVENTIONS:
SOFA 24 hours, SOFA 48 hours, mean, and highest SOFA scores were correlated with survival at 12 and 24 months. Wilcoxon tests were used to analyze differences in variables. Multivariate logistic regressions and likelihood ratio test were used to access the predictive modeling. Receiver operating characteristic curves were used to assess accuracy of the variables in separating survivor from nonsurvivors.
MEASUREMENTS AND MAIN RESULTS:
Lower SOFA scores have better survival rates at 12 and 24 months. Highest SOFA and SOFA at 48 hours showed to be better predictors of outcome and to have higher accuracy in distinguishing survivors from nonsurvivors than initial SOFA and mean SOFA. A decreasing score during the first 48 hours had mortality rates of 4.9%, while an unchanged or increased score was associated with a mortality rate of 5.7%.
CONCLUSIONS:
SOFA score in the ICU after cardiac surgery correlated with survival at 12 and 24 months. Patients with lower SOFA scores had higher survival rates. Differences in survival at 12 months were better correlated with the absolute value at 48 hours than with its variation. SOFA score may be useful to predict long-term outcomes and to stratify patients with higher probability of mortality.
Keywords: cardiac surgery, intensive care, organ dysfunction, Sequential Organ Failure Assessment, severity score
Cardiac surgery is associated with an acute inflammatory response that disrupts homeostasis resulting in important postoperative organ dysfunction (1, 2). Inflammation is heterogeneous and subsequent organ dysfunction is usually subclinical, but in its extreme forms, the systemic inflammatory syndrome can lead to major organ dysfunction and death (2, 3).
The presence of persistent organ dysfunction has been associated with worse clinical outcome and increased risk of death or disability (4). Indeed, patients with persistent organ dysfunction have increased rates of morbidity and mortality, with a higher length of stay in the ICU and the hospital, accounting for a high proportion of the ICU resources and budget (4, 5).
The Sequential Organ Failure Assessment (SOFA) score is a six-organ dysfunction/failure score measuring multiple organ failure daily, created in 1994 in a consensus meeting of the European Society for Intensive Care Medicine, and then revised in 1996. Each organ is graded from 0 (normal) to 4 (the most abnormal), providing a daily score of 0 to 24 points (6). Therefore, the SOFA score has been designed to report morbidity and to objectively quantify the degree of dysfunction/failure of each organ daily in critically ill patients (7). SOFA score is validated to be used after cardiac surgery and can be safely used to predict the degree of severity of postoperatory morbidity without specific adaptations (6–8).
Outcome prediction is important in the management of patients in the ICU, especially for therapeutic decision and resource allocation (5). Several risk models have been developed in the past decades to analyze postoperative data and predict outcome (8). Some of these models designed for and in use in general ICU patients also accurately predict mortality after cardiac surgery (6, 9, 10), with the SOFA score generally demonstrating the best performance (8).
However, few risk models predict long-term outcome. It may be specifically important in cardiac surgery, since long-term survival, rather than immediate outcomes (such as ICU length of stay) was previously demonstrated as a more adequate outcome to evaluate, due to the curative nature of the procedure (11). Although SOFA has been used as a reliable independent predictor of ICU mortality after cardiac surgery, few studies correlate early postoperative SOFA score with long-term survival. The impact of organ dysfunction after cardiac surgery on long-term outcome remains unknown.
The purpose of this study was to evaluate the correlation between early postoperative SOFA score and mortality at 12 and 24 months, understanding if SOFA can be an important tool to stratify patients in follow-up programs and allocate resources more efficiently.
PATIENTS AND METHODS
Study Population
Single-center retrospective study including 1,379 consecutive patients submitted to cardiac surgery between January 1, 2017, and December 31, 2019. Patients 1) who died during ICU stay, 2) were transferred to other ICUs after surgery or readmitted to ICU during the hospital stay, and 3) without SOFA calculated during ICU stay were excluded. After ICU stay, patients were transferred to the cardiothoracic surgical ward. No intermediate care unit was available. We used our institution registry database, combined with medical records to access information. This study was approved by the local ethics committee (Comissão Ética Centro Hospitalar Lisboa Norte—Ref. N.º 386/21) and followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Baseline Characteristics
Preoperative variables evaluated in this study included age, gender, weight, height, body mass index, comorbidities including hypertension, dyslipidemia, diabetes mellitus, chronic renal insufficiency (including dialysis dependence), cerebrovascular disease, peripheral vascular disease, smoking, respiratory disease, ischemic cardiomyopathy, previous cardiac surgery, and surgical reference diagnosis. Operative variables included the surgical procedure performed, surgical urgency (elective, urgent, emergent, salvage), cardiopulmonary bypass (CPB) time, and cross-clamp time. European System for Cardiac Operative Risk Evaluation (EuroSCORE) II were collected preoperatively for each patient, as previously published (12).
SOFA Calculation
SOFA score was calculated in the ICU every 24 hours, starting on the first postoperative day, as previously described, until discharge or for a maximum of 5 days. Only the scores of the first 5 postoperative days were included in this study since we aimed at correlating the initial postoperative SOFA score with the long-term outcome. SOFA was calculated considering the variables previously published (Supplementary Table 1, http://links.lww.com/CCX/A975). The assumed Glasgow Coma Scale values were used in sedated patients until demonstrated otherwise. To analyze the outcome prediction, we have considered the initial SOFA score (total SOFA score recorded on postoperative day 1), SOFA at 48 hours (total SOFA score recorded on postoperative day 2), mean SOFA (mean SOFA score of the total score recorded between postoperative day 1 and 5 in the ICU stay), and the highest SOFA (the highest total score recorded during the ICU stay between postoperative day 1 and 5). All these variables were correlated with survival at 12 and 24 months.
Statistics
Continuous variables are expressed as median with interquartile range. Categorical variables are reported in frequencies or percentages. We compared categorical variables with the chi-square test or Fisher exact test. All reported p values are two-sided, and p values of less than 0.05 were considered statistically significant. GraphPad Prism (GraphPad Software, San Diego, CA), Version 9 and Statistical Package for the Social Sciences (IBM, New York, NY) for Macintosh was used for this statistical analysis.
For continuous variables, nonparametric tests were performed either for small groups or for when the data did not fulfill the requirements of normality and homoscedasticity. If fulfilled, a parametric approach was always chosen.
Multivariate logistic regression analysis was conducted to assess associations between SOFA score and mortality, adjusted for possible confounding factors. Mortality was the dependent variable, while gender, diagnosis, procedure, comorbidities, left ventricular function, timing of surgery, and previous cardiac surgery were used as nominal independent variables, and age as covariable, using log-likelihood ratio test. To assess the ability to determine if the variables related with SOFA have a predictive value regarding mortality, receiver operating characteristic (ROC) curves were performed. We performed five-fold cross validation in a stratified fashion in order to keep the proportions of the dependent variable across splits. We repeated the procedure 40 times, randomizing the fold splits. We calculated mean and sd for the area under the curves of each split. Procedure was performed in python using packages numpy (1.20.1), statsmodels (0.12.2), and sklearn (1.0.1).
RESULTS
Patient Demographic Data
During the study period, a total of 1,379 patients submitted to cardiac surgery and admitted to the ICU were included in this study. Demographic data from all patients are presented in Table 1. Table 1 also presents a comparison of demographic data between survival and nonsurvival at 12 months. No differences were observed between both groups, except for the presence of chronic kidney disease and timing of surgery. Table 1 also presents information regarding the surgical procedure. Most of the patients were submitted to a single noncoronary artery bypass graft procedure (47.1%), with 9% of the patients having concomitant thoracic aortic surgery.
TABLE 1.
Demographic Data
| Variable | All Patients | Survival 12 mo | Death 12 mo | p |
|---|---|---|---|---|
| n | 1,379 | 1,318 | 61 | |
| Age, yr, median (IQR) | 69 (62–76) | 69 (62–76) | 71 (66–78) | 0.076 |
| Male sex, n (%) | 902 (65.4) | 860 (65.3) | 42 (68.9) | 0.679 |
| Hypertension, n (%) | 1,117 (81) | 1,069 (81.1) | 48 (78.7) | 0.618 |
| Diabetes mellitus, n (%) | 430 (31.2) | 413 (31.3) | 17 (27.9) | 0.672 |
| Dyslipidemia, n (%) | 840 (60.9) | 802 (60.9) | 38 (62.3) | 0.894 |
| Chronic kidney disease, n (%) | 356 (25.8) | 323 (24.5) | 33 (54) | < 0.0001 |
| Peripheral vascular disease, n (%) | 98 (7.1) | 95 (7.2) | 3 (8.2) | 0.797 |
| Cerebrovascular disease, n (%) | 96 (7) | 88 (6.7) | 8 (13.1) | 0.067 |
| Chronic lung disease, n (%) | 158 (11.5) | 147 (11.2) | 11 (18) | 0.102 |
| Ischemic cardiopathy, n (%) | 384 (27.8) | 371 (28.1) | 13 (21.3) | 0.306 |
| Previous cardiac surgery, n (%) | 26 (1.9) | 26 (2) | 0 (0) | 0.626 |
| Preserved LV function, n (%) | 1,107 (80.3) | 1,062 (80.6) | 45 (73.8) | 0.19 |
| Moderate LV function (31–50%), n (%) | 132 (9.6) | 127 (9.6) | 5 (8.2) | > 0.999 |
| Poor LV function (21–30%), n (%) | 100 (7.2) | 93 (7) | 7 (11.5) | 0.202 |
| Very poor LV function (< 20%), n (%) | 40 (2.9) | 36 (2.7) | 4 (6.6) | 0.097 |
| Elective | 1,264 (91.7) | 1,218 (92.4) | 46 (75.4) | < 0.0001 |
| Urgent | 69 (5) | 62 (4.7) | 7 (11.5) | 0.029 |
| Emergent | 46 (3.3) | 38 (2.9) | 8 (13.1) | 0.0006 |
| European System for Cardiac Operative Risk Evaluation II (IQR) | 1.64 (1.11–3.07) | 1.62 (1.08–2.98) | 3.03 (1.65–5.89) | < 0.0001 |
| Isolated CABG | 387 (28.1) | 377 (28.6) | 10 (16.4) | 0.041 |
| Single non-CABG | 650 (47.1) | 622 (47.2) | 28 (45.9) | 0.896 |
| Two procedures | 283 (20.5) | 266 (20.2) | 17 (27.9) | 0.147 |
| Three procedures | 59 (4.3) | 53 (4) | 6 (9.8) | 0.042 |
| Thoracic aorta surgery | 124 (9) | 116 (8.8) | 8 (13.1) | 0.249 |
| Cardiopulmonary bypass time, min (IQR) | 63 (43.8–88) | 62 (43–86) | 85 (62–104) | 0.0009 |
| Cross-clamp time, min (IQR) | 50 (34–68.3) | 50 (34–67) | 58 (44–77) | 0.018 |
CABG = coronary artery bypass graft, IQR = interquartile range, LV = left ventricular.
SOFA at the ICU
SOFA score was calculated for all patients during the first 5 days (Table 2). Only 202 of the patients (14.6%) were discharged from the ICU after 5 days. SOFA at 48 hours and was calculated in 968 patients (70.2%) since 411 (29.8%) were discharged from the ICU in the first 48 hours.
TABLE 2.
Sequential Organ Failure Assessment Description
| Sequential Organ Failure Assessment, n (%) | 0–1 | 2–3 | 4–5 | 6–7 | 8–9 | 10–11 | > 11 | Total |
|---|---|---|---|---|---|---|---|---|
| 24 hr | 535 (38.8) | 347 (25.2) | 256 (18.6) | 118 (8.6) | 64 (4.6) | 27 (2) | 32 (2.3) | 1,379 |
| 48 hr | 275 (28.4) | 281 (29) | 203 (21) | 113 (11.7) | 50 (5.2) | 22 (2.3) | 24 (2.5) | 968 |
| Mean | 495 (35.9) | 486 (35.2) | 241 (17.5) | 89 (6.5) | 35 (2.5) | 15 (1.1) | 18 (1.3) | 1,379 |
| Highest | 441 (32) | 372 (27) | 253 (18.3) | 160 (11.6) | 75 (5.4) | 34 (2.5) | 44 (3.2) | 1,379 |
Most of the patients had SOFA values between 0 and 1 (535 patients, 38.8%) and 2–3 (347 patients, 25.2%) at 24 hours after surgery. SOFA values between 2 and 3 were the most frequent value at 48 hours of stay at the ICU (29%). The majority of patients had a mean and highest SOFA of 0–1 (35.9% and 32%, respectively).
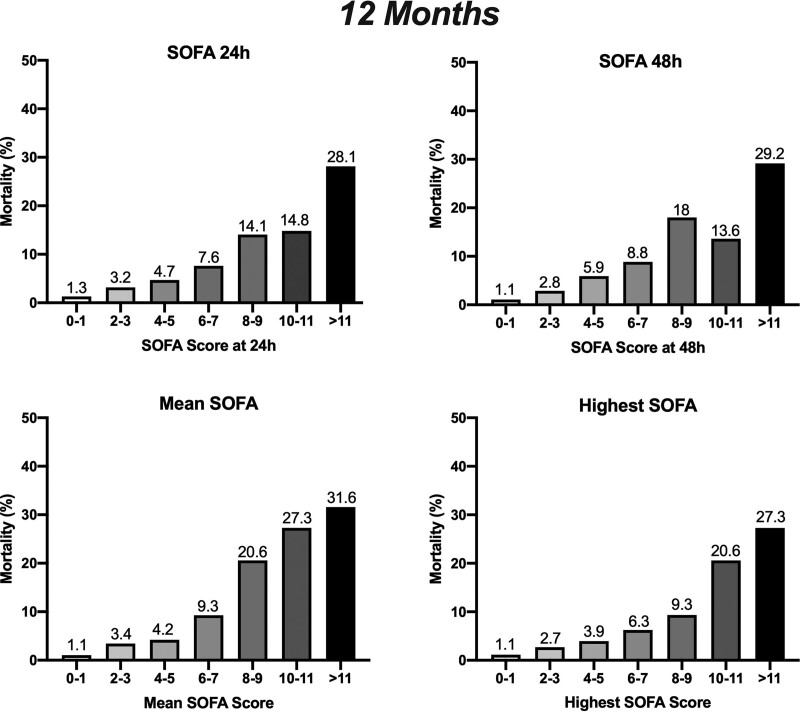
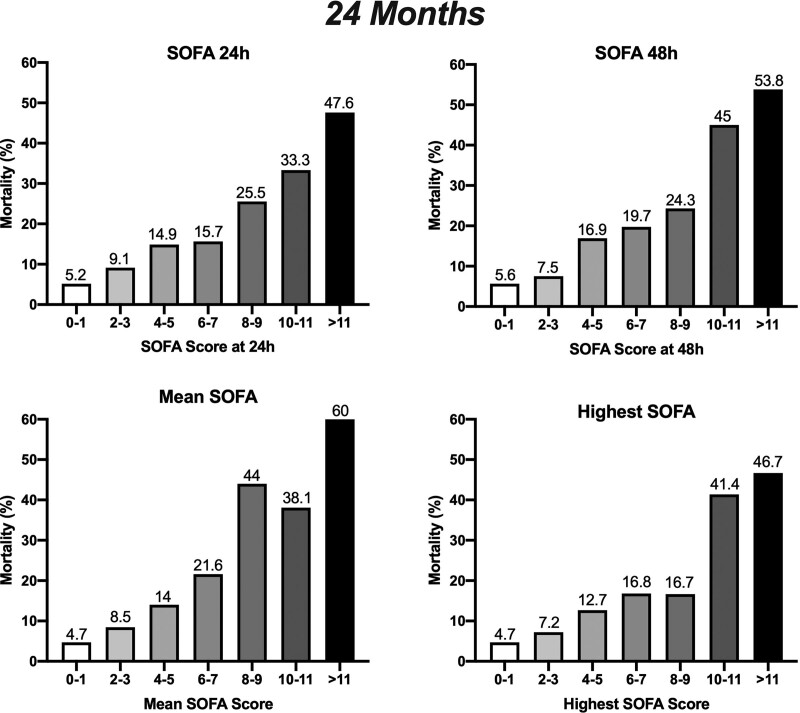
We have then accessed mortality at 12 and 24 months considering the calculated SOFA at the ICU. Patients with lower SOFA scores have better survival rates at 12 and 24 months (Figs. 1 and 2 and Table 3). At 12 months, patients with a postoperative SOFA values at 24 hours of 0–1 had mortality rates of 1.3%; SOFA 2–3 3.2%, SOFA 4–5 4.7%, SOFA 6–7 7.6%, SOFA 8–9 14.1%, SOFA 10–11 14.8%, and SOFA greater than 11 28.1%. The trend was similar at 24 months. We also observed a correlation between SOFA at 48 hours and survival at 12 and 24 months. Regarding the outcome at 12 months, SOFA at 48 hours of 0–1 had a mortality rate of 1.1%, SOFA 2–3 2.8%, SOFA 4–5 5.9%, SOFA 6–7 8.8%, SOFA 8–9 18%, SOFA 10–11 13.6%, and SOFA greater than 11 29.2% (Fig. 1). At 24 months, patients with SOFA at 48 hours of 0–1 had a mortality rate of 5.6%, SOFA 2–3 7.5%, SOFA 4–5 16.9%, SOFA 6–7 19.7%, SOFA 8–9 24.3%, SOFA 10–11 45%, and SOFA greater than 11 53.8% (Fig. 2).
Figure 1.
Correlation of Sequential Organ Failure Assessment (SOFA) at 24 hr, SOFA at 48 hr, mean SOFA, and highest SOFA at the ICU with mortality proportions of patients at 12 mo.
Figure 2.
Correlation of Sequential Organ Failure Assessment (SOFA) at 24 hr, SOFA at 48 hr, mean SOFA, and highest SOFA at the ICU with mortality proportions of patients at 24 mo.
TABLE 3.
Sequential Organ Failure Assessment Description
| Variable | Events (n) | Are Under the Curve (%) (95% CI) | se | Cutoffb | Sensitivity % (95% CI) | Specificity % (95% CI) | p | OR (95% CI) | p |
|---|---|---|---|---|---|---|---|---|---|
| 12 mo | |||||||||
| SOFA at 24 hra | 1,379 | 81.6 (75.1–88.1) | 3.3 | 4.5 | 62.3 (49.8–73.4) | 74 (71.5–76.3) | < 0.0001 | 1.203 (1.103–1.313) | < 0.001 |
| SOFA at 48 hra | 968 | 85.2 (79.8–90.6) | 2.7 | 4.5 | 73.1 (59.8–83.2) | 70.9 (67.8–73.7) | < 0.0001 | 1.211 (1.105–1.327) | < 0.001 |
| Mean SOFAa | 1,379 | 82.7 (76.1–89.3) | 3.4 | 4.55 | 55.7 (43.3–67.5) | 84.5 (82.4–86.3) | < 0.0001 | 1.266 (1.157–1.385) | < 0.001 |
| Highest SOFAa | 1,379 | 82.7 (76.2–89.2) | 3.3 | 5.5 | 59 (46.5–70.5) | 78.9 (76.7–81.1) | < 0.0001 | 1.224 (1.131–1.325) | < 0.001 |
| 24 mo | |||||||||
| SOFA at 24 hra | 899 | 73.3 (67.5–79.2) | 2.9 | 4.5 | 59.1 (48.7–68.8) | 71.4 (67.7–74.9) | < 0.0001 | 1.130 (1.001–1.276) | 0.048 |
| SOFA at 48 hra | 652 | 75.7 (69.4–82.1) | 3.3 | 4.5 | 67.1 (55.9–76.6) | 71.9 (67.6–75.9) | < 0.0001 | 1.158 (1.024–1.310) | 0.020 |
| Mean SOFAa | 899 | 73.8 (67.7–79.9) | 3.1 | 3.23 | 63.6 (53.2–72.9) | 69.8 (66.1–73.3) | < 0.0001 | 1.184 (1.038–1.350) | 0.043 |
| Highest SOFAa | 899 | 74.3 (68.4–80.2) | 3 | 4.5 | 65.9 (55.5–74.9) | 66.6 (62.7–70.2) | < 0.0001 | 1.136 (1.016–1.270) | 0.025 |
OR = odds ratio, SOFA = Sequential Organ Failure Assessment.
aAdjusted for age, gender, diagnosis, procedure, comorbidities (hypertension, dyslipidemia, diabetes mellitus, ischemic cardiopathy, peripheral artery disease, cerebrovascular disease, chronic kidney disease, pulmonary disease), left ventricular function, timing of surgery, and previous cardiac surgery.
bOptimal cutoff value for the best sensitivity and specificity.
Considering mean SOFA to analyze the outcome at 12 months, patients with a mean SOFA 0–1 had mortality rates of 1.1%, SOFA 2–3 3.4%, SOFA 4–5 4.2%, SOFA 6–7 9.3%, SOFA 8–9 20.6%, SOFA 10–11 27.3%, and SOFA greater than 11 31.6% (Fig. 1). The trend was similar when the outcome was accessed at 24 months, with mortality rates of 4.7% for SOFA 0–1, 8.5% for SOFA 2–3, 14% for SOFA 4–5, 21.6% for SOFA 6–7, 44% for SOFA 8–9, 38.1% for SOFA 10–11, and 60% for SOFA greater than 11 (Fig. 2). The trend for 12 and 24 months was similar for the highest SOFA score, with a significant correlation between values and mortality.
Prior to ROC curves, we assessed whether there was any confounding effect caused by the any variable and none was significant beside the presence of chronic kidney disease and the timing of surgery (data not shown). Then, we assessed whether the variables related with SOFA showed differences between survivors and nonsurvivors, thus having predictive potential. In this context, only the presence of chronic kidney disease and the timing of surgery had also predictive power to assess mortality at 12 and 24 months, as previously described, beside SOFA score. Taking this result into consideration, an adjustment for these variables was performed for ROC curves analysis.
Considering the ROC curves for the outcome at 12 months (Supplementary Fig. 1, http://links.lww.com/CCX/A976), SOFA at 48 hours showed the best potential to model outcome, since it showed the best balance between sensitivity and specificity; mean and highest SOFA also showed a good predictive power, with a similar strength between them. Considering the outcome at 24 months (Supplementary Fig. 2, http://links.lww.com/CCX/A976), SOFA at 48 hours shows, once again, the best power to model outcome, although with a reduced strength compared with 12 months. The other variables have a slightly reduced values of area under the curve but with considerably lower values of sensitivity. In fact, this trend is observed in most variables at 24 months, with a reduced ability to identify true positives. Subsequent cross-validation was performed in order to assess the out-of-sample performance of the model (Supplementary Fig. 3, http://links.lww.com/CCX/A976). Out-of-sample performance is slightly lower than in-sample performance, as expected, but still good, especially for the 24-month prediction.
Considering the patients with SOFA calculated at 48 hours, 299 (30.9%) increased the value between the first and second postoperative day, 302 (31.2%) had an unchanged value, and 367 (37.9%) a decreased score. Patients with a decreasing score during the first 48 hours had mortality rates of 4.9% at 12 months, while an unchanged or increased score was associated with a mortality rate of 5.7% (p = 0.662).
DISCUSSION
Here, we show an important correlation between SOFA score at the ICU and long-term outcome, in terms of mortality at 12 and 24 months. SOFA score has already been validated for cardiac surgery patients, and it has been used as a predictor of ICU and 30-day mortality (7, 8). However, to our knowledge, postoperative SOFA score has not been correlated with long-term mortality.
Multivariable risk models have been used in cardiac surgery for over 30 years. Risk models such as the EuroSCORE II; Society for Thoracic Surgeons 2008 Cardiac Surgery Risk Model score; and Age, Creatinine, Ejection Fraction score are widely use to predict in hospital and 30-day mortality, especially to identify high-risk patients.
Preoperative risk models consider multiple individual factors such as comorbidities, left ventricular function, clinical status before the procedure, and the procedure performed. However, they ignore anatomic and surgical individual complexity, the performance of the surgeon and perioperative events and complications. All of these situations can induce organ dysfunction, leading to increased postoperative morbidity and mortality. Furthermore, preoperative risk models are not dynamic and are unable to represent patient evolution in ICU.
Cardiac surgery patients have a particularity that predisposes to organ dysfunction: the use of CPB. CPB is essential to perform the majority of cardiac surgeries but is a major contributor for postoperative morbidity. CPB leads to a systemic inflammatory process with endothelial and vascular dysfunctions (13). Practically all organs and systems are affected by CPB. If we consider the six systems evaluated with SOFA, it is not difficult to understand how postoperative organ dysfunction covers all fields.
Systemic inflammation and pulmonary reperfusion during CPB contribute to lung injury, with reduction of end-expiratory lung volumes that lead to ventilation-perfusion mismatch and hypoxemia (14, 15). CPB is also responsible for myocardial dysfunction and injury, with ventricular impairment (16, 17) that increases the need for vasoactive and inotropic drugs at the ICU, increasing mortality and morbidity, with longer postoperative ICU stay and mechanical ventilation time (18, 19). Coagulation is also impaired after cardiac surgery, with hemodilution, acidosis, and hypothermia affecting platelet function during cardiac surgery (20). Not only platelets count in circulating blood decrease but the function of the remaining platelets is also impaired (21). Furthermore, neurologic complications (including postoperative cognitive dysfunction, delirium, and stroke) are frequent after cardiac surgery, affecting 25% to 50% of patients (22), and have an important functional impairment. Neurologic complications are multifactorial, with important contributions of hypoxia, reperfusion injuries, and CPB, with inflammation and endothelial dysfunction (13, 22). Even patients without any documented lesion can have an altered mental status due to cerebral edema caused by heart failure fluid overload (23). Postoperative neurologic changes are not included in preoperative risk models but are reflected in SOFA. In view of the renal system, preoperative renal function is included in almost all risk models. However, changes in renal function and renal support therapies (e.g., hemofiltration) have an important impact in the postoperative course and must also be considered. Acute kidney injury is independently associated with three- to eight-fold higher perioperative mortality and prolonged in ICU and in hospital length of stay (24).
The SOFA score was originally created to evaluate organ dysfunction in noncardiac critically ill patients (25), predicting the prognosis during ICU stay (5, 25). It was lately validated as a predictor of mortality and length of stay in cardiac surgical patients (6).
The SOFA score has the advantage of being a dynamic score, with daily evaluation, that reflect and represent the perioperative evolution and clinical status of patients at the ICU. For example, an increase of vasopressors use or worsening of an acute kidney injury will increase SOFA score and establish a higher risk of in-hospital and 30-day mortality. However, little is known on how organ dysfunction can affect long-term survival and outcome, and SOFA has not been evaluated to predict late mortality.
Our purpose was to analyze whether organ dysfunction, accessed by SOFA during the first postoperative days at the ICU, would predict mortality at 12 and 24 months. We selected a cohort of patients submitted to cardiac surgery, with an heterogeneous and complete population, with multiple comorbidities, procedures, cross-clamp, CPB times, and disease evolution and recovery to strengthen our observation.
Our study shows that highest SOFA and SOFA at 48 hours correlate with survival at 12 and 24 months, and patients with lower scores have higher survival rates. Probably SOFA at 48 hours represents the best variable to predict outcome, since a significant percentage of patients (29.8%) are discharged within the first 48 hours after surgery, and further evaluations are impossible to perform. The obtained ROC curves establish the best cutoffs between SOFA 3–4. This is an important consideration since short-term mortality (30 d) is one of the most frequent outcome measures to establish follow-up programs. Some cardiac surgery centers have follow-up programs up to 120–150 days since it has been shown that mortality after cardiac surgery matches mortality in the general population only after 140 days (26, 27). However, cause of death of patients submitted to cardiac surgery differs from the background population throughout the first postoperative year, especially due to heart failure (27). Our findings support this data and bring new questions regarding postoperative organ dysfunction. Possibly, organ dysfunction is not entirely reversible and may persist more than ICU or hospital stay. Reaching a specific level of organ dysfunction might have an important role in long-term mortality. Using SOFA to access long-term outcomes may be essential in identifying and stratifying patients with a higher probability of mortality. It will allow the design of more efficient and personalized follow-up programs. Thus, patients with higher SOFA and SOFA at 48 hours scores, who have a more significant postoperative organ dysfunction, may have an extended and more complete follow-up program. Nevertheless, using these variables for prediction has some downsides, since the values of sensitivity shows that they are maybe too conservative and could present an over-protective approach.
Furthermore, differences in survival at 12 months were better correlated with the SOFA scores at 48 hours than with its variation during the first 48 hours. Previous studies have showed that the highest SOFA score during the first 3 postoperative days and a greater increase in SOFA score during first 3 postoperative days had a significant correlation with 30-day mortality (6). However, for long-term outcome, the absolute value seems to be more accurate than its variation.
Our cutoffs demonstrate that SOFA can be a useful tool to stratify patients submitted to cardiac surgery and to predict long-term outcome. Future studies may assess whether long-term stratification using SOFA score can efficiently allocate resources, since the patients who are identified as requiring a more personalized follow-up are indeed the ones who need them. Nevertheless, there is a relevant number of cases our cutoffs seem to miss. Thus, SOFA should not be used as “one-size-fits-all” and other variables should be also considered. Future studies should look into finding these variables, exploring SOFA parameters and influence of the degree of dysfunction of each organ.
This study has several limitations, including the retrospective methodology. It is a single-center study, and our findings are related to our population and may not be applicable to other populations. The number of patients included is also a limitation.
CONCLUSIONS
SOFA in the ICU after cardiac surgery was correlated with survival at 12 and 24 months, with SOFA at 48 hours and mean SOFA showing the best potential to model outcome. Patients with lower scores had higher survival rates. Furthermore, differences in survival at 12 months were better correlated with the absolute SOFA score at 48 hours than with its variation during the first 48 hours. SOFA at 48 hours may be an important tool to identify and stratify patients with a higher probability of mortality, designing more efficient follow-up programs to improve patient monitoring. Prospective studies and bigger cohorts are needed to validate these findings.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Hill A, Clasen KC, Wendt S, et al. : Effects of vitamin C on organ function in cardiac surgery patients: A systematic review and meta-analysis. Nutrients 2019; 11:2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warltier DC, Laffey JG, Boylan JF, et al. : The systemic inflammatory response to cardiac surgery implications for the anesthesiologist. Anesthesiology 2002; 97:215–52 [DOI] [PubMed] [Google Scholar]
- 3.Day JR, Taylor KM: The systemic inflammatory response syndrome and cardiopulmonary bypass. Int J Surg 2005; 3:129–140 [DOI] [PubMed] [Google Scholar]
- 4.Stoppe C, McDonald B, Benstoem C, et al. : Evaluation of persistent organ dysfunction plus death as a novel composite outcome in cardiac surgical patients. J Cardiothorac Vasc Anesth 2016; 30:30–38 [DOI] [PubMed] [Google Scholar]
- 5.Ferreira FL, Bota DP, Bross A, et al. : Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001; 286:1754–1758 [DOI] [PubMed] [Google Scholar]
- 6.Pätilä T, Kukkonen S, Vento A, et al. : Relation of the Sequential Organ Failure Assessment score to morbidity and mortality after cardiac surgery. Ann Thorac Surg 2006; 82:2072–2078 [DOI] [PubMed] [Google Scholar]
- 7.Ceriani R, Mazzoni M, Bortone F, et al. : Application of the Sequential Organ Failure Assessment score to cardiac surgical patients. Chest 2003; 123:1229–1239 [DOI] [PubMed] [Google Scholar]
- 8.Howitt SH, Caiado C, McCollum C, et al. : Validation of three postoperative risk prediction models for intensive care unit mortality after cardiac surgery. Thorac Cardiovasc Surg 2018; 66:651–660 [DOI] [PubMed] [Google Scholar]
- 9.Hekmat K, Doerr F, Kroener A, et al. : Prediction of mortality in intensive care unit cardiac surgical patients. Eur J Cardiothorac Surg 2010; 38:104–109 [DOI] [PubMed] [Google Scholar]
- 10.Badreldin A, Elsobky S, Lehmann T, et al. : Daily-mean-SOFA, a new derivative to increase accuracy of mortality prediction in cardiac surgical intensive care units. Thorac Cardiovasc Surg 2012; 60:43–50 [DOI] [PubMed] [Google Scholar]
- 11.Falcoz PE, Chocron S, Stoica L, et al. : Open heart surgery: One-year self-assessment of quality of life and functional outcome. Ann Thorac Surg 2003; 76:1598–1604; discussion 1604 [DOI] [PubMed] [Google Scholar]
- 12.Nashef SA, Roques F, Sharples LD, et al. : EuroSCORE II. Eur J Cardiothorac Surg 2012; 41:734–744; discussion 744–745 [DOI] [PubMed] [Google Scholar]
- 13.Giacinto O, Satriano U, Nenna A, et al. : Inflammatory response and endothelial dysfunction following cardiopulmonary bypass: Pathophysiology and pharmacological targets. Recent Pat Inflamm Allergy Drug Discov 2019; 13:158–173 [DOI] [PubMed] [Google Scholar]
- 14.Ochs M, Nenadic I, Fehrenbach A, et al. : Ultrastructural alterations in intraalveolar surfactant subtypes after experimental ischemia and reperfusion. Am J Respir Crit Care Med 1999; 160:718–724 [DOI] [PubMed] [Google Scholar]
- 15.Dodd-o JM, Welsh LE, Salazar JD, et al. : Effect of bronchial artery blood flow on cardiopulmonary bypass-induced lung injury. Am J Physiol Heart Circ Physiol 2004; 286:H693–H700 [DOI] [PubMed] [Google Scholar]
- 16.Prasad A, Stone GW, Holmes DR, et al. : Reperfusion injury, microvascular dysfunction, and cardioprotection: The “dark side” of reperfusion. Circulation 2009; 120:2105–2112 [DOI] [PubMed] [Google Scholar]
- 17.Yellon DM, Hausenloy DJ: Myocardial reperfusion injury. N Engl J Med 2007; 357:1121–1135 [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki Y, Oba K, Matsui Y, et al. : Vasoactive-inotropic score as a predictor of morbidity and mortality in adults after cardiac surgery with cardiopulmonary bypass. J Anesth 2018; 32:167–173 [DOI] [PubMed] [Google Scholar]
- 19.Shahin J, DeVarennes B, Tse CW, et al. : The relationship between inotrope exposure, six-hour postoperative physiological variables, hospital mortality and renal dysfunction in patients undergoing cardiac surgery. Crit Care 2011; 15:R162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kjellberg G, Holm M, Lindvall G, et al. : Platelet function analysed by ROTEM platelet in cardiac surgery after cardiopulmonary bypass and platelet transfusion. Transfus Med 2020; 30:369–376 [DOI] [PubMed] [Google Scholar]
- 21.Warren OJ, Smith AJ, Alexiou C, et al. : The inflammatory response to cardiopulmonary bypass: Part 1–mechanisms of pathogenesis. J Cardiothorac Vasc Anesth 2009; 23:223–231 [DOI] [PubMed] [Google Scholar]
- 22.Cropsey C, Kennedy J, Han J, et al. : Cognitive dysfunction, delirium, and stroke in cardiac surgery patients. Semin Cardiothorac Vasc Anesth 2015; 19:309–317 [DOI] [PubMed] [Google Scholar]
- 23.Anderson RE, Li TQ, Hindmarsh T, et al. : Increased extracellular brain water after coronary artery bypass grafting is avoided by off-pump surgery. J Cardiothorac Vasc Anesth 1999; 13:698–702 [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Bellomo R: Cardiac surgery-associated acute kidney injury: Risk factors, pathophysiology and treatment. Nat Rev Nephrol 2017; 13:697–711 [DOI] [PubMed] [Google Scholar]
- 25.Vincent JL, Moreno R, Takala J, et al. : The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707–710 [DOI] [PubMed] [Google Scholar]
- 26.Siregar S, Groenwold RH, de Mol BA, et al. : Evaluation of cardiac surgery mortality rates: 30-day mortality or longer follow-up? Eur J Cardiothorac Surg 2013; 44:875–883 [DOI] [PubMed] [Google Scholar]
- 27.Hansen LS, Sloth E, Hjortdal VE, et al. : Follow-up after cardiac surgery should be extended to at least 120 days when benchmarking cardiac surgery centers. J Cardiothorac Vasc Anesth 2015; 29:984–989 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.