Abstract
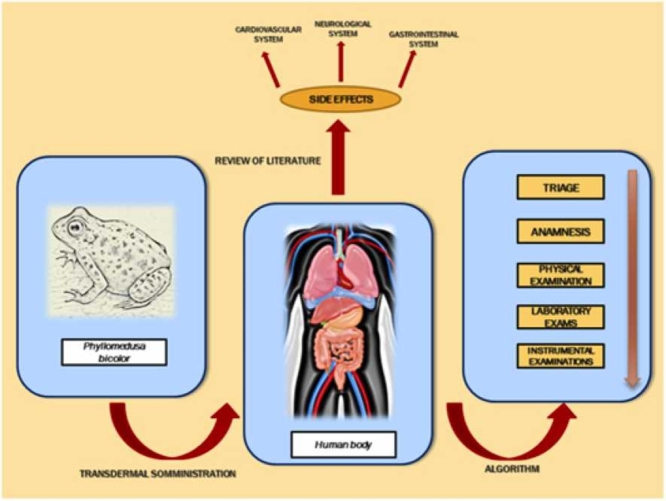
Kambo is the name of a natural substance derived from the glandular secretions of the amphibian Phyllomedusa bicolor, a species native to regions in South America. The communities living in these areas administer the substance generally transdermally during rituals for religious-purifying purposes, producing small skin burns. The scientific literature has reported some cases of intoxication following the use of Kambo but this aspect is still poorly understood. In fact, no shared therapy protocols exist for these events nor any real legislation on Kambo. The purpose of this work was to examine all cases of acute intoxication resulting from the administration of Kambo and published over the last 10 years, illustrating clinical signs, laboratory findings, instrumental tests, and therapy. The several cases identified in our review confirm that acute Kambo intoxication can occur, with serious and life-threatening effects. We developed a protocol aimed at the early diagnosis of cases of suspected acute intoxication by creating a treatment algorithm. The study aims to investigate the pathophysiology of these events in humans, proposing a protocol for the diagnosis and treatment of these cases that can be used by healthcare professionals.
Keywords: Kambo, Intoxication, Phyllomedusa bicolor, Forensic toxicology, Sapo
Graphical Abstract
Highlights
-
•
The use of Kambo has spread for distribution worldwide through numerous websites.
-
•
The literature review confirmed that acute Kambo intoxication include possible side effects.
-
•
Intoxication may affect various systems with laboratory or instrumental alterations.
-
•
Intoxication may be reversible if promptly diagnosed and treated.
-
•
An algorithm with adequate triage can support diagnosis and treatment.
1. Introduction
Toxins include a wide variety of molecules, enzymes and polypeptides that may cause dangerous effects on humans after contact and absorption, due to interaction with enzymes or cells [1], [2]. In nature, various biological organisms synthetize toxins such as bacteria, fungi, plants, or animals. Biotoxins include necrotoxins (causing cell death), cytotoxins (causing cellular damage), mycotoxins (causing food contamination), neurotoxins (disturbing ion channels conductance) [3]. Especially in animals, toxins are used for predation or defense strategies. Several mechanisms are responsible of the poisoning effects like the action on cell receptors or ion channels, resulting in production of neuropeptides, neuromuscular effects, cytoskeletal injury, increase of vasoactive molecules with coagulation activation. For example, toxins produced by insects like bees, beetles or wasps, such as phylanthotoxins may stimulate ligand gated channels; apamin can activate potassium channels and release neurotrasmittors; assassin bug saliva stimulates calcium channels [4], [5], [6]. Despite their toxicity, pharmacological study of these compounds is very useful to evaluate their potential for biomedical applications, because of their concomitant antimicrobial or anticancer properties. For instance, wasp venom contains different compounds like mastoparan, decoralin, phospholipase A2 with anticancer and anti-inflammatory properties [2]. Scorpion venom (Androctonus amoreux) has also shown in vitro anti-proliferative effects, in solid and liquid tumors, resulting in reduction of cancer volume/size and changes in VEGF and Ki67 expression [4]; bee venom showed in vivo and in vitro anticancer properties [7]. Amphibians, like frogs or salamanders can also secrete numerous de novo peptides or proteins, thanks to their skin glands or using diet or microorganisms as sources.
Kambo is a substance derived from the natural secretions of a frog (Phyllomedusa bicolor) typically found in regions of South America, such as the Amazon and Brazil [8]. In these geographical areas, Kambo is used for recreational and purifying purposes during sessions characterized by a strong spiritual component [9]. The substance is generally administered transdermally with a hot stick during the rite by a shaman, generating small circular burns on the skin. The injection sites are typically the upper limbs in men and the lower limbs in women [10]. The use of this substance, typical of South America, has subsequently spread to other geographical regions, including Europe and the United States [11]. As its dissemination increased, so has the interest in this product on the part of the scientific community. In particular, several papers have been published that examined the biochemical and molecular composition of Kambo [12]. To date, Kambo is the subject of discussion on several websites that encourage its use through guides and video tutorials, as well as online e-commerce platforms that make it available for distribution worldwide. Numerous websites also emphasize the psychological benefits of the substance, suggesting particular methods of preparation on the days prior to its administration. Kambo contains various peptides with potential biomedical applications, especially for pain management. Hesselink suggested the use of ceruletide, an agonist of cholecystokinin (CCK), for pain treatment in cancer, colics, headache with promising clinical applications [12]. Also, intrathecal use of dermorphin was suggested in post-operative pain or for palliative aims [13]. Antimicrobial properties of dermaseptins, a group of polycationic peptides produced from skin glands of Phyllomedusa bicolor have been widely proved [14]. Byard reported that various Web sites encourage the use of Kambo in contexts of alternative and integrative medicine for prevention and treatment of neurological diseases (Alzheimer, Parkinson, meningitis, stroke) but also for chronic fatigue syndrome, hepatitis and diabetes [9]. In this sense, Kambo is known as “toad vaccine” for its likely potential in preventing diseases.
In parallel, the literature has also illustrated some cases of side effects related to the administration of Kambo, although this aspect is still poorly understood. However, there are no reviews that have systematically examined the case studies available in the literature regarding the toxic effects of Kambo, nor are shared therapy protocols available for these cases. The purpose of this work was to analyze all clinical cases of acute intoxication with Kambo published over the last 10 years, the period of greatest scientific production on this topic. This review aims to provide the most comprehensive view possible on the cases of acute intoxication studied so far, deepening the understanding of the pathophysiology of these adverse events and the effects on organs and systems and proposing a management algorithm that can be used by doctors in these cases. The proposed analysis, with examination of signs, symptoms, laboratory findings and instrumental tests, could prove useful in supporting the early diagnosis of acute Kambo intoxication cases and their treatment.
2. Materials and methods
We performed a literature review using NCBI’s PubMed search engines by entering the keywords “kambo” or “Phyllomedusa bicolor”. Studies published between 2010 and 2021 that focused on clinical cases in which adverse effects occurred after the use of Kambo were investigated. As a selection criterion, the full text of articles whose title and abstract contained the word Kambo or Phyllomedusa were read.
The articles were excluded by title, abstract or full text if they did not meet our inclusion criteria. Particular exclusion criteria were: studies focusing exclusively on the pharmacological properties of individual peptides or discursive reviews on the topic without description of a clinical case of intoxication managed by the authors. Table 1, Table 2, Table 3, Table 4, Table 5.
Table 1.
Results of the literature review (part one).
| Robalino Gonzaga et al. [15] | Alamos et al. [16] | de la Vega et al. [17] | Campodónico et al. [18] | Agüero-González et al. [19] | Roy et al. [20] | Kumachev et al. [21] | Li et al.[22] | Aquila et al. [23] | Pogorzelska et al. [24] | Leban et al. [25] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | F | F | F | F | F | F | F | F | M | M | F |
| Age | 62 | 41 | 33 | 41 | 33 | 32 | 24 | 42 | 34 | 44 | |
| Weight | 88 | Overweight | 72 | ||||||||
| Comorbidity | Depression, alcohol, and nicotine dependence | Depression | None | Hypothyroidism, personality disorder | None | None | Moderate coronary atheroma, left ventricular hypertrophy | Previous consumption of alcohol and cannabis | None | ||
| Onset of symptoms after administration | Few minutes | 12 h | 6 h | 1 h | 30 min | 3 h | |||||
| Site of administration | Skin (ointment) | Leg | Shoulder | arm | leg | Arm and heel | Ankle | arm | Abdomen-back | Shoulder | |
| Water intake after administration | 6 litres | 3,5 litres | 4 litres | ||||||||
| Type of consumer | Occasional | Chronic | Occasional | Occasional | chronic | Cronic | chronic | Occasional | |||
| Identification of the substance at toxicological screening | No | No | No | No | yes | No | |||||
| Toxicological findings | +BDZ | + cannabinoids | + cannabinoids | + deltorphin A | |||||||
| Tachycardia | Yes | yes | No | No | yes | ||||||
| Heart rate | 110 bpm | 60 | 82 | 90 | |||||||
| Blood pressure (mmHg) | 110/74 | 92/55 | 112/85 | 160/100 | |||||||
| Respiratory rate (min) | 14 | 26 | 20 | ||||||||
| O2 saturation | 96 | 100 | |||||||||
| Temperature | Yes | ||||||||||
| Dyspnea | Yes | Yes | |||||||||
| Nausea | yes | yes | yes | yes | yes | yes | yes | ||||
| Abdominal discomfort | yes | yes | yes | ||||||||
| Results of the literature review (part two) | |||||||||||
| Vomit | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| Diarrhea | Yes | ||||||||||
| Muscle ailments | Yes | Yes | Yes | Yes | Yes | ||||||
| Rhabdomyolysis | Yes | Yes | Yes | ||||||||
| Psychiatric disorders | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| Epilepsy | Yes | Yes | Yes | Yes | Yes | ||||||
| Muscle markers | Elevate | Elevate | Elevate | Elevate | Elevate | ||||||
| Transaminases | Elevate | Elevate | Elevate | Elevate | |||||||
| Leukocytosis | Yes | Yes | Yes | ||||||||
| Lactic acidosis | Yes | Yes | |||||||||
| pH | 7.52 | 7.38 | |||||||||
| LDH | Elevate | ||||||||||
| Amylase | Normal | ||||||||||
| Lipase | Normal | ||||||||||
| Natremia | Reduced | Reduced | Reduced | ||||||||
| Kaliemia | Reduced | Reduced | Reduced | ||||||||
| Magnesemia | Reduced | ||||||||||
| Phosphatemia | Reduced | ||||||||||
| Number of platelets | Reduced | ||||||||||
| CT / MRI investigation | Pneumothorax, pneumomediastinum and pleural effusions | Normal brain imaging | Normal brain imaging | Normal brain imaging | No evidence of abnormalities on brain imaging (CT) | ||||||
| EEG | Normal | Altered | Altered | ||||||||
| EMG | Altered | ||||||||||
| Related pathology | Rupture of gullet from severe vomiting | Acute renal failure | Dermatomyosis | Syndrome of Inappropriate ADH Secretion (SIADH) | Syndrome of Inappropriate ADH Secretion (SIADH) | Psychosis | Acute gastrointestinal poisoning | Syndrome of Inappropriate ADH Secretion (SIADH) | Death secondary to acute heart failure | Toxic hepatitis | Syndrome of Inappropriate ADH Secretion (SIADH) |
| Duration of symptoms | 7 days | 3 months | 7 days | 5 days | 9 days | 1 h | 1 day | 10 days | Three days | ||
| Therapy | Surgical intervention | Mechanical ventilation, Valproic acid, Hydration | Prednisone | Dexmedetomidine, Sodium bicarbonate, Reduction in water intake | Midazolam, Calcium Gluconate, Magnesium Sulphate, Potassium Chloride, Dexmedetomidine | Risperidone | Ondansetron, Ketorolac, Naloxone | Haloperidol, diphenhydramine, Lorazepam | Lactulose, Silymarin, Ornithine | Diazepam, Sodium chloride 0.9%, Water restriction | |
Table 2.
Features of acute intoxication cases in the reviewed literature.
| Number of cases | Sex | Age range | Mage | Systems affected by signs/ symptoms | Related pathologies |
|---|---|---|---|---|---|
| Acute poisoning N = 11 |
M:F = 2:9 | 24–62 years | 38,6 years | Gastrointestinal system = 8 Nervous system = 7 Musculoskeletal system = 7 Cardiovascular system = 4 |
Syndrome of Inappropriate ADH Secretion: 5 Acute renal failure: 1 Dermatomyositis: 1 Rupture of esophagus: 1 Severe psychosis: 1 Toxic hepatitis: 1 Death: 1 |
Table 3.
Toxicological examination, collection and storage of samples.
| Collection of biological material on living beings | Collection of biological material from corpse | Method of conservation | Instrumental examination | Main families of bioactive peptides to be researched |
|---|---|---|---|---|
| Peripheral blood Central blood Urine Hair |
Peripheral blood Central blood Vitreous humor Bile Urine Hair Skin biopsy |
-80 °C | Mass spectrometry | Bradykinins Phyllokinin and Phyllomedusine Ceruleine and Sauvagine Dermorphins and Deltorfins Adenoreguline |
Table 4.
Peptides’formula and pharmacodynamics.
 |
Table 5.
Protocol for early diagnosis and treatment of cases of suspected acute intoxication by Kambo.
 |
Subsequently, a table containing the selected cases was generated, with the creation of a medical record for each case in which all available parameters were noted, including:
-
–
anamnestic data (sex, age, clinical history);
-
–
method of administration and onset of intoxication;
-
–
symptoms and signs of intoxication and their relative duration;
-
–
pathological findings in laboratory tests, including toxicological tests;
-
–
results of instrumental tests;
-
–
pathologies associated with the use of Kambo;
-
–
therapies administered by health professionals;
-
–
patient hospital discharge times.
The data were examined and compared with each other and a management algorithm was created for the patient with suspected acute Kambo intoxication.
3. Results
3.1. Cases examined
The search retrieved 50 studies published between 2010 and 2021. The analysis of titles, abstracts and full texts led to the final selection of 11 cases of acute intoxication [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25].
The cases examined involved 9 female subjects (81.8% of cases) and 2 male subjects (18.2%) ranging in age from 24 to 62 years, with a mean age of 38.6 years. The comorbidities identified in the medical history included mood disorders, previous consumption of substances such as alcohol, cannabis or nicotine, overweight, and structural cardiac alterations with coronary atheroma.
3.2. Administration of the substance
The limbs were the typical injection sites, with more frequent administration to the upper limbs (45.4%) than the lower limbs (36.3%). However, administration can also take place in other parts of the body, including the abdomen and back. In 3 cases, the administration of Kambo was followed by the intake of several liters of water (27.2%). The onset of symptoms after intake ranged from a few minutes to a maximum of 12 h.
3.3. Toxicological investigations
Toxicological investigations in the patients examined only identified the presence of benzodiazepines and cannabinoids. The substance Kambo was only isolated in one case, with post-mortem investigation identifying the peptide deltorphin A [23].
3.4. Signs and symptoms
The series showed the presence of the following signs and symptoms: heart rhythm alterations, nausea and vomiting, diarrhea, abdominal discomfort, dyspnea, neuropsychiatric disorders such as epilepsy, muscle disorders such as weakness or cramps, mood disturbances, and hydroelectrolytic imbalance.
3.5. Laboratory investigations
The following pathological findings were identified by laboratory investigation: elevation of muscle markers, elevation of transaminases, leukocytosis, lactic acidosis, hyponatremia, hypokalemia, hypomagnesemia, hypophosphatemia, and thrombocytopenia. In cases with high water intake and subsequent development of syndrome of inappropriate antidiuretic hormone secretion (SIADH), marked alteration of plasma and urine osmolarity was also found.
3.6. Instrumental examinations
Among the instrumental tests, alterations in the electroencephalogram (EEG) and electromyography (EMG)were identified. In one case, X-ray and CT examination of the chest revealed the development of pneumothorax in a patient with severe vomiting following the intake of Kambo. Brain imaging examination by CT or MRI showed no alterations in any of the cases.
3.7. Pathologies associated with the use of Kambo
The following associated pathologies were described: SIADH (45.45%); acute renal failure (9%) dermatomyositis (9%); rupture of esophagus (9%); severe psychosis (9%); toxic hepatitis: (9%), and death (9%).
The systems affected by intoxication that exhibited pathological signs and symptoms or laboratory or instrumental alterations were the gastrointestinal system (72.72%); nervous system (63.63%); musculoskeletal system (63.63%), and cardiovascular system (36.36%).
3.8. Therapies and discharge times
The following therapies were carried out in the cases described:
-
-
benzodiazepines;
-
-
antipsychotics;
-
-
mood stabilizers
-
-
naloxone;
-
-
stabilization of the hydroelectrolytic balance;
-
-
hepatic protectors;
-
-
antihistamine;
-
-
antiemetics;
-
-
respiratory assistance;
-
-
surgery.
The time of hospital discharge ranged from 1 h to 3 months, with an average hospital stay of one week.
4. Discussion
Kambo, also known by the name Sapo, is a substance derived from the natural secretions of an amphibian belonging to the Phyllomedusa family [13]. Kambo is developed in the context of a local ritual during which Phillomedusa bicolor is tied up and a stick is rubbed on its skin. After a few days, the frog is released and the shaman, after mixing the secretions with saliva, administers the substance transdermally. A hot stick containing the secretions is then applied to the human skin producing small circular burns, usually on the upper limbs in men and lower limbs in women [23]. The described ritual has a strong religious and spiritual component in these geographical areas. The applications of this substance have therefore also extended to other countries. An online survey carried in Western countries has reported various reasons for Kambo application, including religious, spiritual, preventive or therapeutic motivations in the context of alternative medicine [26].
The first studies on this substance, endemic in some regions of South America, can be found in the PubMed search engine by the late 1960 s [27]. Since then, various polypeptides present in amphibian extracts have been examined and studied by the research group of Prof. Erspamer, both their biochemical composition and their pharmacological and biological properties through animal experiments [28]. Subsequently, using immunohistochemistry and electron microscopy, Lacombe et al. identified the presence of three different types of glands on the frog’s skin, each of which is capable of producing different substances with biological properties [29].
Today it is known that the secretions of Phyllomedusa bicolor are composed of molecules with different biological activities, including antimicrobial peptides such as dermaseptins, and peptides with opioid properties such as dermorphins and deltorphins. Also known are peptides such as phyllocaerulein (with action on smooth muscles of the gastrointestinal system and analgesic effect), phyllokinin (a bradykinin with hypotensive action), phyllomedusin (a tachykinin with vasodilating action), and sauvagine (a peptide with peripheral vasodilating action) [30], [31].
These peptides have been individually examined for their molecular structure and effects. The possible use of some of these peptides, such as cerulein, for therapeutic purposes has been evaluated due to their intrinsic analgesic properties [12]. Although the individual molecules have been extracted and analyzed in various laboratory assays, the overall in vivo effects of the substance Kambo, i.e., the concomitant administration of all of these peptides to humans, still appear uncertain. The physiopathology of acute intoxication in humans has not yet been fully defined, nor have specific antidotes or therapeutic protocols been developed in these cases. In recent years, several cases of acute Kambo intoxication have been described as a result of the widespread commercialization of the substance. Therefore, our literature review systematically evaluated individual cases of intoxication from a clinical point of view in order to facilitate comparison of the symptoms related to Kambo and the therapies proposed by different health facilities in an attempt to develop a uniform protocol.
The several cases identified in our review confirm that acute Kambo intoxication can occur, with serious and life-threatening effects [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]. In the series evaluated, intoxication was more common among female subjects than among males, with a rather low mean age. The transdermal route of administration with a stick was used in all cases, which generated characteristic circular burns. In some cases, a cream was also used. The onset of intoxication was rather rapid and occurred within a few hours and in some cases almost immediately. After intoxication the substance containing different bioactive peptides (over one hundred) could reach and act on different anatomical systems such as neurological, cardiovascular, gastrointestinal system. Various cytotoxin effects in different systems have been described in literature even if the precise cellular mechanism of the peptide mixing is not yet fully understood [16], [17]. For instance, deltorphins and dermorphins are opioids with agonist action on delta and mu receptors which can determine respiratory depression acting on the nervous cells and causing dysphoria and analgesia [16], [17], [18]. Also, in rats, deltorphin administration have been associated with motor activity. Sauvagine is a neuropeptide and corticotropin realizing factor. Several studied have described active action on adrenal cortex thus causing production of catecholamines, cortisol, beta-endorphins with hypotensive effects, responsive tachycardia, and gastrointestinal effects like diarrhea. Blood pressure falling and vasodilatation of mesenteric vascular system have been reported in animals i.e., rats, rabbits and dog after sauvagine administration. Also, phyllocaerulein is a peptide hormone with a cholecystokinin-like action that can stimulate adrenal cortex and pituitary gland, thus causing hypotension with tachycardia. Also, vomiting, sweating and urge for defecation have been associated with phyllocerulein. Phyllomedusin is a tachykinin that can increase neuronal excitation with psychoactive and behavioral responses but also it acts on smooth muscle cells causing their contractions. The severity of toxic effects after Kambo intoxication could vary among subjects, also depending on dose administered, and even if cytotoxic effects are not yet clearly known, the analysis of various pathological conditions reported in literature like acute renal failure, dermatomyositis, rupture of esophagus, psychosis, toxic hepatitis suggest that the combined action of the different bioactive peptides in Kambo can result in acute adverse events on humans.
Regarding the intake of the substance, we emphasize in particular the danger related to the concomitant ingestion of large volumes of water, which has been associated with SIADH and strong hydroelectrolytic imbalances, with alterations in plasma and urine osmolarity, as well as hypokalemia, hypomagnesemia and hypophosphatemia. Campodónico et al. hypothesized that hyponatremia may be due to the central effects of peptides on the nervous system resulting in SIADH that can be worsened by the ingestion of water [18]. The electrolyte imbalances would also be exacerbated by the powerful action of Kambo on the gastrointestinal system. In fact, many authors have described symptoms such as nausea, incoercible vomiting, and profuse diarrhea that would explain the loss of electrolytes. For esophageal rupture, Robalino Gonzaga et al. reported that the pathological mechanisms would be related to the combined action of different vasoactive peptides, such as opioid peptides, phyllocaerulein, sauvagine, phyllomedusin and phyllokinin which can cause smooth muscle contraction and vasodilatation. The combined effect would result in increase of intra-esophageal pressure with respect to the intra-thoracic pressure with possible rupture, probably due to the action of peptide mix on smooth muscle contraction [15]. Finally, De la Vega et al. analyzed the possibility of drug-induced dermatomyosis with unknown mechanisms. The series also demonstrated a hepatotoxic action of the toxins, with an increase of liver damage markers (transaminases) and cholestasis indices in numerous cases. In one case, severe toxic hepatitis accompanied by the development of jaundice was also described in a regular user of Kambo.
From a neurological point of view, the data confirm that during the pathophysiology of the acute event, Kambo possesses strong neuroactive properties, inducing tonic-clonic epileptic seizures as well as serious personality alterations such as anxiety, paranoia, psychosis, and personality and consciousness disorders. It is known that the substance contains centrally acting peptides such as sauvagine and phyllocaerulein as well as opioid peptides which, together, could explain the overall neurotoxic action. These effects were evaluated using instrumental tests that demonstrated wave alterations in the EEG, although no acute changes in brain imaging (CT and MRI) were found. Roy et al. reported in patients with post-traumatic stress disorder and psychosis increased levels of CRF with respects of patients without psychosis that had normal levels of CRF. So, the psychosis could be because of the various neuropeptides and in particular to molecules which have an activity linked to CRF levels. Aquila et al. also hypothesized the possibility of an increasing lethal effect of the peptide mix, in particular on blood-brain barrier permeability which could allow to other peptides to arrive on the nervous system.
The series confirmed the action of Kambo on the peripheral vascular system and heart rhythm, with a hypotensive effect and tachycardia. These properties could be associated with the known tachykinins and bradykinins present in the secretions, which are responsible for the peripheral vasodilatory effect and concomitant rebound change in the heart rhythm, with immediate flushing effects on the face. These mechanisms of action on the cardiovascular system should be monitored and considered potentially risky, especially in subjects with previous cardiac risk factors such as coronary atheroma or structural cardiac alterations, in the light of a case of death confirmed at autopsy and by post-mortem toxicological investigations, which occurred approximately 30 min after administration. Death would be related to the action of peptide mixing on myocardial perfusion, and to the effects of biopeptides on the vascular cells which result in increasing risk of ischemic disease.
It is also interesting to consider a powerful action on the skeletal muscle system which, in some cases, resulted in muscle damage characterized by an increase of muscle markers (CK, LDH), lactic acidosis, rhabdomyolysis, and renal failure. Such muscle damage could be exacerbated by the development of tonic-clonic seizures, which should be treated promptly. Among the possible laboratory alterations, we also report leukocytosis and thrombocytopenia, which suggest a plausible action of toxins on the hematopoietic and coagulation systems.
Among the possible therapies, the authors described the use of fluid therapy, psychotropic drugs, electrolyte balance correctors, liver protectors and antiemetics, depending on the symptoms present and the laboratory data. In particular, we emphasize the need to study naloxone as a possible antidote, which was successfully administered in a case of acute gastrointestinal intoxication that resolved within an hour. These benefits may be due to the pharmacodynamics of naloxone, which acts as a non-selective and competitive antagonist of opioid receptors, with maximum affinity for the μ-opioid receptor. All cases described, except one case of sudden death, were resolved because of the initiation of therapy within a few days (average hospital stay of 7 days). Therefore, it is important to emphasize the potential reversibility of acute intoxication if promptly diagnosed and treated.
For this purpose, we developed a protocol aimed at the early diagnosis of cases of suspected acute intoxication by creating a treatment algorithm. Our protocol consists of assigning a triage code when the patient arrives in the emergency room and immediate hemodynamic stabilization in the event of a red code, i.e., absolute emergency. Cases of mild-moderate urgency (green-yellow triage) require the following diagnostic steps:
-
1)
Anamnesis: evaluation of the route of administration and time when the substance was taken; possible concomitant use of other exogenous substances (alcohol, drugs, psychotropic drugs); possible water intake during the rite; collection of all signs and symptoms reported by the patient, and evaluation of the patient’s comorbidities.
-
2)
Physical examination: identification of the site of administration, with analysis of upper and lower limbs as well as chest and abdomen; evaluation of vital parameters (blood pressure, SO2, body temperature, heart rate, respiratory rate), and neurological, psychiatric, cardiovascular, and abdominal examination. We believe that already at this stage it is appropriate to introduce a first-level empirical therapy aimed at stabilizing acute symptoms, pending the results of the laboratory and instrumental investigations.
-
3)
Laboratory investigations:
-
-
complete blood count with leukocyte formula and electrolytes;
-
-
markers of cardiac and skeletal muscle damage (troponins, CK, LDH);
-
-
indices of liver damage and cholestasis (transaminases, direct and indirect bilirubin, gamma-glutamyl transferase);
-
-
complete urinalysis;
-
-
collection of blood and urine for toxicological investigations, with at least one skin biopsy being obtained from the site of administration of the substance.
Treatment should be initiated as soon as the laboratory findings are available.
-
4)
Instrumental examinations:
-
-
ECG;
-
-
abdominal ultrasound exam;
-
-
EEG and EMG;
-
-
total body CT.
At the end of this phase, it would be appropriate to modulate the therapy depending on the instrumental results, with the help of specialists and based on the circumstances.
Certainly, benefit risk-ratio should be further assessed for this substance, through the comparison of favorable effects and potential harmful events, also with future clinical reports and pharmacological studies. This analysis will have necessarily to balance the potential benefits of biopeptides with severity, longevity and preventability of risk due to Kambo administration.
Although one limitation of our review is the small number of cases analyzed, the study suggests that Kambo cannot be considered a simple natural medicine. The substance contains numerous bioactive peptides that are still not fully known, with effects on various organs and systems, and that are possibly lethal. Although some studies suggest an analgesic potential of individual peptides, the combined intake of these molecules may have significant side effects. We therefore emphasize the importance of strict surveillance of the websites that encourage the use of this substance and urge greater control of e-commerce or illicit trafficking of animals and secretions, including through the dark web.
From a toxicological point of view, we highlight the need to increase the isolation of peptides from biological samples obtained from these patients, proposing the storage of fluids and skin biopsy at −80 °C and their analysis at clinical toxicology institutes by mass spectrometry to identify the peptides.
Funding
This research did not receive any specific grant.
CRediT authorship contribution statement
Sacco Matteo Antonio: Conceptualization, Writing – original draft. Zibetti Angelica: Methodology. Bonetta Carlo Filippo: Validation. Scalise Carmen: Visualization. Abenavoli Ludovico Data curation. Guarna Francesca: Formal analysis. Gratteri Santo: Writing – original draft. Ricci Pietrantonio: Writing – original draft. Aquila Isabella: Investigation, Writing – original draft, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
M.A. Sacco, Email: matteoantoniosacco@gmail.com.
A. Zibetti, Email: angelicazibetti@gmail.com.
C.F. Bonetta, Email: carlo.filippob@yahoo.it.
C. Scalise, Email: scalisecarmen@libero.it.
L. Abenavoli, Email: l.abenavoli@unicz.it.
F. Guarna, Email: francy.guarna@gmail.com.
S. Gratteri, Email: gratteri@unicz.it.
P. Ricci, Email: ricci@unicz.it.
I. Aquila, Email: isabella.aquila@unicz.it.
References
- 1.Agahi F., Juan C., Font G., Juan-García A. Neurotoxicity of zearalenone’s metabolites and beauvericin mycotoxins via apoptosis and cell cycle disruption. Toxicology. 2021 30;456 doi: 10.1016/j.tox.2021.152784. [DOI] [PubMed] [Google Scholar]
- 2.Abd El-Wahed A., Yosri N., Sakr H.H., Du M., Algethami A.F.M., Zhao C., Abdelazeem A.H., Tahir H.E., Masry S.H.D., Abdel-Daim M.M., Musharraf S.G., El-Garawani I., Kai G., Al Naggar Y., Khalifa S.A.M., El-Seedi H.R. Wasp venom biochemical components and their potential in biological applications and nanotechnological interventions. Toxins (Basel) 2021 12;13(3):206. doi: 10.3390/toxins13030206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernández M., Juan-García A., Moltó J.C., Mañes J., Juan C. Evaluation of mycotoxins in infant breast milk and infant food, reviewing the literature data. Toxins (Basel) 2021 30;13(8):535. doi: 10.3390/toxins13080535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salem M.L., Shoukry N.M., Teleb W.K., Abdel-Daim M.M., Abdel-Rahman M.A. In vitro and in vivo antitumor effects of the Egyptian scorpion androctonus amoreuxi venom in an Ehrlich ascites tumor model. Springerplus. 2016 10;5:570. doi: 10.1186/s40064-016-2269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Naggar Y., Giesy J.P., Abdel-Daim M.M., Javed Ansari M., Al-Kahtani S.N., Yahya G. Fighting against the second wave of COVID-19: Can honeybee products help protect against the pandemic? Saudi J. Biol. Sci. 2021;28(3):1519–1527. doi: 10.1016/j.sjbs.2020.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaldam M.A., Yahya G., Mohamed N.H., Abdel-Daim M.M., Al, Naggar Y. In silico screening of potent bioactive compounds from honeybee products against COVID-19 target enzymes. Environ. Sci. Pollut. Res Int. 2021;28(30):40507–40514. doi: 10.1007/s11356-021-14195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Bassiony M.N., Mahfouz H.M., Hussein A.S., El-Hamamy M.M., Abdel Daim M.M., Bufo S.A. Effect of honey bee venom on cancer in rats model. J. Entomol. 2016;13:72–83. [Google Scholar]
- 8.Leban V., Kozelj G., Brvar M. The syndrome of inappropriate antidiuretic hormone secretion after giant leaf frog (Phyllomedusa bicolor) venom exposure. Toxicon. 2016;120:107–109. doi: 10.1016/j.toxicon.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Byard R.W. Is voluntary envenomation from the kambô ritual therapeutic or toxic? Forensic Sci. Med Pathol. 2020;16(2):205–206. doi: 10.1007/s12024-019-00192-5. [DOI] [PubMed] [Google Scholar]
- 10.Silva F.V.A.D., Monteiro W.M., Bernarde P.S. “Kambô” frog (Phyllomedusa bicolor): Use in folk medicine and potential health risks. Rev. Soc. Bras. Med Trop. 2019;52 doi: 10.1590/0037-8682-0467-2018. [DOI] [PubMed] [Google Scholar]
- 11.Daly J.W., Caceres J., Moni R.W., Gusovsky F., Moos M., Jr., Seamon K.B., Milton K., Myers C.W. Frog secretions and hunting magic in the upper Amazon: identification of a peptide that interacts with an adenosine receptor. Proc. Natl. Acad. Sci. USA. 1992;89(22):10960–10963. doi: 10.1073/pnas.89.22.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keppel Hesselink J.M. Rediscovery of Ceruletide, a CCK Agonist, as an analgesic drug. J. Pain. Res. 2020;13:123–130. doi: 10.2147/JPR.S232714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hesselink J.M.K., Schatman M.E. Rediscovery of old drugs: The forgotten case of dermorphin for postoperative pain and palliation. J. Pain. Res. 2018;11:2991–2995. doi: 10.2147/JPR.S186082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartels E.J.H., Dekker D., Amiche M. Dermaseptins, multifunctional antimicrobial peptides: A review of their pharmacology, effectivity, mechanism of action, and possible future directions. Front. Pharm. 2019;10:1421. doi: 10.3389/fphar.2019.01421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robalino Gonzaga E.S., Chamorro M., Ganti L., Schneider R. Kambo frog poison as a cause of esophageal rupture. Cureus. 2020;12(9) doi: 10.7759/cureus.10677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alamos M.F., Walker R.H., Miranda M. Life-threatening risk of using Kambó in alternative medicine. Neurol. Clin. Pr. 2020;10(4):e35–e37. doi: 10.1212/CPJ.0000000000000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Vega M., Maldonado G., Kraus A. Dermatomyositis induced by the secretion of Phyllomedusa bicolor or Kambô frog - A case report. Toxicon. 2020;184:57–61. doi: 10.1016/j.toxicon.2020.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Campodónico J., Aedo P., Montané M.I., Rojas A., Aveiga A., Silva L., Ríos J.C., Solís I. Hiponatremia grave secundaria a la exposición a veneno de Phyllomedusa bicolor (Rana Kambó). Caso clínico [Severe hyponatremia secondary to Phyllomedusa bicolor (Kambó frog) poisoning. Report of one case] Rev. Med. Chil. 2019;147(7):935–939. doi: 10.4067/S0034-98872019000700935. [DOI] [PubMed] [Google Scholar]
- 19.Agüero-González D.L., Pané-Vila A., Gil V., Castro P. Severe hyponatremia after a purification ritual using an Amazonian frog poison (Kambô) Emergencias. 2019;31(5):368–369. [PubMed] [Google Scholar]
- 20.Roy R., Baranwal A., Espiridion E.D. Can overuse of Kambô cause psychosis? Cureus. 2018;10(6) doi: 10.7759/cureus.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumachev A., Zipursky J.S., Weinerman A.S., Thompson M. Poisoning from the Kambô ritual. CJEM. 2018;20(6):962–964. doi: 10.1017/cem.2018.58. [DOI] [PubMed] [Google Scholar]
- 22.Li K., Horng H., Lynch K., Smollin C.G. Prolonged toxicity from Kambo cleansing ritual. Clin. Toxicol. (Philos.) 2018;56(11):1165–1166. doi: 10.1080/15563650.2018.1457153. [DOI] [PubMed] [Google Scholar]
- 23.Aquila I., Gratteri S., Sacco M.A., Fineschi V., Magi S., Castaldo P., Viscomi G., Amoroso S., Ricci P. The biological effects of Kambo: Is there a relationship between its administration and sudden death? J. Forensic Sci. 2018;63(3):965–968. doi: 10.1111/1556-4029.13641. [DOI] [PubMed] [Google Scholar]
- 24.Pogorzelska J., Łapiński T.W. Toxic hepatitis caused by the excretions of the Phyllomedusa bicolor frog - A case report. Clin. Exp. Hepatol. 2017;3(1):33–34. doi: 10.5114/ceh.2017.65228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leban V., Kozelj G., Brvar M. The syndrome of inappropriate antidiuretic hormone secretion after giant leaf frog (Phyllomedusa bicolor) venom exposure. Toxicon. 2016;120:107–109. doi: 10.1016/j.toxicon.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Majić T., Sauter M., Bermpohl F., Schmidt T.T. Connected to the spirit of the frog: An Internet-based survey on Kambô, the secretion of the Amazonian Giant Maki Frog (Phyllomedusa bicolor): Motivations for use, settings and subjective experiences. J. Psychopharmacol. 2021;35(4):421–436. doi: 10.1177/0269881121991554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anastasi A., Bertaccini G., Cei J.M., De Caro G., Erspamer V., Impicciatore M. Structure and pharmacological actions of phyllocaerulein, a caerulein-like nonapeptide: its occurrence in extracts of the skin of Phyllomedusa sauvagei and related Phyllomedusa species. Br. J. Pharm. 1969;37(1):198–206. doi: 10.1111/j.1476-5381.1969.tb09538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erspamer V., Erspamer G.F., Improta G., Negri L. de Castiglione R. Sauvagine, a new polypeptide from Phyllomedusa sauvagei skin. Occurrence in various Phyllomedusa species and pharmacological actions on rat blood pressure and diuresis. Naunyn Schmiede Arch. Pharm. 1980;312(3):265–270. doi: 10.1007/BF00499156. [DOI] [PubMed] [Google Scholar]
- 29.Lacombe C., Cifuentes-Diaz C., Dunia I., Auber-Thomay M., Nicolas P., Amiche M. Peptide secretion in the cutaneous glands of South American tree frog Phyllomedusa bicolor: an ultrastructural study. Eur. J. Cell Biol. 2000;79(9):631–641. doi: 10.1078/0171-9335-00085. [DOI] [PubMed] [Google Scholar]
- 30.Erspamer V., Erspamer G.F., Severini C., Potenza R.L., Barra D., Mignogna G., Bianchi A. Pharmacological studies of ‘sapo’ from the frog Phyllomedusa bicolor skin: a drug used by the Peruvian Matses Indians in shamanic hunting practices. Toxicon. 1993;31(9):1099–1111. doi: 10.1016/0041-0101(93)90125-3. [DOI] [PubMed] [Google Scholar]
- 31.Junior V.H., Martins I.A. KAMBÔ: An Amazonian enigma. J. Venom. Res. 2020;10:13–17. [PMC free article] [PubMed] [Google Scholar]