Abstract
The host immune response to foreign materials is a major hurdle for implanted medical devices. To control this response, modulation of macrophage behavior has emerged as a promising strategy, given their prominent role in inflammation and wound healing. Towards this goal, we explore the effect of biomimetic multi-scale wrinkles on macrophage adhesion and expression of phenotype markers. We find that macrophages elongate along the direction of the uniaxial wrinkles made from shape memory polymers, and express more arginase-1 and IL-10, and less TNF-α, suggesting polarization towards an alternatively activated, anti-inflammatory phenotype. Materials were further implanted in the subcutaneous space of mice and tissue surrounding the material evaluated by histology and immunohistochemistry. We found that material surface topography altered the distribution of collagen deposition in the adjacent tissue, with denser collagen tissue observed near flat materials when compared to wrinkled materials. Furthermore, cells surrounding wrinkled materials exhibited higher arginase-1 expression. Together these data suggest that wrinkled material surfaces promote macrophage alternative activation, and may influence the foreign body response to implants.
Graphical and Textual Abstract
Biomimetic multi-scale wrinkles alters macrophage cell shape and promotes anti-inflammatory activation in vitro, and modulates the host response in vivo, suggesting a potential benefit for wrinkled material surfaces to promote tissue healing and remodeling.
The host response to biomaterials remains a major challenge for implanted medical devices1. Chronic inflammatory activation caused by the presence of a foreign body often leads to the formation of a thick fibrous capsule, which ultimately prevents the function of the device. Recently, approaches to actively modulate the immune system have emerged as a promising strategy to minimize inflammation and achieve appropriate wound healing response in response to biomaterial implants. Towards this goal, macrophages have been an attractive target for immunomodulation given their role in inflammation and wound healing 2–4. These cells are capable of polarizing towards a pro-inflammatory (classically activated) or anti-inflammatory (alternatively activated) phenotype depending on their microenvironment 5, 6. In an inflammatory environment containing damage or pathogen associated molecular patterns including LPS and IFN-γ, macrophages polarize towards an inflammatory phenotype, and release cytokines and reactive species to promote inflammation. However, during wound healing and in the presence of Th2 cytokines including IL-4 and IL-13, the same cells acquire an anti-inflammatory phenotype and express IL-10 and arginase-1 (in mice), which are involved in dampening inflammation and mediating tissue regeneration. While the molecular mediators of macrophage phenotype polarization have been well-documented, less is known about how physical properties presented by a biomaterial surface might modulate their behavior.
Recent studies suggest that biomaterial size and shape, rather than surface chemistry, play a dominant role in the host response7, 8. At the surface scale, it is thought that roughness or topographical features modulate macrophage behavior9–11, with surface features generally eliciting lower levels of inflammation when compared to flat surfaces of the same chemistry. However, the mechanism underlying topography-induced modulation of macrophage phenotype remains unclear, and precise design criteria to leverage topography-mediated changes in the immune response have not been established. In addition, methods that specifically promote an anti-inflammatory phenotype have remained elusive. Our laboratory has demonstrated that macrophage elongation induced by surface adhesive micropatterns leads to an increase in expression of markers associated with an alternatively activated phenotype12. Since surface grooves have been shown to elicit cell elongation in macrophages as well as many other cell types13–15, we postulated that topological features could be leveraged to control macrophage shape and polarization, and therefore the foreign body response to an implanted material. We recently showed using deep etched titanium surfaces that 0.5–1 μm grooves optimally induced macrophage elongation and alternative activation16. This work suggested that topography may be used to modulate macrophage phenotype, and surface features may be designed to mitigate the foreign body response to implanted materials.
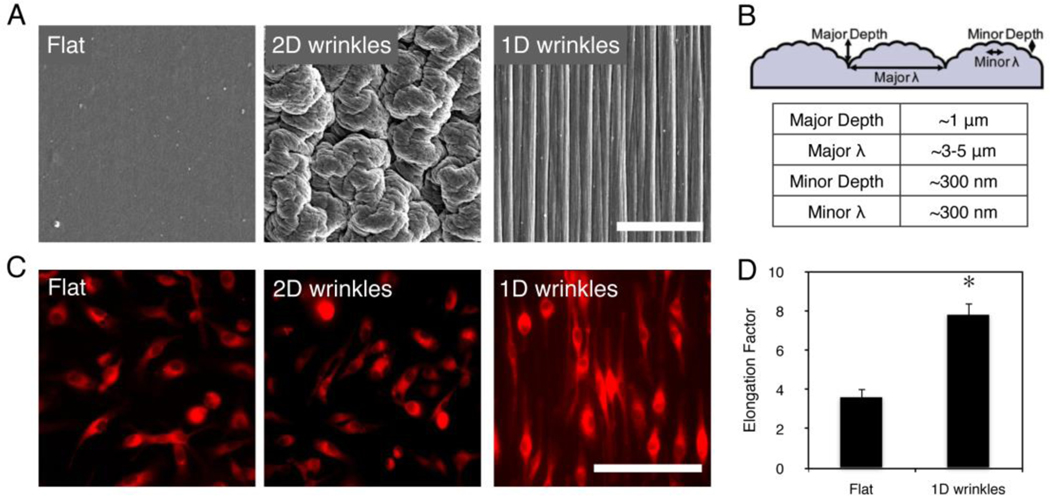
In this study, we sought to determine (1) whether multi-scale wrinkled surfaces on a polymeric material produced by shrink film methods could also induce cell shape and phenotypic changes in macrophages, and (2) whether changes in macrophage behavior in vitro correlated with a change in the in vivo macrophage response. Self-assembled wrinkles by shape memory polymers has been shown to create biomimetic wrinkles15, 17. This facile fabrication technique leveraging shrink film offers a low-cost, high throughput approach to create micro- to nano-scale topological features on biomaterials. This general technique can be applied to thermally-induced shape-memory polymers, and can further be molded using thermoset polymers including poly(dimethyl siloxane) (PDMS). Materials were fabricated using methods as previously described15 and further elaborated in Supplemental Information. One-dimensional (1D) wrinkles were generated by creating a stiff layer via plasma treating and then confining the material along one direction upon heat induced shrinkage in order to constrain shrinkage of the material in the perpendicular direction, whereas two-dimensional (2D) wrinkles were generated by shrinking under unconfined conditions. The wrinkled surfaces were imaged by scanning electron microscopy (SEM, Figure 1A) and confirmed to have dimensions of approximately 1 nm – 5 μm by atomic force microscopy (summarized in Figure 1B).
Figure 1. Macrophages elongate on shrink-induced multi-scale wrinkles.
(A) Scanning electron microscopy micrographs of flat surface, 2D wrinkles, and 1D wrinkles. (B) Schematic of depth and width of 1D wrinkle surfaces as measured by atomic force microscopy. (C) Fluorescent micrographs of BMDM on flat, 2D wrinkle, and 1D wrinkle surfaces. Cells were stained using CellTracker Red CMTPX dye for monitoring cell shape. (D) Quantification of cell elongation on flat, 2D wrinkle, and 1D wrinkle surfaces. Mean ± SEM, astericks denotes p < 0.05 compared to Flat surfaces by paired Student’s t-test, n = 3. Scale bar = 50 μm.
Murine bone marrow derived macrophages were seeded onto 1D wrinkles, 2D wrinkles, and flat materials for 24 hours and stained with Cell Tracker Red for visualization by fluorescence microscopy. Macrophages seeded on 1D wrinkled materials elongated and oriented along the direction of the groove, whereas macrophages on 2D wrinkles were not elongated and similar in morphology to cells on flat surfaces (Figure 1C). We quantified the degree of elongation by measuring the long axis of the cell and dividing by the width across the nucleus, and found that this ratio or “elongation factor” was approximately 8, which is similar to the level of elongation that we have previously observed in IL-4/IL-13 treated alternatively activated macrophages, as well as macrophages seeded on lined micropatterns12. Cells seeded on flat surfaces were on average less elongated with a ratio of approximately 3–4 (Figure 1D). On 2D wrinkled surfaces, the periphery of the cells were difficult to ascertain by fluorescence microscopy because of the undulating nonplanar features, and therefore elongation was not quantified. These data suggest that wrinkled surfaces lead to adhesive changes in macrophages that result in cell elongation.
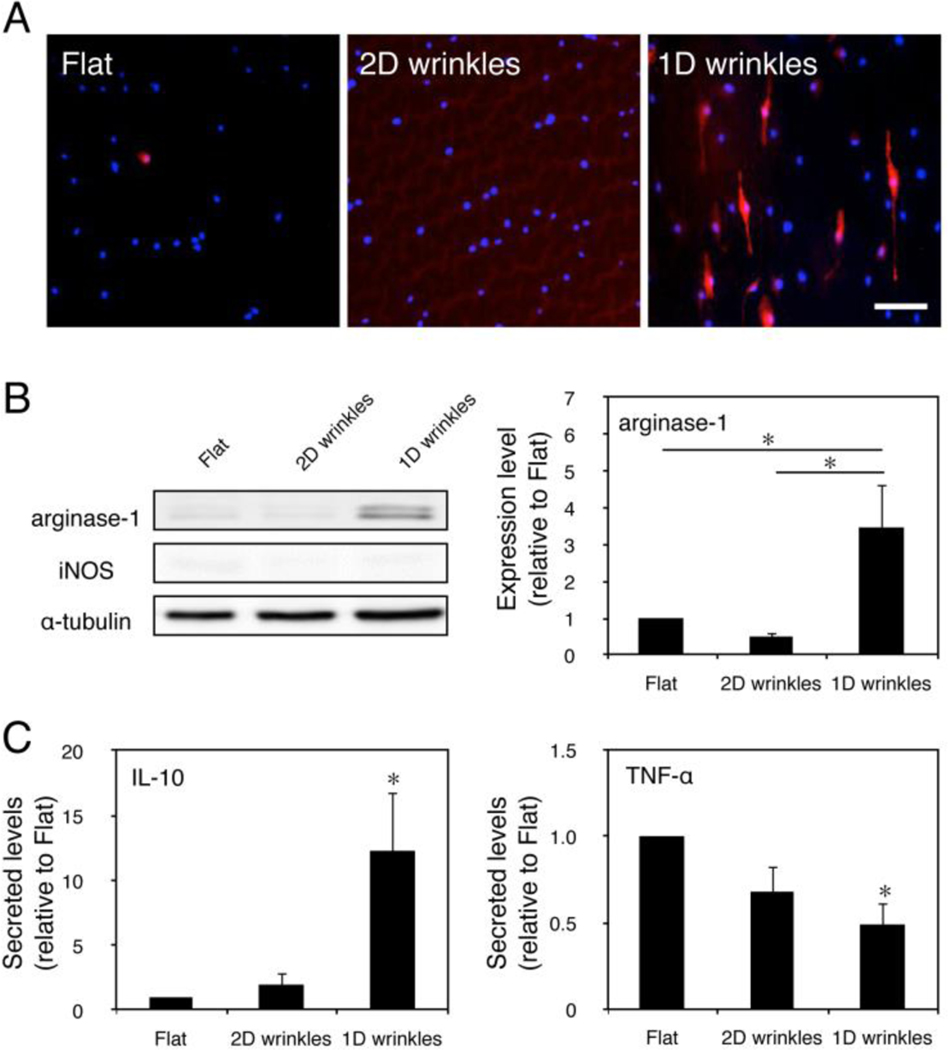
To determine whether adhesion to surfaces of varied topologies altered the polarization state of macrophages, cells were seeded on 1D wrinkles, 2D wrinkles, and flat surfaces for 24 hours and assayed for markers of arginase-1 and iNOS, markers of alternative and classical activation, respectively. We found that several cells on 1D wrinkles exhibited strikingly high arginase-1 staining intensity, which was not observed on flat or 2D wrinkles (Figure 2A). The shape of the high expressing cells as visualized by fluorescence microscopy was elongated. This was further confirmed and quantified by Western blot, where arginase-1 levels were significantly higher in macrophages cultured on 1D wrinkles when compared to cells cultured on flat surfaces or 2D wrinkles (Figure 2B). In contrast, levels of iNOS, a marker of inflammatory polarization was undetectable in cells cultured on all three types of substrates; α-tubulin was used as a loading control and was not statistically different across the three culture conditions. The arginase-1 levels in macrophages cultured on wrinkled surfaces were similar in magnitude to the levels observed in elongated cells cultured on micropatterned adhesive lanes, which was more moderate than that caused by stimulation of Th2 cytokines IL-4 and IL-1312.
Figure 2. Surface topology regulates macrophage phenotype and cytokine secretion.
(A) Immunofluorescence images of arginase-1 on flat, 2D wrinkle, and 1D wrinkle surfaces. Scale bar = 50 μm. (B) Representative Western blot of arginase-1, iNOS, and α-tubulin on flat, 2D wrinkle, and 1D wrinkle surfaces (left) and quantification of arginase-1 expression of flat, 2D wrinkle, and 1D wrinkle surfaces (right). Mean ± SEM, astericks denotes p < 0.05; one-way ANOVA with Tukey’s posthoc test, n = 3. (C) Secretion of IL-10 (left) and TNF- α (right) by BMDM cultured on flat, 2D wrinkle, and 1D wrinkle surfaces for 24 hours. Mean ± SEM, astericks denotes p < 0.05 compared to flat surfaces; one-way ANOVA with Sidak’s posthoc test, n = 3.
To further examine whether surface topography altered macrophage function, we examined the presence of an anti-inflammatory cytokine, IL-10 and a pro-inflammatory cytokine, TNF-α, in the supernatants of macrophages after culture on substrates for 24 hours (Figure 2C). We found that secretion of IL-10 was significantly greater and TNF-α level was significantly lower from macrophages on 1D wrinkles when compared to macrophages on flat surfaces. Together with immunostaining of arginase-1, these data suggest that 1D wrinkled surface topology induce murine macrophages towards an alternatively activated, anti-inflammatory phenotype. These changes were not attributed to changes in surface composition associated with material shrinkage, since 2D wrinkles that also undergo a shrink procedure did not elicit arginase-1 expression or IL-10 secretion. Interestingly, while 1D wrinkled surfaces did not alter iNOS expression, the levels of TNF-α were moderately less compared to flat surfaces. These data suggest that while surface topological features such as those present on 2D wrinkles might inhibit inflammatory activation, grooved surfaces created on 1D wrinkles most significantly depress inflammatory activation.
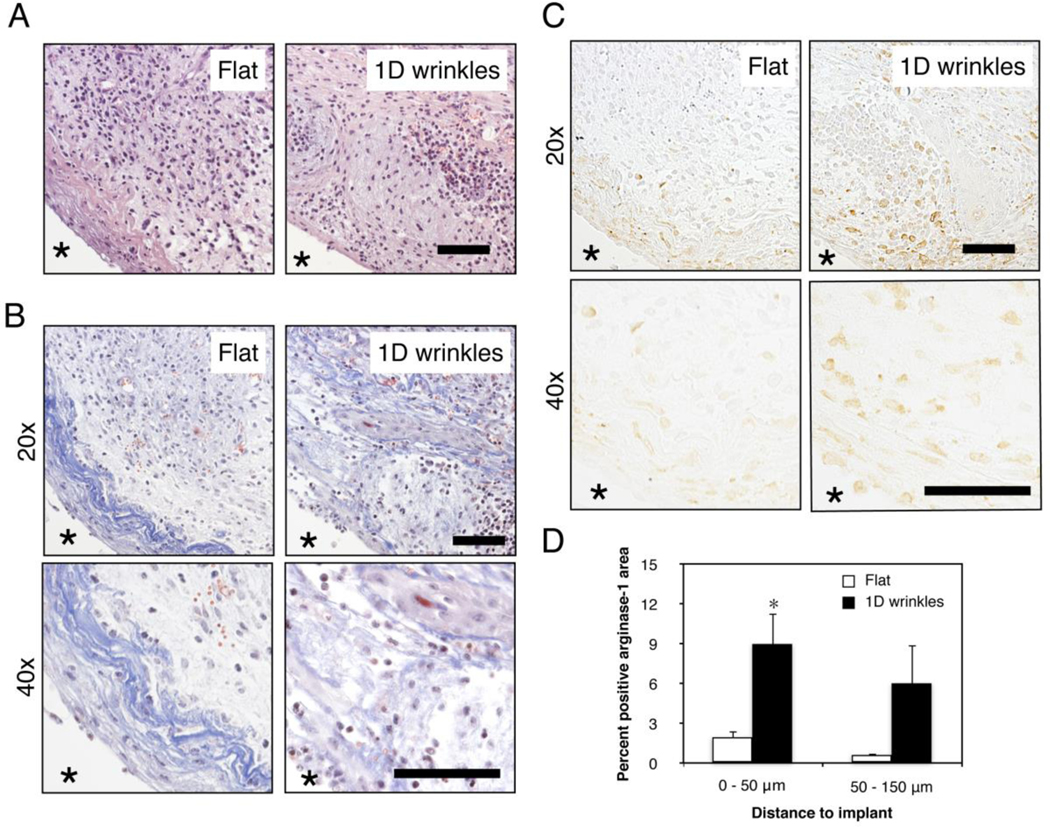
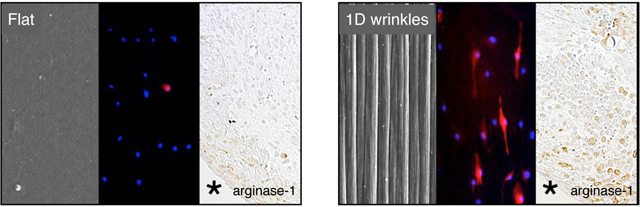
To demonstrate whether surface topography influences the macrophage response to a biomaterial in vivo, we surgically implanted wrinkled and flat materials in the subcutaneous space of C576BL/6J mice. Tissue surrounding the material was excised one week after material implantation, and evaluated by histology and immunohistochemistry (Figure 3). It appeared that the extent of inflammatory infiltration was similar in the tissue surrounding the flat material compared to the tissue surrounding the wrinkled material, as indicated by H&E staining (Figure 3A). However, Masson’s trichrome staining revealed the highest extent of fibrillar collagen deposition in the tissue directly adjacent to the flat material (Figure 3B). Tissue surrounding the wrinkled material also exhibited some fibrillar collagen but the density was not as high or localized. Higher magnification images also show changes in collagen fibril structure, although the heterogeneity of fibril deposition particularly in the wrinkled material condition made it difficult to quantify. Many newly formed blood vessels were found in the tissue directly around both implants materials. The most striking difference was observed by immunohistochemistry for arginase-1, which showed higher percentage of positively stained arginase-1 area in the inflammatory tissue around wrinkled materials compared to flat materials, confirming the results from in vitro macrophage experiments (Figure 3C and 3D). Interestingly, the positive staining of arginase-1 was observed not only in the tissue directly adjacent to the material, but also 50 – 150 μm away from the implant surface, suggesting that paracrine effects from cells adhering to the material may potentiate the response. In addition, arginase-1 expression appeared to be co-localized with collagen deposition in both implant conditions. Expression of F4/80 also appeared to be higher in the tissue surrounding the wrinkled implants when compared to flat implants, but expression of the inflammatory marker iNOS was reduced (Supplemental Figure 2). Together, these data demonstrate that surface topography modulates the recruitment and activity of local macrophages. It is likely that these cells work in concert with other topography-sensitive cell types including fibroblasts to alter tissue remodeling response in vivo18, 19.
Figure 3. Surface wrinkles modulates arginase-1 expression in vivo.
Representative (A) H&E stained, (B) Masson’s Trichrome at 20x and 40x magnification, and (C) arginase-1 stained 20x and 40x magnification images of Flat and 1D wrinkle surface implants at 7 days after subcutaneous implantation in C57BL/6J mice. Astericks denotes location of material. Scale bar = 50 μm. (D) Quantification of percent positive arginase-1 area at indicated distances from the implant. Mean ± SEM, astericks denotes p < 0.05 compared to control flat surfaces; unpaired Student’s t-test, n = 3.
In this study, we demonstrate that wrinkled surfaces modulate murine macrophage cell shape phenotype, specifically promoting their elongation and alternative activation. Wrinkles fabricated from shrink films offers a low cost and high throughput method of generating topologies containing micro- and nano-scale features. 1D wrinkled materials that contain grooves led to higher macrophage expression of arginase-1 both in vitro and in vivo. In addition, macrophages secreted higher levels of IL-10 and lower levels of TNF-α on 1D wrinkles when compared to 2D wrinkles or flat surfaces in vitro, and also promoted blood vessel formation in vivo. Together these data suggest that surface wrinkles promote alternative activation of macrophages and may be used to modulate the foreign body response to implants. However, it is still not clear whether wrinkled surface-induced changes in macrophage function will lead improved wound healing, as it has recently been shown that heightened alternative activation may in fact lead to fibrosis20, 21. Nevertheless, topological features have long been clinically used as a strategy to mitigate the foreign body response to biomaterial implants22, 23, and studies have suggested that material topography or architecture influences macrophages and the foreign body response10, 13, 24. Our work may provide a cellular and molecular mechanism underlying improved response to roughened surfaces, and suggests that topological features including wrinkled surfaces promote changes in macrophage cell shape, which in turn modulate their phenotypic activation state. Further work examining additional markers and using human cell systems will be needed to elucidate the translational potential of topological regulation of the foreign body response.
Conclusions
We demonstrate that shrink-film multi-scale wrinkled materials modulate the shape and function of macrophages. Wrinkled materials induced macrophage elongation along the direction of 1D wrinkles, and led to increases in arginase-1 expression and IL-10 secretion. Furthermore, implanted 1D wrinkled materials increased arginase-1 and reduced iNOS expression in tissue around the material. Together these results suggest the potential use of material topography to control the macrophage response to biomaterial implants. Further studies will be necessary to determine the long term fibrotic response to materials of varied topographies.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (NIH) National Institute of Dental and Craniofacial Research (NIDCR) Grant DP2DE023319. T.U.L. was supported by a California Institute of Regenerative Medicine (CIRM) Training Fellowship (TG2-01152) and M.K. from NIH New Innovator Grant 1DP2OD007283. All protocols involving animals were approved by University of California Irvine’s Institutional Animal Care and Use Committee, which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALACi).
References
- 1.Anderson JM, Rodriguez A. and Chang DT, Semin Immunol, 2008, 20, 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kou PM and Babensee JE, J Biomed Mater Res A, 2011, 96, 239–260. [DOI] [PubMed] [Google Scholar]
- 3.Kim YS, Lee WH, Choi EJ, Choi JP, Heo YJ, Gho YS, Jee YK, Oh YM and Kim YK, J Immunol, 2015, 194, 3361–3368. [DOI] [PubMed] [Google Scholar]
- 4.Kzhyshkowska J, Gudima A, Riabov V, Dollinger C, Lavalle P. and Vrana NE, J Leukoc Biol, 2015, 98, 953–962. [DOI] [PubMed] [Google Scholar]
- 5.Mosser DM and Edwards JP, Nat Rev Immunol, 2008, 8, 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantovani A. and Garlanda C, Nat Immunol, 2013, 14, 768–770. [DOI] [PubMed] [Google Scholar]
- 7.Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, Hollister-Lock J, Aresta-Dasilva S, Bochenek M, Mendoza-Elias J, Wang Y, Qi M, Lavin DM, Chen M, Dholakia N, Thakrar R, Lacik I, Weir GC, Oberholzer J, Greiner DL, Langer R. and Anderson DG, Nat Mater, 2015, 14, 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE and Ratner BD, Proc Natl Acad Sci U S A, 2010, 107, 15211–15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma QL, Zhao LZ, Liu RR, Jin BQ, Song W, Wang Y, Zhang YS, Chen LH and Zhang YM, Biomaterials, 2014, 35, 9853–9867. [DOI] [PubMed] [Google Scholar]
- 10.Bota PC, Collie AM, Puolakkainen P, Vernon RB, Sage EH, Ratner BD and Stayton PS, J Biomed Mater Res A, 2010, 95, 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg K, Pullen NA, Oskeritzian CA, Ryan JJ and Bowlin GL, Biomaterials, 2013, 34, 4439–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McWhorter FY, Wang T, Nguyen P, Chung T. and Liu WF, Proc Natl Acad Sci U S A, 2013, 110, 17253–17258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S, Jones JA, Xu Y, Low HY, Anderson JM and Leong KW, Biomaterials, 2010, 31, 3479–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yim EK, Darling EM, Kulangara K, Guilak F. and Leong KW, Biomaterials, 2010, 31, 1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen A, Lieu DK, Freschauf L, Lew V, Sharma H, Wang J, Nguyen D, Karakikes I, Hajjar RJ, Gopinathan A, Botvinick E, Fowlkes CC, Li RA and Khine M, Adv Mater, 2011, 23, 5785–5791. [DOI] [PubMed] [Google Scholar]
- 16.Luu TU, Gott SC, Woo BWK, Rao MP and Liu WF, ACS Appl. Mater. Interfaces, 2015, DOI: 10.1021/acsami.5b10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luna JI, Ciriza J, Garcia-Ojeda ME, Kong M, Herren A, Lieu DK, Li RA, Fowlkes CC, Khine M. and McCloskey KE, Tissue Eng Part C Methods, 2011, 17, 579–588. [DOI] [PubMed] [Google Scholar]
- 18.Wojciak-Stothard B, Denyer M, Mishra M. and Brown RA, In Vitro Cell. Dev. Biol., 1997, 33, 110–117. [DOI] [PubMed] [Google Scholar]
- 19.Dalby MJ, Riehle MO, Yarwood SJ, Wilkinson CDW and Curtis ASG, Experimental Cell Research, 2003, 284, 272–280. [DOI] [PubMed] [Google Scholar]
- 20.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ and Wynn TA, The Journal of Immunology, 2001, 167, 6533–6544. [DOI] [PubMed] [Google Scholar]
- 21.Knipper JA, Willenborg S, Brinckmann J, Bloch W, Maass T, Wagener R, Krieg T, Sutherland T, Munitz A, Rothenberg ME, Niehoff A, Richardson R, Hammerschmidt M, Allen JE and Eming SA, Immunity, 2015, 43, 803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakelius L. and Ohlsén L, Plast Reconstr Surg, 1992, 90, 247–254. [PubMed] [Google Scholar]
- 23.Sridharan R, Cameron AR, Kelly DJ, Kearney CJ and O’Brien FJ, Materials Today, 2015, 18, 313–325. [Google Scholar]
- 24.Cao H, McHugh K, Chew SY and Anderson JM, J Biomed Mater Res A, 2010, 93, 1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.