Abstract
In this provocative commentary, we consider several questions posed by the late chronic myeloid leukaemia (CML) expert Prof. Michele Baccarani, which he challenged us to address after his death. He noted only a small proportion of people with chronic phase CML receiving tyrosine kinase-inhibitor (TKI)-therapy are likely to achieve sustained therapy-free remission (TFR) and even fewer are likely to be cured. Persons most likely to fail TKItherapy can be identified at diagnosis or soon after starting TKI-therapy. These persons are likely to need lifetime TKI-therapy with attendant risks of adverse events, cost and psychological consequences. Allogeneic transplants achieve much higher rates of leukaemia-free survival compared with TKI-therapy but are associated with transplant-related adverse events including an almost 20 percent risk of transplant-related deaths within 1 year post-transplant and a compromised quality-of-life because of complications such as chronic graft-versus-host disease. Subject-, disease- and transplant-related co-variates associated with transplant outcomes are known with reasonable accuracy. Not everyone likely to fail TKI-therapy is a transplant candidate. However, in those who candidates are physicians and patients need to weigh benefits and risks of TKI-therapy versus a transplant. We suggest transplants should be more often considered in the metric when counseling people with chronic phase CML unlikely to achieve TFR with TKI-therapy. We question whether we are discounting a possible important therapy intervention; we think so.
Subject terms: Medical research, Haematological diseases
Scientists who fall deeply in love with their hypothesis are proportionately unwilling to take no as an experimental answer.
Sir Peter Medawar
Introduction
Before the development of imatinib and other tyrosine kinase-inhibitors (TKIs) allogeneic haematopoietic cells transplants were a common intervention in chronic phase chronic myeloid leukaemia (CML) in appropriate persons and were the only approach to cure. With the remarkable success of TKI-therapy transplants for chronic phase CML became rare with less than 300 reported to the Centre for International Blood and Marrow Research (CIBMTR) in 2014–2016. However, it’s become clear that despite excellent survivals with TKI-therapy in many but not all countries only a small proportion of people are likely to achieve therapy-free remission (TFR) and even fewer cured. There is also considerable debate over the most appropriate target of TKI-therapy. Should it be population-adjusted survival, TFR or cure? When population-adjusted survival is the target transplants are unlikely to be better than TKI-therapy in most, but not all persons such as those failing to respond to TKI-therapy and those with some ABL1 mutations, high-risk additional cytogenetic abnormalities (ACAs) and/or with other signs of leukaemia progression. Also, when the goal of TKI-therapy is TFR or cure transplants may be appropriate for some persons. In this Perspective, we present 10 questions for future research on the roles of TKI-therapy and transplants in chronic phase CML, questions raised by the late CML expert Prof. Michele Baccarani.
What is the appropriate goal of CML therapy?
The optimal goal of CML therapy is cure resulting in normal sex- and age-adjusted survival with a normal quality-of-life (QoL) [1–3]. Unfortunately, cure is achieved in few people with CML [4, 5]. An intermediate goal is achieving near normal age- and sex-matched adjusted survival off tyrosine kinase inhibitor (TKIs)-therapy referred to as therapy-free remission (TFR) [2–10].
Are TKI therapy goals changing and which TKI is best to achieve which goal?
Several TKIs are commercially available to treat CML in many but not all countries and at considerably different costs [11]. Imatinib, nilotinib, dasatinib, bosutinib and, in Korea, radotinib are approved for initial therapy, and ponatinib and asciminib in the US for 2nd and 3rd-line therapies [2, 7–9, 12–14]. Imatinib is less potent and does not inhibit several BCR::ABL1 mutations many of which are sensitive to the other TKIs, except BCR::ABL1T315I which is inhibited only by ponatinib and asciminib [14–16]. All TKIs cause adverse events, with some clinically relevant differences particularly for cardio-vascular and pulmonary complications. Imatinib is the safest [17]. Safety profiles of TKIs are considered largely manageable with favourable benefit-to-risk ratios. Cost and compliance are also important considerations and often influence TKI choice, especially in resource-poor geospaces [6, 17–21].
The therapeutic strategy for CML when imatinib was the only approved TKI was simple. After nilotinib and dasatinib were approved for initial therapy and bosutinib and ponatinib for subsequent therapy, several different strategies were developed, followed by debate and competition [22–32]. This competition is mainly over which TKI is the best initial therapy in the context of faster, deeper molecular responses obtainable with 2nd-generation TKIs (2G-TKIs; nilotinib, dasatinib, bosutinib) and over the switch from imatinib to 2G-TKIs if there is a sub-optimal response to imatinib [2, 6–9, 19]. This is important because molecular response, particularly major molecular response (MMR; BCR::ABL1 ≤ 0.1% on the International Scale) is widely considered the best surrogate for survival [31, 32]. However, there are no convincing data supporting the initial use of a 2G-TKI being associated with better progression-free survival (PFS), probability of achieving TFR or survival [2, 7–9, 23]. Consequently, whether the advantage of 2G-TKIs over imatinib in achieving faster and deeper molecular responses translates into a higher rate of TFR and operational cures remains unproven and can only be tested in a randomized controlled trial [2, 7–9, 21]. Such a trial is unlikely to be done.
Are current recommendations for TKI-therapy appropriate?
CML therapy recommendations are continuously modified with success inviting to more ambitious goals. Five-year survival of persons with CML is now 80–90% in the European geospaces, with about one-half of deaths occurring from unrelated causes [1, 33]. Survival is like that of sex- and age-adjusted people without CML in Europe, but not in the US and certainly not in resource-poor countries [33]. Considerable data suggest people with CML achieving a stable deep molecular response (DMR; ≥MR4; 4-log BCR::ABL1 transcript decrease from the standardized baseline, corresponding to a transcript level ≤0.01% on the International Scale) can discontinue therapy, about one-half of whom achieving TFR [34–49]. The clinical advantage of TFR over lifelong TKI-therapy is obvious, but the road to achieving this goal is not simple, cheap or rewarding for everyone. Some people choose not to stop TKI therapy for diverse reasons, usually fear of leukaemia recurrence [50].
There is controversy on how to best use TKIs. Which are the best and most cost-effective strategies to achieve TFR, to optimize survival and improve QoL [2, 7–9, 35, 36, 38, 41–46]? Which strategy(ies) should be used when someone does not meet proposed TKI stopping criteria or fails because of molecular, cytogenetic and/or haematologic leukaemia recurrence? How can we limit adverse events (AEs) associated with lifelong TKI-therapy and complications of more intensive therapies aimed at achieving TFR? Put otherwise, the main issues are: which TKI, at what dose and for how long, alone or with other drugs? But there is another important consideration. TKI-therapy rarely cures CML, as we discussed elsewhere [5]. If so, should the only therapy of CML be TKIs?
How quickly is deep molecular response achieved with TKI-therapy?
Achieving a stable DMR (BCR::ABL1 ≤ 0.01% on the International Scale) is widely considered to be necessary before stopping TKI-therapy [2, 7–9, 22, 26, 27, 34–37, 43–46]. DMR rates in 30 cohorts of newly-diagnosed subjects receiving different TKIs at different doses, alone or with other drugs such as interferon-alfa (IFNα) or low-dose cytarabine, are displayed in Table 1. These rates, typically reported as probability of achieving a DMR within a specified interval rather than as proportion of subjects achieving a DMR, over-estimate the proportion of subjects eligible to discontinue TKI therapy. These studies report rates of 20–70% with imatinib-based regimes and 60–80% with 2G-TKIs. Comparably, 5-year rates of MR4.5 are 5–35% and 35–70% (Table 2). Interestingly, although achieving MR4 is universally considered as a critical target, reported DMR rates vary widely with the same therapy and despite of standardization of real-time quantitative polymerase chain reaction (RT-qPCR), used for response assessment.
Table 1.
MR4 response rates (percentage) by or at 3-, 5- and 10-years from initial TKI therapy. Only studies with ≥ 3-year follow-up are displayed. All rates are ‘by’ except for those reported for Guilhot et al. [27].
| Ref. | Study | Initial TKI | N | Median age (y) | 3-y MR4 rate | 5-y MR4 rate | 10-y MR4 rate |
|---|---|---|---|---|---|---|---|
| De Lavallade et al. [88] | Hammersmith | IM 400 | 204 | 46 | 15 | 20 | NR |
| Castagnetti et al. [89] | GIMEMA | IM 400/800 | 559 | 52 | 25a | 61b | NR |
| O’Brien et al. [29] | UK SPIRIT 2 | IM 400 | 407 | 53 | NR | 57 | NA |
| Hochhaus et al. [24], Kantarjian et al. [26] | ENESTnd | IM 400 | 283 | 46 | 26 | 42 | 50 |
| Zhang et al. [69] | Peking | IM 400 | 1379 | 40 | NR | NR | 54c |
| Guilhot et al. [27] | French SPIRIT | IM 400 | 223 | 50 | 36 | 37 | 40 |
| Hehlmann et al. [30, 31] | German Study IV | IM 400 | 400 | 53 | 49 | 66 | 81 |
| Guilhot et al. [27] | French SPIRIT | IM 400 + LDAC | 172 | 51 | 35 | 41 | 48 |
| Hehlmann et al. [30, 31] | German Study IV | IM 400 + LDAC | 158 | 51 | 49 | 68 | 86 |
| Guilhot et al. [27] | French SPIRIT | IM 400 + IFNα | 221 | 55 | 44 | 48 | 40 |
| Hehlmann et al. [30, 31] | German Study IV | IM 400 + IFNα | 430 | 53 | 51 | 67 | 83 |
| Guilhot et al. [27] | French SPIRIT | IM 600 | 171 | 51 | 36 | 49 | 50 |
| Hehlmann et al. [30, 31] | German Study IV | IM 800 | 420 | 51 | 59 | 69 | 81 |
| Geelen et al [90] | Dutch | IM 400, NIL 600, DAS 100 | 434 | 58 | 41a | 69d | NA |
| O’Brien et al. [29] | UK SPIRIT 2 | DAS 100 | 407 | 52 | NR | 78 | NA |
| Hochhaus et al. [24], Kantarjian et al. [26] | ENESTnd | NIL 600 | 282 | 47 | 50 | 66 | 70 |
| Gugliotta et al. [91] | GIMEMA | NIL 600/800 | 472 | 52 | 76 | NR | NA |
| Gugliotta et al. [92] | GIMEMA | NIL800 | 73 | 51 | 70 | 76 | 83 |
| Hochhaus et al. [24], Kantarjian et al. [26] | ENESTnd | NIL 800 | 281 | 47 | 44 | 63 | 68 |
| Masarova et al. [93] | MDACC | NIL 800 | 122 | 51 | 66 | 73 | 82 |
TKI doses are in mg/d. Percentages are rounded.
MR4 BCR::ABL1 ≤ 0.01%IS, IM imatinib, NIL nilotinib, DAS dasatinib, IFNα interferon-α, LDAC low dose cytarabine, NR not reported, NA not available, GIMEMA Gruppo Italiano Malattie Ematologiche dell’Adulto, JALSG Japan Adult Leukemia Study Group, MDACC MD Anderson Cancer Center.
a2-y, b6-y, c7-y, d4-y.
Table 2.
MR4.5 response rates (percentage) by or at 3-, 5- and 10-years. Only studies with ≥3 years follow-up. All rates are ‘by’ except for those reported for Guilhot et al. [27].
| Ref. | Study | Initial TKI | N | Median age (y) | 3-y MR4.5 rate | 5-y MR4.5 rate | 10-y MR4.5 rate |
|---|---|---|---|---|---|---|---|
| De Lavallade et al. [88] | Hammersmith | IM 400 | 204 | 46 | 4f | 8f | NR |
| Branford et al. [22] | Adelaide | IM 400/600/800 | 423 | NR | NR | NR | 52a |
| Cortes et al. [25] | DASISION | IM 400 | 260 | 49 | 13 | 33 | NA |
| Hochhaus et al. [24], Kantarjian et al. [26] | ENESTnd | IM 400 | 283 | 46 | 15 | 31 | 39 |
| Zhang et al. [69] | Peking | IM 400 | 1373 | 41 | NR | NR | 43b |
| Guilhot et al. [27] | French SPIRIT | IM 400 | 223 | 50 | 24 | 23 | 27 |
| Hehlmann et al. [30, 31] | German Study IV | IM 400 | 401 | 53 | 35 | 49 | 67 |
| Guilhot et al. [27] | French SPIRIT | IM 400 + LDAC | 172 | 51 | 22 | 22 | 34 |
| Hehlmann et al. [30, 31] | German Study IV | IM 400 + LDAC | 158 | 51 | 31 | 50 | 70 |
| Guilhot et al. [27] | French SPIRIT | IM 400 + IFNα | 221 | 55 | 26 | 33 | 29 |
| Hehlmann et al. [30, 31] | German Study IV | IM 400 + IFNα | 430 | 53 | 38 | 54 | 74 |
| Guilhot et al. [27] | French SPIRIT | IM 600 | 171 | 51 | 24 | 31 | 36 |
| Hehlmann et al. [30, 31] | German Study IV | IM 800 | 399 | 52 | 43 | 58 | 71 |
| Etienne et al. [43] | French | IM 400, DAS 100,NIL 600 | 398 | 62 | 31e | 40e | 52e |
| Geelen et al. [90] | Dutch | IM 400, DAS 100, NIL 600 | 434 | 58 | 30c | 56d | 57 |
| Cortes et al. [25] | DASISION | DAS 100 | 259 | 46 | 20 | 42 | NA |
| Matsumura et al. [28] | JALSG | DAS 100 | 227 | 53 | 45 | NA | NA |
| Matsumura et al. [28] | JALSG | NIL 600 | 227 | 53 | 41 | NA | NA |
| Hochhaus et al. [24], Kantarjian et al. [26] | ENESTnd | NIL 600 | 282 | 47 | 32 | 54 | 61 |
| Hochhaus et al. [24], Kantarjian et al. [26] | ENESTnd | NIL 800 | 281 | 47 | 28 | 52 | 61 |
| Masarova et al. [93] | MDACC | NIL 800 | 122 | 51 | 61 | 72 | 75 |
TKI doses are in mg/d. Percentages are rounded.
MR4.5 BCR::ABL1 ≤ 0.0032%IS, other abbreviations as in Table 1.
a8-y, b7-y, c2-y, d4-y, esustained (at least 24 months), frates of ‘complete molecular response (CMR)’ defined as two consecutive samples with no detectable transcripts.
How many people can successfully discontinue TKI therapy?
Expert consensus statements and clinical practice guidelines recommend >5 years of imatinib and >3 to 5 years of a 2G-TKI, with a response ≥ MR4 for ≥2 years [2, 7–9, 38, 44, 46, 48, 49]. Convincing data supporting these recommendations are lacking [10]. If applying these criteria, only about 45% of people receiving imatinib might achieve MR4 at 3 years. Assuming they remain in MR4 for other 2 years it can be estimated that about 45% would become eligible to stop TKI therapy at ≥ 5 years. In persons receiving 2G-TKIs alone or with other drugs, this estimate is only slightly higher, about 50%. Combining these data only 10–25% of people will be eligible to stop TKI-therapy, which can be estimated to be successful in about one-half of people or about 10% of everyone with chronic phase CML (see below).
Many studies have reported the rate of TFR on > 2000 subjects cumulatively, but the real rate of successful TKI-stopping in persons with newly-diagnosed chronic phase CML is rarely reported. We estimate this proportion in Table 3 along with the proportion still in TFR at last contact at only 10–25%.
Table 3.
Percentage of newly-diagnosed CML patients meeting TKI discontinuation criteria and achieving stable TFR. Discontinuation and TFR criteria are arbitrarily defined, differ between studies and are often not pre-specified. Data are from retrospective analyses.
| Ref | Study | Initial TKI | N | Median follow-up (y) | Met discontinuation criteria | Discontinued | Achieved Stable TFR |
|---|---|---|---|---|---|---|---|
| Branford et al. [22] | Adelaide | IM 400/600/800 | 423 | 8 | 37% | NR | NR |
| Geelen et al. [90] | Dutch | IM 400 (75%), 2GTKIs (25%) | 382 | 10 | 31% | 10% | NR |
| Flygt et al. [48] | Swedish | Mainly IM 400 | 548 | 9 | NR | 23% | 12% |
| Etienne et al. [43] | French | Mainly IM 400 | 398 | 7 | 10%–55% | 46% | 12% |
| Kantarjian et al. [26] | ENESTnd | IM 400 | 283 | 10 | 30% | NR | NR |
| Guilhot et al. [27] | French SPIRIT | IM 400 + LDAC or + IFNα or IM 600 | 787 | 13.5 | NR | 44% | 18% |
| Gugliotta et al. [92] | GIMEMA | NIL 800 | 73 | 10 | NR | 33% | 25% |
| Kantarjian et al. [26] | ENESTnd | NIL 600 | 282 | 10 | 49% | NR | NR |
| Kantarjian et al. [26] | ENESTnd | NIL 800 | 281 | 10 | 47% | NR | NR |
TKI doses are in mg/d. Percentages are rounded.
Abbreviations as in Table 1. 2GTKIs: second-generation TKIs.
Are survival results of TKI-therapy adequate?
Survival from diagnosis is the most reliable study endpoint because it requires no further definition and time-to-event data are evaluable in almost all subjects. In contrast, definitions of other endpoints such as failure-free survival (FFS), progression-free survival (PFS) and CML-related survival differ between studies. For example, identifying the cause(s) of death may be subjective and difficult to accurately ascertain in retrospective analyses. Survival data of newly-diagnosed people initially treated with TKIs are reasonably consistent with 1- and 2-year survivals of >95% and 3-, 5- and 10-year survivals >80% in persons receiving imatinib or a 2G-TKI as initial therapy (Table 4).
Table 4.
Survival of subjects receiving TKI therapy.
| Ref. | Study | Initial TKI | N | Median age (y) | 3-y (%) | 5-y (%) | 10-y (%) |
|---|---|---|---|---|---|---|---|
| Castagnetti et al. [94] | EUTOS | IM 400 | 236 | 60 | 93 | 85 | NA |
| Castagnetti et al. [89] | GIMEMA | IM 400/800 | 559 | 52 | NR | 89b | NA |
| de Lavallade et al. [88] | Hammersmith | IM 400 | 204 | 46 | 96 | 83 | NA |
| Hochhaus et al. [32] | IRIS | IM 400 | 553 | 50 | 92 | 89 | 83 |
| Guilhot et al. [27] | French SPIRIT | IM 400 | 223 | 50 | 95 | 95 | 90 |
| O’Brien et al. [29] | UK SPIRIT 2 | IM 400 | 407 | 53 | NR | 91 | NR |
| Hehlmann et al. [30, 31] | German Study IV | IM 400 | 400 | 53 | 96 | 88 | 80 |
| Hochhaus et al. [24], Kantarjian et al. [26] | ENESTnd | IM 400 | 283 | 46 | 94 | 92 | 88 |
| Zhang et al. [69] | Peking | IM 83%, 2G-TKI 17% | 1373 | 40 | NR | 94c | NA |
| Cortes et al. [25] | DASISION | IM 400 | 260 | 49 | 95d | 90 | NR |
| Guilhot et al. [27] | French SPIRIT | IM 400 + LDAC | 172 | 55 | 95 | 91 | 85 |
| Hehlmann et al. [30, 31] | German Study IV | IM 400 + LDAC | 158 | 51 | NR | 86 | 84 |
| Hehlmann et al. [30, 31] | German Study IV | IM 400 + IFNα | 430 | 53 | 95 | 88 | 84 |
| Guilhot et al. [27] | French SPIRIT | IM 400+ IFNα | 221 | 51 | 95 | 95 | 89 |
| Kalmanti et al. [95] | German Study IV | IM 400 ± LDAC or + IFNα or IM 800 | 120 | 16–29a | NR | 97 | NR |
| Kalmanti et al. [95] | German Study IV | IM 400 ± LDAC or + IFNα or IM 800 | 383 | 30–44a | NR | 94 | NR |
| Pfirrmann et al [96] | EUTOS | IM 400 > 80% | 2290 | 51 | NR | NR | 89e |
| Geelen et al. [90] | Dutch | IM 77%, 2G-TKI 23% | 382 | 58 | 92d | 85f | NA |
| Etienne et al. [43] | French | IM 73%, 2G-TKI 27% | 398 | 64 IM, 54 2G-TKI | NR | 90 | 81 |
| Jain et al. [23] | MDACC | IM 57%, NIL 21%, DAS 21% | 197 | 14–44a | 98 | 96 | 87 |
| O’Brien et al. [29] | UK SPIRIT 2 | DAS 100 | 407 | 53 | NR | 92 | NA |
| Matsumura et al. [28] | JALSG | DAS 100 | 227 | 53 | 99 | NA | NA |
| Cortes et al. [25] | DASISION | DAS 100 | 259 | 46 | 95d | 91 | NA |
| Hochhaus et al. [24], Kantarjian et al. [26] | ENESTnd | NIL 600 | 282 | 47 | 95 | 94 | 88 |
| Matsumura et al. [28] | JALSG | NIL 600 | 227 | 53 | 99 | NA | NA |
| Hochhaus et al. [24], Kantarjian et al. [26] | ENESTnd | NIL 800 | 281 | 47 | 97 | 96 | 95 |
| Masarova et al. [93] | MDACC | NIL 800 | 122 | 51 | 97 | 93 | 88 |
| Gugliotta et al. [92] | GIMEMA | NIL 800 | 73 | 51 | 97 | 96 | 95 |
TKI dose in mg/d. Percentages rounded.
Some data are estimated from graphs (±1%). Abbreviations as in Table 1.
aAge intervals instead of median, b6-y, c7-y, d2-y, e8-y, f4-y.
What are results of allogeneic haematopoietic cell transplants and have they improved?
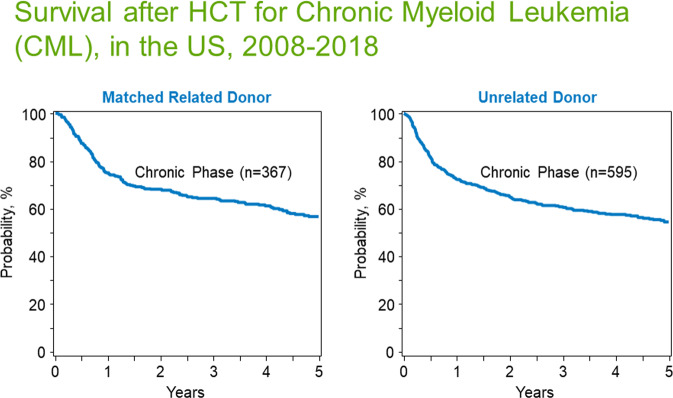
CML transplants, once the most common transplant indication, are now uncommon. In 2020, <200 of >10,000 allotransplants reported to the CIBMTR were for CML, done mostly in persons in accelerated or blast phases. Outcomes from several transplant centres and registries of transplant outcomes in persons with chronic phase CML mostly done before 2012 are displayed in Table 5. A 5-year survival, not leukaemia-free survival (LFS), of about 60% is reported by the CIBMTR in 1,445 subjects with CML in chronic phase receiving transplants from HLA-identical siblings (Fig. 1). Goldman et al. [51] reported data from 2,221 persons in chronic phase CML receiving transplants from HLA-identical siblings (N = 1,692) or HLA-matched unrelated donors (N = 639) alive and leukaemia-free at 5 years posttransplant. Ten- and 15-year posttransplant LFS were 91% (95% Confidence Interval [CI], 90, 92%) and 83% (81, 85%). Comparable cumulative incidences of relapse (CIR) were 4% (3, 5%) and 7% (5, 8%). There was a slow but steady relapse risk after 5 years posttransplant with the latest relapse at 18 years. These data indicate a high cure in persons alive and without relapse at 5 years posttransplant.
Table 5.
Survival after an allotransplant for CML in 1st chronic phase.
| Interval | N | Median age (y) | Conditioning | Donor | 1-y | 2-y | 3-y | 5-y | 10-y | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Millot et al. [97] | SGFMTC | 1982–1998 | 42 | 14 | MA | REL | 87% | 85% | 77% | 73% | 73% |
| Cwynarski et al. [98] | EBMT | 1985–2001 | 156 | 14 | NR | REL | 78% | 75% | 75% | 72% | 70% |
| Arora et al. [99] | CIBMTR | 1988–2003 | 3514 | 36 | MA | REL | 74% | 65% | 63% | 63% | 60% |
| Arora et al. [99] | CIBMTR | 1988–2003 | 531 | 37 | MA | UNR | 70% | 63% | 58% | 55% | 50% |
| Radich et al. [100] | Seattlea | 1995–2000 | 131 | 43 | MA | REL | 91% | 86% | 86% | NA | NA |
| Gratwohl et al. [61] | German Study IIIa | 1997–2004 | 151 | 38 | MA | REL | 90% | 85% | 82% | 78% | 76% |
| Gratwohl et al. [61] | German Study III | 1997–2004 | 148 | 41 | MA | UNR | 97% | 85% | 77% | 76% | 76% |
| Bacher et al. [62] | German Registry | 1998–2004 | 1084 | 40 | MA 62% | REL 61% | 67% | 65% | 65% | 64% | 64% |
| Ohashi et al. [101] | Japanese Registry | 2000–2009 | 531 | 40 | MA 89% | UNR 51% | 87% | 86% | 85% | 85% | 78% |
| Chaudury et al. [102] | CIBMTR | 2001–2010 | 224 | 24 | MA | REL | 90% | 88% | 85% | 83% | NA |
| Chaudury et al. [102] | CIBMTR | 2001–2010 | 225 | 24 | MA | UNR | 80% | 76% | 72% | 68% | NA |
| Lee et al. [103] | Koreana | 2001–2012 | 47 | 32 | MA 77% | UNR 43% | 88% | 86% | 86% | NA | NA |
| Lee et al. [103] | Korean | 2001–2012 | 50 | 33 | MA 48% | UNR 42% | 90% | 86% | 80% | NA | NA |
| Koenecke et al. [104] | EBMT | 2002–2005 | 193 | 31 | MA | REL | 90% | 87% | 86% | 85% | 84% |
| Saussele et al. [105] | German Study IVa | 2003–2008 | 19 | 35 | MA 79% | REL 53% | 95% | 88% | 88% | NA | NA |
| Saussele et al. [105] | German Study IV | 2003–2008 | 37 | 38 | MA 65% | UNR 70% | 95% | 95% | 94% | NA | NA |
aData are estimated from graphs (±1%). SGFMTC Société Française de Greffe de Moelle et de Thérapie Cellulaire, EBMT European Group for Marrow and Blood Transplantation, CIBMTR Center for International Blood and Marrow Transplantation, MA myelo-ablative, REL related donor, UNR unrelated donor, NR not reported.
Fig. 1. Survival after allogeneic transplants (2008–18) for chronic myeloid leukemia in chronic phase (from Phelan, R., Arora, M., Chen, M. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides, 2020).
Left panel shows overall survival of patients with chronic myeloid leukemia in chronic phase transplanted from a matched related donor; right panel shows overall survival after allogeneic transplants from an unrelated donor. n number of patients transplanted.
Because these data are predominately from the pre-TKI era, we analyzed CIBMTR data from the 238 transplants done between 2014 and 2016 in persons with CML in 1st chronic phase from all donors. One-year non-relapse mortality (NRM) was 17% (12, 23%). Five-year CIR was 18% (13, 23%) with almost all relapses with the 1st year posttransplant. 5-year survival was 68% (61, 74%). These outcomes very likely reflect strong selection biases operating in both directions. First, persons responding poorly to TKI-therapy are more likely to receive a transplant than good responders. In contrast, transplants were likely done in young persons with a good performance score, well-matched donors and few co-morbidities. Consequently, these summary outcomes data should be viewed cautiously.
There are several recent transplant advances including: (1) a donor such as an HLA-haplotype-matched relative for almost everyone; (2) increasing use of blood cells over bone marrow grafts; (3) development of less intensive pretransplant conditioning regimens (termed reduced-intensity condition [RIC]) applicable to older persons; (4) use of posttransplant cyclophosphamide as well as anti-lymphocyte globulin (ATG/ATLG) reducing risks of acute and chronic graft-versus-host disease (GvHD) seemingly without increasing relapse risk (although this has not been critically tested in CML) [52–54]; (5) better supportive care; and others. These advances have decreased transplant-related deaths by about 20% and increased survival by about 10% [55]. Whether these advances apply to transplants done for chronic phase CML is unknown.
As indicated, leukaemia recurrence is uncommon after allotransplants for chronic phase CML [51, 55–57]. Much of this anti-leukaemia efficacy results from an allogeneic effect [54, 58]. Transplants from genetically-identical twins, T-cell-depleted grafts and transplants in persons without GvHD have substantially higher CIRs, reflecting immune-mediated anti-leukaemia effect. Early relapses are often successfully treated by stopping posttransplant immune suppression, giving donor lymphocyte infusions (DLIs) and/or giving TKIs [59]. Late relapses are rare, but relapse risk continues indefinitely [51]. Allotransplants done in chronic phase result in about 80% 15-year LFS [51]. However, some persons develop chronic GvHD or other complications which compromise QoL and are sometimes fatal. Other considerations which are incompletely resolved are the impact of pre- and posttransplant TKI-therapy on transplant outcomes.
Who should be considered for a transplant in chronic phase?
The question of who should receive a transplant in chronic phase is complex and controversial. Probably the clearest indication is in drug compliant persons failing to respond to TKI-therapy and those with some BCR::ABL1 mutations, high-risk additional cytogenetic abnormalities (ACAs) and/or with other signs of leukaemia progression [60]. There are persons who cannot tolerate TKI therapy, or who develop severe adverse events which cannot be managed by dose adjustment of switching to a different TKI. They are a minimal part of patients. But they are.
A more complicated question is whether a transplant is an appropriate option in a person likely to have good survival but unlikely to achieve TFR and who therefore require lifelong TKI-therapy. The first issue is whether such persons can be accurately identified and when. Several predictive models have been developed which predict failure of TKI-therapy but none has a Concordance (C)-statistic >0.80. The next issue is whether it’s possible to accurately predict transplant outcomes. Again, several predictive models have been developed with similar C-statistics. A third issue is suitability of someone to receive a transplant including age, co-morbidities, donor availability and fiscal resources.
There cannot be an uniform correct answer. For example, a younger person is more likely to accept the immediate survival disadvantage of transplants for a substantial probability of cure whereas an older person may not. Another consideration is a personal satisfaction/dis-ratification with remaining on lifelong TKI-therapy. There are also fiscal considerations. In some resource-poor geospaces there may be a substantial cost saving to receiving a transplant. And one should not ignore the important impact of patient and physician risk-taking attitude which we discuss below.
Aren’t most people with CML too old to receive a transplant?
Most studies of CML therapy including transplants are in resource-rich geospaces where median age at diagnosis is about 60 years [61–64]. However, in some Asian and African countries median age at diagnosis is <50 years [65–67]. In an international review of >40,000 subjects with newly diagnosed CML, the rate of adults <50 years old in Asia and Africa was about 70% compared with 35% in Europe, increasing the proportion of persons with CML who might be considered for a transplant [64]. Transplant studies are obviously skewed towards younger persons.
Do we need to reconsider use of transplant in chronic phase CML?
Despite recent progress, few persons with chronic phase CML receiving TKI therapy achieve TFR, and even fewer, if any, are cured [68]. Most persons failing to achieve arbitrarily specified TKI-therapy response goals can be reasonably accurately identified at diagnosis or soon after starting TKI therapy [69]. Rates of remaining leukaemia-free are certainly lower and cure rates higher in persons receiving a transplant. However, there are important caveats when interpreting these data: (1) few transplants have been done for CML recently, limiting the certainty of estimating outcomes; (2) there are subject selection biases favouring transplants including younger age, better performance score and fewer co-morbidities in transplant recipients compared with persons receiving TKIs. For example, median age of the CIBMTR cohort we describe above is 46 years, substantially younger than the median age of persons with CML of predominately European descent; (3) selection biases against transplant recipients who are more likely to have had a worse prognosis at diagnosis or soon thereafter compared with those receiving only TKI therapy; and (4) the almost 20% 1-year mortality associated with transplants and risk of transplant-related complications such as chronic GvHD.
At diagnosis, most physicians and persons with chronic phase CML are understandably reluctant to accept a 1-year TRM of almost 20% without a trial of TKI-therapy to determine whether the person is amongst the small proportion of those likely to achieve TFR and possibly cure. However, there are several time-dependent predictive and prognostic models and scores which enable physicians to estimate the likelihood of success of TKI therapy in achieving TFR reasonably early after starting TKI therapy. At this point, in persons who are potential transplant candidates, physicians and patients must choose between probable lifetime TKI therapy with attendant medical, physical and psychological costs versus likelihood of success and risks of a transplant [11, 70–73]. On the TKI therapy side of the calculus are considerations such as estimating the likelihood of adverse events, costs and risk tolerance. On the transplants side of the calculus are co-variates correlated with outcomes such as age, co-morbidities, donor HLA-matching, graft-type, pretransplant conditioning and posttransplant immune suppression regimens and others [74–76]. Of note, subject-, disease- and transplant-related predictive and prognostic co-variates previously operating in persons receiving and possibly failing TKI-therapy need confirmation.
A critical comparison of LFS or survival between TKI-therapy and transplants in comparable persons can only come from randomized controlled trials. Such a trial has not and will not be done. Also, the issue is not whether one or the other therapy is better but which therapy is more appropriate for different persons at different times after CML diagnosis and after observing response to TKI therapy [22, 23, 26, 27, 43, 44, 49, 77]. Both therapies have worse outcomes in older people, people with a poor performance score and those with co-morbidities, but these gradients are steeper for transplant recipients compared with persons receiving TKIs. Also older persons receiving TKI therapy are less likely to be therapy compliant, achieve TFR and remain on lifelong TKI therapy with attendant impacts on QoL. This is especially true for 2G-TKIs [2, 5–8].
A transplant is an increasingly relevant consideration in persons with a non-optimal response to TKI-therapy. Many of these persons can be identified by cytogenetic and molecular analyses, especially those with high-risk additional chromosome abnormalities (ACAs) and/or a 2nd BCR::ABL1 or mutations in TP53 and/or epigenetic modifier genes [78–84].
When the best therapy is controversial, physicians often rely on expert consensus statements and clinical practice guidelines. We discussed limitations of these tools elsewhere [85, 86]. However, our point is that panellists should consider adding transplants in persons with chronic phase CML during their deliberations [77, 87].
Conclusion
This Perspective is a series of questions awaiting answers. They reflect questions Prof. Baccarani after a lifetime of CML research thought needed to be answered by the next generation of physicians interested in CML. Some of these questions can be answered by appropriately designed clinical trials. Others could theoretically be answered in clinical trials but for diverse reason such trials will not or cannot be done. Lastly, there are questions to which there is no one answer and certainly not one correct answer.
Medicine is an art, not a science. As the distinguished English, Canadian, American physician and medical educator Sir William Osler noted: Medicine is a science of uncertainty and an art of probability – Prof. Baccarani practiced a perfect blend of the science and art of medicine, of balancing uncertainty and probability. More mistakes are made by those who think they know the answer compared with those admitting uncertainty. Prof. Baccarani leaves us with these questions and challenges us to provide answers or at least to try. He was never afraid to challenge dogma or challenge answers to questions others thought answered. As Thomas Paine said: He who dares not to offend cannot be honest. Omnia munda mundis.
Acknowledgements
Profs. Rudiger Hehlmann (Univ. Heidelberg) and Andreas Hochhaus (Univ. Jena) kindly reviewed the typescript. FB, FC, GG, SS, GR acknowledge support from AIL BOLOGNA ODV. FB acknowledges support from Ministero della Salute-Ricerca Corrente. RPG acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme. The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014–20–1–2705 and N00014–20–1–2832 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Accenture; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies Corporation; Adienne SA; Allovir, Inc.; Amgen, Inc.; Astellas Pharma US; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Fate Therapeutics; Gamida-Cell, Ltd.; Gilead; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Kadmon; Karius; Karyopharm Therapeutics; Kiadis Pharma; Kite Pharma Inc; Kite, a Gilead Company; Kyowa Kirin International plc; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Medac GmbH; Medexus; Merck & Co.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OncoImmune, Inc.; Oncopeptides, Inc.; OptumHealth; Orca Biosystems, Inc.; Ossium Health, Inc; Pfizer, Inc.; Pharmacyclics, LLC; Priothera; Sanofi Genzyme; Seagen, Inc.; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Tscan; Vertex; Vor Biopharma; Xenikos BV.
Author contributions
MB conceived and designed the study but died shortly before the finalization of the typescript leaving us with these questions to pose on his behalf. The other authors contributed to the development of the study and typescript, take responsibility for the content and approved submitting the typescript for publication.
Data availability
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
Competing interests
FB received speaker fees from NEOVII Biotech, JAZZ pharmaceuticals, Novartis, Celgene, MSD, Pfizer and Amgen. SS received speaker fees from Incyte Biosciences and Bio-Rad and is a consultant to Incyte Biosciences and Cepheid. FC received speaker fees and is a consultant to Novartis, Pfizer, Bristol-Myers Squibb and Incyte Biosciences. GG received speaker fees from Novartis and Incyte Biosciences. WS reports no conflict of interests. NEM reports no conflict of interests. GR received speaker fees from and is a consultant to Novartis, Pfizer, Bristol-Myers Squibb and Incyte Biosciences. RPG is a consultant to BeiGene Ltd., Fusion Pharma LLC, LaJolla NanoMedical Inc., Mingsight Parmaceuticals Inc. CStone Pharmaceuticals, NexImmune Inc. and Prolacta Bioscience; advisor to Antengene Biotech LLC, Medical Director, FFF Enterprises Inc.; partner, AZAC Inc.; Board of Directors, Russian Foundation for Cancer Research Support; and Scientific Advisory Board: StemRad Ltd.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Deceased: Michele Baccarani.
References
- 1.Bower H, Bjorkholm M, Dickman PW, Hoglund M, Lambert PC, Andersson TM. Life Expectancy of Patients With Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J Clin Oncol. 2016;34:2851–7. doi: 10.1200/JCO.2015.66.2866. [DOI] [PubMed] [Google Scholar]
- 2.Baccarani M, Abruzzese E, Accurso V, Albano F, Annunziata M, Barulli S, et al. Managing chronic myeloid leukemia for treatment-free remission: a proposal from the GIMEMA CML WP. Blood Adv. 2019;3:4280–90. doi: 10.1182/bloodadvances.2019000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radivoyevitch T, Weaver D, Hobbs B, Maciejewski JP, Hehlmann R, Jiang Q, et al. Do persons with chronic myeloid leukaemia have normal or near normal survival? Leukemia. 2020;34:333–5. doi: 10.1038/s41375-019-0699-y. [DOI] [PubMed] [Google Scholar]
- 4.Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Bartley PA, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia. 2010;24:1719–24. doi: 10.1038/leu.2010.185. [DOI] [PubMed] [Google Scholar]
- 5.Baccarani M, Gale RP. Why chronic myeloid leukaemia cannot be cured by tyrosine kinase-inhibitors. Leukemia. 2021;35:2199–204. doi: 10.1038/s41375-021-01272-8. [DOI] [PubMed] [Google Scholar]
- 6.Malhotra H, Radich J, Garcia-Gonzalez P. Meeting the needs of CML patients in resource-poor countries. Hematol Am Soc Hematol Educ Program. 2019;2019:433–42. doi: 10.1182/hematology.2019000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84. doi: 10.1038/s41375-020-0776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith G, Apperley J, Milojkovic D, Cross NCP, Foroni L, Byrne J, et al. A British Society for Haematology Guideline on the diagnosis and management of chronic myeloid leukaemia. Br J Haematol. 2020;191:171–93. doi: 10.1111/bjh.16971. [DOI] [PubMed] [Google Scholar]
- 9.Deininger MW, Shah NP, Altman JK, Berman E, Bhatia R, Bhatnagar B, et al. Chronic Myeloid Leukemia, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020;18:1385–415. doi: 10.6004/jnccn.2020.0047. [DOI] [PubMed] [Google Scholar]
- 10.Saglio G, Gale RP. Prospects for achieving treatment-free remission in chronic myeloid leukaemia. Br J Haematol. 2020;190:318–27. doi: 10.1111/bjh.16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang Q, Larson RA, Gale RP. Economics influences therapy decisions in chronic myeloid leukaemia: should it? J Cancer Res Clin Oncol. 2021;147:3693–8. doi: 10.1007/s00432-021-03607-5. [DOI] [PubMed] [Google Scholar]
- 12.Kwak JY, Kim SH, Oh SJ, Zang DY, Kim H, Kim JA, et al. Phase III clinical trial (RERISE study) results of efficacy and safety of radotinib compared with imatinib in newly diagnosed chronic phase chronic myeloid leukemia. Clin Cancer Res. 2017;23:7180–8. doi: 10.1158/1078-0432.CCR-17-0957. [DOI] [PubMed] [Google Scholar]
- 13.Cortes JE, Gambacorti-Passerini C, Deininger MW, Mauro MJ, Chuah C, Kim DW, et al. Bosutinib Versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia: Results From the Randomized BFORE Trial. J Clin Oncol. 2018;36:231–7. doi: 10.1200/JCO.2017.74.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes TP, Mauro MJ, Cortes JE, Minami H, Rea D, DeAngelo DJ, et al. Asciminib in chronic myeloid leukemia after ABL. N. Engl J Med. 2019;381:2315–26. doi: 10.1056/NEJMoa1902328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–12. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, et al. Ponatinib in refractory philadelphia chromosome-positive leukemias. N. Engl J Med. 2012;367:2075–88. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steegmann JL, Baccarani M, Breccia M, Casado LF, Garcia-Gutierrez V, Hochhaus A, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648–71. doi: 10.1038/leu.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Q, Liu ZC, Zhang SX, Gale RP. Young age and high cost are associated with future preference for stopping tyrosine kinase inhibitor therapy in Chinese with chronic myeloid leukemia. J Cancer Res Clin Oncol. 2016;142:1539–47. doi: 10.1007/s00432-016-2159-7. [DOI] [PubMed] [Google Scholar]
- 19.Padula WV, Larson RA, Dusetzina SB, Apperley JF, Hehlmann R, Baccarani M, et al. Cost-effectiveness of tyrosine kinase inhibitor treatment strategies for chronic myeloid leukemia in chronic phase after generic entry of imatinib in the United States. J Natl Cancer Inst. 2016;108. 10.1093/jnci/djw003. [DOI] [PMC free article] [PubMed]
- 20.Shih YT, Cortes JE, Kantarjian HM. Treatment value of second-generation BCR-ABL1 tyrosine kinase inhibitors compared with imatinib to achieve treatment-free remission in patients with chronic myeloid leukaemia: a modelling study. Lancet Haematol. 2019;6:e398–e408. doi: 10.1016/S2352-3026(19)30087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Branford S, Yeung DT, Ross DM, Prime JA, Field CR, Altamura HK, et al. Early molecular response and female sex strongly predict stable undetectable BCR-ABL1, the criteria for imatinib discontinuation in patients with CML. Blood. 2013;121:3818–24. doi: 10.1182/blood-2012-10-462291. [DOI] [PubMed] [Google Scholar]
- 23.Jain P, Kantarjian H, Nazha A, O’Brien S, Jabbour E, Romo CG. et al. Early responses predict better outcomes in patients with newly diagnosed chronic myeloid leukemia: results with four tyrosine kinase inhibitor modalities. Blood. 2013;121:4867–74. 10.1182/blood-2013-03-490128. [DOI] [PMC free article] [PubMed]
- 24.Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–54. doi: 10.1038/leu.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boque C, et al. Final 5-year study results of dasision: the dasatinib versus imatinib study in treatment-naive chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333–40. doi: 10.1200/JCO.2015.64.8899.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kantarjian HM, Hughes TP, Larson RA, Kim DW, Issaragrisil S, le Coutre P, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. 2021;35:440–53. doi: 10.1038/s41375-020-01111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilhot F, Rigal-Huguet F, Guilhot J, Guerci-Bresler AP, Maloisel F, Rea D, et al. Long-term outcome of imatinib 400 mg compared to imatinib 600 mg or imatinib 400 mg daily in combination with cytarabine or pegylated interferon alpha 2a for chronic myeloid leukaemia: results from the French SPIRIT phase III randomised trial. Leukemia. 2021;35:2332–45. doi: 10.1038/s41375-020-01117-w. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura I, Ohtake S, Atsuta Y, Kurata M, Minami Y, Takahashi N, et al. Nilotinib vs dasatinib in achieving MR4.5 for newly diagnosed chronic myeloid leukemia. Results of the prospective randomized phase 3 study, JALSG CML212. Blood. 2020;136:40–1. doi: 10.1182/blood-2020-134168. [DOI] [Google Scholar]
- 29.O’Brien S, Cork L, Bandeira V, Bescoby R, Foroni L, Alaily L, et al. Spirit 2: final 5 year analysis of the uk national cancer research institute randomized study comparing imatinib with dasatinib in patients with newly diagnosed chronic phase cml. Blood. 2018;132:457. doi: 10.1182/blood-2018-99-110128. [DOI] [Google Scholar]
- 30.Hehlmann R, Muller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32:415–23. doi: 10.1200/JCO.2013.49.9020. [DOI] [PubMed] [Google Scholar]
- 31.Hehlmann R, Lauseker M, Saussele S, Pfirrmann M, Krause S, Kolb HJ, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31:2398–406. doi: 10.1038/leu.2017.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long- N. Engl J Med. 2017;376:917–27. doi: 10.1056/NEJMoa1609324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunner AM, Campigotto F, Sadrzadeh H, Drapkin BJ, Chen YB, Neuberg DS, et al. Trends in all-cause mortality among patients with chronic myeloid leukemia: a Surveillance, Epidemiology, and End Results database analysis. Cancer. 2013;119:2620–9. doi: 10.1002/cncr.28106. [DOI] [PubMed] [Google Scholar]
- 34.Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–35. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 35.Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17–23. doi: 10.1182/blood-2016-01-694265. [DOI] [PubMed] [Google Scholar]
- 36.Saussele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30:1638–47. doi: 10.1038/leu.2016.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campiotti L, Suter MB, Guasti L, Piazza R, Gambacorti-Passerini C, Grandi AM, et al. Imatinib discontinuation in chronic myeloid leukaemia patients with undetectable BCR-ABL transcript level: A systematic review and a meta-analysis. Eur J Cancer. 2017;77:48–56. doi: 10.1016/j.ejca.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 38.Etienne G, Faberes C, Bauduer F, Adiko D, Lifermann F, Dagada C, et al. Relevance of treatment-free remission recommendations in chronic phase chronic leukemia patients treated with frontline tyrosine kinase inhibitors. Cancer Med. 2021;10:3635–45. doi: 10.1002/cam4.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A, et al. Long-Term Follow-Up of the French Stop Imatinib (STIM1) Study in Patients With Chronic Myeloid Leukemia. J Clin Oncol. 2017;35:298–305. doi: 10.1200/JCO.2016.68.2914. [DOI] [PubMed] [Google Scholar]
- 40.Baccarani M. Treatment-free remission in chronic myeloid leukemia: floating between expectation and evidence. Leukemia. 2017;31:1015–6. doi: 10.1038/leu.2017.20. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki K, Kantarjian H, O’Brien S, Ravandi F, Konopleva M, Borthakur G, et al. Prediction for sustained deep molecular response of BCR-ABL1 levels in patients with chronic myeloid leukemia in chronic phase. Cancer. 2018;124:1160–8. doi: 10.1002/cncr.31187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baccarani M, Rosti G, Soverini S. Chronic myeloid leukemia: the concepts of resistance and persistence and the relationship with the BCR-ABL1 transcript type. Leukemia. 2019;33:2358–64. doi: 10.1038/s41375-019-0562-1. [DOI] [PubMed] [Google Scholar]
- 43.Etienne G, Dulucq S, Bauduer F, Adiko D, Lifermann F, Dagada C, et al. Incidences of deep molecular responses and treatment-free remission in de novo cp-cml patients. Cancers (Basel). 2020;12. 10.3390/cancers12092521. [DOI] [PMC free article] [PubMed]
- 44.Rea D. Handling challenging questions in the management of chronic myeloid leukemia: when is it safe to stop tyrosine kinase inhibitors? Blood Adv. 2020;4:5589–94. doi: 10.1182/bloodadvances.2020002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atallah E, Schiffer CA. Discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia: when and for whom? Haematologica. 2020;105:2738–45. doi: 10.3324/haematol.2019.242891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross DM, Hughes TP. Counterpoint: There is a best duration of deep molecular response for treatment-free remission, but it is patient-specific, and that is the challenge. Br J Haematol. 2021;192:24–7. doi: 10.1111/bjh.17111. [DOI] [PubMed] [Google Scholar]
- 47.Shanmuganathan N, Pagani IS, Ross DM, Park S, Yong ASM, Braley JA, et al. Early BCR-ABL1 kinetics are predictive of subsequent achievement of treatment-free remission in chronic myeloid leukemia. Blood. 2021;137:1196–207. doi: 10.1182/blood.2020005514. [DOI] [PubMed] [Google Scholar]
- 48.Flygt H, Sandin F, Dahlen T, Dremaine A, Lubking A, Markevarn B, et al. Successful tyrosine kinase inhibitor discontinuation outside clinical trials - data from the population-based Swedish chronic myeloid leukaemia registry. Br J Haematol. 2021;193:915–21. doi: 10.1111/bjh.17392. [DOI] [PubMed] [Google Scholar]
- 49.Kim DDH, Novitzky-Basso I, Kim TS, Atenafu EG, Forrest D, Savoie L, et al. Optimal duration of imatinib treatment/deep molecular response for treatment-free remission after imatinib discontinuation from a Canadian tyrosine kinase inhibitor discontinuation trial. Br J Haematol. 2021;193:779–91. doi: 10.1111/bjh.17447. [DOI] [PubMed] [Google Scholar]
- 50.Jiang Q, Yu L, Gale RP. Patients’ and hematologists’ concerns regarding tyrosine kinase-inhibitor therapy in chronic myeloid leukemia. J Cancer Res Clin Oncol. 2018;144:735–41. doi: 10.1007/s00432-018-2594-8. [DOI] [PubMed] [Google Scholar]
- 51.Goldman JM, Majhail NS, Klein JP, Wang Z, Sobocinski KA, Arora M, et al. Relapse and late mortality in 5-year survivors of myeloablative allogeneic hematopoietic cell transplantation for chronic myeloid leukemia in first chronic phase. J Clin Oncol. 2010;28:1888–95. doi: 10.1200/JCO.2009.26.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 53.Bonifazi F, Bandini G, Stanzani M, Palandri F, Giannini B, Arpinati M, et al. In vivo T-cell depletion with low-dose ATG is effective in reducing cGVHD after peripheral blood stem cell myeloablative sibling transplants in CML: results from a prospective phase II study. Bone Marrow Transpl. 2005;35:1025–6. doi: 10.1038/sj.bmt.1704940. [DOI] [PubMed] [Google Scholar]
- 54.Gale RP, Fuchs EJ. Is there really a specific graft-versus-leukaemia effect? Bone Marrow Transpl. 2016;51:1413–5. doi: 10.1038/bmt.2016.183. [DOI] [PubMed] [Google Scholar]
- 55.McDonald GB, Sandmaier BM, Mielcarek M, Sorror M, Pergam SA, Cheng GS, et al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: comparing 2003-2007 versus 2013-2017 cohorts. Ann Intern Med. 2020;172:229–39. doi: 10.7326/M19-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Passweg JR, Baldomero H, Chabannon C, Basak GW, Corbacioglu S, Duarte R, et al. The EBMT activity survey on hematopoietic-cell transplantation and cellular therapy 2018: CAR-T’s come into focus. Bone Marrow Transpl. 2020;55:1604–13. doi: 10.1038/s41409-020-0826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kongtim P, Adekola K, Milton DR, Ramlal R, Jimenez A, Chen J, et al. Donor type, in addition to transplantation in chronic phase and myeloablative conditioning, influence transplant survival for patients with advanced chronic myeloid leukemia in the era of tyrosine kinase inhibitors. Leukemia. 2017;31:1654–7. doi: 10.1038/leu.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62. doi: 10.1182/blood.V75.3.555.555. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt S, Liu Y, Hu ZH, Williams KM, Lazarus HM, Vij R, et al. The Role of Donor Lymphocyte Infusion (DLI) in Post-Hematopoietic Cell Transplant (HCT) Relapse for Chronic Myeloid Leukemia (CML) in the Tyrosine Kinase Inhibitor (TKI) Era. Biol Blood Marrow Transpl. 2020;26:1137–43. doi: 10.1016/j.bbmt.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hehlmann R, Voskanyan A, Lauseker M, Pfirrmann M, Kalmanti L, Rinaldetti S, et al. High-risk additional chromosomal abnormalities at low blast counts herald death by CML. Leukemia. 2020;34:2074–86. doi: 10.1038/s41375-020-0826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gratwohl A, Pfirrmann M, Zander A, Kroger N, Beelen D, Novotny J, et al. Long-term outcome of patients with newly diagnosed chronic myeloid leukemia: a randomized comparison of stem cell transplantation with drug treatment. Leukemia. 2016;30:562–9. doi: 10.1038/leu.2015.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bacher U, Klyuchnikov E, Zabelina T, Ottinger H, Beelen DW, Schrezenmeier H, et al. The changing scene of allogeneic stem cell transplantation for chronic myeloid leukemia-a report from the German Registry covering the period from 1998 to 2004. Ann Hematol. 2009;88:1237–47. doi: 10.1007/s00277-009-0737-3. [DOI] [PubMed] [Google Scholar]
- 63.Hoffmann VS, Baccarani M, Hasford J, Lindoerfer D, Burgstaller S, Sertic D, et al. The EUTOS population-based registry: incidence and clinical characteristics of 2904 CML patients in 20 European Countries. Leukemia. 2015;29:1336–43. doi: 10.1038/leu.2015.73. [DOI] [PubMed] [Google Scholar]
- 64.Baccarani M, Castagnetti F, Gugliotta G, Rosti G, Soverini S, Albeer A, et al. The proportion of different BCR-ABL1 transcript types in chronic myeloid leukemia. An international overview. Leukemia. 2019;33:1173–83. doi: 10.1038/s41375-018-0341-4. [DOI] [PubMed] [Google Scholar]
- 65.Au WY, Caguioa PB, Chuah C, Hsu SC, Jootar S, Kim DW, et al. Chronic myeloid leukemia in Asia. Int J Hematol. 2009;89:14–23. doi: 10.1007/s12185-008-0230-0. [DOI] [PubMed] [Google Scholar]
- 66.Faye BF, Dieng N, Seck M, Gadji M, Gueye YB, Sy D, et al. Pattern of chronic myeloid leukemia in the imatinib era in a Sub-Saharan African setting. Ann Hematol. 2016;95:1603–10. doi: 10.1007/s00277-016-2745-4. [DOI] [PubMed] [Google Scholar]
- 67.Ganesan P, Kumar L. Chronic Myeloid Leukemia in India. J Glob Oncol. 2017;3:64–71. doi: 10.1200/JGO.2015.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Innes AJ, Milojkovic D, Apperley JF. Allogeneic transplantation for CML in the TKI era: striking the right balance. Nat Rev Clin Oncol. 2016;13:79–91. doi: 10.1038/nrclinonc.2015.193. [DOI] [PubMed] [Google Scholar]
- 69.Zhang XS, Gale RP, Huang XJ, Jiang Q. Is the Sokal or EUTOS long-term survival (ELTS) score a better predictor of responses and outcomes in persons with chronic myeloid leukemia receiving tyrosine-kinase inhibitors? Leukemia. 2021 doi: 10.1038/s41375-021-01387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 1998;352:1087–92. doi: 10.1016/s0140-6736(98)03030-x. [DOI] [PubMed] [Google Scholar]
- 71.Passweg JR, Walker I, Sobocinski KA, Klein JP, Horowitz MM, Giralt SA, et al. Validation and extension of the EBMT risk score for patients with chronic myeloid leukaemia (CML) receiving allogeneic haematopoietic stem cell transplants. Br J Haematol. 2004;125:613–20. doi: 10.1111/j.1365-2141.2004.04955.x. [DOI] [PubMed] [Google Scholar]
- 72.Innes AJ, Apperley JF. Chronic myeloid leukemia-transplantation in the tyrosine kinase era. Hematol Oncol Clin North Am. 2014;28:1037–53. doi: 10.1016/j.hoc.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 73.Osman AEG, Deininger MW. Chronic Myeloid Leukemia: Modern therapies, current challenges and future directions. Blood Rev. 2021;49:100825.. doi: 10.1016/j.blre.2021.100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chhabra S, Ahn KW, Hu ZH, Jain S, Assal A, Cerny J, et al. Myeloablative vs reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chronic myeloid leukemia. Blood Adv. 2018;2:2922–36. doi: 10.1182/bloodadvances.2018024844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu B, Lin X, Lee HC, Huang X, Tidwell RSS, Ahn KW, et al. Timing of allogeneic hematopoietic cell transplantation (alloHCT) for chronic myeloid leukemia (CML) patients. Leuk Lymphoma. 2020;61:2811–20. doi: 10.1080/10428194.2020.1783444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Copelan EA, Avalos BR, Ahn KW, Zhu X, Gale RP, Grunwald MR, et al. Comparison of outcomes of allogeneic transplantation for chronic myeloid leukemia with cyclophosphamide in combination with intravenous busulfan, oral busulfan, or total body irradiation. Biol Blood Marrow Transpl. 2015;21:552–8. doi: 10.1016/j.bbmt.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Craddock CF. We do still transplant CML, don’t we? Hematol Am Soc Hematol Educ Program. 2018;2018:177–84. doi: 10.1182/asheducation-2018.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamilton A, Helgason GV, Schemionek M, Zhang B, Myssina S, Allan EK, et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood. 2012;119:1501–10. doi: 10.1182/blood-2010-12-326843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yeung DT, Tang C, Vidovic L, White DL, Branford S, Hughes TP, et al. KIR2DL5B genotype predicts outcomes in CML patients treated with response-directed sequential imatinib/nilotinib strategy. Blood. 2015;126:2720–3. doi: 10.1182/blood-2015-07-655589. [DOI] [PubMed] [Google Scholar]
- 80.Branford S, Wang P, Yeung DT, Thomson D, Purins A, Wadham C, et al. Integrative genomic analysis reveals cancer-associated mutations at diagnosis of CML in patients with high-risk disease. Blood. 2018;132:948–61. doi: 10.1182/blood-2018-02-832253. [DOI] [PubMed] [Google Scholar]
- 81.Radich J, Larson R, Kantajian HM, Deininger M, Pinilla-Ibarz J, De Angelo D, et al. Gene expression signature predicts deep molecular response (DMR) in chronic myeloid leukemia (CML): an exploratory biomarker analysis from ENESTnd. Blood. 2019;134:665. doi: 10.1182/blood-2019-124716. [DOI] [Google Scholar]
- 82.Branford S, Kim DDH, Apperley JF, Eide CA, Mustjoki S, Ong ST, et al. Laying the foundation for genomically-based risk assessment in chronic myeloid leukemia. Leukemia. 2019;33:1835–50. doi: 10.1038/s41375-019-0512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Branford S. Why is it critical to achieve a deep molecular response in chronic myeloid leukemia? Haematologica. 2020;105:2730–7. doi: 10.3324/haematol.2019.240739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mughal TI, Psaila B, DeAngelo DJ, Saglio G, Van Etten RA, Radich JP. Interrogating the molecular genetics of chronic myeloproliferative malignancies for personalized management in 2021. Haematologica. 2021;106:1787–93. doi: 10.3324/haematol.2020.267252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barosi G, Gale RP. Is there expert consensus on expert consensus? Bone Marrow Transpl. 2018;53:1055–60. doi: 10.1038/s41409-018-0128-2. [DOI] [PubMed] [Google Scholar]
- 86.Gale RP, Barosi G. Transplant indications, guidelines and recommendations: Caveat Emptor. Bone Marrow Transpl. 2021 doi: 10.1038/s41409-021-01510-8. [DOI] [PubMed] [Google Scholar]
- 87.Gale RP, Phillips GL, 2nd, Lazarus HM. New cancer therapies. Are haematopoietic cell transplants a dead duck? Bone Marrow Transpl. 2021;56:1086–9. doi: 10.1038/s41409-020-01151-3. [DOI] [PubMed] [Google Scholar]
- 88.de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26:3358–63. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- 89.Castagnetti F, Gugliotta G, Breccia M, Stagno F, Iurlo A, Albano F, et al. Long-term outcome of chronic myeloid leukemia patients treated frontline with imatinib. Leukemia. 2015;29:1823–31. doi: 10.1038/leu.2015.152. [DOI] [PubMed] [Google Scholar]
- 90.Geelen IGP, Thielen N, Janssen J, Hoogendoorn M, Roosma TJA, Willemsen SP, et al. Treatment outcome in a population-based, ‘real-world’ cohort of patients with chronic myeloid leukemia. Haematologica. 2017;102:1842–9. doi: 10.3324/haematol.2017.174953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gugliotta G, Castagnetti F, Breccia M, Iurlo A, D’Adda M, Stagno F, et al. Outcome of 472 chronic myeloid leukemia patients treated with frontline nilotinib: A gimema CML WP analysis. Blood. 2018;132:458. doi: 10.1182/blood-2018-99-119182.. [DOI] [Google Scholar]
- 92.Gugliotta G, Castagnetti F, Breccia M, Levato L, Intermesoli T, D’Adda M, et al. Ten-year follow-up of patients with chronic myeloid leukemia treated with nilotinib in first-line: final results of the gimema CML 0307 trial. Blood. 2019;134:4145. doi: 10.1182/blood-2019-126163. [DOI] [Google Scholar]
- 93.Masarova L, Cortes JE, Patel KP, O’Brien S, Nogueras-Gonzalez GM, Konopleva M, et al. Long-term results of a phase 2 trial of nilotinib 400 mg twice daily in newly diagnosed patients with chronic-phase chronic myeloid leukemia. Cancer. 2020;126:1448–59. doi: 10.1002/cncr.32623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castagnetti F, Di Raimondo F, Vivo A, Spitaleri A, Gugliotta G, Fabbiano F, et al. A population-based study of chronic myeloid leukemia patients treated with imatinib in first line. Am J Hematol. 2017;92:82–7. doi: 10.1002/ajh.24591. [DOI] [PubMed] [Google Scholar]
- 95.Kalmanti L, Saussele S, Lauseker M, Proetel U, Muller MC, Hanfstein B, et al. Younger patients with chronic myeloid leukemia do well in spite of poor prognostic indicators: results from the randomized CML study IV. Ann Hematol. 2014;93:71–80. doi: 10.1007/s00277-013-1937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pfirrmann M, Baccarani M, Saussele S, Guilhot J, Cervantes F, Ossenkoppele G, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30:48–56. doi: 10.1038/leu.2015.261. [DOI] [PubMed] [Google Scholar]
- 97.Millot F, Esperou H, Bordigoni P, Dalle JH, Michallet M, Michel G, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia in childhood: a report from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC) Bone Marrow Transpl. 2003;32:993–9. doi: 10.1038/sj.bmt.1704255. [DOI] [PubMed] [Google Scholar]
- 98.Cwynarski K, Roberts IA, Iacobelli S, Biezen A, Brand R, Devergie A, et al. Stem cell transplantation for chronic myeloid leukemia in children. Blood. 2003;102:1224–31. doi: 10.1182/blood-2002-12-3637. [DOI] [PubMed] [Google Scholar]
- 99.Arora M, Weisdorf DJ, Spellman SR, Haagenson MD, Klein JP, Hurley CK, et al. HLA-identical sibling compared with 8/8 matched and mismatched unrelated donor bone marrow transplant for chronic phase chronic myeloid leukemia. J Clin Oncol. 2009;27:1644–52. doi: 10.1200/JCO.2008.18.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Radich JP, Gooley T, Bensinger W, Chauncey T, Clift R, Flowers M, et al. HLA-matched related hematopoietic cell transplantation for chronic-phase CML using a targeted busulfan and cyclophosphamide preparative regimen. Blood. 2003;102:31–5. doi: 10.1182/blood-2002-08-2619. [DOI] [PubMed] [Google Scholar]
- 101.Ohashi K, Nagamura-Inoue T, Nagamura F, Tojo A, Miyamura K, Mori T, et al. Effect of graft sources on allogeneic hematopoietic stem cell transplantation outcome in adults with chronic myeloid leukemia in the era of tyrosine kinase inhibitors: a Japanese Society of Hematopoietic Cell Transplantation retrospective analysis. Int J Hematol. 2014;100:296–306. doi: 10.1007/s12185-014-1632-9. [DOI] [PubMed] [Google Scholar]
- 102.Chaudhury S, Sparapani R, Hu ZH,, Nishihori T, Abdel-Azim H, Malone A, et al. Outcomes of allogeneic hematopoietic cell transplantation in children and young adults with chronic myeloid leukemia: A CIBMTR cohort analysis. Biol Blood Marrow Transpl. 2016;22:1056–64. doi: 10.1016/j.bbmt.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee SE, Choi SY, Kim SH, Jang EJ, Bang JH, Byeun JY, et al. Prognostic factors for outcomes of allogeneic stem cell transplantation in chronic phase chronic myeloid leukemia in the era of tyrosine kinase inhibitors. Hematology. 2014;19:63–72. doi: 10.1179/1607845413Y.0000000100. [DOI] [PubMed] [Google Scholar]
- 104.Koenecke C, Heim D, van Biezen A, Heuser M, Aljurf M, Kyrcz-Krzemien S, et al. Outcome of patients with chronic myeloid leukemia and a low-risk score: allogeneic hematopoietic stem cell transplantation in the era of targeted therapy. A report from the EBMT. Bone Marrow Transpl. 2016;51:1259–61. doi: 10.1038/bmt.2016.97. [DOI] [PubMed] [Google Scholar]
- 105.Saussele S, Lauseker M, Gratwohl A, Beelen DW, Bunjes D, Schwerdtfeger R, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. 2010;115:1880–5. doi: 10.1182/blood-2009-08-237115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.