Abstract
Purpose
Acquired brain injury causing spasticity, pain and loss of function is a major cause of disability and lower quality of life. Sacral 1 (S1) neurectomy claims promising outcomes in spastic hemiparesis. This cadaveric study was conducted to study the surgical anatomy, surgical approach and feasibility of S1 neurectomy and contralateral S1 (cS1) transfer.
Methods
This study was conducted over a period of 10 months and 10 cadavers (age 18–60 years, 7 male and 3 female) were included in the study. 2 cadavers underwent endoscopic S1 neurectomy and 8 cadavers underwent open S1 neurectomy. Mean S1 root length and diameter were recorded using Schirmer tear strips and Vernier calliper. Feasibility of transfer was also assessed by measuring the length of donor nerve and distance between distal ends to proximal end of recipient nerve.
Results
Mean thickness of right S1 root was 4.02 ± 1.5 mm and left S1 was 3.89 ± 1.18 mm. Mean length of right S1 root was 24.9 ± 4.56 mm and left S1 was 23.6 ± 2.86 mm. Endoscopically dissected length of S1 was much less as compared to open technique.
Conclusion
S1 neurectomy is simple procedure to reduce spasticity in lower limb without any permanent deficit. It can be done by open as well as with endoscopic approach while for contralateral S1 transfer open approach need to be used.
Keywords: S1 neurectomy, Spastic hemiparesis, Cadaveric, cS1transfer
1. Introduction
Lower limb spasticity due to brain insult causes pain, loss of function leads to disability and lower quality of life.1 About 30% patients develop spasticity few weeks after the brain insult. For upper limb spasticity C7 neurectomy has been advocated to reduce spasticity and same concept can be applied for reduction of spasticity in lower limb by performing S1 neurectomy.2 Though there are some experimental studies where S1 neurectomy with contralateral S1 transfer claims promising outcomes3 The main results of these studies showed that S1 root does not contribute to the any specific motor action and after sacrifice of S1 roots, neighbouring roots can compensate for the lost functions. S1 nerve transection results in temporary weakness of ankle plantar flexion which recovers gradually
However; there is paucity of human studies assessing the feasibility of S1 neurectomy and cS1 transfer. This cadaveric study was conducted to study the surgical anatomy, surgical approach and feasibility of S1 neurectomy and contralateral S1 transfer to serve as a basis for future endeavours.
2. Materials and methods
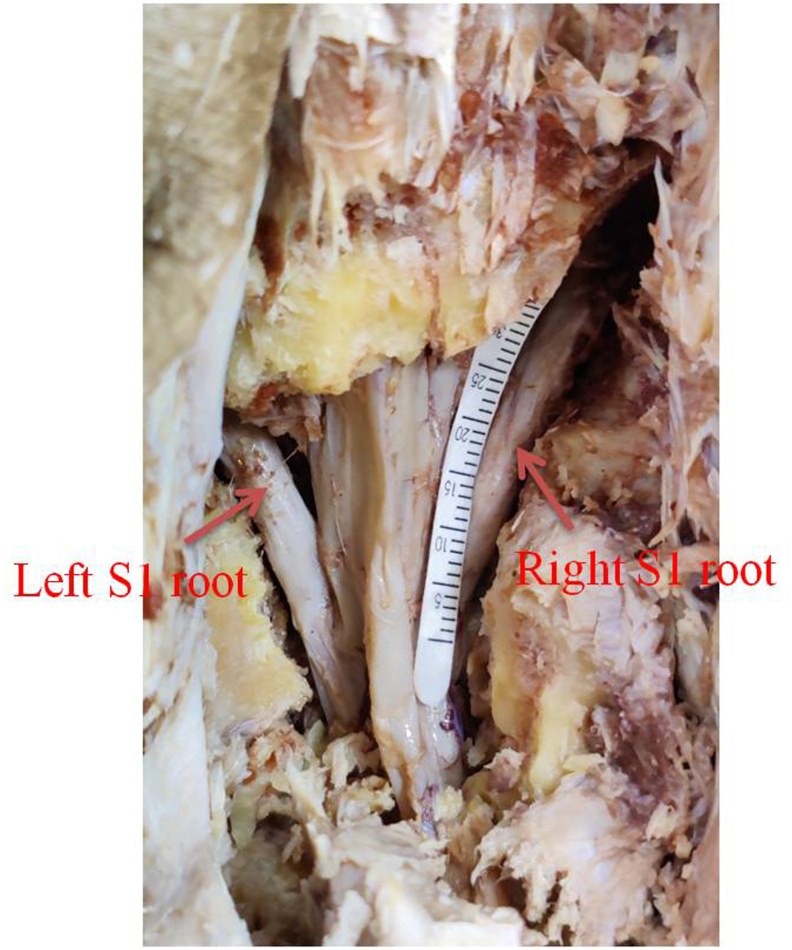
This study was conducted in the Plastic Surgery Unit, Department of surgery and Department of Anatomy in a tertiary referral centre over a period of 10 months from January 2020 to October 2020. Before starting the study Institutional Ethical Committee approval was obtained. 10 cadavers (age 18–60 years, 7 male and 3 female) were included in the study. Cadavers with pelvic injury, lumbosacral plexus injury, scar mark over back, history of any back trauma or surgery were excluded. 2 cadavers underwent endoscopic S1 neurectomy using Karl Storz Easy Go spinal endoscopic system and 8 cadavers underwent open S1 neurectomy. Mean S1 root length and diameter were recorded using Schirmer tear strips and Vernier calliper. (Fig. 1) Feasibility of cS1 transfer was also assessed by measuring the distance between distal ends of donor nerve to proximal end of recipient nerve.
Fig. 1.
Right S1 root length being measured with Schirmer test strip.
Apart from this cadaveric study the thorough literature search was also performed to look for the relevant articles in Pub Med, Cochrane database and Google scholar. All experimental/clinical studies reporting results of cS1 nerve transection and transfer for lumbosacral plexus injury, donor site morbidity, and clinical improvement in lower limb were included. Two authors (PA, DS) independently analyzed the studies and conclusions were drawn.
3. Operative technique –
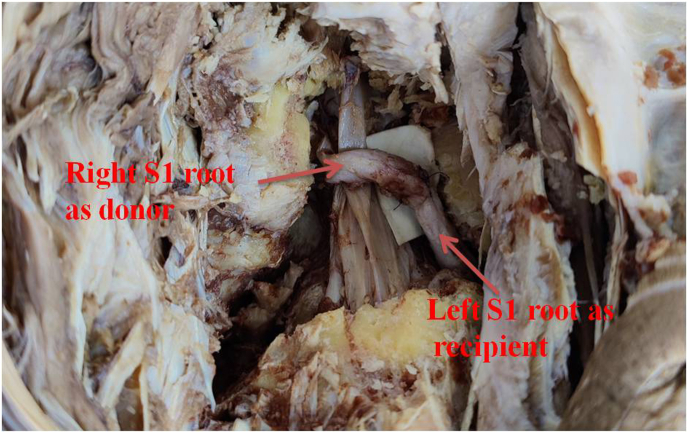
Open Approach- Cadaver was placed in prone position and L5-S1 localization was done by manual palpation. Midline vertical incision was given over L5-S1 region and lumbar fascia was incised in midline. Bilateral muscles were dissected subperiosteally and bilateral laminectomy was done at L5-S1. Ligamentum flavum was excised and medial facetectomy of superior facet of S1 was next done on both sides. S1 root was dissected from the dural sleeve up to the facet joint on both sides and its length and thickness were measured. If extended length of S1 root is required, it was done by removal of bilateral inferior facet of L5 and superior facet of S1 (remaining lateral portion). S1 root of one side was transposed to opposite side and cS1 to S1 anastomosis was done using monofilament polyamide 8-0 suture. (Fig. 2)
Fig. 2.
cS1 transfer performed using Right S1 as donor root and Lt S1 as recipient.
Endoscopic Approach- Cadaver was placed in prone position and L5-S1 localization was done by manual palpation. Left paramedian vertical incision was given over L5-S1 region and Lumbar fascia was incised in midline. Muscles were dissected subperiosteally and Karl-Storz Easy Go endoscopic system was introduced. Under vision bilateral Laminectomy of L5-S1 was done with undercutting of the spinous process. Ligamentum flavum was excised with medial facetectomy of superior facet of S1 on both sides. S1 root was dissected from the dural sleeve up to the facet joint on both sides and length and thickness were measured using Schirmer test strip and Vernier caliper.
4. Results -
Mean thickness of right S1 root was 4.02 ± 1.5 mm (male - 4.286 ± 1.49 mm, female - 3.4 ± 0.4 mm) and left S1 was 3.89 ± 1.183 mm (male - 4.014 ± 1.329 mm, female - 3.6 ± 0.53 mm). Mean length of right S1 root was 24.9 ± 4.565 mm (male −25.428 ± 4.6 mm, female −23.667 ± 4.163 mm) and left S1 was 23.6 ± 2.86 mm (male −23.286 ± 2.99 mm, female −24.33 ± 2.31 mm). There was no significant difference between S1 length on right and left side (p −0.672, 0.486, respectively) and male and female cadavers. (p- 0.148, 0.065 respectively) However, right S1 thickness was significantly higher in males as compared to females. (p- 0.020) (Table 1)
Table 1.
S1root length/thickness in males and females.
| Right S1 root in mm |
Left S1 root in mm |
|||
|---|---|---|---|---|
| Length | Thickness | Length | Thickness | |
| Male | 25.43 ± 4.60 | 4.28 ± 1.49 | 23.28 ± 2.99 | 4.01 ± 1.33 |
| Female | 23.66 ± 4.16 | 3.4 ± 0.4 | 24.33 ± 2.30 | 3.6 ± 0.53 |
| Mean | 24.9 ± 4.56 | 4.02 ± 1.5 | 23.6 ± 2.86 | 3.89 ± 1.18 |
Endoscopically dissected length of right S1 was 7.5 ± 1.4 mm while left S1 was 10.5 ± 1.4 mm and mean length was 9 ± 1.82 mm. Endoscopically dissected S1 length was significantly lower as compared to open approach (p = 0.014)
The extended length of S1 can be obtained by removing inferior facet of L5 and superior facet of S1 and 42.37% and 40.16% additional length was obtained on left and right side respectively. Extended length of right S1 was 33.6 ± 2.86 mm and left S1 was 34.9 ± 4.56 mm.
Mean operative time for S1 neurectomy by endoscopic approach was 92 min ± 8 and for open approach was 28 ± 5 min. Mean time required to perform S1 to cS1 anastomosis was 17 ± 2 min.
5. Discussion
Our cadaveric study measuring the thickness and length of S1 nerve roots shows the anatomical feasibility of S1 neurectomy and contralateral S1 transfer advocated for reducing the incapacitating spasticity after hemiparesis.
The neurological basis of spasticity is disconnection of negative feedback loop between the upper motor neuron and g-neuron circuit leading to uninhibited g-neuron circuit.4 Incapacitating spasticity can lead to pain, diminished joint mobility, decreased muscle flexibility, deformities and contractures and cause significant difficulty in daily activities and reduction in quality of life.5 Different medical and surgical modalities have been tried for treatment of spasticity; however, disappointing and conflicting results have been reported and optimal treatment remains elusive. The goal is improvement in function or in comfort by reducing the degree of spasticity by interruption of the stretch reflex at various points in the peripheral nervous system, or decreasing the degree of excitation of hypertonic circuits in the central nervous system. Caution and precise technique is needed, particularly with ablative techniques, because of the potential for increasing sensory and motor neurological deficits.
C7 neurectomy and contralateral C7 root transfer have been advocated for upper limb spasticity as transection of the affected C7 interrupts the g-neuron circuit and lessens flexor spasticity.2,6 Similarly; S1 neurectomy reduces the lower limb spasticity. Formation of brachial and lumbosacral plexuses are similar and C7 and S1 roots form the central root of each plexus with considerable cross-innervation with upper and lower roots. Therefore, after sacrifice of C7 and S1 roots, neighbouring roots take over their respective functions.7,8 S1 neurectomy is safe because S1 root does not contribute to the any specific motor action that is controlled solely by S1. S1 mainly innervates the peroneus longus, gluteus medius/maximus, biceps femoris, medial/lateral gastrocnemius and extensor digitorum brevis. However, innervation by S1 to each muscle is not its sole supply but is supplemented by L4, L5, S2 and S3.9 When S1 root is injured; fibres from the L4, L5, S2 and S3 nerve roots can regenerate and re-innervate the affected muscles. The highest percentage supplied by S1 is 51.38% to the lateral gastrocnemius.3 S1 nerve transection results in temporary weakness of ankle plantar flexion, possible weakness of toe flexion, loss of sensation on the lateral edge of the foot and loss of Ankle reflex. However, both sensory and motor functions recover clinically and electro-physiologically within 1 year.10 Another advantage of cS1 as a suitable donor are the diameter of ventral root of S1 which is 2.95 ± 0.57 mm; which is quite suitable for anastomosis as it contains large number (4154 ± 3036) of nerve fibres providing a good source of axons.11
These results have been seen in both animal experiments and in human patients, confirming the neurological basis of S1 neurectomy.1,3,12, 13, 14, 15 cS1 transfer has been shown to improve motor power in patients with traumatic lumbosacral plexus injury.3 In clinical studies cS1 has been transferred to inferior gluteal nerve and the branch of the sciatic nerve innervating the hamstrings; with M3 motor function of hamstrings and the glutei so patients could stand and walk without support.16,17 Reinnervation of the muscles was demonstrated by electromyography and there was no residual deficit in function in contralateral donor limb. Half cS1 root transfer has been done in order to reduce donor site complications; however, transfer of the entire root is shown to achieve significantly better recovery.18, 19, 20 Functional improvement after neurectomy may be due to the immediate reduction of spasticity with effect augmented by physical therapy.21 However, to improve the motor function further cS1 transfer has been advocated; which improves the interconnection between two halves of the brain through corpus callosum.6 Patient selection for this procedure is important as presence of lower limb spasticity may be useful for compensating for loss of motor power. Patients with extreme spasticity (rigidity) not responding to conservative treatment, causing pain and contractures/deformities may be ideal candidates for this procedure.
This is the first cadaveric study which shows the anatomical feasibility and surgical approach of S1 neurectomy and cS1 transfer. The main outcome measures of the study include: Mean thickness of right S1 root was 4.02 mm and left S1 was 3.89 mm. Mean length of right S1 root was 24.9 mm and left S1 was 23.6 mm. There was no significant difference between S1 length on right and left side and male and female cadavers.
Endoscopically dissected length of right S1 was 7.5 mm while left S1 was 10.5 mm and mean length was 9 mm. Endoscopically dissected S1 length was significantly lower as compared to open approach. The extended length of S1 can be obtained by removing inferior facet of L5 and superior facet of S1 and 42.37% and 40.16% additional length was obtained on left and right side respectively. Extended length of right S1 was 33.6 mm and left S1 was 34.9 mm.
Mean operative time for S1 neurectomy by endoscopic approach was 92 min and for open approach was 28 min. Mean time required to perform S1 to cS1 anastomosis was 17 min.
Our study found that both endoscopic as well as open approaches are suitable for performing S1 neurectomy however if we want to do cS1 transfer then open approach need to be used. For cS1, the average S1 length obtained by open approach was greater (24.25 mm in open Vs 9 mm in endoscopy) than the average transverse distance between the first anterior sacral foramina (30.48 mm)22 If required, further length can be obtained by dividing the donor root as distally as possible while recipient root can be divided as proximally as possible. Additional length (34.25 mm) can be obtained by removing inferior facet of L5 vertebra and superior facet of S1 vertebra to perform a tension free anastomosis.
We have shown the anatomical feasibility of S1 neurectomy and cS1 transfer. The clinical implications of this study is that patients with brain injury S1 neurectomy reduces the spasticity and cS1 transfer help in improving function in lower extremity. cS1 transfer to inferior gluteal nerve and the branch of the sciatic nerve can also be used to improve motor power in patients with lumbosacral plexus avulsion injury so patients could stand and walk without support.
Limitations of the study include cadaveric study and the actual outcome was not measured in vivo. In cadavers there are significant changes in the biophysical properties of tissues, making an ex vitro to vivo comparison complex.
6. Conclusion
S1 neurectomy is simple procedure to reduce spasticity in lower limb without any permanent deficit. It can be done by open as well as with endoscopic approach while for contralateral S1 transfer open approach need to be used. cS1 transfer can also be used to improve motor power in patients with lumbosacral plexus avulsion injury. More human studies are needed in future to verify the clinical benefits of S1 neurectomy with or without cS1 transfer.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Ethical approval
Ethical approval for this study was granted by the research and ethics committee at the study centre. All procedures performed in studies involving cadavers were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.Informed consent
Not required for this work.
Author contributions
PA: Study design, Data acquisition, Manuscript preparation SN- Data acquisition, Manuscript preparation, NA: Study design, Data acquisition. JB- Data acquisition, Manuscript preparation VP- Data acquisition, Manuscript preparation YRY- Data acquisition, Manuscript preparationDS- Study design, Data acquisition, Manuscript preparationAll authors read and approved the final version of the manuscript.
ICMJE conflict of interest statement
None declared.
References
- 1.Zong Haiyang, Ma Fenfen, Zhang Laiyin, et al. Hindlimb spasticity after unilateral motor cortex lesion in rats is reduced by contralateral nerve transfer. Biosci Rep. 2016 doi: 10.1042/BSR20160412. Portland press limited. 36/art:e 00430/doi10.1042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mou Xianong, Zheng, et al. Trial of contralateral seventh cranial nerve transfer for spastic arm paralysis. N Engl J Med. 2017;20:72–74. doi: 10.1056/NEJMoa1615208. [DOI] [PubMed] [Google Scholar]
- 3.Zhu L., Zhang F., Yang D., Chen A. The effect of severing a normal s1 nerve root to use for reconstruction of an avulsed contralateral lumbosacral plexus. The bone joint J. 2014:358–365. doi: 10.1302/0301-620X.97B3.34330. [DOI] [PubMed] [Google Scholar]
- 4.Bishop B. Spasticity: its physiology and management. Part II. Neurophysiology of spasticity: current concepts. Phys Ther. 1977;57:377–384. doi: 10.1093/ptj/57.4.377. [DOI] [PubMed] [Google Scholar]
- 5.Kischka U. Neurological rehabilitation and management of spasticity. Medicine. 2008;36:616–619. [Google Scholar]
- 6.Zheng M.X., Hua X.Y., Feng J.T., et al. Trial of contralateral seventh cervical nerve transfer for spastic arm paralysis. N Engl J Med. 2018;378:22–34. doi: 10.1056/NEJMoa1615208. [DOI] [PubMed] [Google Scholar]
- 7.Lin H., Xu Z., Liu Y., Chen A., Hou C. The effect of severing L6 nerve root of the sacral plexus on lower extremity function: an experimental study in rhesus monkeys. Neurosurgery. 2012;70:170–177. doi: 10.1227/NEU.0b013e31822c4b39. [DOI] [PubMed] [Google Scholar]
- 8.Gu Y.D., Zhang G.M., Chen D.S., Yan J.G., Cheng X.M., Chen L. Seventh cervical nerve root transfer from the contralateral healthy side for treatment of brachial plexus root avulsion. J Hand Surg. 1992;17:518–521. doi: 10.1016/s0266-7681(05)80235-9. [DOI] [PubMed] [Google Scholar]
- 9.Phillips L.H., 2nd, Park T.S. Electrophysiologic mapping of the segmental anatomy of the muscles of the lower extremity. Muscle Nerve. 1991;14:1213–1218. doi: 10.1002/mus.880141213. [DOI] [PubMed] [Google Scholar]
- 10.Gruener G., Dyck P.J. Quantitative sensory testing: methodology, applications, and future directions. J Clin Neurophysiol. 1994;11:568–583. [PubMed] [Google Scholar]
- 11.Liu Y., Zhou X., Ma J., Ge Y., Cao X. The diameters and number of nerve fibres in spinal nerve roots. J Spinal Cord Med. 2015;38:532–537. doi: 10.1179/1079026814Z.000000000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L., Gu Y.D. Lowest number of brachial plexus nerve roots required for maintaining normal limb function—an experimental study. Hand Surg. 2001;6:37–45. doi: 10.1142/s0218810401000412. [DOI] [PubMed] [Google Scholar]
- 13.Lin H., Xu Z., Liu Y., Chen A., Hou C. The effect of severing L6 nerve root of the sacral plexus on lower extremity function: an experimental study in rhesus monkeys. Neurosurgery. 2012;70:170–177. doi: 10.1227/NEU.0b013e31822c4b39. [DOI] [PubMed] [Google Scholar]
- 14.Lin H., Chen A., Hou C. Contralateral L-6 nerve root transfer to repair lumbosacral plexus root avulsion: experimental study in rhesus monkeys. J Neurosurg. 2013;119:714–719. doi: 10.3171/2013.5.JNS121218. [DOI] [PubMed] [Google Scholar]
- 15.Shonnard N., Wakefield C. Reinnervation of the gastrocnemius muscle by the contralateral sl nerve root. Exp Neurol. 1983;80:418–426. doi: 10.1016/0014-4886(83)90293-5. [DOI] [PubMed] [Google Scholar]
- 16.Li Y., Lin H., Zhao L., Chen A. Unaffected contralateral S1 transfer for the treatment of lumbosacral plexus avulsion. Injur y. 2014;45:1015–1018. doi: 10.1016/j.injury.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Zhu L., Zhang F., Yang D., Chen A. The effect of severing a normal S1 nerve root to use for reconstruction of an avulsed contralateral lumbosacral plexus: a pilot study. Bone Joint Lett J. 2015;97-B:358–365. doi: 10.1302/0301-620X.97B3.34330. [DOI] [PubMed] [Google Scholar]
- 18.Yu B.F., Qiu Y.Q., Du M.X., et al. Contralateral hemi-fifth-lumbar nerve transfer for unilateral lower limb dysfunction due to incomplete traumatic spinal cord injury: a report of two cases. Microsurgery. 2020;40:234–240. doi: 10.1002/micr.30470. [DOI] [PubMed] [Google Scholar]
- 19.Gao K.M., Lao J., Guan W.J., Hu J.J. Is it necessary to use the entire root as a donor when transferring contralateral C7 nerve to repair median nerve? Neural Regen Res. 2018;13:94–99. doi: 10.4103/1673-5374.224376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal P. Sharma D. Contralateral sacral 1 root transection and transfer for lumbosacral plexus avulsion injuries: a systemic review. J Orthop, Trauma and Rehabilit. (Under communication).
- 21.Spinner Robert J., shin Alexander Y., Allen T., Bishop Rewiring to regain function in patients with spastic hemiplegia. (Editorial) N Engl J Med. 2017:5–6. doi: 10.1056/NEJMe1713313. [DOI] [PubMed] [Google Scholar]
- 22.Kalantarian B., Rice D.C., Tiangco D.A., Terzis J.K. Gains and losses of the XII-VII component of the “baby-sitter” procedure: a morphometric analysis. J Reconstr Microsurg. 1998;14:459–471. doi: 10.1055/s-2007-1000208. [DOI] [PubMed] [Google Scholar]