Summary
Background
Cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) are innovative small target molecules that, in combination with endocrine therapy, have recently been employed in the treatment of patients with HR+/HER2− metastatic breast cancer (mBC). In this prospective study, we investigate the impact of CDK4/6i on the immune profile of patients with HR+/HER2− mBC.
Methods
Immune cell subsets were analysed using flow cytometry of peripheral blood mononuclear cells (PBMCs) isolated from patients with HR+/HER2− mBC, both before and during treatment. Regulatory T cells (Tregs) were identified using the markers CD4, CD25, CTLA4, CD45RA, and intracellular FOXP3. Monocytic and polymorphonuclear myeloid-derived suppressor cells (M-MDSCs and PMN-MDSCs) and other immune populations were analysed using CD45, CD14, CD66b, CD11c, HLA-DR, CD3, CD8, CD28, CD137, PD1, CD45RA, CCR7, and Ki67.
Findings
The percentage of circulating Tregs and M/PMN-MDSCs was significantly downregulated from baseline during CDK4/6i-treatment (p<0.0001 and p<0.05, respectively). In particular, the effector Treg subset (CD4+CD25+FOXP3highCD45RA−) was strongly reduced (p<0.0001). The decrease in Treg levels was significantly greater in responder patients than in non-responder patients. Conversely, CDK4/6i treatment was associated with increased levels of CD4+ T cells and anti-tumour CD137+CD8+ T cells (p<0.05).
Interpretation
CDK4/6i treatment results in downregulation of Tregs, M-MDSCs, and PMN-MDSCs, thus weakening tumour immunosuppression. This decrease is associated with response to treatment, highlighting the importance of unleashing immunity in cancer treatment efficacy. These results suggest a novel mechanism of immunomodulation in mBC and provide valuable information for the future design of novel treatments combining CDK4/6i with immunotherapy in other cancer settings.
Funding
Sapienza University of Rome.
Keywords: CDK4/6i, Regulatory T cells, Treg, Immune modulation, HR+/HER2- metastatic breast cancer, MDSCs
Abbreviations: CDK4/6i, Cyclin-dependent-kinase 4/6 inhibitors; HR+, Hormone Receptor-positive; mBC, metastatic Breast Cancer; Treg, Regulatory T cells; M-MDSCs, monocytic-myeloid derived suppressor cells; PMN-MDSCs, Polymorphonuclear-myeloid derived suppressor cells; CDKs, cyclin-dependent-kinase; HT, Hormone Therapy; AI, Aromatase Inhibitors; PFS, progressive free survival; DCs, Dendritic Cells; CTLA4, cytotoxic T-lymphocyte antigen-4; HDs, Healthy Donors; PBMCs, Peripheral Blood Mononuclear Cells; R, Responder Patients; SD, Stable Disease Patients; PD, Progression Disease Patients; MVA, multivariate logistic regression analysis
Research in context.
Evidence before this study
CDK4/6 inhibitors (CDK4/6i) have dramatically changed the landscape of treatment in HR+/HER2− metastatic breast cancer (HR+/HER2− mBC). Palbociclib, abemaciclib, and ribociclib, in combination with hormone therapy (HT), have improved survival outcomes and quality of life for patients with mBC and can also delay the use of chemotherapy in this setting. The on-target effect of these drugs is inhibition of the kinase activity of CDK4/6 that arrests progression through the G1/S phase of the cell cycle, potentially causing apoptosis or permanent arrest in the G0 phase. Preclinical studies have demonstrated that CDK4/6 inhibition in mouse models can selectively reduce regulatory T cells (Tregs), which seem to preferentially utilise CDK4/6 to progress through the cell cycle. Tregs are a CD4+ T cell subpopulation that induce immunological tolerance and suppress the immune response. In the tumour microenvironment, Tregs are involved in tumour development and progression, and high Treg levels are associated with poor survival in several cancer settings.
Added value of this study
Our aim was to investigate the effect of CDK4/6i on the immune system of patients with HR+/HER2− mBC. We evaluated the immune profile of patients with mBC from baseline until the first clinical evaluation by analysing circulating myeloid-derived suppressor cells (MDSCs) and distinct T cell subsets. We demonstrated for the first time that CDK4/6i treatment led to an early reduction in the Treg population from baseline, whereas CD4+ T cells increased. Moreover, we found that CDK4/6i also impacts other immunosuppressive cells such as MDSCs. Most importantly, we showed that a significant reduction in circulating Tregs was associated with an objective response to treatment. In fact, only responder patients had a significant reduction in circulating Tregs compared to patients with stable or progressive disease.
Implications of all the available evidence
To the best of our knowledge, our study provides the first evidence for the impact of CDK4/6i on the immune system of patients with HR+/HER2− mBC by strongly down-modulating immunosuppressive cell subsets. CDK4/6i-induced Treg suppression was associated with objective response, and this finding will be evaluated as a predictive biomarker in larger prospective trials. Finally, these results make a strong argument for the use of CDK4/6i in other tumours with a strong immunosuppressive environment, with the objective of selectively targeting immunosuppression and preparing the patient for successful immunotherapy.
Alt-text: Unlabelled box
Introduction
Breast cancer (BC) is one of the most common tumours in women worldwide.1 Among the various subgroups of advanced BC, hormone receptor-positive (HR+)/human epidermal growth factor receptor 2 negative (HER2−) metastatic BC (HR+/HER2− mBC) is the most common.2 Dysregulation of the cell cycle is a key hallmark of cancer cells,3 making it a rational and important target for the new era of targeted therapy in anticancer treatment. The mammalian cell cycle is a highly regulated pathway driven by a group of proteins called cyclins, which bind and activate cyclin-dependent kinases (CDKs). CDKs 2, 4, and 6 (CDK2/4/6) are essential drivers of the cell cycle. Cyclin E-CDK2 and cyclin D-CDK4/6 promote progression from the G1-phase to the S-phase by triggering phosphorylation of several intracellular transduction cascades. The most important event is Rb protein hyperphosphorylation, which releases the E2F transcription factor, promoting progression through the cell cycle.4
Clinical trials led to the approval of CDK4/6 inhibitors (CDK4/6i; ribociclib, abemaciclib, and palbociclib) in combination with endocrine therapy (ET) by the Food and Drug Administration (FDA) and European Medicines Agency (EMA), after showing a significant improvement in progression-free survival (PFS) in first- or second-line treatment of both premenopausal and postmenopausal patients with HR+/HER2− mBC.5,6 Recently, all three clinically available CDK4/6i demonstrated a benefit in overall survival (OS) in combination with fulvestrant in aromatase inhibitor (AI)-resistant patients.7, 8, 9
Evidence of the physiological roles of CDK4/6 in immune cells has been demonstrated in vivo in mouse models. Preclinical studies have shown a critical role for these kinases in T-cell proliferation and differentiation. In particular, CDK4/6 inhibition led to the preferential effect of depleting regulatory T cells while promoting cell differentiation.10
Regulatory T cells (Tregs) are a CD4+ T cell subpopulation that enforce immunological tolerance by suppressing the immune response and maintaining immunological homeostasis. They are characterised by high expression of the CD25 receptor, which binds to the cytokine IL-2, controlling T cell homeostasis and dysregulation. The FOXP3 transcription factor is a Treg-specific marker that functions as a key regulator of immune tolerance and modulates T cell differentiation. In the tumour microenvironment, Tregs are involved in tumour development and progression. They cooperate with tumour cells by suppressing anti-cancer immune responses through several mechanisms, such as inhibiting dendritic cell (DC)-derived costimulatory CD80/CD86 signals via cytotoxic T-lymphocyte antigen-4 (CTLA4),11 IL-2 consumption by high CD25 expression, and direct killing of effector T cells.12,13
From a clinical point of view, a high concentration of Treg cells in the tumour microenvironment is associated with poor survival in several cancers.14,15 Thus, better comprehension of the Treg subsets and the development of specific therapeutic strategies to target them are envisaged to improve anti-cancer immunotherapy.
The aim of this study was to investigate whether CDK4/6i treatment affects the immune system of patients with mBC. We analysed Treg and other immune cell subsets before (T0) and within the first six months of treatment (>T0) and assessed their association with clinical response. Moreover, modulation of myeloid-derived suppressor cells (MDSCs) was analysed in a subset of patients. These results suggest that CDK4/6i can modulate the immune system, affecting the immunosuppressive Treg and MDSC subpopulations, while promoting the activated T cells.
Materials and methods
Patients’ characteristics
From September 2019 to May 2021, 50 consecutive women with HR+/HER2− mBC were enrolled in this prospective monocentric study. Clinical staging was performed at baseline using contrast-enhanced computed tomography (CT) and, if clinically indicated, magnetic resonance imaging (MRI) or positron emission tomography (PET). All patients were treated with a CDK4/6i agent, palbociclib, ribociclib, or abemaciclib, in combination with a hormone therapy (HT), aromatase inhibitor (AI) or fulvestrant, according to the respective approved schedule until unacceptable toxicities or disease progression was observed. Endocrine sensitivity and resistance were defined following ESMO guidelines.16 Patients with primary or secondary endocrine resistance were treated with CDK4/6i plus fulvestrant, while others received first-line CDK4/6i plus AI as per clinical practice. Pre- or peri-menopausal women received a gonadotropin-releasing hormone agonist (leuprorelin or triptorelin). Patients received at least one month of treatment before being considered for statistical evaluation. Baseline conditions were defined using the ECOG Performance Status (PS). Response evaluation was performed every 12-16 weeks or as clinically indicated and defined according to response evaluation criteria in solid tumours (RECIST 1.1)17 and PET response criteria in solid tumours (PERCIST),18 if needed. All patients had reached at least the first radiological evaluation. The ‘best response’ was defined as the best radiological objective response (OR) according to RECIST 1.1 criteria, obtained from the patients during the treatment. An OR was defined as the presence of complete or partial response (CR or PR), and patients who obtained an OR were classified as responders (R). Those who showed a worsening according to the criteria were classified as having progressive disease (PD), while those who did not fall into these categories were classified as having stable disease (SD). Written informed consent was obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki.
Peripheral blood sample collection and isolation of Peripheral Blood Mononuclear Cells (PBMCs)
Peripheral blood samples were collected from 20 healthy donors (HDs) and 50 female patients with mBC at baseline, before the first administration of CDK4/6i (defined as T0) and during treatment (defined as >T0). The first on-treatment blood sample (>T0) was collected after the first 28-day treatment cycle for 41 patients (82%). However, due to difficulties during the COVID-19 pandemic, for nine patients the blood samples were taken any way within six months from baseline; therefore, all >T0 samples were collected before the first radiological evaluation. Blood samples were processed within 1 h of blood sampling. Every patient signed a specific informed consent form, and clinical data were collected from the medical reports and transferred to an anonymous database for statistical processing.
Peripheral blood samples of mBC patients and healthy donors were collected using ethylenediaminetetraacetic acid (EDTA) anticoagulated vacutainers (BD Biosciences). Before separation, blood was mixed with the same volume of PBS and then overlaid on a Ficoll-Hypaque gradient (1,077 g/mL; Cedarlane, Cat CL5020). Density centrifugation was performed at room temperature at 1800 rpm for 30 min with no brake. Peripheral blood mononuclear cells (PBMCs) were collected and stored at -196°C (1 × 107 cells in 10% DMSO + 90% FCS) or were freshly analysed in case of MDSCs.
Immune phenotypic profiles
The following immune cell subsets were analysed using flow cytometry; T-cell subsets: anti-CD3-BV510 (BD, Cat: 564713, clone HIT3a), CD8-APC-H7 (BD, Cat: 560179, clone SK1), CD45RA-BB515 (BD, Cat: 564552, clone HI100), CCR7-PE (BioLegend, Cat: 353204, clone G043H7), CD28-PeCy7 (BD, Cat: 560684, clone CD28.2), CD137 (4-1BB)-APC (BD, Cat: 550890clone 4B4-1), PD1-BB700 (BD, Cat: 566460, clone EH12.1), intracellular Ki67-BV420 (BD, Cat. 562899, clone B56); Treg cells: anti–CD4-APC-H7 (BD, Cat: 560158, clone RPA-T4); CD25-PE (BioLegend, Cat: 356134, M-A251), CD45RA-BB15 (BD, Cat: 564552, clone HI100), CTLA-4-PerCp-Cy5.5 (BD, Cat: 561717, BNI3); FOXP3OX-APC (Thermo Fisher Scientific, Cat: 17-4776-42; PCH101); MDSCs and DC CD14-BB700 (Biolegend, Cat: 566465, MΦP9); CD66b-PeCy7 (BioLegend, cat: 305116, G101F5); HLA-DR-FITCH (BioLegend, Cat: 307604, L243); CD45-AF700 (BioLegend, Cat: 368514 2D1); CD11c-BV421 (BioLegend, Cat: 301628, 3.9). Cell autofluorescence and fluorescence minus one (FMO) were used as negative controls. Flow cytometric acquisition was performed using a FACSCantoII flow cytometer running FACS Diva data acquisition. FACS DIVA analysis software (version 8.0.2, BD Biosciences) and FlowJo (version 10.8.8, BD) were used to analyse the data.
Gating strategy
To characterise the different T cell subpopulations, lymphocytes were first identified on FSC-A and SSC-A and then selected for the expression of the CD3 marker.
CD3 naïve T cells were gated through the double positive expression of the CD45RA and CCR7 markers and then analysed for CD4 and CD8 expression.
To analyse the CD28+ subpopulation, CD3+ T cells were evaluated for the expression of CD28 markers. CD3+CD28+ T cells were positively selected for the expression of CD45RA and CCR7 markers to identify CD3+CD28+ naïve T cells, which were subsequently characterised for CD4 and CD8 markers. A similar gating strategy was used to select the CD3+CD137+ T cell subpopulation, which was then evaluated for the CD4 and CD8 T cell subgroups. The gating strategy for Tregs and MDSCs is explained in Supplementary Figure 5.
Statistical analysis
Data are expressed as the average and standard error of the mean (SEM). The Shapiro-Wilk test was used to study the normalisation of groups. The Wilcoxon matched-pairs signed-rank test and paired t-test were used to analyse the mBC group and compare T0 to >T0, using nonparametric and parametric tests, respectively. Patients with mBC were stratified according to the best response at treatment into three groups: R, SD, or PD, and one-way ANOVA was used to compare these populations. Student's t-test or Mann-Whitney test or was employed to compare parametric and non-parametric groups/ subgroups, respectively. Chi-square test was performed to compare response rate among subgroups. Fold change represents the ratio between the values obtained at >T0 and T0 (>T0/T0).
The multivariate logistic regression analysis (MVA) was implemented to assess the early predictor of patient response based on the investigated biomarkers, while considering the confounding factors. The variables resulted associated to the patient response was inserted in the MVA as confounding factor but not retained in the final model. The treatment response was used as the gold standard for non-parametric clustered receiver operating characteristic (ROC) analysis to evaluate the predictive utility of a single variable or MVA model. By comparing observed and calculated response, sensitivity and specificity were plotted in ROC19 form and 95% Confidence interval (CI). When a perfect correlation of predicted versus observed response was found, the area under the curve (AUC) was equal to 1, whereas random assignment of outcome led to a ROC/AUC of 0.5.20 A p-value <0.05 was considered statistically significant.
Ethics
The study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. The protocol was approved by the Institutional Ethics Committee of the two “Sapienza” Hospital Centres (Ethical Committee approval, protocol n° 805/16, RIF.CE: 4181). Written informed consent was obtained from all patients.
Role of the funding source
The study was funded by Sapienza (RM120172B803DB14, RM1181643132016E, and RP120172A7DF1B76). The funding sources had no role in the study design or in the collection, analysis, and interpretation of data.
Results
Patients’ characteristics
Fifty female patients with histologically and clinically confirmed HR+/HER2− mBC who received CDK4/6i plus HT were enrolled in this trial. The baseline clinical and pathological characteristics are summarised in Table 1. The median age was 62 years (range, 36–80 years). Forty (80%) patients had a baseline Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0. 36 patients were postmenopausal. No-special type (NTS) ductal carcinoma was the most common histology (86.8%), and LUM B-like was the most common BC subtype in the studied population (74%). All patients tested positive for estrogen receptor (ER+), while 36 patients tested positive for progesterone receptor (PR+) (72%). More than half of the patients (54%) received adjuvant HT. Bone was the most common site of metastasis (68%), followed by lymph nodes (40%). Palbociclib, ribociclib, and abemaciclib were administered in 26%, 40%, and 34% of patients, respectively. Almost all patients were treated with the combination CDK4/6i plus HT as the first-line treatment (88%). CDK4/6i plus AI was the most common combination (66%). Data regarding the response to CDK4/6i + HT are summarised in Table 1.
Table 1.
Summarisation of baseline clinical and pathological characteristics.
| Characteristics | Patients N | % |
|---|---|---|
| Menopausal status | ||
| pre | 14 | 28% |
| post | 36 | 72% |
| Previous adjuvant ET | ||
| yes | 27 | 54% |
| no | 23 | 46% |
| Histology | ||
| IDC | 43 | 86% |
| ILC | 7 | 14% |
| Luminal-like subtype | ||
| A | 13 | 26% |
| B | 37 | 74% |
| Sites of metastases | ||
| Bone | 34 | 68% |
| Lymph nodes | 20 | 40% |
| Liver | 10 | 20% |
| Skin/soft tissues | 11 | 22% |
| Lung | 7 | 14% |
| CNS | 2 | 4% |
| Other | 3 | 6% |
| Treatment Line | ||
| First | 44 | 88% |
| Second or subsequent | 6 | 12% |
| CDK 4/6i | ||
| palbociclib | 13 | 26% |
| ribociclib | 20 | 40% |
| abemaciclib | 17 | 34% |
| HT companion | ||
| AI | 33 | 66% |
| Fulvestrant | 17 | 34% |
| Best Response | ||
| CR | 2 | 4% |
| PR | 21 | 42% |
| SD | 16 | 32% |
| PD | 10 | 20% |
| NV | 1 | 2% |
IDC: invasive ductal carcinoma; ILC: invasive lobular carcinoma; CNS: central nervous system; ET: endocrine therapy; CDK4/6i: CDK 4/6 inhibitors; HT: hormone therapy; AI: aromatase inhibitors; CR: complete response; PR: partial response; SD stable disease; PD: progressive disease; HT: hormone therapy; NV: Not Valuable.
The best response to CDK4/6i was evaluable in 49 out of 50 patients; except for one patient that underwent surgery (NV). The objective response rate (ORR) was 46%, with two patients achieving a CR (4%) and 21 achieving a PR (42%). Sixteen patients (32%) experienced SD, while ten patients (20%) had PD as the best response to CDK4/6i. Subgroup analysis revealed that 59% of patients who received CDK4/6i plus AI (CDK4/6i+AI) as a first-line treatment showed a CR or PR, whereas 14% of patients treated with CDK4/6i plus fulvestrant (CDK4/6i+F) as second-line therapy were considered responders. This difference was statistically significant (chi-square test, p=0.012). No statistical difference was found according to menopausal status.
CDK4/6i reduce immunosuppression by downregulating Tregs
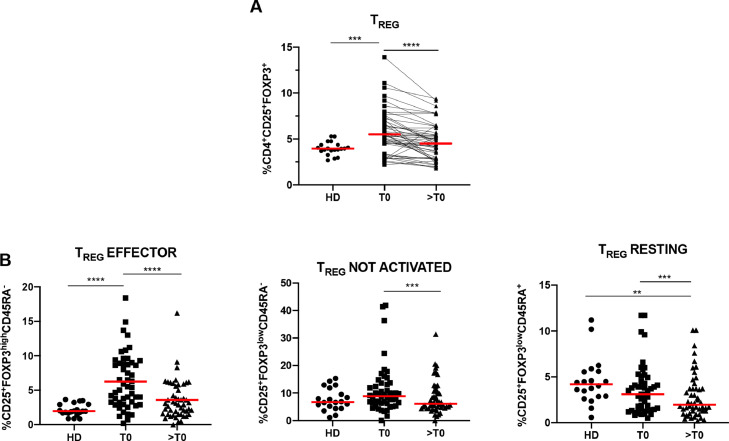
In this study, PBMC samples from 50 patients with HR+/HER2− mBC were analysed at baseline (T0) and during treatment (>T0). Immune phenotype analysis showed that the percentage of circulating Treg cells (CD4+CD25+FOXP3+) was significantly lower after CDK4/6i administration (p<0.0001; Figure 1A). Tregs can be stratified according to the pattern of expression of CD45RA and FOXP3 into the distinct functional subsets; effector (or activated), non-suppressive (or not activated), and naïve (or resting).21 CDK4/6i treatment was accompanied by downregulation of each Treg subset and was particularly remarkable for the CD25+FOXP3highCD45RA− (effector) subpopulation (p<0.0001; Figure 1B), which are effective T suppressor cells. There was no difference between the three CDK4/6i (palbociclib, ribociclib, and abemaciclib) in terms of their impact on Tregs (Supplementary Figure 1A). Moreover, Treg reduction was not influenced by the line of treatment (first or second line) or the hormone therapy administered, aromatase inhibitors or fulvestrant (Supplementary Figure 1B and 1C).
Figure 1.
Regulatory T cells decrease during CDK4/6i treatment in patients with metastatic breast cancer (mBC). (A) Scatter plots show the percentage of circulating Tregs in 20 healthy donors (HDs) and 50 patients with mBC at baseline (T0) and during CDK4/6i treatment (>T0). Red line indicates the mean percentage of Tregs, evaluated as CD4+CD25+FOXP3+ cells (mean values: HDs: 3.97%; mBC, at T0: 5.75% and at >T0: 4.70%). Immune cells were evaluated using flow cytometry and analysed by FlowJo software. (B) Scatter plots show the percentages of circulating Treg subpopulations in 20 HDs compared to 50 patients with mBC as evaluated at T0 and >T0. Red line indicates the mean values of the following Treg cell subsets: effector Tregs (median values: HDs 2.13%, mBC, at T0 6.26% and at >T0 3.59%), not activated/not suppressive Tregs (median values: HDs 7.65%, mBC, at T0 11.02% and at >T0 8.26%), naïve/resting Tregs (median values: HDs 4.52%, mBC, at T0 3.57% and at >T0 2.89%). To analyse the Treg subpopulations, the CD4+CD25+FOXP3+ cells were analysed for expression of the CD45RA and FOXP3 markers. The effector Tregs were identified as CD25+FOXP3highCD45RA−not activated/not suppressive Tregs as CD25+FOXP3lowCD45RA− and naïve/resting Tregs as CD25+FOXP3lowCD45RA+. The gating strategy for the different Treg subsets is shown in Supplementary Figure 5A. Statistical significance was determined by t-test: * p<0.05; **p<0.01; ***p<0.001; **** p<0.0001.
The median percentage of circulating Treg cells in patients with mBC was 5.75% (SEM±0.35), which was significantly higher than that in HDs (3.98% [SEM±0.16]) (p<0.001, Figure 1A). Interestingly, following CDK4/6i treatment (>T0), the percentage of circulating Tregs in patients with mBC decreased to the same percentage as observed in HDs. These results were also consistent with those of the effector Treg subsets (p<0.0001, Figure 1B).
Modulation of circulating Treg levels correlates with clinical outcome
We evaluated the association between the best response to CDK4/6i + HT treatment and circulating Treg variation (summarised in Table 1). Flow cytometry analysis revealed that, regardless of the clinical outcome, the R and SD patient groups had significantly higher levels of Tregs at baseline (T0) compared to HDs (Figure 2A; Treg: HDs vs. R p<0.01; vs SD p<0.01). During treatment, R and SD patients showed a significant decrease in the overall Treg population (Figure 2A; R p<0.0001; SD p<0.01). Moreover, only R patients reached levels similar to those observed in HD patients.
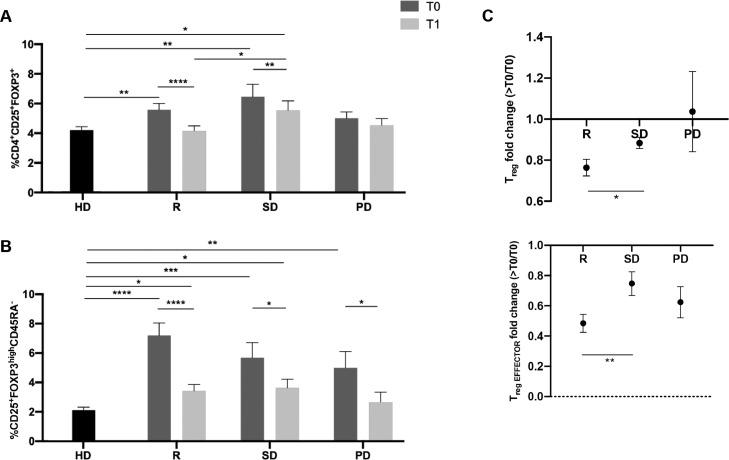
Figure 2.
Responder patients have a greater reduction in Tregs than do patients with stable or progressive disease. (A) Histograms show the mean percentage ±SEM of circulating Tregs (CD4+CD25+FOXP3+) in 20 HD controls and 49 patients with mBC at T0 and >T0. Patients with mBC were analysed according to their best response to CDK4/6i treatment. 23 patients were considered responders (R), 16 patients had stable disease (SD) and ten patients had progressive disease (PD). The Treg mean values ±SEM of each group at T0 are as follows: for HDs: 3.97%±0.16%; for mBC, R: 5.57%±0.43%, SD: 6.47%±0.84%, and PD: 5.00%±0.43%. The Treg mean values ±SEM at >T0 are, for R: 4.15%±0.34%, SD: 5.54%±0.64%, and PD: 4.53%±0.44%. (B) Histograms show the mean values ±SEM of the percentage of circulating effector Tregs (CD25+FOXP3highCD45RA−) in 20 HD controls (2.13%±0.20%) and 49 patients with mBC at T0 and >T0. Patients with mBC were divided into responders (R: T0 7.20±0.85%; >T0 3.41±0.45%), patients with stable disease (SD) (T0: 5.69±102%; >T0: 3.62±0.59%) and those with progressive disease (PD) (T0: 4.99±1.12%; >T0: 2.66±0.66%). (C) Histograms show the mean value±SEM of the Treg and effector Treg fold change evaluated in 49 patients with mBC. The fold change was calculated by dividing the percentage of Tregs at >T0 with that obtained at T0, considering the three different best responses to treatment (R, SD, and PD). Statistical significance was determined by non-parametric t-test. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Furthermore, these results were confirmed with fold change analysis, which revealed significantly less circulating Tregs and effectors in R patients than those with SD (p<0.05 and p<0.01; Figure 2C). In contrast, the PD group did not show this variation.
The effector Treg subset in patients with HR+/HER2− mBC displayed a distribution with higher values for every patient in each group at baseline compared to the control HD group (Figure 2B; HDs vs. R p<0.0001; vs SD p<0.001; vs PD p<0.01).
It is interesting to note that only in R patients, the decrease in Tregs observed during treatment was mainly due to a decrease in the effector Treg cells (p<0.0001; Figure 2), while in the resting and non-activated Treg subsets there was a slightly significant downregulation (Supplementary Figure 3). Surprisingly, SD patients showed a lower level of resting Tregs, both at T0 and >T0, compared to R patients, which displayed a similar level to that of HDs (Supplementary Figure 3).
In the SD patient group, a significant reduction in the number of effector Tregs was observed during treatment (p<0.05; Figure 2B). Nevertheless, in SD and R patients at >T0 the effector Treg levels were still higher than that in the HD controls (p<0.05; Figure 2B). In patients with PD, a significant reduction in the effector Treg subset was observed (p<0.05; Figure 2B); nevertheless, the overall Treg levels were not modified by the treatment.
At the univariate analysis, the only confounding factor resulted associated as trend to the patient response was the subtype (p=0.07). This variable was inserted in the MVA as confounding factor but not retained in the final model. The MVA revealed that Treg levels at >T0 and Treg effector at T0 were significant prognostic factors of response to therapy. The MVA model allows correctly classifying 74% of mBC patients according to their response (Supplementary Figure 4, Table 2). The area under the ROC curve was 0.701 (95%CI=0.553 to 0.823, p= 0.008; Supplementary Figure 4). The MVA model was also applied to CDK4/6i+AI and CDK4/6i+F subgroups. The model result was confirmed in the CDK4/6i+AI with AUC/ROC of 0.714 (95%CI=0.534 to 0.855, p =0.015), but not in the CDK4/6i+F subgroup, likely due to the limited number of responders (n=2 R).
The time delay between the end of the treatment cycle and blood sampling was analysed to assess the robustness of the study findings. In the BC group, Treg at >T0 was not statistically different in mBC patients with blood sample collection performed as planned or delayed (p=0.1392). Analysing responder BC vs. HDs, the difference of values of Treg, Resting, and Not Activated at >T0 was not statistically significant in HDs and R cancer patients versus irrespective of the blood sample collection. The Treg effector values at >T0 were statistically significantly different between HD and responder patients with blood samples collection performed as planned (p= 0.042; Figure 2B), while the values showed a trend (p= 0.062) in the subgroup of responder cancer patients with delayed blood samples collection. The last trend is likely due to the limited number of responder patients (i.e., 2) in this subgroup. Due to the low number of responder patients of this subgroup, further analyses were not carried out.
In summary, these results indicate a direct effect of CDK4/6i in reducing the Treg population, and this effect appears to be significantly associated with an objective response to treatment in patients with HR+/HER2− mBC. Moreover, the MVA suggests that Treg at >T0 and Treg effector at T0 could be considered as reliable predictors of response in the investigated mBC patients.
CDK4/6i treatment correlates with a decrease in the MDSC population in patients with HR+/HER2− mBC
We also conducted an exploratory study to evaluate the impact of CDK4/6i treatment on MDSCs, analysing both freshly separated monocytic (M-MDSC, CD14+HLA-DR−) and polymorphonuclear (PMN-MDSC; CD14−CD66b+HLA-DR−) subsets. The MDSC population plays a crucial role in cancer development by suppressing the anti-cancer immune response. The circulating MDSC levels were analysed in 22 patients with HR+/HER2− mBC at baseline (T0) and at >T0. Both subsets of MDSCs showed a remarkable numerical decrease during therapy (PMN-MDSCs T0 vs. >T0, p<0.05; M-MDSCs T0 vs. >T0, p<0.05, Figure 3A, and 3B).
Figure 3.
The percentage of M-MDSCs and PMN-MDSCs decreases after CDK4/6i treatment in patients with mBC. (A) Cytofluorimetric analysis of PMN-MDSC and M-MDSC populations at T0 and >T0 (gating strategies can be seen in Supplementary Figure 5). PMN-MDSC cell subpopulations were identified as CD66b+HLA-DR−, while M-MDSCs were identified as CD66b−CD14+HLADR−. (B) The histogram groups represent the mean percentages±SEM of circulating PMN-MDSCs, M-MDSCs, and DCs of 22 mBC patients, as analysed using flow cytometry (PMN-MDSCs T0 0.98±0.35% and >T0 0.44±0.15; M-MDSCs T0 3.57±0.72% and 1.88±0.59; DCs T0 2.13±0.30 and >T0 2.73±0.40). Statistical significance was determined by non-parametric t-test. * p<0.05; **p<0.01; ***p<0.001; **** p<0.0001.
CDK4/6i modulate T cell activation
To investigate whether CDK4/6i could influence effector T cells, T cell immunophenotyping was performed by analysing costimulatory profile expression and T cell subsets. As expected, CDK4/6i treatment affected intracellular Ki67 protein levels in all T cell subsets. However, the overall percentage of circulating CD8+ and CD4+ T cells was not reduced during therapy. In contrast, CD4+ T cells showed a significant increase (p<0.001), while the number of CD8+ T cells remained unchanged (Supplementary Figure 2A). When each T cell subset was analysed, a significant increase in naïve CD3+ T cells (CD45RA+CCR7+) was observed (p<0.001), and CD4+ naïve T cells appeared to predominantly contribute to this upregulation (T0 18.43% vs. >T0 21.39%; p<0.001), while a mild increase was observed for CD8+ naïve T cells (T0 5.62% vs. >T0 6.16%; p<0.01; Figure 4A).
Figure 4.
CDK4/6i modulate the anti-cancer immune response by increasing naïve T cells, as well as CD28+ and CD137+ lymphocytes. (A) Histograms show the mean percentages±SEM of naïve cells (CD45RA+CCR7+) in CD3, CD8, and CD4 T cell subsets, as evaluated at T0 and >T0 (CD3 naïve: T0 24.46%±1.79% and >T0 27.61%±1.88%; CD8 naïve T0 5.62±0.59% and >T0 6.16±0.64%; CD4 naïve T0 18.43±1.61% and >T0 21.39±1.67). (B) Histograms show the mean percentages±SEM of CD3, CD8, and CD4 naïve T cells expressing the CD28 molecule at T0 and at >T0 (CD3+CD28+ naïve T0 23.75±1.84% and >T0 27.67±1.97%; CD8+CD28+ naïve T0 6.77±0.70% and >T0 7.31±0.74%; CD4+ CD28+ naïve T0 22.12±1.97% >T0 25.02±2.01%). (C) Histograms show the mean percentages±SEM of CD3, CD8, and CD4 T cells expressing the CD137 molecule at T0 and >T0 (CD3+CD137+ T0 1.16±0.14 vs >T0 1.56±0.30; CD8+CD137+ T0 0.76±0.09 vs >T0 1.09±0.21; CD4+ CD137+ T0 0.35±0.06 vs >T0 0.42±0.10). D) The before and after plots show the ratio between the percentages of CD8+CD137+ and Tregs in 50 patients with mBC. Statistical significance was determined by parametric (CD3CD28 naïve and CD3CD4CD28 naïve) or non-parametric t-test. * p<0.05; **p<0.01; ***p<0.001; **** p<0.0001.
Interestingly, the increase in naïve CD3+, naïve CD3+CD4+, and CD3+CD8+ cells was associated with an increase in CD28 expression in these cell subsets (Figure 4B). Our data revealed that CD8+CD28− T cells were significantly downregulated by CDK4/6i (p<0.05) (Supplementary Figure 5A). In contrast, CD3+T cells showed increased CD28 surface expression with therapy (p<0.05) (Supplementary Figure 5B).
In addition, the CD3+CD137+ cell subset was analysed, as these cells are regarded as anti-tumour effector T cells.22 During CDK4/6i treatment, a trend of increased CD137 expression was observed for CD3+ cells (p=0.07), but most importantly, CD8+CD137+ cell levels significantly increased (p<0.01). These results suggest that CDK4/6i treatment favours the overall activation of both subsets of CD3+ effector cells involving distinct co-stimulatory mechanisms. These results were also demonstrated by combining the change in the immune activation and immunosuppressive T cell subsets by dividing the percentage of CD8+CD137+ cells with Treg subsets at T0 and >T0. This ratio significantly increased after the first month of therapy (p<0.0001) (Figure 4D).
These results support the hypothesis that CDK4/6i treatment impacts the immune system, decreasing immunosuppressive T cells, augmenting the anti-tumour CD8+CD137+ T cells and stimulating the renewal of naïve T cells subset ready to activate the anti-cancer immune response.
Discussion
To the best of our knowledge, our data demonstrated for the first time, an in vivo correlation between CDK4/6i treatment and the modulation of the immune system in patients with HR+/HER2− mBC, characterised by a reduction in immunosuppressive Treg cells and activation of T effector cells.
To date, the capacity of CDK4/6i to reduce Treg levels has been described only in preclinical studies,10,23 and the results have never been translated in human patients or clinical practice. Here, we conducted the first analysis in patients with HR+/HER2− mBC, describing the changes in Treg balance during treatment with CDK4/6i (palbociclib, ribociclib, and abemaciclib). The Treg subset exerts an immunosuppressive function, promoting cancer immune evasion. The transcription factor FOXP3 is of critical importance in the development and function of CD4+ regulatory T cells. These suppressive cells are composed of three phenotypically and functionally distinct subpopulations: (1) CD45RA+FOXP3low resting Tregs, (2) CD45RA−FOXP3high effector Tregs, and (3) CD45RA−FOXP3low non-activated Tregs.21 Our data demonstrated an overall reduction of Treg cells (CD4+CD25+FOXP3+) and, in particular, the effector Treg subset (CD4+CD25+FOXP3highCD45RA−), which actually exerts an immunosuppressive function. For the majority of patients (82%), we collected the >T0 blood sample after the first cycle of CDK4/6i; the samples of the remaining nine patients were collected later, but always before the first radiological evaluation (within six months of therapy). Consequently, according to our results, it can be assumed that Treg reduction occurs early during treatment with CDK4/6i.
At baseline, the levels of circulating Tregs detected in patients with mBC were higher than those detected in HD controls. This observation is in accordance with experimental evidence that the increased levels of Tregs in several cancers, including BC, directly contribute to tumour growth, progression, and metastasis, suppressing the anti-tumour T cell response21,24, 25, 26, 27 and making the Treg cell subset an appealing target for immunotherapy.
The substantial CDK4/6i-induced downregulation of the effector Treg subset, as well as the overall Treg population, appeared to be independent of the CDK4/6i molecule administered as therapy (i.e. palbociclib, ribociclib, or abemaciclib). These CDK4/6i have distinct affinities, although they target the same pathway.28, 29, 30
We found that Tregs were significantly reduced to HD levels only in R patients, while remaining high in SD and PD patients. Moreover, total and effector Treg appeared to be reliable predictors of response in the investigated mBC patients. To the best of our knowledge, this is the first time that Treg modulation has been associated with the clinical response to CDK4/6i treatment. Interestingly, the effector Treg subset decreased after treatment, independent of the clinical response. This suggests that other molecular mechanisms could play a role in the interrelation among cancer cells, immune cells, and the tumour microenvironment. We hypothesise that, in order to lead to clinical response and disease control, the effects of CDK4/6i on Treg cells must synergise with the direct effect on cancer cells. To date, it is hypothesised that primary and secondary resistance to CDK4/6i may be caused by remodelling the cell cycle pathway.31, 32, 33 Considering the urgent clinical need to identify patients with rapid and durable response or resistance to treatment, CDK4/6i-induced immune modulation could be further evaluated as an innovative predictive factor. Indeed, changes in the circulating immune profile and tumoural immune microenvironment have been shown to be strongly related34, 35, 36 and immune-profiling can become a powerful tool to provide prompt aid to therapeutic choice.36 We cannot exclude the fact that the tumour burden reduction induced by CDK4/6i treatment could indirectly account for the Treg decrease observed in this study, by generally relieving the immunosuppressive status. A more focused study would be necessary to confirm the mechanism underlying our results.
In a mouse model, it was shown that CDK4/6i treatment does not affect naïve CD3 T cells due to reprogramming of the cell cycle through activation of CDK2.37,38 In our study, we found that CDK4/6i reduced the expression of the proliferative Ki67 marker in each of the analysed T cell subsets. Nevertheless, Ki67 reduction was not associated with a decrease in the percentage of circulating T cells. Indeed, during treatment, CD8+ T cells remained unchanged, while a significant percentage increase was observed in the CD4+ T and the total naïve T cell. Furthermore, the increase in naïve T cells during treatment was accompanied by augmented expression of the activation marker CD28. CD28 is a potent co-stimulatory receptor expressed by activated and naïve T cells39 and its absence is a typical sign of T-cell senescence.40 CD8+CD28− T cells appear to promote Tregs during aging41,42 and play an immunosuppressive role in cancer.42 High peripheral levels of CD8+CD28− T cells were observed in patients with lung cancer43 and were correlated with a worse prognosis in NSCLC.44 In addition, a lower PFS was associated with high levels of circulating CD8+CD28− T cells in mBC.45 We found that CDK4/6i reduced this immunosuppressive CD8+CD28− T cell subset.
It is well known that regulatory and senescent T cells are not the only anti-cancer immunosuppressive cells. MDSCs are a heterogeneous population of immature monocytic (M-MDSCs) and polymorphonuclear (PMN-MDSCs) cells that play a crucial role in inhibiting innate and adaptive immunity in the cancer microenvironment. MDSCs can inhibit T cells, NK cells, and DCs by stimulating immune regulators, such as Tregs and tumour-associated macrophages (TAMs).46 In patients with BC, circulating MDSCs are increased, particularly in metastatic disease.47 In our study, treatment with CDK4/6i was associated with a reduction of the circulating MDSC population. Interestingly, myeloid cell development is driven entirely by CDK6.48 Although these results should be considered preliminary due to the small sample size, they strongly suggest that CDK4/6i exerts a strong and synergic immunomodulatory effect on distinct immunosuppressive subsets such as Tregs, CD8+CD28− T cells, and MDSCs.
While immunosuppression is switched off, it is crucial to activate and sustain specific anti-tumour immune effectors for the effectiveness of the anti-tumour immune response. Recently, CD3+CD137+ T cells have been shown to play a fundamental role in efficacious anti-tumour immunity. The CD137 receptor (4-1BB, TNFRSF) is a member of the tumour necrosis factor receptor (TNFR) family that has a tumour-specific activation function22 and is expressed by CD8+ and CD4+ T cells upon activation. Indeed, these cells have attracted particular interest regarding their potential role in various immunotherapy strategies owing to their unique ability to kill tumour cells.49,50 We showed that CDK4/6i treatment promoted the expansion of the CD8+CD137+ T cell population in patients, independent of the clinical response. Interestingly, the CD137+ T cell population is increased during treatment with other targeted drugs that exhibit an off-target effect on the immune system.36,51,52
These results highlight an important immune effect of CDK4/6i, which could remodel and re-educate the anti-cancer immune response in patients with mBC. In fact, the downregulation of immunosuppressive cell subsets was accompanied by an improvement in its immune-activated counterpart. Further studies on a larger population and with extensive analysis of the tissue and circulating immunological profile will be necessary to confirm our results. To the best of our knowledge, this is the first study to report the modulation of the immune system by CDK4/6i in patients with mBC.
Our study had some limitations. First, the population is quite heterogeneous, including patients treated with three different CDK4/6i. However, we found no significant difference between the three different CDK4/6i in terms of Treg modulation (Supplementary Figure 1A). Moreover, patients in the first-line and second-line subgroups showed no difference in baseline Treg or global CD3 population (Supplementary Figure 1B and 1C), and the hormone therapy administered with CDK4/6i, IA, or fulvestrant did not affect the immune profile during treatment. Second, the first on-treatment blood sample (>T0) was collected later than planned for nine patients, mainly due to difficulties related to the COVID-19 pandemic. Nevertheless, all samples were collected within six months of treatment and before the first radiological evaluation. Finally, our sample size was limited, and thus, our preliminary results should be confirmed in a larger population in multicentre prospective trials. Longer follow-up is needed to evaluate survival outcomes (PFS and OS).
In summary, our explorative study indicated that CDK4/6i downregulate Treg cells while safeguarding other T cell subsets. Furthermore, we demonstrated that CDK4/6i affected the anti-cancer immune response by tipping the balance towards immune activation, rather than immunosuppression. Moreover, an objective response to CDK4/6i was associated with Treg reduction, highlighting the importance of immune activation in tumour shrinkage. CDK4/6i-mediated immune modulation may be a novel predictive factor. A preclinical study showed that the combination of CDK4/6i and anti-PD1/PD-L1 therapy led to complete tumour regression and immunological memory.10,23,53 The results reported herein may support the use of such therapeutic approaches in other cancer settings.
Indeed, a high immunosuppression burden in the tumour microenvironment is one of the most important impairments to the efficacy of current immunotherapy. The immune effect of CDK4/6i highlighted in our study could be used in patients with cold tumours to reduce immunosuppression and trigger a robust immune response to checkpoint inhibitors.
Contributors
F.S., S.S., conceptualization, methodology, writing-original draft, writing-review and editing, data curation. A.B., A.D.F, Conceptualization, data curation, formal analysis, validation, methodology. I.G.Z., C.N., A.R. conceptualization, resources, writing review and editing. L.S. data curation, formal analysis, validation. S.T, E.C. writing-review and editing. P.M., M.N., conceptualization, resources, supervision, funding acquisition, writing-review and editing. All authors had access to the raw data and had responsibility for the decision to submit the article for publication. All authors approved the final version of the manuscript. F.S., S.S, M.N. and A.B. can verify the accuracy of the raw data.
Data sharing statement
Individual participant data that underlie the results reported in this article, after de-identification, study protocol, informed consent form and statistical analysis plan will be available immediately following publication, with no end date, with researchers who provide a methodologically sound proposal, to achieve aims in the approved proposal. Proposals should be directed to marianna.nuti@uniroma1.it to gain access; data requestors will need to sign a data access agreement.
Declaration of interests
The authors declare no potential conflicts of interest regarding this work. Paolo Marchetti (P.M.) has/had a consultant/advisory role for BMS, Roche Genentech, MSD, Novartis, Amgen, Merck Serono, Pierre Fabre, and Incyte. Marianna Nuti (M.N.) reports research grants from Incyte and IPSEN. Simone Scagnoli (S.S) has/had consulting fees with Pfizer, Novartis, Lily. Andrea Botticelli (A.B.) has/had a consultant/advisory role for MSD, Pfizer, Novartis, Lily and Roche.
Acknowledgements
We are really grateful to Dr. Pace Angelica for her helpful suggestions. This research was partly supported by Sapienza (RM120172B803DB14, RM1181643132016E and RP120172A7DF1B76). We thank the "Fondazione per la Medicina Personalizzata" (FMP) for the support of the study. F.S. is fellow of the PhD course “Network Oncology and Precision Medicine”; Dpt. Experimental Medicine, “Sapienza” University of Rome.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104010.
Appendix. Supplementary materials
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Schettini F., Buono G., Cardalesi C., Desideri I., De Placido S., Del Mastro L. Hormone receptor/human epidermal growth factor receptor 2-positive breast cancer: where we are now and where we are going. Cancer Treat Rev. 2016;46:20–26. doi: 10.1016/j.ctrv.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation Elsevier enhanced reader. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. PMID: 21376230. [DOI] [PubMed] [Google Scholar]
- 4.Harbour J.W., Luo R.X., Dei Santi A., Postigo A.A., Dean D.C. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98(6):859–869. doi: 10.1016/S0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 5.Schettini F., Giudici F., Giuliano M., et al. Overall survival of CDK4/6-inhibitor-based treatments in clinically relevant subgroups of metastatic breast cancer: systematic review and meta-analysis. J Natl Cancer Inst. 2020;112(11):1089–1097. doi: 10.1093/jnci/djaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hortobagyi G.N., Stemmer S.M., Burris H.A., et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–1748. doi: 10.1056/nejmoa1609709. 3. [DOI] [PubMed] [Google Scholar]
- 7.Slamon D.J., Neven P., Chia S., et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514–524. doi: 10.1056/nejmoa1911149. [DOI] [PubMed] [Google Scholar]
- 8.Turner N.C., Slamon D.J., Ro J., et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–1936. doi: 10.1056/nejmoa1810527. [DOI] [PubMed] [Google Scholar]
- 9.Sledge G.W., Toi M., Neven P., et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy - MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116–124. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng J., Wang E.S., Jenkins R.W., et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 2018;8(2):216–233. doi: 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueda H., Howson J.M.M., Esposito L., et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423(6939):506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 12.Ohue Y., Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 2019;110(7):2080–2089. doi: 10.1111/cas.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zizzari I.G., Martufi P., Battisti F., et al. The macrophage galactose-type C-type lectin (MGL) modulates regulatory T cell functions. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132617. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito T., Nishikawa H., Wada H., et al. Two FOXP3+CD4+ T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22(6):679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 15.Fridman W.H., Zitvogel L., Sautès-Fridman C., Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 16.Cardoso F., Paluch-Shimon S., Senkus E., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz L.H., Litière S., De Vries E., et al. RECIST 1.1 - update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahl R.L., Jacene H., Kasamon Y., Lodge M.A. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venkatraman ES, Begg CB. A distribution-free procedure for comparing receiver operating characteristic curves from a paired experiment. Biometrika. 1996;83(4):835–848. doi: 10.1093/biomet/83.4.835. [DOI] [Google Scholar]
- 20.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. PMID: 3203132. [PubMed] [Google Scholar]
- 21.Miyara M., Yoshioka Y., Kitoh A., et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Ugolini A., Nuti M. CD137+ T-cells: protagonists of the immunotherapy revolution. Cancers (Basel) 2021;13(3):456. doi: 10.3390/cancers13030456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goel S., Decristo M.J., Watt A.C., et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548(7668):471–475. doi: 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahador A., Hadjati J., Hassannejad N., et al. Frequencies of CD4+ T regulatory cells and their CD25high and FoxP3high subsets augment in peripheral blood of patients with acute and chronic brucellosis. Osong Public Health Res Perspect. 2014;5(3):161–168. doi: 10.1016/j.phrp.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langhans B., Nischalke H.D., Krämer B., et al. Role of regulatory T cells and checkpoint inhibition in hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68(12):2055–2066. doi: 10.1007/s00262-019-02427-4. 682055-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plitas G., Konopacki C., Wu K., et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity. 2016;45:1122–1134. doi: 10.1016/j.immuni.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joshi N.S., Akama-Garren E.H., Lu Y., et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity. 2015;43 doi: 10.1016/j.immuni.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen P., Lee N.V., Hu W., et al. Spectrum and degree of CDK drug interactions predicts clinical performance. Mol Cancer Ther. 2016;15(10):2273–2281. doi: 10.1158/1535-7163.MCT-16-0300. [DOI] [PubMed] [Google Scholar]
- 29.Kim S., Tiedt R., Loo A., et al. The potent and selective cyclin-dependent kinases 4 and 6 inhibitor ribociclib (LEE011) is a versatile combination partner in preclinical cancer models. Oncotarget. 2018;11(14):1289. doi: 10.18632/oncotarget.26215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells C.I., Vasta J.D., Corona C.R., et al. Quantifying CDK inhibitor selectivity in live cells. Nat Commun. 2020;11(1):2743. doi: 10.1038/s41467-020-16559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caldon C.E., Sergio C.M., Kang J., et al. Cyclin E2 overexpression is associated with endocrine resistance but not insensitivity to CDK2 inhibition in human breast cancer cells. Mol Cancer Ther. 2012;11(7):1488–1499. doi: 10.1158/1535-7163.MCT-11-0963. [DOI] [PubMed] [Google Scholar]
- 32.Dean J.L., Thangavel C., McClendon A.K., Reed C.A., Knudsen E.S. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene. 2010;29(28):4018–4032. doi: 10.1038/onc.2010.154. [DOI] [PubMed] [Google Scholar]
- 33.Roberto M., Astone A., Botticelli A., et al. Cdk4/6 inhibitor treatments in patients with hormone receptor positive, her2 negative advanced breast cancer: potential molecular mechanisms, clinical implications and future perspectives. Cancers (Basel) 2021;13(2):332. doi: 10.3390/cancers13020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giraldo N.A., Becht E., Vano Y., et al. Tumor-infiltrating and peripheral blood T-cell immunophenotypes predict early relapse in localized clear cell renal cell carcinoma. Clin Cancer Res. 2017;23:4416–4428. doi: 10.1158/1078-0432.CCR-16-2848. [DOI] [PubMed] [Google Scholar]
- 35.Giraldo N.A., Sanchez-Salas R., Peske J.D., et al. The clinical role of the TME in solid cancer. Br J Cancer. 2019;120:45–53. doi: 10.1038/s41416-018-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zizzari I.G., Di Filippo A., Botticelli A., et al. Circulating CD137+ T cells correlate with improved response to anti-PD1 immunotherapy in patients with cancer. Clin Cancer Res. 2022;28(5):1027–1037. doi: 10.1158/1078-0432.CCR-21-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ameratunga M., Kipps E., Okines A.F.C., Lopez J.S. To cycle or fight-CDK4/6 inhibitors at the crossroads of anticancer immunity. Clin Cancer Res. 2019;25:21–28. doi: 10.1158/1078-0432.CCR-18-1999. [DOI] [PubMed] [Google Scholar]
- 38.Ajchenbaum F., Ando K., DeCaprio J.A., Griffin J.D. Independent regulation of human D-type cyclin gene expression during G1 phase in primary human T lymphocytes. J Biol Chem. 1993;268(6):4113–4119. doi: 10.1016/s0021-9258(18)53587-0. [DOI] [PubMed] [Google Scholar]
- 39.Ping W.N., An A., Goronzy J. CD28- T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30(7):306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dey M., Huff W.X., Kwon J.H., Henriquez M., Fetcko K. The evolving role of CD8+CD28- immunosenescent T cells in cancer immunology. Int J Mol Sci. 2019;20(11):2810. doi: 10.3390/ijms20112810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Q., Zheng H., Chen X., et al. Human mesenchymal stromal cells enhance the immunomodulatory function of CD8+ CD28- regulatory T cells. Cell Mol Immunol. 2015;12(6):708–718. doi: 10.1038/cmi.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiser J., Banerjee A. Effector, memory, and dysfunctional CD8+ T cell fates in the antitumor immune response. J Immunol Res. 2016;2016 doi: 10.1155/2016/8941260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karagoz B., Bilgi O., Gümüs M., et al. CD8+CD28- cells and CD4+CD25+ regulatory T cells in the peripheral blood of advanced stage lung cancer patients. Med Oncol. 2010;27(1):29–33. doi: 10.1007/s12032-008-9165-9. [DOI] [PubMed] [Google Scholar]
- 44.Liu C., Jing W., An N., et al. Prognostic significance of peripheral CD8+CD28+ and CD8+CD28-T cells in advanced non-small cell lung cancer patients treated with chemo(radio)therapy. J Transl Med. 2019;17(1):344. doi: 10.1186/s12967-019-2097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song G., Wang X., Jia J., et al. Elevated level of peripheral CD8+CD28- T lymphocytes are an independent predictor of progression-free survival in patients with metastatic breast cancer during the course of chemotherapy. Cancer Immunol Immunother. 2013;62(6):1123–1130. doi: 10.1007/s00262-013-1424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz-Montero C.M., Salem M.L., Nishimura M.I., Garrett-Mayer E., Cole D.J., Montero A.J. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malumbres M., Sotillo R., Santamaría D., et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118(4):493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe K., Suzuki S., Kamei M., et al. CD137-guided isolation and expansion of antigen-specific CD8 cells for potential use in adoptive immunotherapy. Int J Hematol. 2008;88(3):311–320. doi: 10.1007/s12185-008-0134-z. [DOI] [PubMed] [Google Scholar]
- 50.Zizzari I.G., Di Filippo A., Scirocchi F., et al. Soluble immune checkpoints, gut metabolites and performance status as parameters of response to nivolumab treatment in NSCLC patients. J Pers Med. 2020;10(4):208. doi: 10.3390/jpm10040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zizzari I.G., Napoletano C., Botticelli A., et al. TK inhibitor pazopanib primes DCs by downregulation of the β-catenin pathway. Cancer Immunol Res. 2018;6(6):711–722. doi: 10.1158/2326-6066.CIR-17-0594. [DOI] [PubMed] [Google Scholar]
- 52.Zizzari I.G., Napoletano C., Di Filippo A., et al. Exploratory pilot study of circulating biomarkers in metastatic renal cell carcinoma. Cancers (Basel) 2020;12(9):2620. doi: 10.3390/cancers12092620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schaer D.A., Beckmann R.P., Dempsey J.A., et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 2018;22(11):2978–2994. doi: 10.1016/j.celrep.2018.02.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.