Abstract
Ethnopharmacological relevance
Alzheimer's disease (AD) as the most common type of dementia, is affecting the life of many senior individuals around the world. Vinca herbacea Waldst. & Kit. (V. herbacea) as a middle east originated plant demonstrated antioxidant and antitumor effects. This plant traditionally used to treat diabetes and hypertension, but its mechanism remains unclear.
Aim of the study
In the present study, post-treatment effects of V. herbacea on learning and memory functions, antioxidant cellular defense and oxidative stress were investigated using the scopolamine rat model of AD.
Materials and methods
Wistar male rats (170–190 g) were administered Scopolamine, an anti-muscarinic drug, (2 mg/kg) for 10 days followed by V. herbacea extract (200, 300 and 400 mg/kg) and/or donepezil (DON; 1 mg/kg, which were administered before behavioral studies for 10 consecutive days. All the rats were then subjected to Morris water maze (MWM) task. Biochemical parameters of oxidative stress were quantified using the whole brain.
Results
Our data showed significant decrease performance in target quadrant in water maze task following administration of scopolamine (SCOP). Also, V. herbacea and DON, did not induce any neurotoxicity and hepatotoxic effects at the highest utilized doses in healthy rats. Treatment with V. herbacea extract (200&400 mg/kg) and DON improved memory performance significantly in comparison with AD rats. In addition, V. herbacea extract in AD rats exhibited a decrease in malondialdehyde (MDA) and protein carbonyl (PCO) levels and an increase in total antioxidant capacity (FRAP) and glutathione (GSH) amounts in brain and liver.
Conclusion
It seems that cholinergic deficits and oxidative stress are consistently associated with Alzheimer's disease (AD). The richness of V. herbacea in case of indole alkaloids and flavonoids confirms the potentials of this herb in management of oxidative stress, resorting synaptic acetylcholine level and improving cellular antioxidant resources.
Keywords: Vinca herbacea extract, Scopolamine, Memory impairment, Neurotoxicity, Hepatotoxicity, Indole alkaloids
Graphical abstract
Vinca herbacea extract; Scopolamine; Memory impairment; Neurotoxicity; Hepatotoxicity; Indole alkaloids.
1. Introduction
Alzheimer's disease (AD) is one of the most common types of dementia affecting the life of two-thirds of cases suffering memory impairment above 65 years old (Kumar et al., 2018). Comorbidity of AD with some special types of psychiatric disorders including depression, anxiety and psychosis, requires certain diagnostic and therapeutic interventions (Naj et al., 2017). The main pathological aspect of AD is aggregation of amyloid β (Aβ) and Tau protein in neural system (Mattsson et al., 2019; Rabinovici, 2019). Accumulation of Aβ leads to extensive synaptic degeneration and neural loss which are assumed as main causes of memory impairment and cognitive dysfunction. Besides, over activation of NMDA receptor causes an increase in the cellular calcium content, and facilitates cellular apoptosis (Pinheiro and Faustino, 2019).
The patients’ abilities to recall and learn are highly affected by cholinergic system mal function presenting itself as declined levels of acetylcholine in synaptic clefts (Nam et al., 2019). Medications belong to acetylcholinesterase inhibitors family, work by recovering acetylcholine levels and improving cognitive performance in patients. Therefore, medicines such as Donepezil known to improve the neural homeostasis. In addition, by modulating BDNF levels, it can recover the cellular endogenous antioxidant system in encountering free reactive oxygen species (Leyhe et al., 2008). Donepezil treatment demonstrated probable neurogenesis effect by increasing the count of BrdU-positive cells in the dentate gyrus which is a critical region of hippocampus for learning and memory (Kwon et al., 2014). Finally, this drug acts as a modulator for expression of Proinflammatory cytokines like IL-1β and COX-2 which leads to improvement in cellular defense against free radicals which in turn prevents cellular apoptosis (Yoshiyama et al., 2010).
Oxidative stress is one of the contributors to the pathological procedure of AD, therefore, the proper use of antioxidants as free radical scavengers has been demonstrated promising results (Lorigooini et al., 2020; Nouri et al., 2020). On the other hand, scavenging reactive nitrogen species reported to interfere with nitric oxide pathway as another factor in AD progression (Pohanka, 2018). Brain receives higher levels of oxygen from blood and is rich of poly unsaturated fatty acids (PUFA) in comparison with other tissues, which makes the brain more susceptible to oxidative stress and neurodegenerative diseases. The high levels of antioxidant enzymes such as superoxide dismutase and hemeoxygenase in composition of the senile plaques demonstrate the important role of oxidative stress in AD pathology (Thapa and Carroll, 2017). Some evidences indicate that mitochondrial damage which leads to oxidative stress, would happen prior to the formation of Aβ and cognitive impairment symptoms. High prevalence of Aβ-induced excitotoxicity, correlates with oxidative stress and abnormal homeostasis of bioactive metals as well as lipid peroxidation, which in turn results in the augmented rate of synaptic loss (Tönnies and Trushina, 2017).
Attention to herbal therapy for neurodegenerative disorders has been grown over the years. Due to complexity and multifactorial aspects of neurodegeneration, herbal therapy has presented promising results by offering multi-targeting pathways in prevention and treatment of diseases such as AD (Geun Kim and Sook Oh, 2012).
Vinca herbacea as a plant from Apocynaceae family mainly found in Mediterranean zone, central Asia and northwest Africa. Rutin trihydrate or rutoside, is the main consisting flavonoid, and ferulic acid is the main phenol in V. herbacea extract which is important in treatment of ocular inflammation and diabetes, as well as some malignancies in traditional medicine (Akhani, 2006; Farahanikia et al., 2011; Heber, 2004; Mozaffarian, 1996; Sezer and Uysal, 2018; Zargari, 1995). The selection of Vinca herbacea (Persian name: Parivash or Periwinkle) is due to its extensive distribution from northern Spain, western France, central and southern Europe to Caucasus with containing of variety of important alkaloid, vincamine (Farahanikia et al., 2011). Oxindole alkaloids like majdine and isomajdine considered to be the main consisting alkaloids of V. herbacea extract which suggested to have free radical scavenging as well as cupric ions reducing abilities and ferrous chelating potential (Farahanikia et al., 2011; Gülçi˙ n et al., 2012; Heber, 2004).
Therefore, we investigated the protective effects of V. herbacea extract towards scopolamine-induced behavioral disturbances and oxidative stress in rats. Since the common administration of this herbal extract is oral, the possible effects of V. herbacea extract on liver needs to be investigated, due to it’s being the main organ for the biotransformation of chemicals within the body.
Besides, the memory performance was analyzed by Morris water maze task and biochemical assays were performed on samples collected from brain and liver of animal models by measuring the non-enzymatic antioxidant Glutathione level (GSH), Total antioxidant capacity level (FRAP), Lipid peroxidation level (MDA) and Protein carbonylation amount.
2. Materials and methods
2.1. Collection, identification and extraction of V. herbacea
V. herbacea was collected from Alborz mountains, and the aerial parts were separated and dried at room temperature. Then, the samples were powdered by mechanical grinding, and the extraction was performed by adding 200 g of the grinded powder to 400 ml of 70% ethanol and the obtained mixture was stored overnight at room temperature. The extracts were then filtered and evaporated using rotary evaporator (IKA-RV05). The final solution was used to prepare the required concentrations of V. herbacea extract with distilled water (Voucher no. ZUMS-1101).
2.2. Animals housing and ethics
Male Wistar rats (Pasteur Institute, Tehran, Iran) in 170–190 g weight range were housed in plastic cages in the standard laboratory conditions after acclimatization. All the procedures such as handling, injections and samplings were approved by the Animal Ethics Committee of Zanjan University of Medical Sciences (Ethics approval code: ZUMS.REC.1399.084) prior to the experiments, complying with the ARRIVE Guidelines for handling the laboratory animals.
2.3. Experimental design
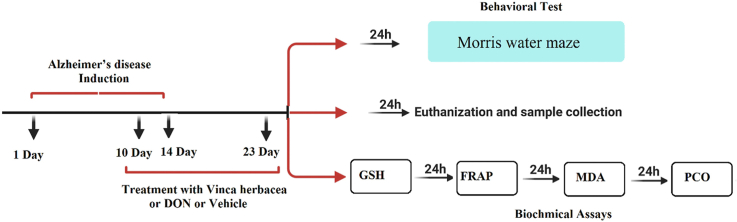
Rats were randomly divided into eight groups each contained 7–8 rats namely, control group which only received intravenous normal saline, VH group (received 200 mg/kg of V. herbacea extract by gavage), DON group (received 1 mg/kg donepezil by IV route), AD group (received 2 mg/kg scopolamine by intraperitoneal route), AD + DON group (received 2 mg/kg IP scopolamine followed by 1 mg/kg IV donepezil) AD + VH groups (first received 2 mg/kg IP scopolamine and then each group treated with 200,300 and 400 mg/kg of V. herbacea extract by gavage for 10 days). 24 hours after the last treatment, the animals were subjected to the behavioral test with Morris water maze (four days of training followed by one day of probe trial). On the following day, the rats were anesthetized and decapitated. Then the target organs (brain and liver) were isolated and stored in -80°c freezer in order to assess the related biochemical parameters.
2.4. Water maze task
One of the approved methods to measure memory and cognitive performance is Morris water maze task. The Morris water maze (MWM; Pishro Andishe Sanat, Tabriz, Iran) applied in this study, was a round black cylinder (round tank) of 150-cm diameter, and 60-cm depth, with an escape platform which was placed on a metal basis (45cm height). The tank was filled with tap water (22–25 °C) so that the escape platform drowned in the water (2 cm below the surface). The maze was fixed in the middle of a room with enough surrounding visual clues. The clues were not rearranged during training or test days as animals can refer and remember the clues for locating where the platform was. An infrared camera, connected with the MWM software on the computer, was used as a tracking system to record the whole swimming paths and latency times, a red light was used to minimize light reflection from the water as tracking software was sensitive to light. On training courses, each animal was allowed to swim freely for at most 60 s and in case of finding the platform, stay on it for 10 s. If the animal was not able to find the platform, they were led by the experimenter towards the platform and let to stay on it for 10 s. The training continued for 4 sequential days. On the 5th day (test day), the platform was removed from the water maze in order to evaluate learning memory of the animals. Each animal was placed randomly on the water to swim freely for 60 s. Since the platform had been placed at Q2 (the quarter circle of platform), the time which animals spent in Q2 zone and the pattern of their swimming were measured (Ahmadi et al., 2021; Vorhees and Williams, 2006).
2.5. Evaluation of lipid peroxidation (LPO)
Peroxidation of polyunsaturated fatty acids is one of the main markers in oxidative stress. The levels of malondialdehyde (MDA) as the main product of LPO were measured using Thiobarbituric acid at 532nm (Amiri et al., 2018; Gutteridge and Halliwell, 1990; Lorigooini et al., 2020). Briefly, 100 mg tissue sample was homogenate in 500 μl KCl (154mM) and then centrifuged at 3500 × g for 10 min. Then, 1.25 mL sulfuric acid (0.05 M) and 1mL TBA reagent [TCA (5%)+HCl (0.5N)+ TBA (0.3%)] 0.2% was added to sediment tubes and put in boiling water (95-100 °C) for 30 min. Afterward, the tubes were moved into ice and 2 mL of n-butanol was added to each tube and then centrifuged at 3500 × g for 10 min. The upper layer (n-butanol phase) was separated and the absorbance was measured at 532 nm. Tetramethoxypropane (TMP) was applied as the standard to gain MDA content in μmol/g tissue.
2.6. Evaluation of the protein carbonyl (PCO)
Oxidative stress could lead to Protein carbonylation, and loss of function in critical proteins The PCO levels were measured in reaction with 2,4-Dinitrophenylhydrazine at 365nm (Ahmadi et al., 2021; Dalle-Donne et al., 2003; Masrour et al., 2018). In brief, 100 mg of tissue was homogenate in 0.5 mL of 20% (w/v) TCA at 4 °C for 15 min. The supernatant was discarded and 0.5 mL DNPH (0.2%) was added to precipitates, as well as 0.5 mL of NaOH (2M) as control. After 1 h incubation, 0.5 mL of TCA (20%) was added so as to precipitate proteins. All samples were centrifuged after three wash with 1mL ethanol-ethyl acetate, they were dissolved in 0.2 mL of guanidine hydrochloride (6 M). Finally, carbonyl content expressing as nmol/g tissue were obtained by reading the absorbance of the samples at 365 nm.
2.7. Evaluation of the reduced glutathione (GSH)
GSH counts as one of the main indigenous antioxidants in neural tissue. The GSH level was measured by DTNB [5,5′-Dithiobis (2-nitrobenzoic acid)] at 412nm (Kianpour et al., 2021; Rahman et al., 2006; Sonei et al., 2017). Briefly, 100 mg of tissue was mixed with 1.5 mL EDTA (0.02M) and 1.5 mL TCA (10%W/V) in a homogenizer. Then, to 0.5 mL of supernatant, 1.25 mL of phosphate buffer (0.4 mol/L) and 0.25 mL of DTNB indicator (0.8 mg/mL; pH = 8.9)was added and vortexed superbly to measure the emergent color at 412 nm.
2.8. Evaluation of the ferric reducing ability of plasma (FRAP)
FRAP assay was used in order to determine the total antioxidant power in tissues using TPTZ [2,4,6-Tri (2-pyridyl)-s-triazine] as the sensitive reagent (Benzie and Strain, 1996; Lorigooini et al., 2020; Yazdinezhad et al., 2017). A blue colored complex is formed by reducing the colorless ferric (Fe3+) tripyridyltriazine ions to ferrous ions (Fe2+) at low pH at 593 nm. Briefly, 100 mg tissue sample was homogenate in 1mL TCA (6%) and then centrifuged at 3500 × g for 10 min. Then 1.5 mL FRAP solution [Sodium acetate (300mM, FeCl3 20mM, TPTZ 10 mM (10:1:1 V/V/V)] was added to 50 μl of supernatant and the absorbance was measured at 593 nm.
2.9. Statistical analysis
All the results were expressed as mean ± SD, and R studio programming software was used for statistical analyses. Comparisons between the groups were carried out using the one-way analysis of variance (ANOVA) followed by Tukey's post hoc tests. P values less than ˂0.05 were considered statistically significant. All the plots were generated using Matplotlib library in Python.
3. Results
3.1. The interventional effects on performance in water maze task
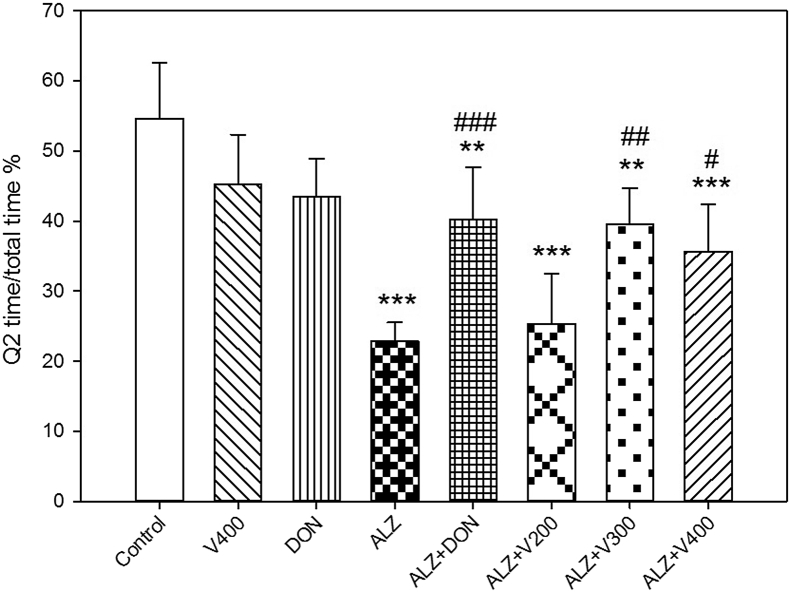
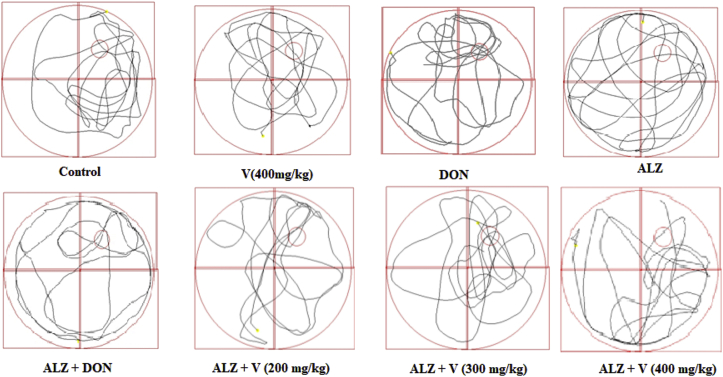
Figure 1 indicated that scopolamine administration induced memory impairment, and decreased animals’ presence in target escape platform compared with control group (P < 0.001). Also, treatment of AD rats with V. herbacea extract (300 and 400 mg/kg) demonstrated to be effective on memory impairment in comparison with AD group (P < 0.01 and P < 0.05 respectively). Similarly, treatment of AD rats with DON, improved the memory performance compared to AD animals (P < 0.001). Our data showed no significant difference between normal treated rats with only V. herbacea extract (400 mg/kg) and DON on memory function (P > 0.05). The swimming pattern for randomly selected rats on MWT is shown in Figure 2. Our data revealed almost similar swimming patterns in the target zone (Q2) in AD rats treated with V (300,400 mg/kg), while swimming pattern for rats received scopolamine noticed to be randomized. Besides the rats in AD group mostly swam at the borders of the tank with lower speeds while the treated rats tried to find the hidden platform and spent their times mostly in Q2 and Q3.
Figure 1.
Effects administration of V. herbacea extract and Donepezil on Q2 time/total time percentage. Values are expressed as the mean ± SD and were analyzed using One-way ANOVA was used followed by Tukey's post hoc test. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001 compared with control group. #P < 0.05 and ##P < 0.01 and ###P < 0.001 compared with CPF treated rats. NS: Non significant with CPF treated rats (n = 8–10).
Figure 2.
Randomized select of swimming patterns in the water maze task.
3.2. The interventional effects on MDA levels in brain and liver
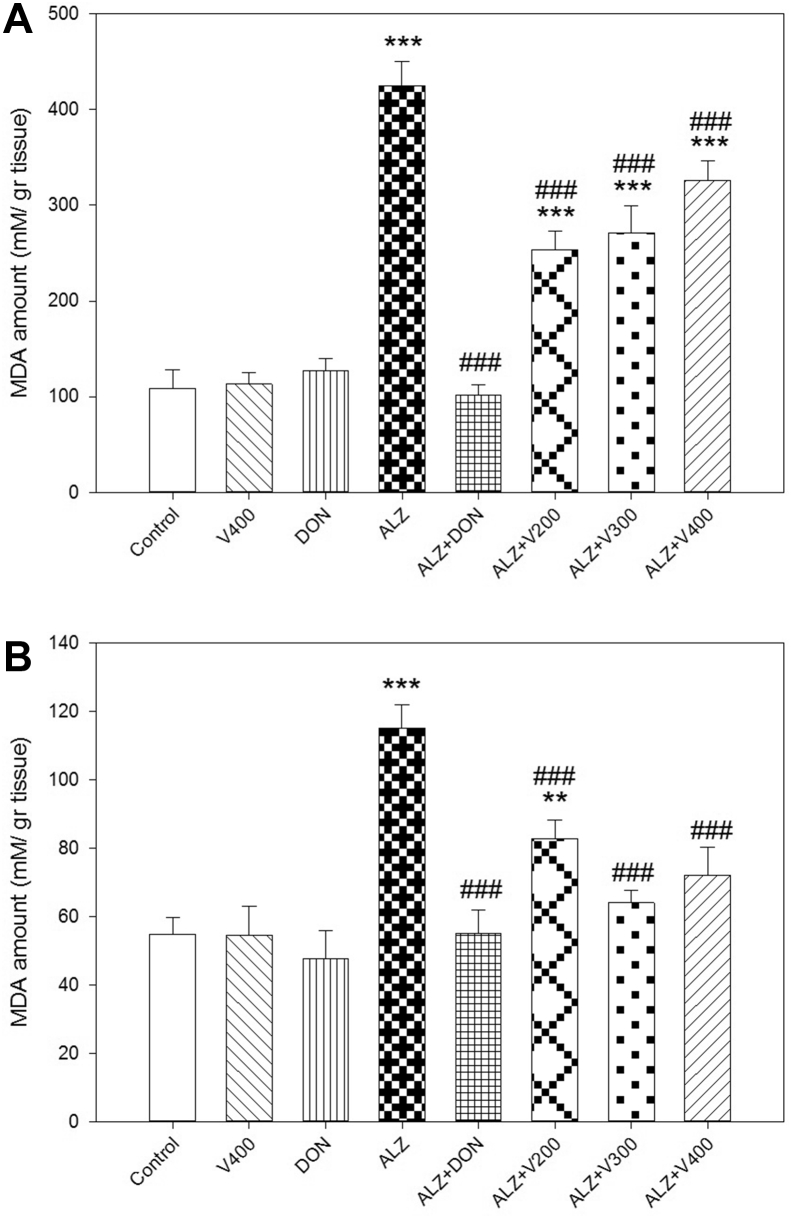
As depicted in Figure 3, scopolamine administration significantly increased MDA levels as an indicator of oxidative stress in both brain and liver tissues compared to control (P < 0.001). Furthermore, Treatment with DON could significantly decreased cellular MDA amount in brain and tissue in comparison with AD rats (P < 0.05). Although, treatment with all three doses of V. herbacea extract has ameliorated MDA levels in both brain and liver compared to AD rats (P < 0.001); MDA levels in treated AD rats didn't demonstrate a significant decrease in brain compared to control group. In liver, only AD rats which were treated with V. herbacea extract (200 mg/kg) showed significant difference with control group (P < 0.01). Also, DON and 400 mg/kg of V. herbacea extract did not affect MDA level compared with control group (P > 0.05).
Figure 3.
Effects administration of V. herbacea extract and Donepezil on MDA amount of (A) Brain samples and (B) Liver samples. Values are expressed as the mean ± SD and were analyzed using One-way ANOVA was used followed by Tukey's post hoc test. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001 compared with control group. #P < 0.05 and ##P < 0.01 and ###P < 0.001 compared with CPF treated rats. NS: Non significant with CPF treated rats (n = 3).
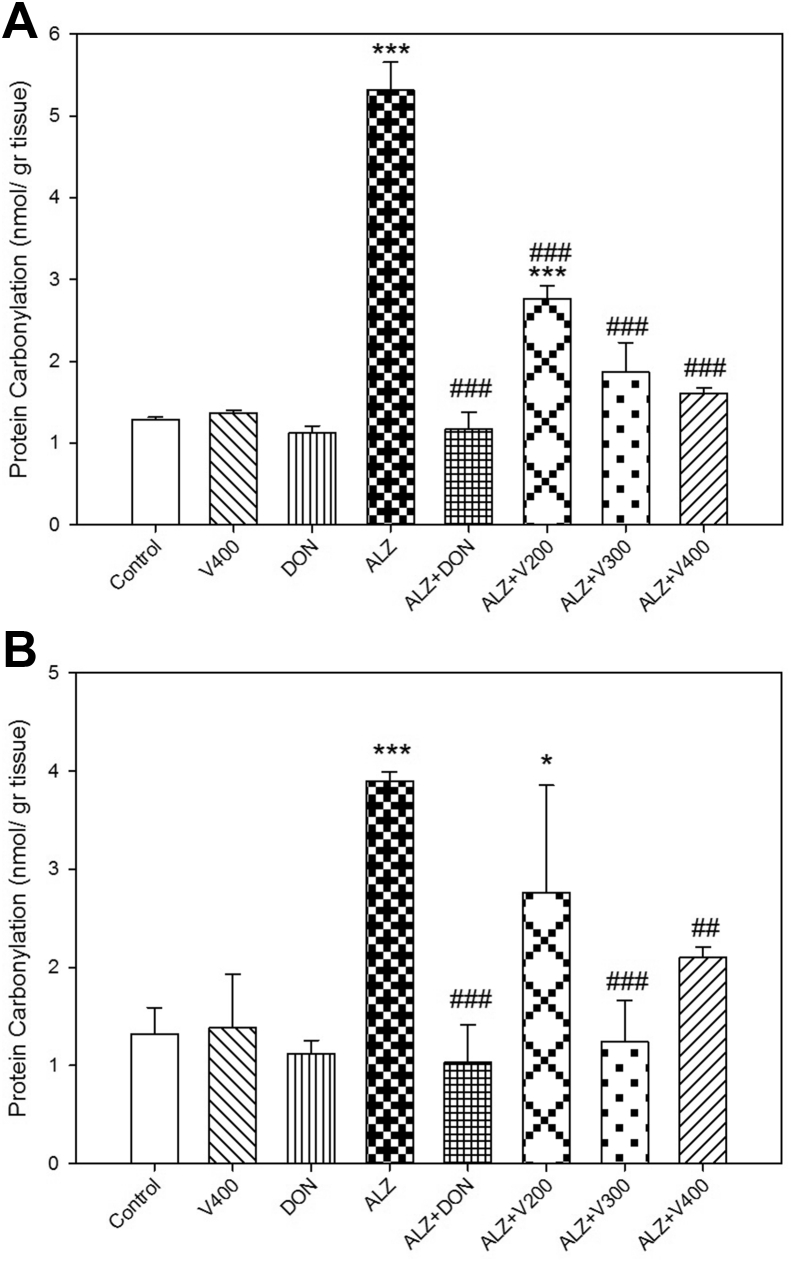
3.3. The interventional effects on PCO level in brain and liver
As shown in Figure.4A-B, there were significant differences in PCO levels between control and scopolamine received rats both in brain and liver (P < 0.001). Following to administration of V. herbacea extract (300–400 mg/kg) in AD rats, the PCO levels were declined significantly in comparison to AD group (P < 0.001) in brain and liver. Our results showed that only 200 mg/kg of V. herbacea extract + AD rats didn't express a significant decline in PCO level compared to control group in both tissues (P > 0.05). Also, the animal treated with DON and V400 groups demonstrated no significant changes in PCO levels compared to normal or control rats (P > 0.05).
Figure 4.
Effects administration of V. herbacea extract and Donepezil on PCO amount of (A) Brain samples and (B) Liver samples. Values are expressed as the mean ± SD and were analyzed using One-way ANOVA was used followed by Tukey's post hoc test. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001 compared with control group. #P < 0.05 and ##P < 0.01 and ###P < 0.001 compared with CPF treated rats. NS: Non significant with CPF treated rats (n = 3).
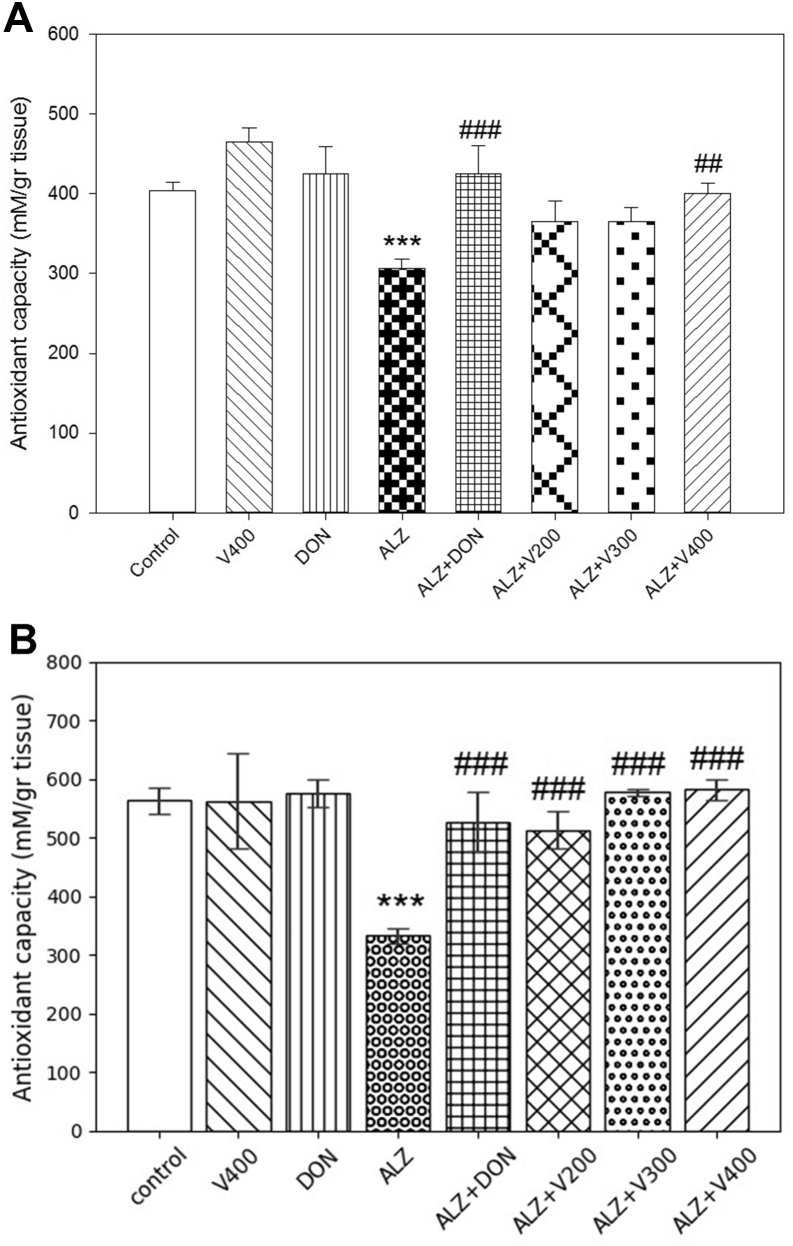
3.4. The interventional effects on FRAP levels in brain and liver
Figure 5A-B revealed that administration of scopolamine had a significant declining effect in FRAP levels compared to control groups in both tissues (P < 0.001). Our data observed significant differences in FRAP concentrations between AD + V (200,300&400 mg/kg) groups compared to AD rats (P < 0.001) in liver. Interestingly, subsequent treatment with DON augmented the total cellular antioxidant capacity levels near the control amount (P > 0.05). On the other hand, our results in brain showed that VH (200&300 mg/kg) treatment didn't result in a significant rise in FRAP level compared to AD rats (P > 0.05). Last but not least, the animals treated only with DON or VH (400 mg/kg) demonstrated no significant changes in FRAP levels compared to normal or control rats (P > 0.05).
Figure 5.
Effects administration of V. herbacea extract and Donepezil on Frap amount of (A) Brain samples and (B) Liver samples. Values are expressed as the mean ± SD and were analyzed using One-way ANOVA was used followed by Tukey's post hoc test. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001 compared with control group. #P < 0.05 and ##P < 0.01 and ###P < 0.001 compared with CPF treated rats. NS: Non significant with CPF treated rats (n = 3).
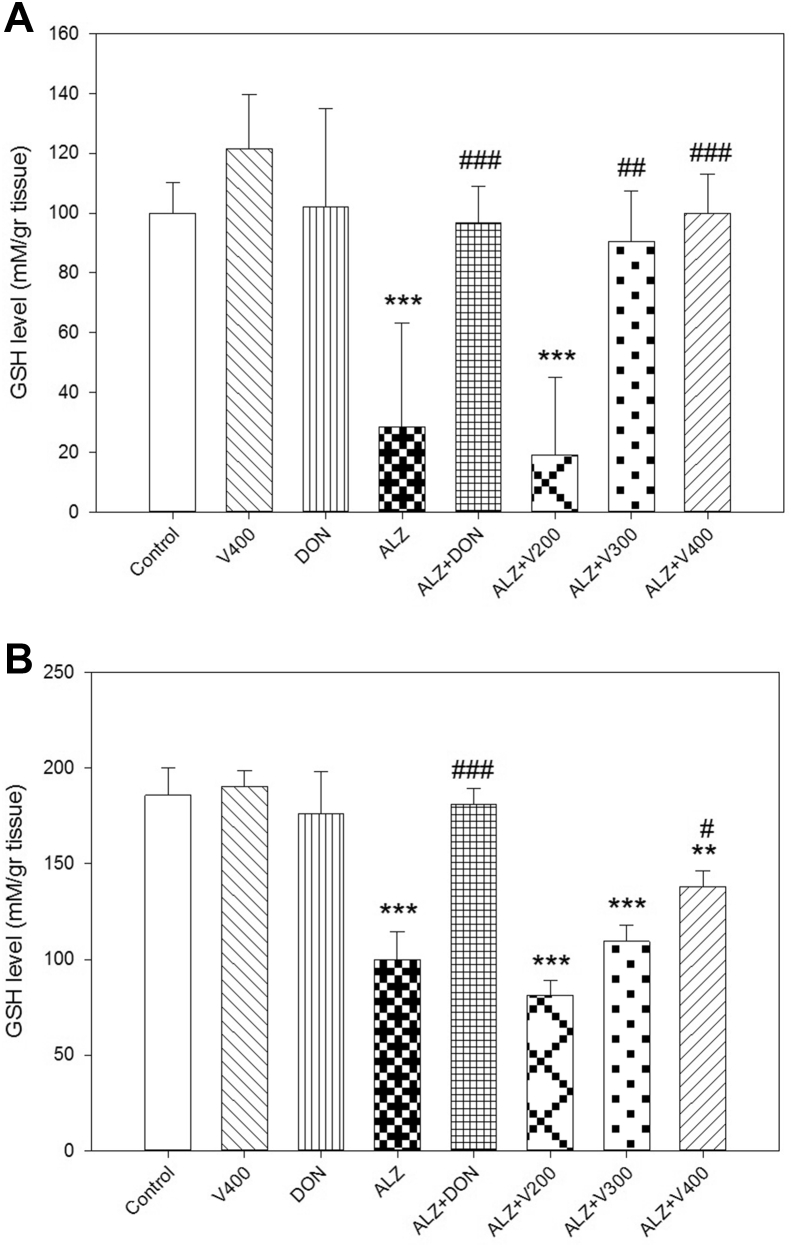
3.5. The intervention effects on GSH level in brain and liver
As shown in Figure 6A-B, our data revealed that the scopolamine treated rats showed significant decline in GSH levels which related to the imbalance between ROS and antioxidant levels, as well as induction of oxidative stress condition in both liver and brain (P < 0.001). The data analysis indicated a significant rise in GSH levels in the brain of AD rats treated with V (300 &400 mg/kg) which was similar to that of control rats (P < 0.05). DON as gold standard drug in AD, managed to reverse GSH amount to normal condition (P < 0.001). In contrary, AD animals treated with V200/V300 demonstrated no significant increase in GSH content when compared to AD rats (P > 0.05). Finally the animals received DON or 400 mg/kg of V. herbacea extract did not express different cellular GSH level with control group (P > 0.05).
Figure 6.
Effects administration of V. herbacea extract and Donepezil on GSH amount of (A) Brain samples and (B) Liver samples. Values are expressed as the mean ± SD and were analyzed using One-way ANOVA was used followed by Tukey's post hoc test. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001 compared with control group. #P < 0.05 and ##P < 0.01 and ###P < 0.001 compared with CPF treated rats. NS: Non significant with CPF treated rats (n = 3).
4. Discussion
The presented study improves our understandings of the probable alternative herbal therapy for treatment of Alzheimer-like disease in rodent models, as the V. herbacea extract managed to improve cellular endogenous antioxidant resources. It can be deducted that, the above mentioned treatment might be capable of memory improvement via inhibiting the over production of free radicals which in turn induces the memory system dysfunction.
In the present study, DON managed to invert the overproduction of free radicals induced by scopolamine administration with a significant suppression effect on PCO and MDA production. It has been reported that Donepezil improves mitochondrial dynamics, ameliorates microglia activation, decreases the Aβ accumulation and finally modulates proinflammatory cytokines interleukin 16 and tumor necrosis factor (Jiang et al., 2019; Ongnok et al., 2021). In addition to DON neuro-protective effects, donepezil managed to recover cellular antioxidant recourses to normal levels, and yet modulates GSH levels as an endogenous antioxidant. In this context, DON's effects on recovering cellular antioxidants suggested to be a result of ameliorating BDNF shortage in neurodegeneration and decreasing synaptic loss which improves neural homeostasis (Klugman et al., 2012). The role of DON in modulating copper and zinc homeostasis could impact the reducing activity of glutamate cysteine ligase as the rate limiting enzyme in GSH production (Giacconi et al., 2019; Maher, 2018).
In this study, Scopolamine injection was utilized as a method for cognitive and memory impairment induction. The obtained results of this study have shown that SCOP administration led to lower memory performances in water maze task which followed by escalations in brain and liver MDA and PCO, as well as a drop in cellular antioxidant capacity. To elaborate more, SCOP administration known to augment IL-1β, IL-4 and TNF-α levels as the main cellular pro-inflammatory cytokines. In accordance with our results, Kim and Choi reported that Scopolamine facilitate neural apoptosis in AD patients by activating p38 and c-Jun N-terminal kinase (JNK) (Kim and Choi, 2010). Besides it has been proved that a significant alteration in calcium reuptake induced by SCOP leads to an increase in the cytosolic calcium, resulted in mitochondrial membrane potential (ΔΨm) dropping which is a critical part of cellular energy production (San Tang, 2019). SCOP also triggers enzymes critical in function of endogenous antioxidants, mainly super-oxide dismutase, glutathione s-transferase, glutathione peroxidase and catalase (Uma and Maheswari, 2014).
In this study V. herbacea extract was selected as the test treatment, which is a rich source of various indole alkaloids (akuamicine, akuamine, herbaine, hervine, and tabersonine), oxindole alkaloids (majdine, isomajdine and carapanaubine) and flavonoids (mainly Rutin and Chlorogenic acid) (Ciorîț;ă et al., 2021; Gagua et al., 2011; Vachnadze et al., 2010). Indole alkaloids known to demonstrate various antidepressant, anticancer and anticholinesterase effects on animal models and cell line studies (Omar et al., 2021). In our study, administration of V. herbacea extract did not induce any toxic effects on liver tissue at any doses. In addition, it did not alter the cellular normal MDA and PCO levels. It was demonstrated that, treatment with 300 and 400 mg/kg of V. herbacea extract improved liver tissue's antioxidant capacity when exposed to scopolamine's hepatoxic effects. Affirmatively, in one study, treatment with V. rosea after diabetic induction with alloxan, managed to recover the degeneration and periportal infiltration in hepatic tissue back to normal level (Ghosh and Suryawanshi, 2001). Based on unique position of liver in circulatory system and biotransformation of many compounds in liver, we decided to investigate the effects of V. herbacea extract in liver when administrated in oral as the common consumption form of beverage and extracts.
Treatment with 300 and 400 mg/kg of V. herbacea significantly improved memory impairments by SCOP in water maze task. Interestingly, the effects of V. herbacea at above mentioned doses was not significantly different from that of Donepezil (P > 0.05). In case of oxidative stress modulation, V. herbacea significantly suppressed the overproduction of MDA and PCO, as the direct indicators of oxidative stress and mitochondrial dysfunction, while recovering the total cellular antioxidants and GSH content. In agreement with our data, some Indole alkaloids have been introduced for possessing the AChE inhibiting properties via formation of hydrogen bonds in His440 and Ser200. In addition, it is suggested that these substances can modulate overproduction of tumor necrosis factor alpha (TNF-α), myeloperoxidase (MPO) and mediators such as nitric oxide (NO) (Hussain et al., 2018). According to a 2021 paper which relatively confirms our results, it can claimed that Rutin as the main flavonoid in V. herbacea extract glycoside, binds to β-sheet secondary structure of amyloid which transforms the polymeric feature of Aβ, and prevents oligomer-induced cytotoxicity of Tau protein (Sun et al., 2021) which can be introduced as a major mechanism of V. herbacea extract. In addition, rutin administration restores the nuclear enzyme poly (ADP-ribose) polymerase-1 (PARP-1) activity, which declines cellular energy consumption as a result of oxidative stress, and via this mechanism, minimizes the chance of energy depletion (Javed et al., 2012). Besides, It has been reported that excessive activation of autophagy leads to intracellular deposition of Aβ, Chlorogenic acid regulates mTOR/TFEB signaling, and decelerates neural injury (Gao et al., 2020).
In an overall view, administration of the V. herbacea extract was capable of remission of memory impairment induced by scopolamine, following the same pattern as donepezil. This effect was mainly due to the improvement in neural defense against free radicals by alleviating the antioxidant system dysfunction.
Ethics approval
All of animal work were in accordance with the National Institute of Health (NIH) Guidelines for the Care and Use of Laboratory Animals (HHS publication 85-23, 1985), legislation for the protection of animals used for scientific purposes (Directive 2010/63/EU) and our institutional guidelines for animal care and use (Department of Pharmacology, School of Pharmacology, Zanjan University of Medical Sciences, Zanjan, Iran).
Declarations
Author contribution statement
Mahdieh Anoush; Mir Jamal Hosseini: Conceived and designed the experiments; Analyzed and interpreted the data; wrote the paper.
Javad Eskandari; Niloofar Mahmoodi; Sorush Bijani; performed the experiments; wrote the paper.
Alireza Yazdinezhad: contributed reagents, materials, analysis tools or data; wrote the paper.
Funding statement
This work was supported by the deputy of research of Zanjan University of Medical Sciences (Grant NO: A-12-323-21).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Dr. Mahdi Tavakkolizadeh for his consultations on this study.
References
- Ahmadi N., Hosseini M.-J., Rostamizadeh K., Anoush M. Investigation of therapeutic effect of curcumin α and β glucoside anomers against Alzheimer’s disease by the nose to brain drug delivery. Brain Res. 2021;1766:147517. doi: 10.1016/j.brainres.2021.147517. [DOI] [PubMed] [Google Scholar]
- Akhani H. Flora Iranica: facts and figures and a list of publications by KH Rechinger on Iran and adjacent areas. Rostaniha. 2006;7(Suppl 2):19–61. [Google Scholar]
- Amiri S., Yousefi-Ahmadipour A., Hosseini M.-J., Haj-Mirzaian A., Momeny M., Hosseini-Chegeni H., Mokhtari T., Kharrazi S., Hassanzadeh G., Amini S.M. Maternal exposure to silver nanoparticles are associated with behavioral abnormalities in adulthood: role of mitochondria and innate immunity in developmental toxicity. Neurotoxicology. 2018;66:66–77. doi: 10.1016/j.neuro.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Ciorîță A., Zăgrean-Tuza C., Moț A.C., Carpa R., Pârvu M. The phytochemical analysis of Vinca L. Species leaf extracts is correlated with the antioxidant, antibacterial, and antitumor effects. Molecules. 2021;26(10):3040. doi: 10.3390/molecules26103040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle-Donne I., Rossi R., Giustarini D., Milzani A., Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 2003;329(1-2):23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Farahanikia B., Akbarzadeh T., Jahangirzadeh A., Yassa N., Ardekani M.R.S., Mirnezami T., Hadjiakhoondi A., Khanavi M. Phytochemical investigation of Vinca minor cultivated in Iran. Iran. J. Pharm. Res. (IJPR) 2011;10(4):777. [PMC free article] [PubMed] [Google Scholar]
- Gagua N., Baghdikian B., Mabrouki F., Elias R., Vachnadze V., Bakuridze A., Ollivier E. HPLC determination of majdine in Vinca herbacea. Nat. Product Commun. 2011;6(12) 1934578X1100601211. [PubMed] [Google Scholar]
- Gao L., Li X., Meng S., Ma T., Wan L., Xu S. Chlorogenic acid alleviates Aβ25-35-induced autophagy and cognitive impairment via the mTOR/TFEB signaling pathway. Drug Des. Dev. Ther. 2020;14:1705. doi: 10.2147/DDDT.S235969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geun Kim H., Sook Oh M. Herbal medicines for the prevention and treatment of Alzheimer's disease. Curr. Pharmaceut. Des. 2012;18(1):57–75. [PubMed] [Google Scholar]
- Ghosh S., Suryawanshi S. 2001. Effect of Vinca Rosea Extracts in Treatment of Alloxan Diabetes in Male Albino Rats. [PubMed] [Google Scholar]
- Giacconi R., Giuli C., Casoli T., Balietti M., Costarelli L., Provinciali M., Basso A., Piacenza F., Postacchini D., Galeazzi R. Acetylcholinesterase inhibitors in Alzheimer's disease influence Zinc and Copper homeostasis. J. Trace Elem. Med. Biol. 2019;55:58–63. doi: 10.1016/j.jtemb.2019.06.001. [DOI] [PubMed] [Google Scholar]
- Gülçi˙ n I.l., Beydemi˙ r Ş., Topal F., Gagua N., Bakuridze A., Bayram R., Gepdiremen A. Apoptotic, antioxidant and antiradical effects of majdine and isomajdine from Vinca herbacea Waldst. and kit. J. Enzym. Inhib. Med. Chem. 2012;27(4):587–594. doi: 10.3109/14756366.2011.604318. [DOI] [PubMed] [Google Scholar]
- Gutteridge J.M., Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem. Sci. 1990;15(4):129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- Heber D. Thomson PDR; 2004. PDR for Herbal Medicines. [Google Scholar]
- Hussain G., Rasul A., Anwar H., Aziz N., Razzaq A., Wei W., Ali M., Li J., Li X. Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. Int. J. Biol. Sci. 2018;14(3):341. doi: 10.7150/ijbs.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed H., Khan M., Ahmad A., Vaibhav K., Ahmad M., Khan A., Ashafaq M., Islam F., Siddiqui M., Safhi M. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience. 2012;210:340–352. doi: 10.1016/j.neuroscience.2012.02.046. [DOI] [PubMed] [Google Scholar]
- Jiang L., Wang Y., Su L., Ren H., Wang C., Chen J., Fu X. Donepezil attenuates obesity-associated oxidative stress and central inflammation and improves memory deficit in mice fed a high-fat diet. Dement. Geriatr. Cognit. Disord. 2019;48(3-4):154–163. doi: 10.1159/000504800. [DOI] [PubMed] [Google Scholar]
- Kianpour F., Mohseni M., Beigmohamadi M., Yazdinezhad A., Ramazani A., Hosseini M.-J., Sharafi A. The protective effects of Ziziphora tenuior L. against chlorpyrifos induced toxicity: involvement of inflammatory and cell death signaling pathways. J. Ethnopharmacol. 2021;272:113959. doi: 10.1016/j.jep.2021.113959. [DOI] [PubMed] [Google Scholar]
- Kim E.K., Choi E.-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2010;1802(4):396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Klugman A., Naughton D.P., Isaac M., Shah I., Petroczi A., Tabet N. Antioxidant enzymatic activities in Alzheimer's disease: the relationship to acetylcholinesterase inhibitors. J. Alzheim. Dis. 2012;30(3):467–474. doi: 10.3233/JAD-2012-120124. [DOI] [PubMed] [Google Scholar]
- Kumar A., Sidhu J., Goyal A., Tsao J.W. 2018. Alzheimer Disease. [Google Scholar]
- Kwon K.J., Kim M.K., Lee E.J., Kim J.N., Choi B.-R., Kim S.Y., Cho K.S., Han J.-S., Kim H.Y., Shin C.Y. Effects of donepezil, an acetylcholinesterase inhibitor, on neurogenesis in a rat model of vascular dementia. J. Neurol. Sci. 2014;347(1-2):66–77. doi: 10.1016/j.jns.2014.09.021. [DOI] [PubMed] [Google Scholar]
- Leyhe T., Stransky E., Eschweiler G., Buchkremer G., Laske C. Increase of BDNF serum concentration during donepezil treatment of patients with early Alzheimer’s disease. Eur. Arch. Psychiatr. Clin. Neurosci. 2008;258(2):124–128. doi: 10.1007/s00406-007-0764-9. [DOI] [PubMed] [Google Scholar]
- Lorigooini Z., Dehsahraei K.S., Bijad E., Dehkordi S.H., Amini-Khoei H. Trigonelline through the attenuation of oxidative stress exerts antidepressant-and anxiolytic-like effects in a mouse model of maternal separation stress. Pharmacology. 2020;105(5-6):289–299. doi: 10.1159/000503728. [DOI] [PubMed] [Google Scholar]
- Maher P. Potentiation of glutathione loss and nerve cell death by the transition metals iron and copper: implications for age-related neurodegenerative diseases. Free Radic. Biol. Med. 2018;115:92–104. doi: 10.1016/j.freeradbiomed.2017.11.015. [DOI] [PubMed] [Google Scholar]
- Masrour F.F., Peeri M., Azarbayjani M.A., Hosseini M.-J. Voluntary exercise during adolescence mitigated negative the effects of maternal separation stress on the depressive-like behaviors of adult male rats: role of NMDA receptors. Neurochem. Res. 2018;43(5):1067–1074. doi: 10.1007/s11064-018-2519-6. [DOI] [PubMed] [Google Scholar]
- Mattsson N., Cullen N.C., Andreasson U., Zetterberg H., Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2019;76(7):791–799. doi: 10.1001/jamaneurol.2019.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian V. Vol. 396. Farhang Moaser; Tehran: 1996. (A Dictionary of Iranian Plant Names). [Google Scholar]
- Naj A.C., Schellenberg G.D., Consortium A.s.D.G. Genomic variants, genes, and pathways of Alzheimer's disease: an overview. Am. J. Med. Genet. Part B: Neuropsychiatric Genetics. 2017;174(1):5–26. doi: 10.1002/ajmg.b.32499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam E., Nam G., Lim M.H. ACS Publications; 2019. Synaptic Copper, Amyloid-β, and Neurotransmitters in Alzheimer’s Disease. [DOI] [PubMed] [Google Scholar]
- Nouri A., Hashemzadeh F., Soltani A., Saghaei E., Amini-Khoei H. Progesterone exerts antidepressant-like effect in a mouse model of maternal separation stress through mitigation of neuroinflammatory response and oxidative stress. Pharmaceut. Biol. 2020;58(1):64–71. doi: 10.1080/13880209.2019.1702704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar F., Tareq A.M., Alqahtani A.M., Dhama K., Sayeed M.A., Emran T.B., Simal-Gandara J. Plant-based indole alkaloids: a comprehensive overview from a pharmacological perspective. Molecules. 2021;26(8):2297. doi: 10.3390/molecules26082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongnok B., Khuanjing T., Chunchai T., Kerdphoo S., Jaiwongkam T., Chattipakorn N., Chattipakorn S.C. Donepezil provides neuroprotective effects against brain injury and Alzheimer's pathology under conditions of cardiac ischemia/reperfusion injury. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2021;1867(1):165975. doi: 10.1016/j.bbadis.2020.165975. [DOI] [PubMed] [Google Scholar]
- Pinheiro L., Faustino C. Therapeutic strategies targeting amyloid-β in Alzheimer’s disease. Curr. Alzheimer Res. 2019;16(5):418–452. doi: 10.2174/1567205016666190321163438. [DOI] [PubMed] [Google Scholar]
- Pohanka M. Oxidative stress in Alzheimer disease as a target for therapy. Bratisl. Lek. Listy. 2018;119(9):535–543. doi: 10.4149/BLL_2018_097. [DOI] [PubMed] [Google Scholar]
- Rabinovici G.D. Late-onset alzheimer disease. Continuum: Lifelong Learn. Neurol. 2019;25(1):14. doi: 10.1212/CON.0000000000000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I., Kode A., Biswas S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006;1(6):3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- San Tang K. The cellular and molecular processes associated with scopolamine-induced memory deficit: a model of Alzheimer's biomarkers. Life Sci. 2019;233:116695. doi: 10.1016/j.lfs.2019.116695. [DOI] [PubMed] [Google Scholar]
- Sezer E.N.Ş., Uysal T. Volatile and phenolic compositions of the leaves of two Vinca L. species from Turkey. Curr. Perspect. Med. Arom. Plants (CUPMAP) 2018;1(2):103–110. [Google Scholar]
- Sonei N., Amiri S., Jafarian I., Anoush M., Rahimi-Balaei M., Bergen H., Haj-Mirzaian A., Hosseini M.-J. Mitochondrial dysfunction bridges negative affective disorders and cardiomyopathy in socially isolated rats: pros and cons of fluoxetine. World J. Biol. Psychiatr. 2017;18(1):39–53. doi: 10.3109/15622975.2016.1149218. [DOI] [PubMed] [Google Scholar]
- Sun X.-y., Li L.-j., Dong Q.-X., Zhu J., Huang Y.-r., Hou S.-j., Yu X.-l., Liu R.-t. Rutin prevents tau pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J. Neuroinflam. 2021;18(1):1–14. doi: 10.1186/s12974-021-02182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa A., Carroll N.J. Dietary modulation of oxidative stress in Alzheimer’s disease. Int. J. Mol. Sci. 2017;18(7):1583. doi: 10.3390/ijms18071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tönnies E., Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheim. Dis. 2017;57(4):1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uma G., Maheswari S. Neuroprotective effects of polyherbal formulation (Indian) on noni scopolamineinduced memory impairment in mice. Int. J. Pharm. Pharmaceut. Sci. 2014;6(1):354–357. [Google Scholar]
- Vachnadze V.Y., Dzhakeli E., Dadeshidze I., Kintsurashvili L. Quantitative spectrophotometric determination of alkaloids in roots of Vinca herbacea. Pharmaceut. Chem. J. 2010;44(4):199–201. [Google Scholar]
- Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdinezhad A., Abbasian M., Hojjat Hosseini S., Naserzadeh P., Agh-Atabay A.H., Hosseini M.J. Protective effects of Ziziphora tenuior extract against chlorpyrifos induced liver and lung toxicity in rat: mechanistic approaches in subchronic study. Environ. Toxicol. 2017;32(9):2191–2202. doi: 10.1002/tox.22432. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y., Kojima A., Ishikawa C., Arai K. Anti-inflammatory action of donepezil ameliorates tau pathology, synaptic loss, and neurodegeneration in a tauopathy mouse model. J. Alzheim. Dis. 2010;22(1):295–306. doi: 10.3233/JAD-2010-100681. [DOI] [PubMed] [Google Scholar]
- Zargari A. Vol. 4. Tehran University Press; Tehran: 1995. (Iranian Medicinal Plants). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.