Abstract
Dietary antibiotics, including antibiotic growth promoters (AGPs), have been commonly used to improve health and growth of poultry. The present study investigated the effects of therapeutic doses of dietary antibiotics, including bacitracin methylene disalicylate (BMD), penicillin G potassium (PP) and an ionophore (salinomycin, SA), on the cecal microbiome of chickens. BMD and SA treatments were given as dietary supplements from d 1 to 35 of age. The SAPP (salinomycin+ penicillin G potassium) group was given SA as a dietary supplement from d 1 to 35 of age and PP was added to drinking water from d 19 to 24 of age to simulate common practices for control of necrotic enteritis in broilers. The cecal contents were collected from all treatment groups on d 10, 24, and 35 of age and DNA was extracted for metagenomic analysis of the cecal microbiome. The results revealed that dietary or water supplementation of therapeutic levels of antibiotics and ionophores to chickens significantly altered the cecal microbial homeostasis during different stages of the chicken life. The alpha diversity analysis showed that BMD, SA, and SAPP treatments decreased diversity and evenness of the cecal microbiome of treated chickens on d 10 of age. Species richness was also reduced on d 35 following treatment with BMD. Beta diversity analyses revealed that SAPP and BMD induced significant changes in the relative abundance of Gram-positive and -negative bacteria on d 10, while no significant differences were observed on d 24. On d 35, the non-treated control group had higher relative abundance of unclassified Gram-positive and -negative bacteria compared to SA, SAPP, and BMD treatment groups. Overall, despite their beneficial role in controlling necrotic enteritis outbreaks, the findings of this study highlight the potential negative effects of dietary supplementation of therapeutic levels of antibiotics on the gut microbiome and suggest that adjusting gut bacteria may be required to restore microbial richness and diversity of the gut microbiome following treatment with these antibiotics.
Key words: microbiome, antibiotics, ionophore, growth promoter, chicken
INTRODUCTION
Antimicrobial growth promoters (AGPs) have been used widely as feed additives to improve livestock health and productivity (Dibner and Richards, 2005). In addition to their health benefits, inclusion of subtherapeutic levels of antibiotics in poultry feed has been shown to enhance feed efficiency and growth performance and to reduce the levels of enteric bacterial pathogens, including Clostridium perfringens (Gadbois et al., 2008; Neumann and Suen 2015). However, due to public health concerns about the impact of antibiotic residues on human health and the potential emergence of antibiotic-resistant bacteria, many countries have taken steps to mitigate these risks by phasing out the use of certain antibiotics in poultry feed (United States Food and Drug Administration 2012; Agunos et al., 2019). For example, measures have been set in Canada to eliminate the preventive use of antimicrobials in poultry feed, including Category I, II, III antibiotics, while Category IV antibiotics such as ionophores that are not being used for humans will not continue to be used in the raised without antibiotic (RWA) programs. The removal of antibiotics from poultry feed has been thought to result in re-emergence of some well-controlled diseases such as necrotic enteritis (NE), consequently leading to the use of antibiotics at therapeutic doses for treatment of these diseases.
Classical AGPs including bacitracin methylene disalicylate (BMD) and ionophores such as salinomycin (SA) are commonly used in poultry production to prevent or control infectious diseases caused by Gram-positive bacteria, particularly C. perfringens-induced NE (Johansen et al., 2007). Administration of penicillin G potassium (Pot-Pen, PP) has also been shown to reduce the incidence of NE in broiler chickens, in addition to its non-specific growth-promoting effect (Gadbois et al., 2008). When subtherapeutic doses are supplemented in diet, these antibiotics exert their effects either directly through inhibition of bacterial cell wall synthesis or disintegration of the cell membrane of Gram-positive bacteria and/or indirectly through inducing significant alterations in the gut mucosal immunity and microbiome composition (Broom, 2017).
In recent years, 16S rRNA surveys, driven by next generation sequencing technology tools, have enabled researchers to distinguish the changes in the gut microbiota profiles in response to dietary changes. In this context, recent studies have shown that dietary supplementation of AGPs results in substantial reduction in the abundance of C. perfringens and other Gram-positive bacteria such as Lactobacilli, Bifidobacteria, and Streptococcus, which make up the majority of beneficial bacteria in the gastrointestinal tract (Broom, 2017), and proliferation of Gram-negative bacteria, including Salmonella and Campylobacter, perhaps due to the lack of competition for available nutrients (Kumar et al., 2019).
While the effects of subtherapeutic doses of antibiotics on the chicken gut microbiome composition and diversity have been extensively studied, relatively little is known about the impact of therapeutic levels of antibiotics on the chicken gut microbiome dynamics. A better understanding of the possible antibiotic-associated dynamic shifts of the gut microbiome will not only aid in the prudent use of antibiotics in poultry production, but will also help in developing potential therapeutics, such as probiotics, to re-shape an impaired gut microbiome following their use. Therefore, this study was undertaken to investigate the effects of different antibiotics, including bacitracin methylene disalicylate, salinomycin, and penicillin G potassium, at therapeutic dosages on the cecal microbiome composition using next generation sequencing.
MATERIALS AND METHODS
Experimental Design
Eighty one-day-old mixed broiler chicks (Ross 708), obtained from a commercial hatchery in Ontario, Canada, were randomly assigned to 4 groups (n = 20/group) and housed in separate floor pens (200 × 215 cm) with fresh litter in the same room at Arkell poultry research station, University of Guelph. Chickens in group 1 were fed a diet containing 0.15% BMD at (150 g/ton) (Bean-Hodgins and Kiarie, 2021) during each phase of growth: starter (d 1 to 10 of age; Table 1), grower (d 11 to 24 of age; Table 2), and finisher (d 25 to 35 of age; Table 3). Chickens in group 2 were fed a diet containing 0.05% SA at (50 g/ton) (Bean-Hodgins and Kiarie, 2021) during the starter, grower and finisher phases. Chickens in group 3 were given a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 g of penicillin g potassium (Pot-Pen)/100 liter of drinking water (SA + PP) from d 19 to 24 of age. Chickens in group 4 served as a negative control group and did not receive any medicated feed or water throughout the entire experiment. The room temperature was maintained at 32°C during the first week of hatch and gradually decreased to reach 27°C by d 17. Birds were exposed to fluorescent lighting in a 23 h of light (20+ lux) for the first 4 days and then a 16 light: 8 dark (10–15 lux) light cycle. Birds in all groups had free access to the food and water during the experiment. The experiment complied with the guidelines of the Canadian Council on Animal Care (CCAC) and was approved by the Animal Care Committee at the University of Guelph.
Table 1.
Starter diet formulation for broilers (d 1–10) fed bacitracin methylene disalicylate or salinomycin or no antibiotics.
| Starter |
||||
|---|---|---|---|---|
| Ingredients, % | Control | BMD | Salinomycin | Salinomycin + Pot Pen1 |
| Corn | 41.98 | 41.83 | 41.93 | 41.93 |
| Soybean meal 46% CP | 32.87 | 32.87 | 32.87 | 32.87 |
| Wheat | 10 | 10 | 10 | 10 |
| Corn gluten meal | 5 | 5 | 5 | 5 |
| Meat & bone meal | 0.4 | 0.4 | 0.4 | 0.4 |
| Choline chloride, 60% | 0.25 | 0.25 | 0.25 | 0.25 |
| Limestone | 1.55 | 1.55 | 1.55 | 1.55 |
| Iodized salt | 0.15 | 0.15 | 0.15 | 0.15 |
| Mono-dicalcium phosphorus | 1.69 | 1.69 | 1.69 | 1.69 |
| Sodium bicarbonate | 0.36 | 0.36 | 0.36 | 0.36 |
| L-Lysine-HCl | 0.35 | 0.35 | 0.35 | 0.35 |
| DL-Methionine | 0.31 | 0.31 | 0.31 | 0.31 |
| L-Threonine | 0.13 | 0.13 | 0.13 | 0.13 |
| Vitamin-mineral premix2 | 1 | 1 | 1 | 1 |
| Soybean oil | 3.96 | 3.96 | 3.96 | 3.96 |
| BMD3, % | 0 | 0.15 | 0 | 0 |
| Salinomycin4, % | 0 | 0 | 0.05 | 0.05 |
| Total | 100 | 100 | 100 | 100 |
| Calculated provisions | ||||
| AMEn (kcal/kg) | 3,000 | 3,000 | 3,000 | 3,000 |
| Crude protein, % | 24.00 | 24.00 | 24.00 | 24.00 |
| Crude fat, % | 6.72 | 6.72 | 6.72 | 6.72 |
| Calcium, % | 0.96 | 0.96 | 0.96 | 0.96 |
| Available phosphorous, % | 0.48 | 0.48 | 0.48 | 0.48 |
| dLysine, % | 1.29 | 1.29 | 1.29 | 1.29 |
| dThreonine, % | 0.87 | 0.87 | 0.87 | 0.87 |
| dMethionine, % | 0.65 | 0.65 | 0.65 | 0.65 |
| dMethionine + Cysteine, % | 0.96 | 0.96 | 0.96 | 0.96 |
| Na, g/kg | 0.16 | 0.16 | 0.16 | 0.16 |
20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SA+PP) from day 19 to 24 of age, Bio Agri Mix.
Vitamin/mineral premix contains the following per kg of diet: trans-retinol, 2.64 mg; cholecalciferol, 83 µg; dl-α-tocopherol, 36 mg; cyanocobalamin, 12.0 mg; menadione, 3.3 mg; niacin, 50.0 mg; choline, 1,200.0 mg; folic acid, 1.0 mg; biotin, 0.22 mg; pyridoxine, 3.3 mg; thiamine, 4.0 mg; calcium pantothenic acid, 15.0 mg; riboflavin, 8.0 mg; manganese, 70.0 mg; zinc, 70.0 mg; iron, 60.0 mg; iodine, 1.0 mg; copper, 10 mg; and selenium, 0.3 mg.
0.15% Medicated Bacitracin Methylene Disalicylate Premix (BMD), BMD 110G, Zoetis Inc.
0.05% Medicated Coxistac 12% Granular, Phibro Animal Health Corporation.
Table 2.
Grower diet formulation for broilers (d 11–24) fed bacitracin methylene disalicylate or salinomycin or no antibiotics.
| Grower |
||||
|---|---|---|---|---|
| Ingredients, % | Control | BMD | Salinomycin | Salinomycin + Pot Pen1 |
| Corn | 47.07 | 46.92 | 47.02 | 47.02 |
| Soybean meal 46% CP | 28.36 | 28.36 | 28.36 | 28.36 |
| Wheat | 10 | 10 | 10 | 10 |
| Corn gluten meal | 4.15 | 4.15 | 4.15 | 4.15 |
| Choline chloride, 60% | 0.22 | 0.22 | 0.22 | 0.22 |
| Limestone | 1.49 | 1.49 | 1.49 | 1.49 |
| Iodized salt | 0.16 | 0.16 | 0.16 | 0.16 |
| Mono-dicalcium phosphorus | 1.61 | 1.61 | 1.61 | 1.61 |
| Sodium bicarbonate | 0.35 | 0.35 | 0.35 | 0.35 |
| L-Lysine-HCl | 0.32 | 0.32 | 0.32 | 0.32 |
| DL-Methionine | 0.28 | 0.28 | 0.28 | 0.28 |
| L-Threonine | 0.11 | 0.11 | 0.11 | 0.11 |
| Vitamin-mineral premix2 | 1 | 1 | 1 | 1 |
| Soybean Oil | 4.88 | 4.88 | 4.88 | 4.88 |
| Pork Meal | 0 | 0 | 0 | 0 |
| BMD3, % | 0 | 0.15 | 0 | 0 |
| Salinomycin4, % | 0 | 0 | 0.05 | 0.05 |
| Total | 100 | 100 | 100 | 100 |
| Calculated provisions | ||||
| AMEn (kcal/kg) | 3,100 | 3,100 | 3,100 | 3100 |
| Crude protein, % | 21.50 | 21.50 | 21.50 | 21.50 |
| Crude fat, % | 7.65 | 7.65 | 7.65 | 7.65 |
| Calcium, % | 0.87 | 0.87 | 0.87 | 0.87 |
| Available phosphorous, % | 0.44 | 0.44 | 0.44 | 0.44 |
| dLysine, % | 1.15 | 1.15 | 1.15 | 1.15 |
| dThreonine, % | 0.77 | 0.77 | 0.77 | 0.77 |
| dMethionine, % | 0.58 | 0.58 | 0.58 | 0.58 |
| dMethionine + Cysteine, % | 0.87 | 0.87 | 0.87 | 0.87 |
| Available phosphorous, % | 0.44 | 0.44 | 0.44 | 0.44 |
| Na, g/kg | 0.16 | 0.16 | 0.16 | 0.16 |
20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SA+PP) from day 19 to 24 of age, Bio Agri Mix.
Vitamin/mineral premix contains the following per kg of diet: trans-retinol, 2.64 mg; cholecalciferol, 83 µg; dl-α-tocopherol, 36 mg; cyanocobalamin, 12.0 mg; menadione, 3.3 mg; niacin, 50.0 mg; choline, 1,200.0 mg; folic acid, 1.0 mg; biotin, 0.22 mg; pyridoxine, 3.3 mg; thiamine, 4.0 mg; calcium pantothenic acid, 15.0 mg; riboflavin, 8.0 mg; manganese, 70.0 mg; zinc, 70.0 mg; iron, 60.0 mg; iodine, 1.0 mg; copper, 10 mg; and selenium, 0.3 mg.
0.15% Medicated Bacitracin Methylene Disalicylate Premix (BMD), BMD 110G, Zoetis Inc.
0.05% Medicated Coxistac12% Granular, Phibro Animal Health Corporation.
Table 3.
Finisher diet formulation for broilers (d 25–35) fed bacitracin methylene disalicylate or salinomycin or no antibiotics.
| Finisher |
||||
|---|---|---|---|---|
| Ingredients, % | Control (%) | BMD (%) | Salinomycin (%) | Salinomycin (%) + Pot Pen1 |
| Corn, % | 55.40 | 55.25 | 55.35 | 55.35 |
| Soybean meal 46% CP | 21.69 | 21.69 | 21.69 | 21.69 |
| Wheat | 7.76 | 7.76 | 7.76 | 7.76 |
| Corn gluten meal | 3.4 | 3.4 | 3.4 | 3.4 |
| Choline chloride, 60% | 0.20 | 0.20 | 0.20 | 0.20 |
| Limestone | 0.89 | 0.89 | 0.89 | 0.89 |
| Iodized salt | 0.25 | 0.25 | 0.25 | 0.25 |
| Mono-dicalcium phosphorus | 0.84 | 0.84 | 0.84 | 0.84 |
| Sodium bicarbonate | 0.16 | 0.16 | 0.16 | 0.16 |
| L-Lysine-HCl | 0.31 | 0.31 | 0.31 | 0.31 |
| DL-Methionine | 0.26 | 0.26 | 0.26 | 0.26 |
| L-Threonine | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin-mineral premix2 | 1 | 1 | 1 | 1 |
| Soybean oil | 5 | 5 | 5 | 5 |
| Pork meal-60% | 2.74 | 2.74 | 2.74 | 2.74 |
| BMD3, % | 0 | 0.15 | 0 | 0 |
| Salinomycin4, % | 0 | 0 | 0.05 | 0.05 |
| Total | 100 | 100 | 100 | 100 |
| Calculated provisions | ||||
| AMEn (kcal/kg) | 3,200 | 3,200 | 3,200 | 3,200 |
| Crude Protein, % | 19.75 | 19.75 | 19.75 | 19.75 |
| Crude Fat, % | 8.18 | 8.18 | 8.18 | |
| Calcium, % | 0.79 | 0.79 | 0.79 | 0.79 |
| dLysine, % | 1.03 | 1.03 | 1.03 | 1.03 |
| dThreonine, % | 0.69 | 0.69 | 0.69 | 0.69 |
| dMethionine (%) | 0.55 | 0.55 | 0.55 | 0.55 |
| dMethionine + Cysteine, % | 0.80 | 0.80 | 0.80 | 0.80 |
| Available phosphorous, % | 0.39 | 0.39 | 0.39 | 0.39 |
| Na, g/kg | 0.16 | 0.16 | 0.16 | 0.16 |
20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SA+PP) from d 19 to 24 of age, Bio Agri Mix.
Vitamin/mineral premix contains the following per kg of diet: trans-retinol, 2.64 mg; cholecalciferol, 83 µg; dl-α-tocopherol, 36 mg; cyanocobalamin, 12.0 mg; menadione, 3.3 mg; niacin, 50.0 mg; choline, 1,200.0 mg; folic acid, 1.0 mg; biotin, 0.22 mg; pyridoxine, 3.3 mg; thiamine, 4.0 mg; calcium pantothenic acid, 15.0 mg; riboflavin, 8.0 mg; manganese, 70.0 mg; zinc, 70.0 mg; iron, 60.0 mg; iodine, 1.0 mg; copper, 10 mg; and selenium, 0.3 mg.
0.15% Medicated Bacitracin Methylene Disalicylate Premix (BMD).
0.05% Medicated Coxistac12% Granular, Phibro Animal Health Corporation.
DNA Extraction and Sequencing
On d 10, 24, and 35 of age, five birds randomly collected from each treatment group and euthanized by CO2 inhalation. Cecal contents were collected from both ceca, snap-frozen on dry ice and then stored at −80°C until further use (Zhou et al., 2007). DNA was extracted from the cecal contents using QIAamp DNA Stool mini kit (Cat. No. / ID:51604) as per manufacturer's instruction (Qiagen, Toronto, Canada). DNA quality and concentration were assessed with a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). The DNA samples with 260/280 ratio between 1.8 and 2 were considered pure and were used for further processing. DNA concentration was measured and adjusted to 10 ng/μL in a volume of 10 μL nuclease-free water. The V4 hypervariable region of the 16S rRNA gene was amplified and sequenced with an Illumina MiSeq sequencer at the Genome Quebec Innovation Center. The pair primer set used in the study was 564F YTGGGYDTAAAGNG and 785R TACNVGGGTATCTAATCC. A total of 1,359,182 sequences were produced with an average number of 22,653 sequences per sample.
Sequence Processing and Bioinformatics Analysis
Processing of sequence data was performed using Mothur (v.1.44.) (Schloss, 2009) software, following the MiSeq SOP (Kozich et al., 2013). Briefly, make.contigs command was applied to assemble the read pairs into contigs. Non-assembled and redundant contigs were removed by screen.seqs and unique.seqs commands, respectively. The sequences that did not align to V4 region of the 16S rRNA gene and had ambiguous bases less than 254 bp or were not classified as bacteria or contained homopolymers of >9 bp removed. No samples were removed due to low reads. SILVA bacteria reference database 138 was used to align the sequences. The redundant aligned sequences created by trimming the ends were removed by unique.seqs command. The chimera.vsearch command was used to remove the chimeric sequences. The dist.seqs (cutoff = 0.03) and cluster commands were used to cluster the sequences into operation taxonomic units (OTUs), and the number of sequences per OTU was determined by using the make.shared command. Taxonomy was assigned against SILVA database (v.138.). Alpha and beta microbial community diversity analysis were performed using standard Mothur pipelines. Bacterial community dissimilarity in the treatments was measured by Nonmetric multidimensional scaling (NMDS) using Bray Curtis dissimilarity index.
Permutational multivariate analysis of variance (PERMANOVA) (‘Adonis’ function, vegan package, R [v.3.5.1.]; 1,000 permutations) was used to analyze statistical comparison of spatial separation on the nMDS. The significant differences between the taxa associated with each treatment were analyzed with the package DESeq2 (Love et al., 2014) in R software.
RESULTS
Alpha Diversity of the Cecal Microbiome
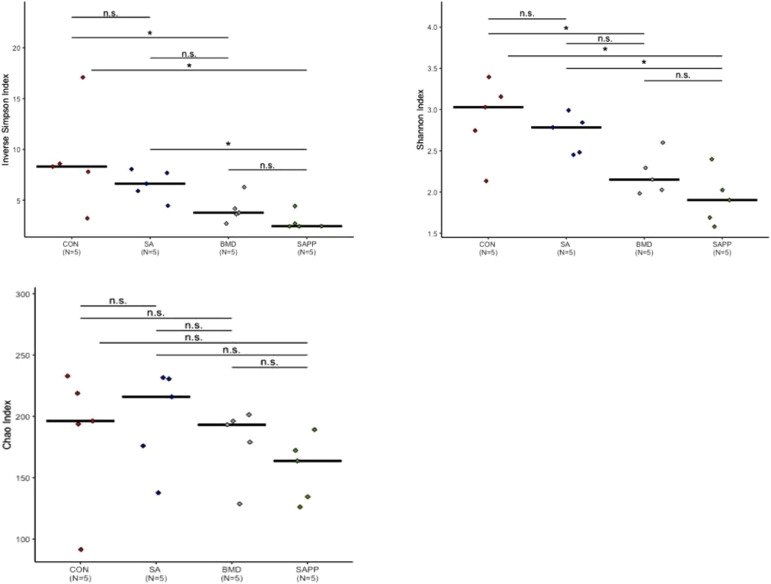
Alpha diversity Chao1 (richness measures), and Shannon and Simpson diversity (evenness measures) were carried out using Mothur software. On d 10 (Figure 1), alpha diversity indexes indicated that chickens in the SA, BMD, and SAPP treatment groups had significantly different cecal microbial evenness and diversity compared to the non-treated control group. The Shannon index showed that chickens in the control group had higher microbial evenness (P < 0.05, ANOVA) compared to those in SAPP group and marginal evenness compared to those in the SA treatment group (Figure 1). Chickens in the SAPP treatment group had lower evenness compared to those in the SA treatment group (Figure 1).
Figure 1.
Alpha diversity analysis illustrating evenness, richness and diversity measures of cecal microbiome of chickens on d 10 of receiving a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the growing phase: starter (d 1 to 10 of age), grower (d 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50 g/ton) during the gowning phase from d 1 to 35 of age, or a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SA + PP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group). ∗ indicates a significant difference at the 0.05 level between CON, SA, SAPP, and BMC that were compared. n.s. indicate a no significant difference at less than 0.05 between CON, SA, SAPP, and BMD that were compared.
Based on inverse Simpson's, chickens in the control group had higher microbial diversity (P < 0.05, ANOVA) in comparison with those in the SAPP treatment group (Figure 1). Further, the Chao diversity index demonstrated that species richness of the cecal bacterial community was not affected by any of the antibiotic treatments (Figure 1).
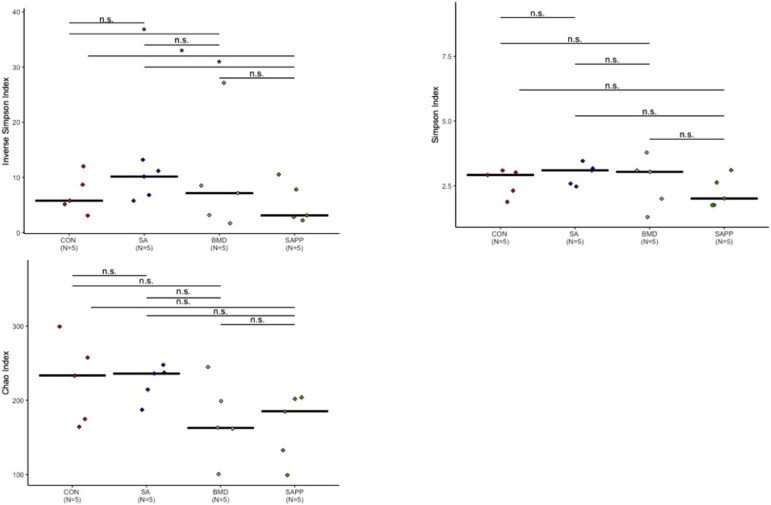
On d 24, the Chao, Shannon, and Inverse Simpson indexes revealed that none of the antibiotic treatments significantly altered either richness or evenness of the cecal bacterial community (Figure 2).
Figure 2.
Alpha diversity analysis illustrating evenness, richness and diversity measures of cecal microbiome of chickens on d 24 of receiving a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (d 1 to 10 of age), grower (d 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50 g/ton) during the gowning phase from d 1 to 35 of age, or a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SA + PP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group). ∗ indicates a significant difference at the 0.05 level between CON, SA, SAPP, and BMC that were compared. n.s. indicate a no significant difference at less than 0.05 between CON, SA, SAPP, and BMD that were compared.
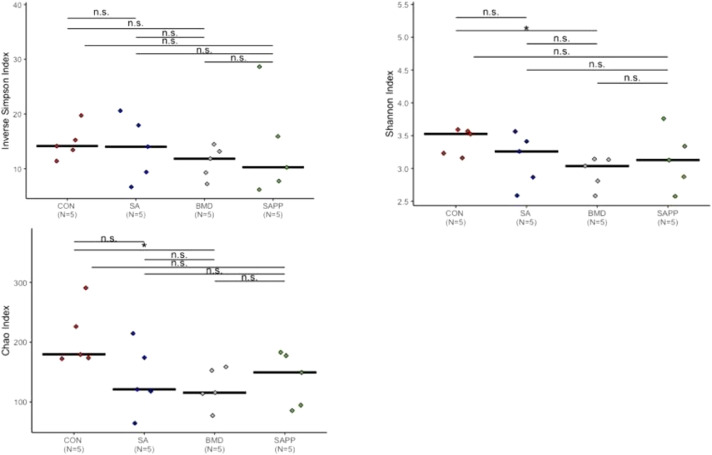
On d 35 (Figure 3), the Chao and Shannon indexes indicated that richness and evenness of the cecal bacteria community in the control group was significantly higher (P < 0.05, ANOVA) than BMD treatment group (Figure 3). The inverse Simpson index did not show any changes in the evenness of the cecal bacterial community by any of the antibiotic treatments (Figure 3).
Figure 3.
Alpha diversity analysis illustrating evenness, richness and diversity measures of cecal microbiome of chickens on d 35 of receiving a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (d 1 to 10 of age), grower (d 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50 g/ton) during the gowning phase from d 1 to 35 of age, or a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SA + PP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group). ∗ indicates a significant difference at the 0.05 level between CON, SA, SAPP, and BMC that were compared. n.s. indicate a no significant difference at less than 0.05 between CON, SA, SAPP, and BMD that were compared.
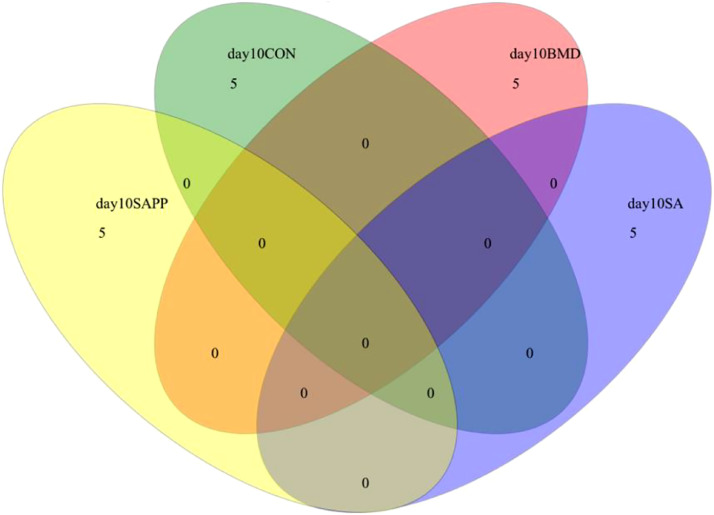
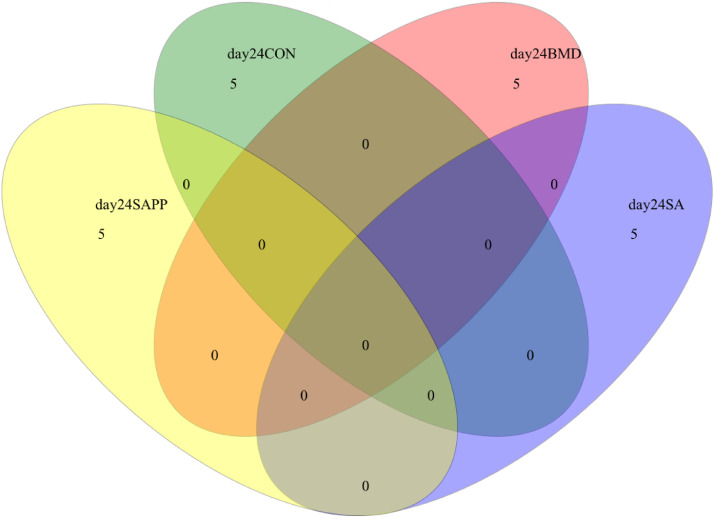
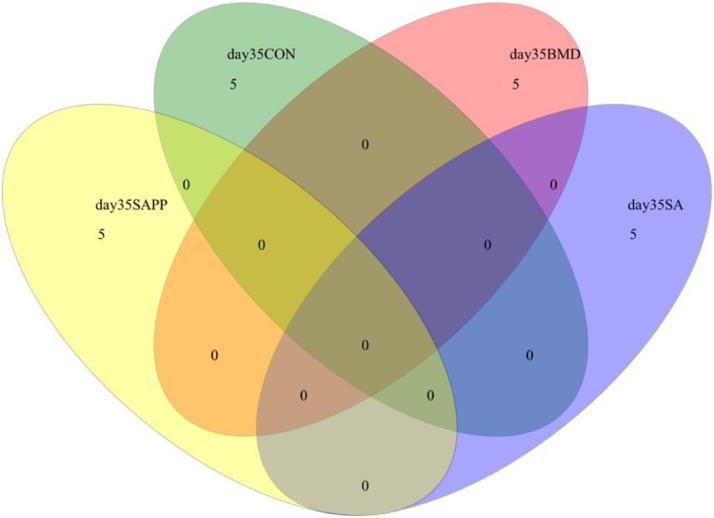
There were not any species shared among the control, SA, and SAPP groups on d 10, 24, and 35 (Figure 4, Figure 5, Figure 6).
Figure 4.
Venn diagram illustrates to compare the microbiome richness shared among chickens on d 10 of receiving a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (d 1 to 10 of age), grower (d 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50 g/ton) during the gowning phase from d 1 to 35 of age, or a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SA+PP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group).
Figure 5.
Venn diagram illustrates to compare the microbiome richness shared among chickens on d 24 of receiving a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (d 1 to 10 of age), grower (d 11 to 24 of age), and finisher (days 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50 g/ton) during the gowning phase from d 1 to 35 of age, or a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SA + PP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group).
Figure 6.
Venn diagram illustrates to compare the microbiome richness shared among chickens on d 35 of receiving a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (d 1 to 10 of age), grower (d 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50 g/ton) during the gowning phase from day 1 to 35 of age, or a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SA + PP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group).
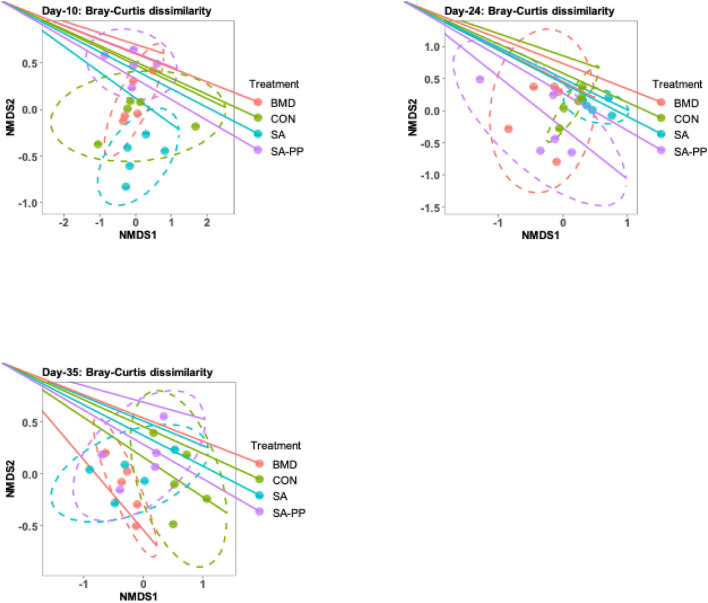
Beta Diversity of the Cecal Microbiome
Beta diversity of the cecal microbiome in chickens was measured in NMDS with Bray Curtis index on d 10, 24, and 35 (Figure 7). Analysis of PERMANOVA was performed to determine the difference in the cecal bacterial communities among treatment groups (Table 4). All pairwise comparisons were significant using ADONIS at 0.05 using Benjimani-Hochberg correction for multiple comparison.
Figure 7.
Nonmetric multidimensional scaling (NMDS) plot illustrating the chicken cecal microbiome beta-diversity on d 10, 24, and 35 following treatment with either a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (d 1 to 10 of age), grower (d 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50 g/ton) during the gowning phase from d 1 to 35 of age, or a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SAPP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group).
Table 4.
ANOVA P-value of treatment groups on d 10, 24, and 35 (iterations = 1000).
| CON | SA | BMD | SA+PP | |
|---|---|---|---|---|
| CONa-D10 | - | - | - | - |
| CON-D24 | - | - | - | - |
| CON-D35 | - | - | - | - |
| SAb-D10 | 0.003 | - | - | - |
| SA-D24 | 0.003 | - | - | - |
| SA-D35 | 0.01 | - | - | - |
| BMDc-D10 | 0.40 | 0.16 | - | - |
| BMD-D24 | 0.50 | 0.12 | - | - |
| BMD-D35 | 0.008 | 0.06 | - | - |
| SA-PPd-D10 | 0.15 | 0.005 | 0.26 | - |
| SA-PP-D24 | 0.12 | 0.015 | 0.86 | - |
| SA-PP-D35 | 0.06 | 0.31 | 0.04 | - |
CON is control treatment.
SA is salinomycin treatment.
BMD is bacitracin methylene disalicylate.
SA-PP is salinomycin plus penicillin G potassium.
On d 10, the cecal microbial community structure of the SA-treated chickens was significantly dissimilar from the control group (Table 4). SA-treated chickens were different in the cecal microbial structure compared to the SAPP group (Table 4).
On d 24, the cecal microbial community structure of the SA-treated chickens was different from that of the SAPP-treated and control groups (Table 4).
On d 35, the cecal microbial community structure of the SA, BMD, and SAPP groups varied significantly from that of the control group (Table 4). The microbial structure of the BMD-treated group was different from that of the SA- and SAPP-treated groups (Table 4).
Differential Enrichment of the Cecal Microbiome
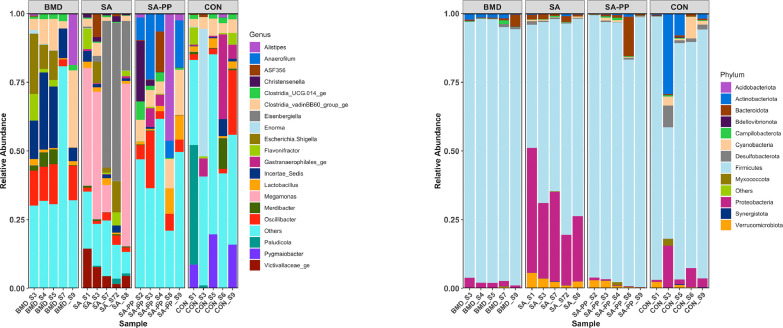
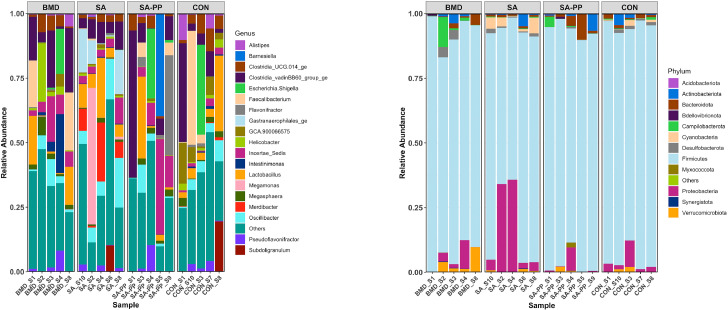
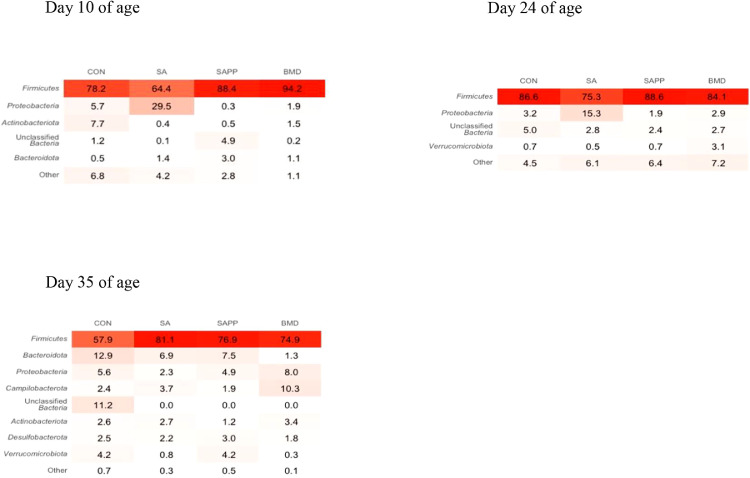
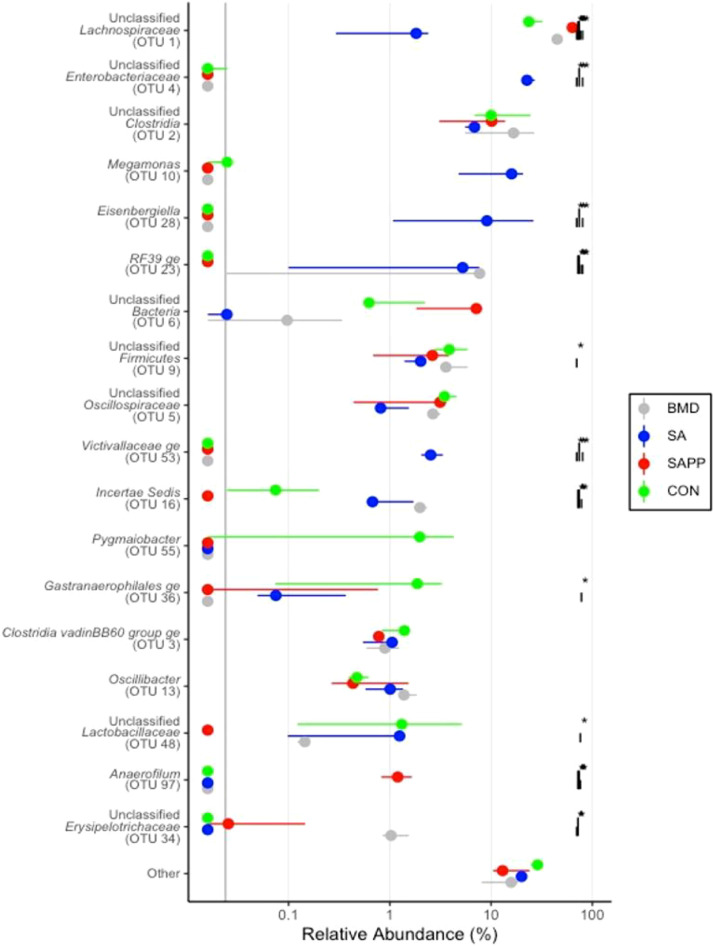
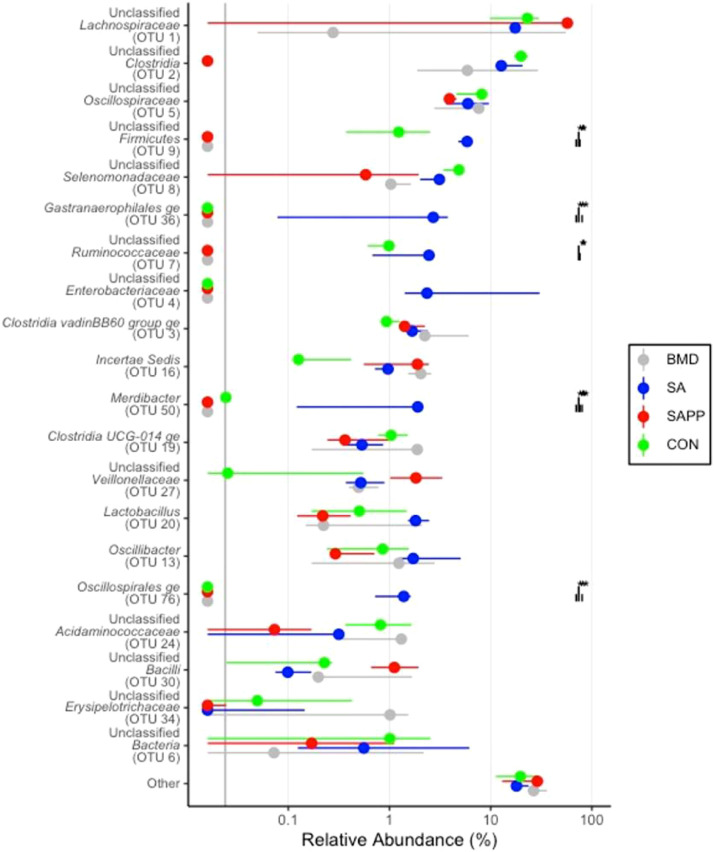
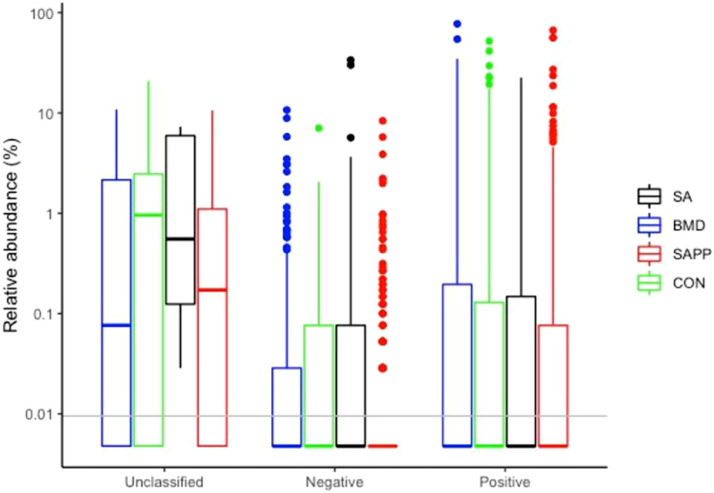
Differences in the cecal microbiome of the antibiotic-treated and control chickens on d 10, 24, and 35 are shown in Figure 8, Figure 9, Figure 10. At the phylum level, on d 10, 24, and 35, the most abundant phylum between all treatments was Firmicutes (Figure 11). Proteobacteria was the most abundant phylum in the SA treatment group on d 10 (29.5%), 24 (15.3%), and 35 (8.0%; Figure 11). Actinobacteria proportion was higher in the control groups compared to other treatment groups on Day 10 (Figure 11). On d 35, the relative abundance of phylum Campilobacterota was higher in the BMD-treated chickens in comparison with other treatments (Figure 11). DESeq2 analysis was used to compare differential enrichment of the cecal microbiome of the antibiotic-treated and control chickens (Love et al., 2014; Figures 12, 14 and 15). On d 10, the cecal microbiome of the SA group was enriched with genera Eisenbergiella, Victivallaceae ge, and Incertae Sedis and family Enterobacteriaceae. The most abundant genus in the SAPP group was Anaerofilum (Figure 12). RF39 ge was also found to be more abundant in SA and BMD treatment groups on d 10 (Figure 12). On the other hand, the SA and SAPP treatments resulted in a significant decrease in the unclassified Firmicutes (Figure 12). The relative abundance of Gram-positive and -negative bacteria on d 10 post-hatch was higher in the SAPP treatment and control groups (Figure 13).
Figure 8.
Differences in the cecal microbiome of the antibiotic-treated and control chickens on d 10 following treatment with either a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (d 1 to 10 of age), grower (d 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50 g/ton) during the gowning phase from day 1 to 35 of age, or a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SAPP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group). (A) Each bar represents the relative abundance of each bacterial taxa of chicken at phylum and genus level.
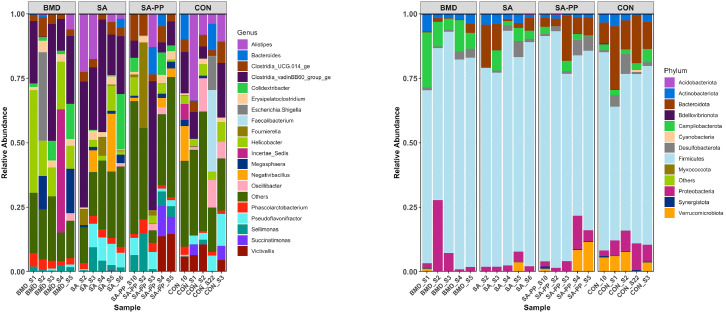
Figure 9.
Differences in the cecal microbiome of the antibiotic-treated and control chickens on d 24 following treatment with either a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (d 1 to 10 of age), grower (d 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50g/ton) during the gowning phase from d 1 to 35 of age, or a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SAPP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group). (A) Each bar represents the relative abundance of each bacterial taxa of chicken at phylum and genus level.
Figure 10.
Differences in the cecal microbiome of the antibiotic-treated and control chickens on d 35 following treatment with either a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (d 1 to 10 of age), grower (d 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50g/ton) during the gowning phase from d 1 to 35 of age, or a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SAPP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group). (A) Each bar represents the relative abundance of each bacterial taxa of chicken at phylum and genus level.
Figure 11.
Heatmap of relative abundance of the phylum-level changes in the cecal microbiome of the antibiotic-treated and control chickens on d 10, 24, and 35 following treatment with either a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (d 1 to 10 of age), grower (d 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50 g/ton) during the gowning phase from day 1 to 35 of age, or a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SAPP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group).
Figure 12.
DESeq2 analysis illustrating differential enrichment of the cecal microbiome of the antibiotic-treated and control chickens on 10 following treatment with either a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (d 1 to 10 of age), grower (d 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50 g/ton) during the gowning phase from d 1 to 35 of age, or a diet containing 0.05% salinomycin from day 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SAPP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group). | indicates a comparison between treatments. ∗ indicates a significant difference at less than 0.05 between CON, SA, SAPP, and BMC that were compared.
Figure 14.
DESeq2 analysis illustrating differential enrichment of the cecal microbiome of the antibiotic-treated and control chickens on d 24 following treatment with either a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (d 1 to 10 of age), grower (d 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50 g/ton) during the gowning phase from d 1 to 35 of age, or a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SAPP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group). | indicates a comparison between treatments. ∗ indicates a significant difference at less than 0.05 between CON, SA, SAPP, and BMC that were compared.
Figure 15.
Differences in the cecal Gram-positive and negative microbiome of the antibiotic-treated and control chickens on d 24 following treatment with either a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (d 1 to 10 of age), grower (d 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50 g/ton) during the gowning phase from d 1 to 35 of age, or a diet containing 0.05% salinomycin from day 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SAPP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group).
Figure 13.
Differences in the cecal Gram-positive and negative microbiome of the antibiotic-treated and control chickens on d 10 following treatment with either a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (d 1 to 10 of age), grower (d 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50 g/ton) during the gowning phase from d 1 to 35 of age, or a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SAPP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group).
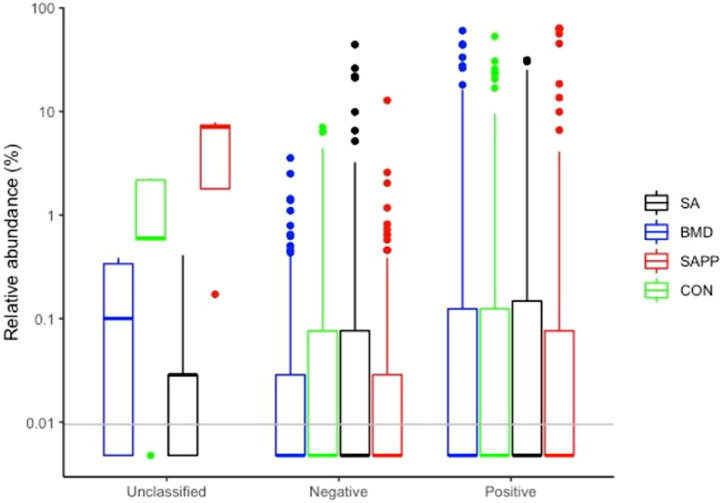
On d 24, the cecal microbiome of the SA treatment and control groups was dominated with the family Ruminococcaceae, a butyrate-producing bacterium (Meehan and Beiko, 2014; Figure 14). The SA treatment group had higher relative abundance of genus Gastranaerophilales ge, Merdibacter and Oscillospirales ge compared to other treatment groups (Figure 14). There was not significant difference in the relative abundance of Gram-positive and -negative bacteria between the control group and SA, BMD, and SAPP treatment groups on d 24 (Figure 15).
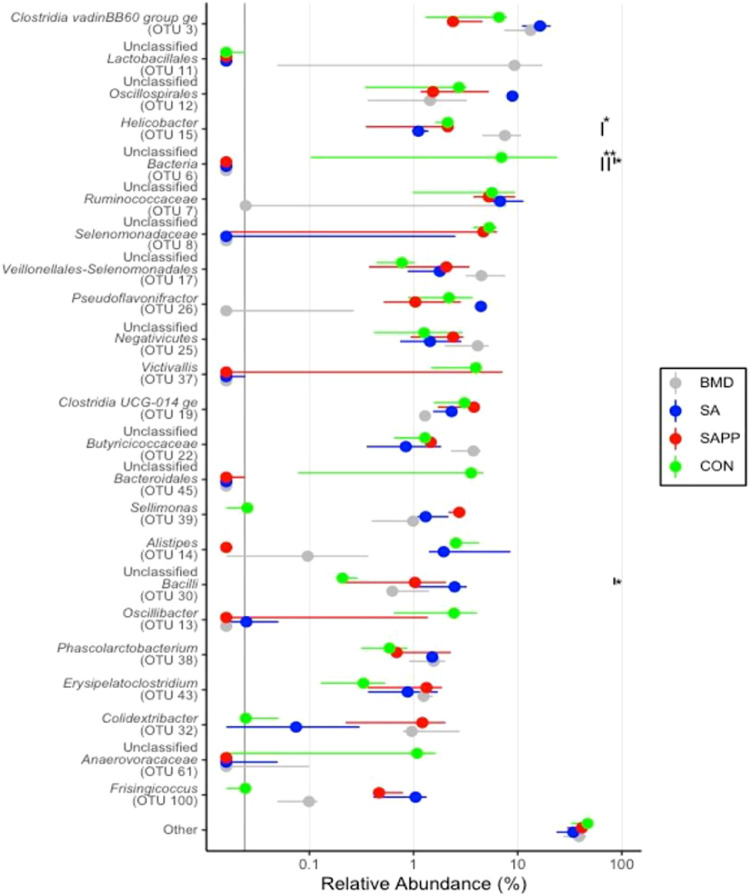
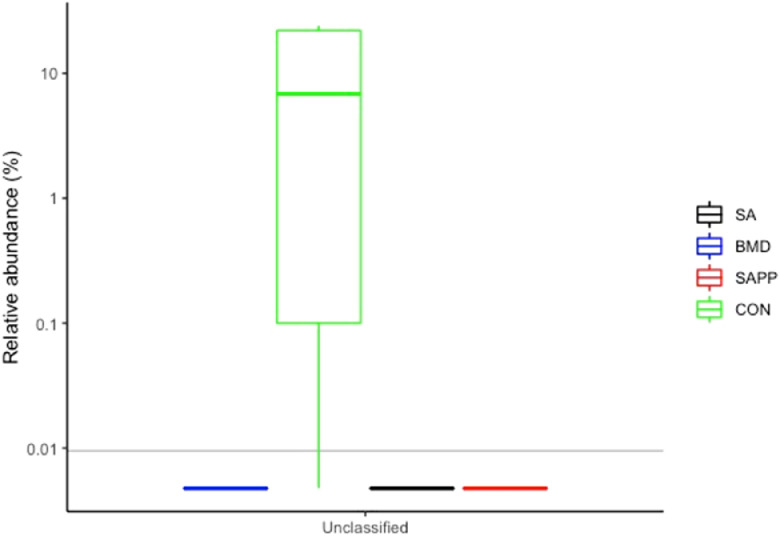
On d 35, the cecal microbiome of the BMD group was significantly enriched with the genus Helicobacter (Figure 16), while the control group was enriched with the unclassified either Gram-positive or Gram-negative bacteria (Figure 16). The SA group had high abundance of unclassified class Bacilli (Figure 16). The cecal microbiome of the control group had significantly higher number of unclassified Gram-positive and -negative bacteria on d 35 (Figure 17).
Figure 16.
DESeq2 analysis illustrating differential enrichment of the cecal microbiome of the antibiotic-treated and control chickens on d 35 following treatment with either a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (days 1 to 10 of age), grower (d 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50 g/ton) during the gowning phase from d 1 to 35 of age, or a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SAPP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group). | indicates a comparison between treatments. ∗ indicates a significant difference at less than 0.05 between CON, SA, SAPP, and BMC that were compared.
Figure 17.
Differences in the cecal Gram-positive and negative microbiome of the antibiotic-treated and control chickens on d 24 following treatment with either a diet containing 0.15% bacitracin methylene disalicylate (BMD) at (150 g/ton) during the gowning phase: starter (d 1 to 10 of age), grower (days 11 to 24 of age), and finisher (d 25 to 35 of age), or a diet containing 0.05% salinomycin (SA) at (50 g/ton) during the gowning phase from d 1 to 35 of age, or a diet containing 0.05% salinomycin from d 1 to 35 of age and had access to medicated water containing 20 grams of penicillin G potassium (Pot-Pen)/100 liter of drinking water (SAPP) from d 19 to 24 of age, or did not receive any medicated feed or water throughout the entire experiment (a negative control group).
DISCUSSION
In recent years, there has been extensive research on the mechanisms of action of AGPs, particularly their role in the modulation of gut microbiome composition (Engberg et al., 2000; Pourabedin et al., 2014; Costea et al., 2017; Proctor and Phillips 2019; Robinson et al., 2019). Despite the general consensus that AGPs can change gut microbial composition either by enhancing or limiting the growth of certain microbial species (Broom, 2017; Torok et al., 2011), there is scarcity of information about the effects of therapeutic doses of antibiotics on richness (number of bacterial species) and evenness (relative distribution) of microbial communities in the chicken gut. As a case in point, while some studies demonstrated that supplementation of diet with AGPs such as avilamycin (Choi et al., 2018) and zinc bacitracin (Díaz Carrasco et al., 2018) decreases the alpha diversity index of the cecal microbiome, other studies revealed that their supplementation has no major effects (Danzeisen et al., 2011; Crisol-Martínez et al., 2017; Proctor and Phillips, 2019). These conflicting data can be attributed to several environmental and experimental factors, including animal age, diets, stressors, and PCR biases (Hume et al., 2003; Yin et al., 2010; Torok et al., 2011; Zou et al., 2018).
In the present study, on d 10, the evenness and diversity of the cecal microbiome were decreased by BMD, SA, and SAPP treatments, while no such changes were observed on d 24 and 35. In addition, supplementation of the diet with these antibiotics decreased species richness compared to the control group on d 24 and 35. This could be attributed to their inhibitory or bactericidal effects against some Gram-positive bacteria which are known to dominate the gut microbiome of chickens during the first week of life (Ballou et al., 2016). In fact, the cecal microbiome of newly hatched chickens is initially dominated by Gram-negative bacteria, which shifts during the first week of age to Gram-positive bacteria (Ballou et al., 2016) and as chickens age, their cecal microbial community changes to a more stable and complex population (Hume et al., 2003; Danzeisen et al., 2011; Torok et al., 2011; Glendinning et al., 2019). The results of the present study are consistent with a previous study by Chen et al. (2020), in which supplementation of the diet with virginiamycin decreased the Chao index of the cecal microbiome. In another study, while supplementation with ionophores (monensin and salinomycin) demonstrated a profound impact on richness and evenness of bacteria in the cecal microbiome of chickens compared to classic antibiotics including bacitracin methylene disalicylate, tylosin, and virginiamycin (Robinson et al., 2019), our results showed that BMD, SAPP, and SA treatments have comparable effects on microbial richness and evenness of the cecal microbiome.
Further, the results of Shannon analyses indicated that treatment with SAPP significantly decreased the diversity of the cecal microbiome, while treatment with BMD marginally decreased the diversity of the cecal microbiome, compared to the control group. Our results revealed that therapeutic levels of either antibiotics or ionophore induced significant alterations in the cecal microbial diversity compared to the subtherapeutic levels of these compounds. For instance, Robinson et al. (2019) observed that subtherapeutic concentrations of BMD, tylosin, and virginiamycin had no significant effects on the diversity of the chicken cecal microbiome. A possible explanation for this might be the below minimum inhibitory concentrations (sub-MIC) effects for the antibiotics used (Broom, 2017).
With respect to their effect on the cecal microbial composition, previous studies have shown that AGPs and ionophores differentially affect the cecal microbial communities (Broom, 2017; Robinson et al., 2019). Consistent with the results of Torok et al. (2011), dietary supplementation with SA resulted in enrichment of family Enterobacteriaceae, which includes many potentially pathogenic bacteria, such as Escherichia coli, Shigella, Salmonella, and Klebsiella, in the cecal microbiome on d 35. The family Enterobacteriaceae constitutes less than 1% of healthy intestinal microbiome (He et al., 2020). Although their presence is important for maintaining immunological balance in the intestine (Scarpa et al., 2011), the increased abundance of members of this family may result in economically significant enteric diseases including mucosal ulceration (He et al., 2020).
On d 10, the family Victivallaceae and Erysipelotrichaceae and the genus Eisenbergiella were most dominant in chickens fed SA. Families Victivallaceae and Erysipelotrichaceae are associated with improving feed conversion ratios in poultry while the genus Incertae Sedis has been associated with poor feed conversation ratios (Singh et al., 2012; Moore and Stanley et al., 2016). Family Victivallaceae, which belongs to the phylum Verrucomicrobiota, is a normal bacterial inhabitant in the human gut and a higher abundance of this family was observed in stool samples of patients with inflammatory bowel disease (IBD) (Bamola et al., 2017). Furthermore, SA treatment increased the relative abundance of genus Eisenbergiella, which belongs to the family Lachnospiraceae. Despite Eisenbergiella’s beneficial role in promoting gut health through expressing enzymes that mediate the production of butyrate, lactate, acetate and succinate (Amir et al., 2014), a positive correlation was observed between the abundance of genus Eisenbergiella and Salmonella enterica serovar Typhimurium in the gut microbiome (Khan and Chousalkar, 2020). Robinson et al. (2019) have also observed that while supplementation of chicken diets with antibiotics, such as bacitracin methylene disalicylate, tylosin and virginiamycin, and ionophores, such as monensin and salinomycin, enriched the cecal microbiome with members of the family Ruminococcaceae, they significantly reduced the abundance of Lachnospiraceae species.
Supplementation of SAPP has been shown to increase the abundance of genus Anaerofilum which belongs to family Ruminococcaceae (Lee et al., 2017). A previous study by Danzeisen and colleagues has demonstrated that administration of coccidiostats, such as monensin and monensin/virginiamycin or tylosin, increases the abundance of Anaerofilum (Danzeisen et al., 2011). Furthermore, SAPP treatment also decreased the relative abundance of Incertae Sedis belonging to the family Ruminococcaceae and the abundance of family Lactobacillaceae, which are both known as butyrate-producing bacteria and have been previously shown to play a crucial role in improving feed efficiency (Meehan and Beiko, 2014; De Cesare et al., 2019). We also noted that on d 24, the cecal microbiome of SA-treated chickens and those in the control groups was enriched with family Ruminococcaceae which belongs to class Clostridia, whose members are known to produce butyrate that possesses anti-inflammatory activities and contributes to the intestinal development of chickens (Onrust et al., 2015).
Experimental evidence indicates that butyrate produced by members of the phylum Firmicutes, during the early stage of the chicken life, is crucial for intestinal cell growth (Polansky et al., 2016). In view of this, the dominance of butyrate-producing bacteria and those expressing enzymes that are involved in butyrate production in the cecal microbiome of SA-, BMD-, and SAPP-treated chickens is indicative of the growth promoting role of these antibiotics.
The genus Gastranaerophilales ge, belonging to phylum Cyanobacteria, was most abundant in the SA treatment group compared to BMD, SAPP and control groups. It is well documented that the abundance of genus Gastranaerophilales ge positively correlates with the average daily gain and some aspects of immune response in birds (Li et al., 2020). Recent studies have shown that members of class Gammaproteobacteria were associated with metabolic disorders and intestinal inflammation (Abaidullah et al., 2019; Gorecki et al., 2019). The birds treated with SA had a higher abundance of genus Merdibacter, belonging to class bacilli, in comparison with other treatments. Merdibacter facilitates the release of energy from food by secreting enzymes that are involved in the pentose phosphate pathways in the microbiome of human intestine (Anani et al., 2019). SA treatment also resulted in an increase in the relative abundance of genus Oscillospirales ge in the cecal microbiome. Adewole and Akinyemi et al. (2021) demonstrated that birds fed with high or normal energy diets treated with BMD had high abundance of genus Oscillospirales ge which was shown to negatively correlate with the population of the pathogenic bacteria, including Streptococcus (Adewole and Akinyemi et al., 2021).
Contrary to a previous observation by Danzeisen et al. (2011) that treatment with monensin and monensin/virginiamycin and monensin/tylosin decreases the abundance of Bacilli on d 7, 14, and 35 age of old, our results revealed that the cecal microbiome of chickens treated with SA had higher relative abundance of class Bacilli compared to the BMD, SAPP and control groups on d 35. These different effects may be attributed to their mode of action in disturbing cation transport across the cell membrane of bacteria; salinomycin stimulates K+ and Na+ ions transportation across the cytoplasmic membrane, while monensin stimulates Na+ and H+ ions (Robinson et al., 2019).
Our study demonstrated a significant reduction in the abundance of family Lactobacillaceae in the cecal microbiome of SAPP-treated chickens and a dominance of this family in the cecal microbiome of SA- and BMD-treated and control chickens on d 10. Robinson et al. (2019) observed a depletion of genus Lactobacillus in the chicken cecal microbiome on d 14 following treatment with antibiotics, including bacitracin methylene disalicylate, tylosin and virginiamycin, and ionophores, including monensin and salinomycin. On the other hand, other studies have demonstrated that supplementation of chickens with SA or BMD decreases the number of Lactobacillus in the chicken intestinal microbiome (Engberg et al., 2000; Crisol-Martínez et al., 2017). To better interpret the variability in these data, comparative metagenomics studies are required to investigate the dynamic changes of the gut microbiome in response to different concentrations of antibiotics in age-matched chickens.
Overall, it appears that dietary supplementation of therapeutic levels of BMD, SA, and SAPP to chickens profoundly affected diversity and community composition of the cecal microbiome as demonstrated by depletion of several classes of beneficial bacteria, including Bacilli (Elshaghabee et al., 2017; Anani et al., 2019), Clostridia (Lopetuso et al., 2013; Onrust et al., 2015), and Actinobacteria (Binda et al., 2018), which all play a pivotal role in maintaining gut health.
Furthermore, supplementation with salinomycin has been shown to increase the abundance of pathogenic bacteria, including order Gastranaerophilales (Li et al., 2020), class Gammaproteobacteria (Gorecki et al., 2019; Abaidullah et al., 2019) and family Enterobacteriaceae (He et al., 2020) that have been previously shown to be associated with metabolic disorders and gastrointestinal diseases.
It is noteworthy that a few members of phyla Bacteroidetes and Firmicutes, particularly butyrate-producing bacteria, were enriched in the cecal microbiome of both antibiotic-and ionophore-treated chickens, albeit not at the same level of diversity and abundance as in the control chickens.
CONCLUSIONS
Dietary or water supplementation of chickens with therapeutic levels of antibiotics and ionophores has been shown to significantly alter the cecal microbial homeostasis during different stages of the chicken life. Given the important role of the gut microbiome in regulating host metabolism and immunity, these findings highlight the importance of prudent use of antibiotics in poultry production and suggest the need for in-feed or water supplementation of chickens with beneficial microbial consortia following treatment with these antibiotics to restore microbial richness and diversity in the gut microbiome. Further investigations are required to determine the functional changes in intestinal immune responses, metabolic activity of the gut microbiome and growth performance of the antibiotic-treated chickens.
ACKNOWLEDGMENTS
Our research team would like to thank the members of the Arkell Research Station, University of Guelph and Dr. Mohsen Mohammadigheisar for their help in this experiment. This research was supported by funds from the Ontario Ministry of Agriculture, Food and Rural Affairs, Ontario Research Funds-Research Excellence, Canadian Poultry Research Council, and Agriculture and Agri-Food Canada.
Availability of Data and Material: The raw amplicon sequencing data from this study is available in the NCBI Sequence Read Archive (SRA; https://www.ncbi.nlm.nih.gov/sra/) with the BioProject identifier PRJNA805262.
Disclosures
The authors declare no conflict of interest.
REFERENCES
- Abaidullah M., Peng S., Kamran M., Yin Z., Song X. Current findings on gut microbiota mediated immune modulation against viral diseases in chicken. Viruses. 2019;11:681. doi: 10.3390/v11080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adewole D., Akinyemi F. Gut microbiota dynamics, growth performance, and gut morphology in broiler chickens fed diets varying in energy density with or without bacitracin methylene disalicylate (BMD) Microorganisms. 2021;9:787. doi: 10.3390/microorganisms9040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agunos A., Gow S.P., Leger D.F., Carson C.A., Deckert A.E., Bosman A.L., Loest D., Irwin R.J., Reid-Smith R.J. Antimicrobial use and antimicrobial resistance indicators - integration of farm-level surveillance data from broiler chickens and turkeys in British Columbia. Canada. Front. Vet. Sci. 2019;6:131. doi: 10.3389/fvets.2019.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir I., Bouvet P., Legeay C., Gophna U., Weinberger A. Eisenbergiella tayi gen. nov., sp. nov., isolated from human blood. Int. J. Syst. Evol. Microbiol. 2014;64:907–914. doi: 10.1099/ijs.0.057331-0. [DOI] [PubMed] [Google Scholar]
- Anani H., Abou Abdallah R., Chelkha N., Fontanini A., Ricaboni D., Mailhe M., Raoult D., Fournier P.E. Draft genome and description of Merdibacter massiliensis gen.nov., sp. nov., a new bacterium genus isolated from the human ileum. Sci. Rep. 2019;9:7931. doi: 10.1038/s41598-019-44343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou A.L., Ali R.A., Mendoza M.A., Ellis J.C., Hassan H.M., Croom W.J., Koci M.D. Development of the chick microbiome: how early exposure influences future microbial diversity. Front. Vet. Sci. 2016;3:103. doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamola V.D., Ghosh A., Kapardar R.K., Lal B., Cheema S., Sarma P., Chaudhry R. Gut microbial diversity in health and disease: experience of healthy Indian subjects, and colon carcinoma and inflammatory bowel disease patients. Microb. Ecol. Health Dis. 2017;28 doi: 10.1080/16512235.2017.1322447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean-Hodgins L., Kiarie E.G. Mandated restrictions on the use of medically important antibiotics in broiler chicken production in Canada: implications, emerging challenges, and opportunities for bolstering gastrointestinal function and health — a review. Can. J. Anim. Sci. 2021;101:602–629. [Google Scholar]
- Binda C., Lopetuso L.R., Rizzatti G., Gibiino G., Cennamo V., Gasbarrini A. Actinobacteria: a relevant minority for the maintenance of gut homeostasis. Dig. Liver. Dis. 2018;50:421–428. doi: 10.1016/j.dld.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Broom L.J. The sub-inhibitory theory for antibiotic growth promoters. Poult. Sci. 2017;96:3104–3108. doi: 10.3382/ps/pex114. [DOI] [PubMed] [Google Scholar]
- Chen Y., Wang J., Yu L., Xu T., Zhu N. Microbiota and metabolome responses in the cecum and serum of broiler chickens fed with plant essential oils or virginiamycin. Sci. Rep. 2020;10:5382. doi: 10.1038/s41598-020-60135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.-H., Lee K., Kim D.-W., Kil D.Y., Kim G.-B., Cha C.-J. Influence of dietary avilamycin on ileal and cecal microbiota in broiler chickens. Poult. Sci. 2018;97:970–979. doi: 10.3382/ps/pex360. [DOI] [PubMed] [Google Scholar]
- Costea P.I., Zeller G., Sunagawa S., Pelletier E., Alberti A., Levenez F., Tramontano M., Driessen M., Hercog R., Jung F.E., Kultima J.R., Hayward M.R., Coelho L.P., Allen-Vercoe E., Bertrand L., Blaut M., Brown J.R.M., Carton T., Cools-Portier S., Daigneault M., Derrien M., Druesne A., De Vos W.M., Finlay B.B., Flint H.J., Guarner F., Hattori M., Heilig H., Luna R.A., Van Hylckama Vlieg J., Junick J., Klymiuk I., Langella P., Le Chatelier E., Mai V., Manichanh C., Martin J.C., Mery C., Morita H., O'toole P.W., Orvain C., Patil K.R., Penders J., Persson S., Pons N., Popova M., Salonen A., Saulnier D., Scott K.P., Singh B., Slezak K., Veiga P., Versalovic J., Zhao L., Zoetendal E.G., Ehrlich S.D., Dore J., Bork P. Towards standards for human fecal sample processing in metagenomic studies. Nat. Biotechnol. 2017;35:1069–1076. doi: 10.1038/nbt.3960. [DOI] [PubMed] [Google Scholar]
- Crisol-Martínez E., Stanley D., Geier M.S., Hughes R.J., Moore R.J. Sorghum and wheat differentially affect caecal microbiota and associated performance characteristics of meat chickens. Peer J. 2017;2017:1–20. doi: 10.7717/peerj.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzeisen J.L., Kim H.B., Isaacson R.E., Tu Z.J., Johnson T.J. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS One. 2011;6:27949. doi: 10.1371/journal.pone.0027949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cesare A., Do Valle I.F., Sala C., Sirri F., Astolfi A., Castellani G., Manfreda G. Effect of a low protein diet on chicken ceca microbiome and productive performances. Poult. Sci. 2019;98:3963–3976. doi: 10.3382/ps/pez132. [DOI] [PubMed] [Google Scholar]
- Díaz Carrasco J.M., Redondo E.A., Pin Viso N.D., Redondo L.M., Farber M.D., Miyakawa M.E.F. Tannins and bacitracin differentially modulate gut microbiota of broiler chickens. Biomed. Res. Int. 2018;2018:1–11. doi: 10.1155/2018/1879168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Elshaghabee F.M.F., Rokana N., Gulhane R.D., Sharma C., Panwar H. Bacillus as potential probiotics: status, concerns, and future perspectives. Front. Microbiol. 2017;8:1–15. doi: 10.3389/fmicb.2017.01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg R.M., Hedemann M.S., Leser T.D., Jensen B.B. Effect of zinc bacitracin and salinomycin on intestinal microflora and performance of broilers. Poult. Sci. 2000;79:1311–1319. doi: 10.1093/ps/79.9.1311. [DOI] [PubMed] [Google Scholar]
- Gadbois P., Brennan J.J., Bruce H.L., Wilson J.B., Aramini J.J. The role of penicillin g potassium in managing clostridium perfringens in broiler chickens. Avian Dis. 2008;52:407–411. doi: 10.1637/8114-091807-Reg. [DOI] [PubMed] [Google Scholar]
- Glendinning L., Watson K.A., Watson M. Development of the duodenal, ileal, jejunal and caecal microbiota in chickens. Anim. Microbiome. 2019;1:17. doi: 10.1186/s42523-019-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorecki A.M., Preskey L., Bakeberg M.C., Kenna J.E., Gildenhuys C., MacDougall G., Dunlop S.A., Mastaglia F.L., Akkari P.Anthony, Koengten F., Anderton R.S. Altered gut microbiome in Parkinson's disease and the influence of lipopolysaccharide in a human α-synuclein over-expressing mouse model. Front. Neurosci. 2019;13:1–13. doi: 10.3389/fnins.2019.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Li C., Cui P., Wang H. Detection of Tn7-like transposons and antibiotic resistance in rnterobacterales from animals used for food production with identification of three novel transposons Tn6813, Tn6814, and Tn6765. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.02049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume M.E., Kubena L.F., Edrington T.S., Donskey C.J., Moore R.W., Ricke S.C., Nisbet D.J. Poultry digestive microflora biodiversity as indicated by denaturing gradient gel electrophoresis. Poult. Sci. 2003;82:1100–1107. doi: 10.1093/ps/82.7.1100. [DOI] [PubMed] [Google Scholar]
- Johansen C.H., Bjerrum L., Pedersen K. Impact of salinomycin on the intestinal microflora of broiler chickens. Acta Vet. Scand. 2007;49:30. doi: 10.1186/1751-0147-49-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Chousalkar K.K. Salmonella Typhimurium infection disrupts but continuous feeding of Bacillus based probiotic restores gut microbiota in infected hens. J. Anim. Sci. Biotechnol. 2020;11:29. doi: 10.1186/s40104-020-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozich J.J., Westcott S.L., Baxter N.T., Highlander S.K., Schloss P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. J. Appl. Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Shang Y., Kim W.K. Insight into dynamics of gut microbial community of broilers fed with fructooligosaccharides supplemented low calcium and phosphorus diets. Front. Vet. Sci. 2019;6:95. doi: 10.3389/fvets.2019.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.C., Kil D.Y., Sul W.J. Cecal microbiome divergence of broiler chickens by sex and body weight. J. Microbiol. 2017;55:939–945. doi: 10.1007/s12275-017-7202-0. [DOI] [PubMed] [Google Scholar]
- Li Y., Guo B., Wu Z., Wang W., Li C., Liu G., Cai H. Effects of fermented soybean meal supplementation on the growth performance and cecal microbiota community of broiler chickens. Animals. 2020;10:1–19. doi: 10.3390/ani10061098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopetuso L.R., Scaldaferri F., Petito V., Gasbarrini A. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog. 2013;5:23. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan C.J., Beiko R.G. A phylogenomic view of ecological specialization in the lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol. Evol. 2014;6:703–713. doi: 10.1093/gbe/evu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R.J., Stanley D. Experimental design considerations in microbiota/inflammation studies. Clin. Transl. Immunol. 2016;5:e92. doi: 10.1038/cti.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann A.P., Suen G. Differences in major bacterial populations in the intestines of mature broilers after feeding virginiamycin or bacitracin methylene disalicylate. J. Appl. Microbiol. 2015;119:1515–1526. doi: 10.1111/jam.12960. [DOI] [PubMed] [Google Scholar]
- Onrust L., Ducatelle R., Van Driessche K., De Maesschalck C., Vermeulen K., Haesebrouck F., Eeckhaut V., Van Immerseel F. Steering endogenous butyrate production in the intestinal tract of broilers as a tool to improve gut health. Front. Vet. Sci. 2015;2:1–8. doi: 10.3389/fvets.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky O., Sekelova Z., Faldynova M., Sebkova A., Sisak F., Rychlik I. Important metabolic pathways and biological processes expressed by chicken cecal microbiota. Appl. Environ. Microbiol. 2016;82:1569–1576. doi: 10.1128/AEM.03473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourabedin M., Zhengxin X., Bushansingh B., Eric C., Xin Z. Effects of mannan oligosaccharide and virginiamycin on the cecal microbial community and intestinal morphology of chickens raised under suboptimal conditions. Can. J. Microbiol. 2014;60:255–266. doi: 10.1139/cjm-2013-0899. [DOI] [PubMed] [Google Scholar]
- Proctor A., Phillips G.J. Differential effects of bacitracin methylene disalicylate (BMD) on the distal colon and cecal microbiota of young broiler chickens. Front. Vet. Sci. 2019;6:114. doi: 10.3389/fvets.2019.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K., Becker S., Xiao Y., Lyu W., Yang Q., Zhu H., Yang H., Zhao J., Zhang G. Differential impact of subtherapeutic antibiotics and ionophores on intestinal microbiota of broilers. Microorganisms. 2019;7:282. doi: 10.3390/microorganisms7090282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa M., Grillo A., Faggian D., Ruffolo C., Bonello E., D'Incà R., Scarpa M., Castagliuolo I., Angriman I. Relationship between mucosa-associated microbiota and inflammatory parameters in the ileal pouch after restorative proctocolectomy for ulcerative colitis. Surgery. 2011;150:56–67. doi: 10.1016/j.surg.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Schloss P.D. A high-throughput DNA sequence aligner for microbial ecology studies. PLoS One. 2009;4:e8230. doi: 10.1371/journal.pone.0008230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.M., Shah T., Deshpande S., Jakhesara S.J., Koringa P.G., Rank D.N., Joshi C.G. High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers. Mol. Biol. Rep. 2012;39:10595–10602. doi: 10.1007/s11033-012-1947-7. [DOI] [PubMed] [Google Scholar]
- Torok V.A., Allison G.E., Percy N.J., Ophel-Keller K., Hughes R.J. Influence of antimicrobial feed additives on broiler commensal posthatch gut microbiota development and performance. Appl. Environ. Microbiol. 2011;77:3380–3390. doi: 10.1128/AEM.02300-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Food and Drug Administration . Food and Drug Administration; Rockland, MD: 2012. The Judicious Use of Medically Important Antimicrobial Drugs in Food-Producing Animals. [Google Scholar]
- Yin Y., Lei F., Zhu L., Li S., Wu Z., Zhang R., Gao G.F., Zhu B., Wang X. Exposure of different bacterial inocula to newborn chicken affects gut microbiota development and ileum gene expression. ISME J. 2010;4:367–376. doi: 10.1038/ismej.2009.128. [DOI] [PubMed] [Google Scholar]
- Zhou H., Gong J., Brisbin J.T., Yu H., Sanei B., Sabour P., Sharif S. Appropriate chicken sample size for identifying the composition of broiler intestinal microbiota affected by dietary antibiotics, using the polymerase chain reaction-denaturing gradient gel electrophoresis technique. Poult. Sci. 2007;86:2541–2549. doi: 10.3382/ps.2007-00267. [DOI] [PubMed] [Google Scholar]
- Zou A., Sharif S., Parkinson J. Lactobacillus elicits a “Marmite effect” on the chicken cecal microbiome. NPJ Biofilms Microbiomes. 2018;4:27. doi: 10.1038/s41522-018-0070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]