Abstract
Background
Studies have shown that patients with non-valvular atrial fibrillation (NVAF) who discontinue oral anticoagulants (OACs) are at higher risk of complications such as stroke.
Objective
This analysis compared the risk of non-persistence with OACs among patients with NVAF.
Methods
Adult patients with NVAF who initiated apixaban, dabigatran, rivaroxaban, or warfarin were identified using 01JAN2013–30JUN2019 data from Centers for Medicare and Medicaid Services and four US commercial claims databases. Non-persistence was defined as discontinuation (no evidence of index OAC use for ≥ 60 days from the last days’ supply) or switch to another OAC. Kaplan–Meier curves were generated to illustrate time to non-persistence along with cumulative incidences of non-persistence. Baseline and time-varying covariates were evaluated, and adjusted Cox proportional hazards models were used to evaluate non-persistence risk.
Results
In total, 363,823 patients receiving apixaban, 57,121 receiving dabigatran, 282,831 receiving rivaroxaban, and 317,337 receiving warfarin were included. Of these, 47–72% discontinued/switched OAC therapy within an average 9-month follow-up. Apixaban was associated with a lower risk of non-persistence than were dabigatran (hazard ratio [HR] 0.62; 95% confidence interval [CI] 0.61–0.62), rivaroxaban (HR 0.76; 95% CI 0.75–0.76), and warfarin (HR 0.74; 95% CI 0.74–0.75). Dabigatran was associated with a higher risk of non-persistence than were warfarin (HR 1.21; 95% CI 1.19–1.22) and rivaroxaban (HR 1.23; 95% CI 1.22–1.25), and rivaroxaban was associated with a lower risk of non-persistence than was warfarin (HR 0.98; 95% CI 0.97–0.98). Clinical events (stroke/systemic embolism and major bleeding [MB]) during follow-up were predictors of non-persistence (stroke HR 1.57; 95% CI 1.53–1.61; MB HR 2.96; 95% CI 2.92–3.00).
Conclusion
In over one million patients with NVAF, our results suggest differences in anticoagulation treatment persistence across OAC agents, even after accounting for clinical events after OAC initiation. It is important for clinicians and patients to take these differences into consideration, especially as non-persistence to OAC therapy is associated with thromboembolic complications.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40256-021-00501-w.
Key Points
| This study examined non-persistence with oral anticoagulants (OACs; apixaban, dabigatran, rivaroxaban, and warfarin) among patients with non-valvular atrial fibrillation (NVAF). OAC non-persistence among patients with NVAF has previously been associated with an increased risk of adverse events. |
| The risk of non-persistence varied among patients with NVAF; baseline characteristics and clinical events occurring after OAC initiation were significant predictors of non-persistence. |
Introduction
Non-valvular atrial fibrillation (NVAF) is the most common heart dysrhythmia diagnosed in the USA and an independent risk factor for stroke [1]. The prevalence of NVAF has been increasing in the USA and is expected to continue to increase substantially because of the aging of the population [2]. Atrial fibrillation (AF), in turn, increases the risk of mortality and morbidities such as major adverse cardiac events, arterial thromboembolism, and ischemic stroke [3, 4].
While vitamin K antagonists (VKAs), such as warfarin, have been the anticoagulant treatment of choice for several years, they have unstable pharmacokinetic profiles, with wide interpatient variability and extensive interactions, requiring routine blood monitoring for dose adjustment [5]. Clinical trials have demonstrated that the reduction in risk of stroke and bleeding with direct oral anticoagulants (DOACs: apixaban, dabigatran, edoxaban, rivaroxaban) is similar or superior to that with warfarin, and DOACs have been approved in the USA to reduce the risk of stroke among patients with NVAF [6–9].
Studies have also shown that patients who discontinue oral anticoagulants (OACs) are at increased risk of adverse cardiovascular effects, thromboembolic events, minor and major bleeds, and all-cause mortality [10, 11]. Gradual declines in persistence after the initial prescriptions among patients with NVAF have been noted, with some studies reporting a wide range of persistence, from 55 to 69%, after 12 months [12]. Although DOACs have fewer drug interactions than warfarin and do not require international normalized ratio (INR) monitoring, real-world evidence rates of discontinuation 12 months after treatment initiation have been estimated at 55% [13]. Results from a meta-analysis of the DOAC randomized controlled trials indicated apixaban had lower discontinuation rates than OACs, whereas warfarin had lower rates than dabigatran and rivaroxaban [14].
While studies have evaluated OAC persistence, real-world studies comparing persistence across DOACs and warfarin in large populations are lacking. Therefore, the objectives of this study were to compare the incidence and risk of non-persistence across DOACs and warfarin and identify predictors of non-persistence among patients with NVAF who initiated OAC therapy.
Methods
Data Sources
In this study, we pooled five large national claims databases with the latest available data at the time of application. They included 100% fee-for-service US Centers for Medicare and Medicaid Services data (1 January 2012–31 December 2017), Truven MarketScan® Commercial Claims and Encounter (1 January 2012–30 June 2019), IMS PharMetrics Plus™ (1 January 2012–31 March 2019), Optum Clinformatics™ Data Mart (1 January 2012–31 March 2019), and the Humana Research Database (1 January 2012–31 March 2019). Of note, patients with Medicare supplemental plans in Truven MarketScan and IMS PharMetrics Plus data were not included in the study to avoid potential duplicates with Medicare Part A and Part B. More details on the datasets and pooling method have been published [15, 16].
Patient Selection
We identified adult patients with NVAF between 1 January 2012 and 30 June 2019 using inpatient and outpatient claims, and selected patients with a pharmacy claim for apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin during the identification period between 1 January 2013 and 30 June 2019. Each patient’s first OAC was labeled as their “index therapy,” and the date of the first OAC prescription claim was labeled as the “index date.” Patients prescribed edoxaban were not included in the final analysis because of the small sample size (N = 1629). Patients were required to have continuous medical and pharmacy health plan enrollment for ≥ 12 months before the index date [17].
We excluded patients with any OAC treatment within 12 months before the index date to ensure they were treatment naïve. We also excluded patients with evidence of valvular heart disease, venous thromboembolism, transient AF (pericarditis, hyperthyroidism, or thyrotoxicity), or heart valve replacement or transplant during the baseline period; pregnancy during the study period; or hip/knee replacement surgery within 6 weeks before the index date. To give all patients the possibility of discontinuing/switching index treatment, we also excluded patients with more than one OAC on the index date and those with fewer than 60 days of follow-up, based on continuous health plan enrollment post-index.
Outcome Measures
Patient data were assessed from the day after the index date until the earliest of the following: end of continuous medical and pharmacy enrollment, death (when available), or end of the study period. The primary outcome of interest was non-persistence, defined as either discontinuation or switch, with discontinuation defined as no evidence of the index OAC prescription for ≥ 60 days from the last day of supply of the last filled prescription and switch defined as filling a prescription for an OAC other than the index drug during the follow-up period within ± 60 days of last days’ supply from the index OAC.
Baseline variables included demographics (age, sex, US geographic region) and clinical characteristics such as clinical risk scores, prior stroke/systemic embolism (SE), prior bleeding, comorbidities, and baseline medication use.
Statistical Methodology
Descriptive analysis of clinical and demographic variables was conducted for patients prescribed DOACs or warfarin. Rates of non-persistence among patients with NVAF who initiated an OAC during the identification period were calculated during the follow-up period overall and at 12 months post-index date. Unadjusted Kaplan–Meier survival curves were generated to illustrate time to non-persistence in addition to cumulative incidence of non-persistence. Cox proportional hazard models with robust sandwich estimates were used to compare the risk of non-persistence across treatment groups while adjusting for patient covariates. Baseline variables included in the model were patient demographics, index OAC prescription, and clinical characteristics such as prior stroke/SE, prior bleeding, comorbidities, and baseline medication use.
A secondary analysis was conducted taking into consideration clinical events occurring after the index date, including stroke/SE hospitalizations, major bleeding (MB) hospitalizations, new acute renal failure (considered new when it occurred for the first time within a timeframe of 6 months), new chronic renal failure (considered new when it occurred for the first time after the index date), new cancer diagnoses (considered new when it occurred for the first time within a timeframe of 6 months), and cardioversions and catheter ablations [18]. The diagnosis codes used for stroke/SE and MB hospitalizations were based on validated administrative claims-based algorithms and the International Society on Thrombosis and Haemostasis definition of MB [19, 20].
We conducted three sensitivity analyses:
Follow-up was limited to the first 12 months after the index date. The shorter follow-up period allowed us to focus on short-term persistence.
Non-persistence was reanalyzed using a ≥ 30-day discontinuation gap instead of ≥ 60 days, which allowed us to observe how a shorter gap period would alter non-persistence outcomes [21].
Warfarin non-persistence was reanalyzed with the inclusion of INR records to extend warfarin treatment lines. Patients receiving warfarin were considered discontinued if the gap between two consecutive warfarin prescriptions or from the last prescription to end of study was longer than 60 days and they did not have INR measurements at least every 42 days [22, 23]. This sensitivity analysis allowed us to take into consideration dose adjustments or possible additional prescriptions.
Results
Baseline Characteristics
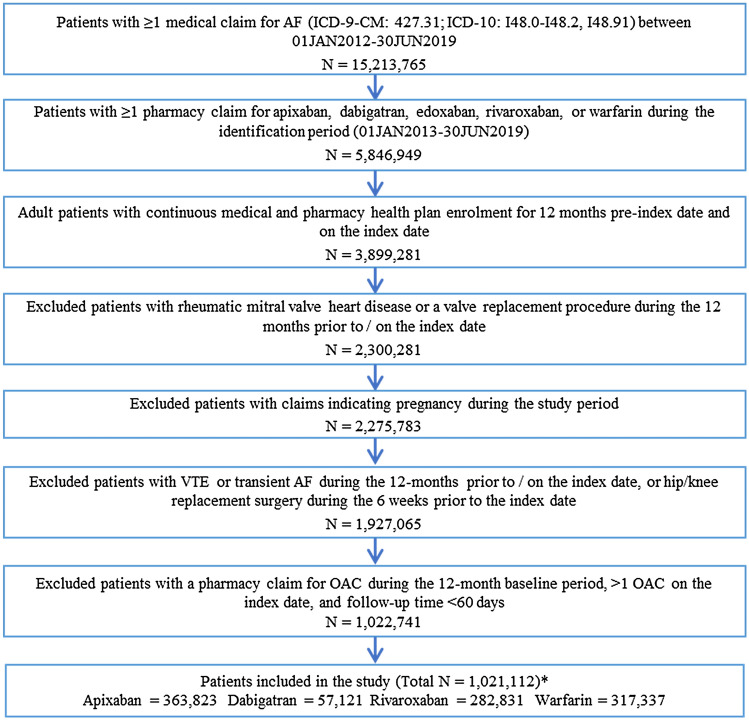
In total, 1,021,112 adults with NVAF were newly prescribed an OAC during the study period (Fig. 1). Of those patients, 363,823 (35.6%) initiated apixaban with a mean ± standard deviation follow-up of 605 ± 410.5 days; 57,121 (5.6%) initiated dabigatran with a mean follow-up of 939.3 ± 566.4 days; 282,831 (27.7%) patients initiated rivaroxaban with a mean follow-up of 799.7 ± 524.4 days; and 317,337 (31.1%) patients initiated warfarin with a mean follow-up of 892 ± 543.7 days. Approximately 53% of patients in all treatment groups combined were male. The mean age of patients receiving apixaban, dabigatran, rivaroxaban, and warfarin was 75.1 ± 10.6, 73.4 ± 10.3, 73.5 ± 10.7, and 76.7 ± 9.3 years, respectively. Patients initiating warfarin were older and were at higher risk in terms of the CHA2DS2-VASc score (4.5 ± 1.8) and HAS-BLED score (3.2 ± 1.4) (see Table 1 for CHA2DS2-VASc and HAS-BLED definitions) and had higher mean Deyo–Charlson Comorbidity Index scores of 3.4 ± 3.0. For those receiving apixaban, dabigatran, or rivaroxaban, 22.6% (2.5 mg), 15.6% (15.2% on 75 mg; 0.4% on 110 mg), and 25.8% (5.4% on 10 mg; 20.4% on 15 mg) had lower dosage regimens, respectively (Table 1).
Fig. 1.
Patient selection criteria. The patient selection criteria yielded > 1,000,000 patients with non-valvular atrial fibrillation prescribed either apixaban, dabigatran, rivaroxaban, or warfarin. Patients were stratified into cohorts based on their index OAC. *Edoxaban was not included in the study because it received US FDA approval in 2015 so the sample size was small (N = 1629). AF atrial fibrillation, ICD-9/10-CM International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification, OAC oral anticoagulant, VTE venous thromboembolism
Table 1.
Baseline characteristics and treatment follow-up
| Characteristics | Warfarin cohort (N = 317,337), reference |
Apixaban cohort (N = 363,823) |
P value | Dabigatran cohort (N = 57,121) |
P value | Rivaroxaban cohort (N = 282,831) |
P value |
|---|---|---|---|---|---|---|---|
| Age, years | 76.7 ± 9.3 | 75.1 ± 10.6 | < 0.001 | 73.4 ± 10.3 | < 0.001 | 73.5 ± 10.7 | < 0.001 |
| 18–54 | 5630 (1.8) | 13,780 (3.8) | < 0.001 | 2735 (4.8) | < 0.001 | 14,352 (5.1) | < 0.001 |
| 55–64 | 17,296 (5.5) | 35,915 (9.9) | < 0.001 | 6169 (10.8) | < 0.001 | 32,520 (11.5) | < 0.001 |
| 65–74 | 104,121 (32.8) | 117,039 (32.2) | < 0.001 | 21,238 (37.2) | < 0.001 | 100,054 (35.4) | < 0.001 |
| ≥ 75 | 190,290 (60.0) | 197,089 (54.2) | < 0.001 | 26,979 (47.2) | < 0.001 | 135,905 (48.1) | < 0.001 |
| Sexa | |||||||
| Male | 165,668 (52.2) | 188,252 (51.7) | < 0.001 | 32,309 (56.6) | < 0.001 | 156,327 (55.3) | < 0.001 |
| Female | 151,669 (47.8) | 175,570 (48.3) | < 0.001 | 24,812 (43.4) | < 0.001 | 126,503 (44.7) | < 0.001 |
| US geographic region | |||||||
| Northeast | 60,061 (18.9) | 60,877 (16.7) | < 0.001 | 11,430 (20.0) | < 0.001 | 50,509 (17.9) | < 0.001 |
| North central | 98,273 (31.0) | 81,283 (22.3) | < 0.001 | 13,225 (23.2) | < 0.001 | 68,069 (24.1) | < 0.001 |
| South | 98,706 (31.1) | 161,072 (44.3) | < 0.001 | 22,184 (38.8) | < 0.001 | 113,057 (40.0) | < 0.001 |
| West | 59,688 (18.8) | 60,182 (16.5) | < 0.001 | 10,148 (17.8) | < 0.001 | 50,634 (17.9) | < 0.001 |
| Other | 609 (0.2) | 409 (0.1) | < 0.001 | 134 (0.2) | 0.035 | 562 (0.2) | 0.552 |
| Baseline comorbidity | |||||||
| Deyo–Charlson Comorbidity Index | 3.4 ± 3.0 | 2.9 ± 2.8 | < 0.001 | 2.5 ± 2.5 | < 0.001 | 2.6 ± 2.6 | < 0.001 |
| CHA2DS2-VASc score | 4.5 ± 1.8 | 4.1 ± 1.9 | < 0.001 | 3.8 ± 1.9 | < 0.001 | 3.8 ± 1.9 | < 0.001 |
| HAS-BLED scoreb | 3.2 ± 1.4 | 3.0 ± 1.4 | < 0.001 | 2.8 ± 1.3 | < 0.001 | 2.9 ± 1.3 | < 0.001 |
| Bleeding history | 71,975 (22.7) | 63,850 (17.5) | < 0.001 | 9339 (16.3) | < 0.001 | 48,054 (17.0) | < 0.001 |
| Congestive heart failure | 108,185 (34.1) | 99,373 (27.3) | < 0.001 | 13,521 (23.7) | < 0.001 | 67,500 (23.9) | < 0.001 |
| Diabetes mellitus | 125,920 (39.7) | 125,450 (34.5) | < 0.001 | 19,679 (34.5) | < 0.001 | 94,611 (33.5) | < 0.001 |
| Hypertension | 270,847 (85.3) | 309,483 (85.1) | 0.001 | 47,463 (83.1) | < 0.001 | 235,415 (83.2) | < 0.001 |
| Renal disease | 88,114 (27.8) | 87,925 (24.2) | < 0.001 | 9026 (15.8) | < 0.001 | 49,075 (17.4) | < 0.001 |
| Liver disease | 17,539 (5.5) | 20,070 (5.5) | 0.850 | 2708 (4.7) | < 0.001 | 14,555 (5.1) | < 0.001 |
| Cancer | 46,240 (14.6) | 48,281 (13.3) | < 0.001 | 7391 (12.9) | < 0.001 | 37,374 (13.2) | < 0.001 |
| Myocardial infarction | 48,548 (15.3) | 46,891 (12.9) | < 0.001 | 5762 (10.1) | < 0.001 | 30,437 (10.8) | < 0.001 |
| Cardioversion and catheter ablations | 7739 (2.4) | 14,005 (3.8) | < 0.001 | 1905 (3.3) | < 0.001 | 9784 (3.5) | < 0.001 |
| Dyspepsia or stomach discomfort | 64,295 (20.3) | 67,744 (18.6) | < 0.001 | 9804 (17.2) | < 0.001 | 51,007 (18.0) | < 0.001 |
| Non-stroke/SE peripheral vascular disease | 86,342 (27.2) | 90,367 (24.8) | < 0.001 | 11,594 (20.3) | < 0.001 | 61,243 (21.7) | < 0.001 |
| Stroke/SE history | 48,714 (15.4) | 44,225 (12.2) | < 0.001 | 6209 (10.9) | < 0.001 | 27,976 (9.9) | < 0.001 |
| Transient ischemic attack | 29,861 (9.4) | 40,381 (11.1) | < 0.001 | 4814 (8.4) | < 0.001 | 22,912 (8.1) | < 0.001 |
| Anemia and coagulation defects | 108,463 (34.2) | 101,299 (27.8) | < 0.001 | 13,261 (23.2) | < 0.001 | 69,496 (24.6) | < 0.001 |
| Alcoholism | 4333 (1.4) | 7139 (2.0) | < 0.001 | 872 (1.5) | 0.003 | 5208 (1.8) | < 0.001 |
| Peripheral artery disease | 84,900 (26.8) | 83,434 (22.9) | < 0.001 | 11,176 (19.6) | < 0.001 | 58,338 (20.6) | < 0.001 |
| Coronary artery disease | 145,663 (45.9) | 155,696 (42.8) | < 0.001 | 22,774 (39.9) | < 0.001 | 111,304 (39.4) | < 0.001 |
| Baseline medication use | |||||||
| ACEI/ARB | 186,500 (58.8) | 218,361 (60.0) | < 0.001 | 33,813 (59.2) | 0.057 | 165,980 (58.7) | 0.504 |
| Amiodarone | 34,585 (10.9) | 40,772 (11.2) | < 0.001 | 5713 (10.0) | < 0.001 | 27,606 (9.8) | < 0.001 |
| β-blocker | 189,302 (59.7) | 224,582 (61.7) | < 0.001 | 33,939 (59.4) | 0.287 | 169,755 (60.0) | 0.004 |
| H2-receptor antagonist | 24,156 (7.6) | 26,202 (7.2) | < 0.001 | 3,548 (6.2) | < 0.001 | 18,177 (6.4) | < 0.001 |
| Proton pump inhibitor | 99,092 (31.2) | 112,992 (31.1) | 0.132 | 16,342 (28.6) | < 0.001 | 83,248 (29.4) | < 0.001 |
| Statin | 190,060 (59.9) | 221,106 (60.8) | < 0.001 | 32,954 (57.7) | < 0.001 | 162,231 (57.4) | < 0.001 |
| Antiplatelets | 57,262 (18.0) | 64,285 (17.7) | < 0.001 | 8992 (15.7) | < 0.001 | 45,541 (16.1) | < 0.001 |
| NSAIDs | 63,271 (19.9) | 86,806 (23.9) | < 0.001 | 13,453 (23.6) | < 0.001 | 70,583 (25.0) | < 0.001 |
| Dose of the index prescription | |||||||
| Standard dosec | 281,476 (77.4) | 48,219 (84.4) | 209,777 (74.2) | ||||
| Low dosed | 82,347 (22.6) | 8902 (15.6) | 73,054 (25.8) | ||||
Data are presented as mean ± standard deviation or N (%) unless otherwise indicated
ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, CHA2DS2-VASc congestive heart failure, hypertension, aged ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, aged 65–74 years, sex category, HAS-BLED hypertension, abnormal renal or liver function, stroke, bleeding, labile international normalized ratios, elderly, drugs or alcohol, INR international normalized ratio, NSAIDs non-steroidal anti-inflammatory drugs, SE systemic embolism
aSex was unknown for one patient in the apixaban cohort and another patient in the rivaroxaban cohort
bAs the INR value was not available in the databases, a modified HAS-BLED score was calculated with a range of 0–8
cStandard dose: apixaban 5 mg, dabigatran 150 mg, rivaroxaban 20 mg
dLower dose: apixaban 2.5 mg, dabigatran 75 mg, dabigatran 110 mg, rivaroxaban 10 mg, rivaroxaban 15 mg; 200 patients treated with dabigatran were prescribed dabigatran 110 mg, and 15,362 patients treated with rivaroxaban were prescribed rivaroxaban 10 mg
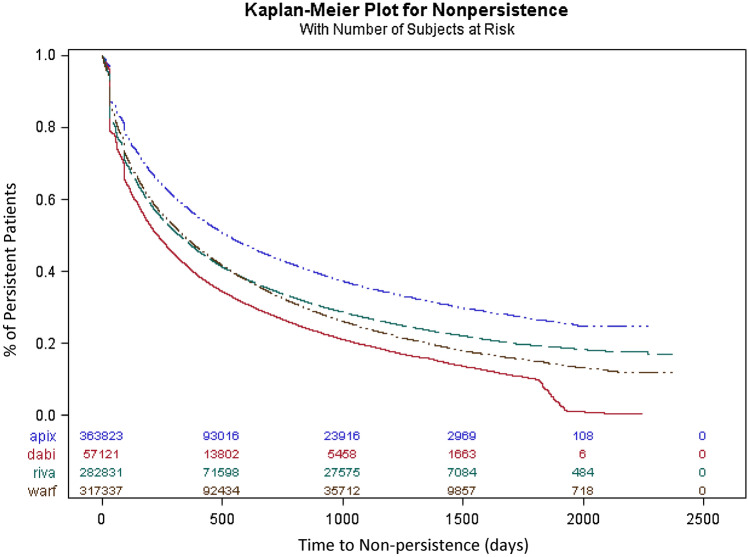
Unadjusted Cumulative Incidence of Non-persistence
The cumulative incidence of non-persistence at 3 months was 21.2, 33.9, 28.8, and 26.7% for patients receiving apixaban, dabigatran, rivaroxaban, or warfarin, respectively (Fig. 2). The cumulative incidence of non-persistence at 12 months was 42.7, 58.9, 52.2, and 51.3% for patients receiving apixaban, dabigatran, rivaroxaban, or warfarin, respectively (Fig. 2). Most of the non-persistence was because of discontinuation of therapy, but 3–9% of patients switched treatment, with dabigatran having the highest switch rate (Table 2).
Fig. 2.
Cumulative incidence of non-persistence to oral anticoagulants. The cumulative incidence of non-persistence to oral anticoagulants during the entire follow-up period was calculated using a 60-day gap. At 3 months, the cumulative incidences of non-persistence were 21.2, 33.9, 28.8, and 26.7% for apixaban, dabigatran, rivaroxaban, and warfarin patients, respectively. Apix apixaban, Dabi dabigatran, Riva rivaroxaban, Warf warfarin
Table 2.
Descriptive outcomes for main analysis
| Outcomes | Warfarin cohort (N = 317,337) |
Apixaban cohort (N = 363,823) |
Dabigatran cohort (N = 57,121) |
Rivaroxaban cohort (N = 282,831) |
|---|---|---|---|---|
| Follow-up time (in days) | 892.0 ± 543.7 | 605.0 ± 410.5 | 939.3 ± 566.4 | 799.7 ± 524.4 |
| Minimum | 62 | 62 | 62 | 62 |
| Q1 | 412 | 269 | 445 | 335 |
| Median | 845 | 521 | 872 | 715 |
| Q3 | 1336 | 867 | 1420 | 1208 |
| Maximum | 2371 | 2319 | 2371 | 2371 |
| Number of prescriptions | ||||
| Pts with one index OAC prescription | 40,192 (12.7) | 41,298 (11.4) | 11,416 (20.0) | 47,499 (16.8) |
| Pts with more than one index OAC prescription | 277,145 (87.3) | 322,525 88.6) | 45,705 (80.0) | 235,332 (83.2) |
| Non-persistent patients | 207,565 (65.4) | 172,574 (47.4) | 41,108 (72.0) | 171,799 (60.7) |
| Type of change in therapy | ||||
| Discontinued | 183,401 (57.8) | 162,455 (44.7) | 35,869 (62.8) | 157,717 (55.8) |
| Time to discontinuation (days) | 278 ± 317.1 | 214 ± 240.2 | 250 ± 317.7 | 223 ± 275.5 |
| Switched | 24,164 (7.6) | 10,119 (2.8) | 5239 (9.2) | 14,082 (5.0) |
| Time to switch (days) | 211 ± 301.3 | 154 ± 213.6 | 208 ± 299.6 | 198 ± 279.4 |
| OAC switched to | ||||
| Apixaban | 10,627 (44.0) | 0 (0.0) | 2016 (38.5) | 6642 (47.2) |
| Dabigatran | 2392 (9.9) | 959 (9.5) | 0 (0.0) | 1170 (8.3) |
| Edoxaban | 55 (0.2) | 60 (0.6) | 8 (0.2) | 58 (0.4) |
| Rivaroxaban | 11,090 (45.9) | 4552 (45.0) | 1727 (33.0) | 0 (0.0) |
| Warfarin | 0 (0.0) | 4548 (44.9) | 1488 (28.4) | 6212 (44.1) |
Data are presented as mean ± standard deviation or N (%) unless otherwise indicated
OAC oral anticoagulant, pts patients, Q quarter
Primary Analyses Controlling for Baseline Characteristics
Figure 3 and Table 3 show the results of primary analyses with Cox models that adjusted for demographic and clinical covariates defined at baseline. When compared with those receiving warfarin, patients initiating apixaban (adjusted hazard ratio [aHR] 0.74; 95% confidence interval [CI] 0.74–0.75; P < 0.001) or rivaroxaban (aHR 0.98; 95% CI 0.97–0.98; P < 0.001) were 26 and 2% less likely to be non-persistent, respectively. Patients receiving dabigatran (aHR 1.21; 95% CI 1.19–1.22; P < 0.001) were 21% more likely to be non-persistent than those receiving warfarin.
Fig. 3.
Risk of non-persistence among oral anticoagulants. Cox proportional hazard models were used to evaluate the risk of non-persistence. Apixaban and rivaroxaban were associated with a lower risk of non-persistence than was warfarin. Dabigatran was associated with a higher risk of non-persistence than were warfarin and rivaroxaban. Apixaban was associated with a lower risk of non-persistence than were dabigatran and rivaroxaban. Large sample sizes and precision mean the 95% confidence intervals (CIs) are narrow and difficult to observe in the figure
Table 3.
Adjusted hazard ratios of non-persistence
| Variable | HRa (95% CI) |
|---|---|
| Age, years | |
| 18–54 (reference) | |
| 55–64 | 0.72 (0.71–0.73)* |
| 65–74 | 0.66 (0.65–0.66)* |
| ≥ 75 | 0.64 (0.63–0.65)* |
| Sex | |
| Male (reference) | |
| Female | 0.96 (0.95–0.96)* |
| US geographic region | |
| Northeast (reference) | |
| Midwest | 1.02 (1.01–1.03)* |
| South | 1.16 (1.15–1.17)* |
| West | 1.13 (1.12–1.14)* |
| Other | 1.42 (1.34–1.51)* |
| Comorbidities | |
| Deyo–Charlson Comorbidity Index | 1.02 (1.02–1.02)* |
| Bleeding history | 1.08 (1.07–1.09)* |
| Congestive heart failure | 0.99 (0.99–1.00) |
| Diabetes mellitus | 1.00 (0.99–1.01) |
| Hypertension | 0.89 (0.88–0.90)* |
| Renal disease | 1.02 (1.01–1.03)* |
| Liver disease | 0.99 (0.97–1.00) |
| Cancer | 0.97 (0.96–0.98)* |
| Myocardial infarction | 1.04 (1.03–1.05)* |
| Cardioversion and catheter ablations | 1.02 (1.01–1.04) |
| Dyspepsia or stomach discomfort | 1.07 (1.06–1.08)* |
| Non-stroke/SE peripheral vascular disease | 0.93 (0.91–0.95)* |
| Stroke/SE | 0.90 (0.89–0.91)* |
| Transient ischemic attack | 0.96 (0.95–0.97)* |
| Anemia and coagulation defects | 1.11 (1.10–1.11)* |
| Alcoholism | 1.11 (1.09–1.13)* |
| Peripheral artery disease | 1.12 (1.09–1.14)* |
| Coronary artery disease | 1.07 (1.06–1.08)* |
| Medication use | |
| ACEI/ARB | 0.94 (0.93–0.94)* |
| Amiodarone | 1.18 (1.17–1.19)* |
| β-blockers | 0.95 (0.95–0.96)* |
| H2-receptor antagonist | 1.01 (1.00–1.02) |
| Proton pump inhibitor | 1.03 (1.02–1.03)* |
| Statins | 0.88 (0.88–0.89)* |
| Antiplatelets | 0.99 (0.98–1.00) |
| NSAIDs | 1.07 (1.06–1.07)* |
ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, CI confidence interval, HR hazard ratio, NSAIDs non-steroidal anti-inflammatory drugs, SE systemic embolism
aModels adjusted for age, sex, region, atrial fibrillation index year, Deyo–Charlson Comorbidity Index, bleeding history, history of congestive heart failure, diabetes mellitus, hypertension, renal disease, liver disease, cancer, myocardial infarction, cardioversion and catheter ablations, dyspepsia or stomach discomfort, non-stroke/SE peripheral vascular disease, stroke/SE, transient ischemic attack, anemia and coagulation defects, alcoholism, peripheral artery disease, coronary artery disease, and baseline medication use
*HR significant at P < 0.001
When compared with patients receiving rivaroxaban, those initiating apixaban (aHR 0.76; 95% CI 0.75–0.76; P < 0.001) were 24% less likely to be non-persistent. Those receiving dabigatran (aHR 1.23; 95% CI 1.22–1.25; P < 0.001) were 23% more likely to be non-persistent than those receiving rivaroxaban. When compared with patients receiving dabigatran, those initiating apixaban (aHR 0.62; 95% CI 0.61–0.62; P < 0.001) were 38% less likely to be non-persistent.
Older age and history of hypertension and stroke/SE were predictors of a lower likelihood of non-persistence (Table 3). Patient use of statins was also a predictor of reduced risk of non-persistence.
Secondary Analyses Controlling for Baseline Characteristics and Time-Dependent Variables
Table 1 in the electronic supplementary material (ESM) shows the proportion of patients with time-varying clinical events after the index date, and Table 4 shows the model results when time-varying covariates were included in Cox models. The HRs for OACs remained consistent after the inclusion of time-varying covariates. All time-varying clinical characteristics were significant predictors of non-persistence: stroke/SE aHR 1.57; 95% CI 1.53–1.61; MB aHR 2.96; 95% CI 2.92–3.00; new acute renal failure aHR 1.44; 95% CI 1.42–1.46; new chronic renal failure aHR 1.14; 95% CI 1.12–1.15, new cancer aHR 1.22; 95% CI 1.20–1.25; and cardioversions and catheter ablations aHR 1.17; 95% CI 1.15–1.19. All HRs were significant at P < 0.001.
Table 4.
Adjusted hazard ratios of non-persistence with time-varying covariates
| HRa (95% CI) | HRa (95% CI) | HRa (95% CI) | |
|---|---|---|---|
| Cohort | |||
| Warfarin | Reference | ||
| Apixaban | 0.76 (0.75–0.76) | 0.76 (0.76–0.77) | 0.62 (0.61–0.62) |
| Dabigatran | 1.23 (1.22–1.24) | 1.25 (1.23–1.26) | Reference |
| Rivaroxaban | 0.99 (0.98–0.99) | Reference | |
| Time-varying covariates | |||
| Stroke/SE (primary discharge) | 1.57 (1.53–1.61) | 1.58 (1.53–1.64) | 1.52 (1.45–1.60) |
| Major bleeding (primary discharge) | 2.96 (2.92–3.00) | 3.31 (3.25–3.37) | 3.12 (3.04–3.20) |
| New acute renal failure | 1.44 (1.42–1.46) | 1.45 (1.43–1.47) | 1.44 (1.41–1.47) |
| New chronic renal failure | 1.14 (1.12–1.15) | 1.17 (1.14–1.19) | 1.13 (1.10–1.16) |
| New cancer | 1.22 (1.20–1.25) | 1.23 (1.21–1.26) | 1.23 (1.19–1.27) |
| Cardioversions and catheter ablations | 1.17 (1.15–1.19) | 1.15 (1.13–1.18) | 1.13 (1.10–1.16) |
Data are presented as hazard ratio (95% confidence interval). All hazard ratios were significant at P < 0.001. Models adjusted for age, sex, region, atrial fibrillation index year, Deyo–Charlson Comorbidity Index, bleeding history, history of congestive heart failure, diabetes mellitus, hypertension, renal disease, liver disease, cancer, myocardial infarction, cardioversion and catheter ablations, dyspepsia or stomach discomfort, non-stroke/SE peripheral vascular disease, stroke/SE, transient ischemic attack, anemia and coagulation defects, alcoholism, peripheral artery disease, coronary artery disease, baseline medication use, and time-varying covariates during the follow-up
HR hazard ratio, CI confidence interval, SE systemic embolism
Sensitivity Analyses
In the first sensitivity analysis, follow-up was limited to the first 12 months after index treatment initiation. The results are shown in Tables 2 and 4 in the ESM. The results were generally consistent with those of the main analysis, except when comparing rivaroxaban and warfarin: when follow-up was limited to the first 12 months, rivaroxaban was associated with a 3% increase in non-persistence compared with warfarin (aHR 1.03; 95% CI 1.02–1.04; P < 0.001).
The effect of a change to the definition of the discontinuation gap from 60 to 30 days is shown in Tables 2, 4 and Fig. 1 in the ESM. As expected, there was an increase in non-persistence for all OACs, but the results for the comparative risk of non-persistence across DOACs did not change significantly.
Finally, the addition of INR records to extend warfarin treatment increased the likelihood of non-persistence with rivaroxaban, as compared with warfarin (aHR 1.02; 95% CI 1.01–1.03; P < 0.001), as shown in Tables 2, 4 and Fig. 2 in the ESM.
Discussion
This is the largest retrospective observational study to date to examine the risk of non-persistence among patients with NVAF initiating OAC treatment. Non-persistence was high across all OAC treatment cohorts; however, patients initiating apixaban or rivaroxaban were less likely than those receiving warfarin to be non-persistent. Patients initiating apixaban were less likely to be non-persistent than those receiving rivaroxaban, whereas patients initiating rivaroxaban were less likely to be non-persistent than those receiving dabigatran.
Our results were generally consistent with those of other studies, including a recently published network meta-analysis that pooled 36 real-world retrospective studies published between 2013 and 2018 and found persistence to be higher among DOAC users than VKA users (odds ratio [OR] 1.44; 95% CI 1.12–1.86; P = 0.005) [12, 24, 25]. Our results for the comparisons between rivaroxaban and apixaban and warfarin were consistent with these observations. Our results were also in line with previous studies, which reported a higher risk of non-persistence among dabigatran users than rivaroxaban and warfarin users [12]. The twice-daily dosing of dabigatran was thought to be a possible explanation for these observations. However, we found that patients receiving apixaban, which also has a twice-daily dosing regimen, had a lower risk of non-persistence than those receiving rivaroxaban and warfarin. This suggests that factors other than the dosing regimen play a major role in the lower persistence associated with dabigatran [12]. In addition, a recent meta-analysis found that apixaban had the best safety profile of the DOACs, another possible explanation for the lower risk of non-persistence among patients receiving apixaban [26].
Our study adds to the literature on persistence with OAC because we evaluated whether differences in the comparative risk of non-persistence across treatment groups remained significant after adjusting for clinical events during follow-up. This is relevant because a Danish study evaluating OAC switch and discontinuation found that half of OAC treatment changes were preceded by a hospitalization, most frequently for stroke/SE, MB, new acute renal failure, new chronic renal failure, new cancer diagnoses, or cardioversions or catheter ablations [18, 27]. To build on this evidence, we tested whether the differences in the risk of non-persistence across treatment groups remained significant after accounting for the differential risk of these events associated with treatment changes. In doing so, we demonstrated that apixaban remained associated with a lower risk of non-persistence after accounting for ischemic and bleeding events and other relevant clinical factors.
Our analyses, including both baseline patient characteristics and time-dependent variables for major clinical events, demonstrated that the occurrence of MB events after treatment initiation was the strongest predictor of non-persistence. However, including the occurrence of major clinical events during follow-up did not significantly change the comparative risk of non-persistence across groups in our model that included time-dependent variables. This may be because of other factors related to the differences in non-persistence risk among OACs, including drug tolerability and meal requirements for certain DOACs [28], which we were unable to ascertain because of data limitations. Among baseline characteristics, increasing age, statin use, and history of hypertension or stroke were associated with a lower risk of non-persistence.
Limitations
As with many real-world studies, our study has several limitations. This study was designed to examine non-persistence among patients with NVAF initiated on OACs and predictors of non-persistence, so we could not evaluate causal relationships. As is the nature with retrospective observational studies, our study was subject to confounders. This study was bound by the limitations of the claims data; variables such as over-the-counter use of aspirin, serum creatinine/creatinine clearance, and laboratory values were unavailable and thus were not controlled for in the model. Codes from the International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification were used to identify baseline characteristics and outcomes, which may lack clinical accuracy. Additionally, we were unable to determine time in therapeutic range for patients prescribed warfarin or jointly assess the relationship between persistence and adherence. Furthermore, we were unable to identify predictors related to cost or access. Our results might in fact have been driven by non-medical reasons, including out-of-pocket costs, formulary changes, insurance changes, physician preferences, and access issues, which we were unable to capture but have been documented as possible indicators for treatment switch or discontinuation [29].
Nevertheless, our study is a major contribution to the literature on real-world persistence with OAC, since it is by far the largest retrospective observational study, with over 1 million patients, examining the comparative risk of non-persistence between OACs. By pooling five datasets and including a comprehensive comparison of the OACs, this study adds supplemental information to the literature and may assist in decisions around treatment selection for stroke prevention among patients with NVAF. The robustness of our findings is evidenced by the similar results observed after limiting follow-up to the initial 12 months after OAC initiation, shortening of the gap length from 60 to 30 days, and the inclusion of INR results in lengthening warfarin treatment line.
Conclusion
In this real-world study of over 1 million patients with NVAF, our results suggest that noteworthy differences in anticoagulation treatment persistence exist across OAC agents, even after accounting for clinical events after OAC initiation. It is important for clinicians and patients to take these differences into consideration, especially as non-persistence to OAC therapy is associated with thromboembolic complications, including stroke, MB, and all-cause mortality.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
This study was sponsored by Bristol Myers Squibb and Pfizer.
Conflict of interest
Amol Dhamane and Mauricio Ferri are paid employees of Bristol Myers Squib. Inmaculada Hernandez is an employee of the University of Pittsburgh. Manuela Di Fusco, Cristina Russ, and Birol Emir are paid employees and shareholders of Pfizer. Allison Keshishian, Cynthia Gutierrez, and Wan-Lun Tsai are paid employees of STATinMED Research, which is a paid consultant to Pfizer and Bristol Myers Squibb. Huseyin Yuce has no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Since this study did not involve the collection, use, or transmittal of individually identifiable data, it was exempt from Institutional Review Board review. Both the datasets and the security of the offices where analysis was completed (and where the datasets are kept) meet the requirements of the Health Insurance Portability and Accountability Act of 1996.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Code availability
Code not publicly available.
Author Contributions
Conceptualization: all authors; Methodology: all authors; Formal analysis and investigation: Allison Keshishian, Cynthia Gutierrez, and Wan-Lun Tsai; Writing - original draft preparation: Allison Keshishian and Cynthia Gutierrez; Writing - review and editing: all authors; Funding acquisition: Amol D Dhamane Manuela Di Fusco, Mauricio Ferri, Cristina Russ, Birol Emir.
References
- 1.Shea JB, Sears SF. Cardiology patient pages. A patient’s guide to living with atrial fibrillation. Circulation. 2008;117(20):340–343. doi: 10.1161/CIRCULATIONAHA.108.780577. [DOI] [PubMed] [Google Scholar]
- 2.Marinigh R, Lip GY, Fiotti N, Giansante C, Lane DA. Age as a risk factor for stroke in atrial fibrillation patients: implications for thromboprophylaxis. J Am Coll Cardiol. 2010;56(11):827–837. doi: 10.1016/j.jacc.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 3.Sankaranarayanan R, Kirkwood G, Visweswariah R, et al. How does chronic atrial fibrillation influence mortality in the modern treatment era? Curr Cardiol Rev. 2015;11(3):190–198. doi: 10.2174/1573403x10666140902143020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pastori D, Menichelli D, Del Sole F, Pignatelli P, Violi F. Long-term risk of major adverse cardiac events in atrial fibrillation patients on direct oral anticoagulants. Mayo Clin Proc. 2021;96(3):658–665. doi: 10.1016/j.mayocp.2020.06.057. [DOI] [PubMed] [Google Scholar]
- 5.Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55–61. doi: 10.15420/aer.2017.50.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 7.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 8.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 9.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 10.Rivera-Caravaca JM, Esteve-Pastor MA, Roldán V, et al. Non-vitamin K antagonist oral anticoagulants: impact of non-adherence and discontinuation. Expert Opin Drug Saf. 2017;16(9):1051–1062. doi: 10.1080/14740338.2017.1351542. [DOI] [PubMed] [Google Scholar]
- 11.Lowres N, Giskes K, Hespe C, Freedman B. Reducing stroke risk in atrial fibrillation: adherence to guidelines has improved, but patient persistence with anticoagulant therapy remains suboptimal. Korean Circ J. 2019;49(10):883–907. doi: 10.4070/kcj.2019.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozaki AF, Choi AS, Le QT, et al. Real-world adherence and persistence to direct oral anticoagulants in patients with atrial fibrillation: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2020;13(3):e005969. doi: 10.1161/CIRCOUTCOMES.119.005969. [DOI] [PubMed] [Google Scholar]
- 13.Wiley V, Franchina-Elder J, Fu AC, et al. Treatment and persistence with oral anticoagulants among newly diagnosed patients with non-valvular atrial fibrillation: a retrospective observational study in a US commercially insured and Medicare Advantage population. BMJ Open. 2018;8:e020676. doi: 10.1136/bmjopen-2017-020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell SA, Simon TA, Raza S, et al. The efficacy and safety of oral anticoagulants in warfarin-suitable patients with nonvalvular atrial fibrillation: systematic review and meta-analysis. Clin Appl Thromb Hemost. 2013;19(6):619–631. doi: 10.1177/1076029613486539. [DOI] [PubMed] [Google Scholar]
- 15.Lip GY, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients: the ARISTOPHANES study. Stroke. 2018;49(12):2933–2944. doi: 10.1161/STROKEAHA.118.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Deitelzweig S, Keshishian A, et al. Effectiveness and safety of apixaban versus warfarin in non-valvular atrial fibrillation patients in “real-world” clinical practice. Thromb Haemost. 2017;117(6):1072–1082. doi: 10.1160/TH17-01-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):141–147. doi: 10.1002/pds.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellfritzsch M, Grove EL, Husted SE, Rasmussen L, Poulsen BK, Johnsen SP, Hallas J, Pottegård A. Clinical events preceding switching and discontinuation of oral anticoagulant treatment in patients with atrial fibrillation. Ep Europace. 2017;19(7):1091–1095. doi: 10.1093/europace/euw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20(6):560–566. doi: 10.1002/pds.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thigpen JL, Dillon C, Forster KB, et al. Validity of international classification of disease codes to identify ischemic stroke and intracranial hemorrhage among individuals with associated diagnosis of atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2015;8(1):8–14. doi: 10.1161/CIRCOUTCOMES.113.000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lip GYH, Pan X, Kamble S, Kawabata H, Mardekian J, Masseria C, Phatak H. Discontinuation risk comparison among ‘real-world’ newly anticoagulated atrial fibrillation patients: Apixaban, warfarin, dabigatran, or rivaroxaban. PLoS ONE. 2018;13(4):e0195950. doi: 10.1371/journal.pone.0195950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290(20):2685–2692. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- 23.Rosendaal FR, Cannegieter SC, Van der Meer FJ, Briet EA. Method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–239. doi: 10.1055/s-0038-1651587. [DOI] [PubMed] [Google Scholar]
- 24.Nelson WW, Song X, Coleman CI, Thomson E, Smith DM, Damaraju CV, Schein JR. Medication persistence and discontinuation of rivaroxaban versus warfarin among patients with non-valvular atrial fibrillation. Curr Med Res Opin. 2014;30(12):2461–2469. doi: 10.1185/03007995.2014.933577. [DOI] [PubMed] [Google Scholar]
- 25.Bancroft T, Lim J, Wang C, Sander SD, Swindle JP. Health care resource utilization, costs, and persistence in patients newly diagnosed as having nonvalvular atrial fibrillation and newly treated with dabigatran versus warfarin in the United States. Clin Ther. 2016;38(3):545–556. doi: 10.1016/j.clinthera.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Menichelli D, Del Sole F, Di Rocco A, et al. Real-world safety and efficacy of direct oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of 605 771 patients. Eur Heart J Cardiovasc Pharmacother. 2021;7(FI1):f11–f19. doi: 10.1093/ehjcvp/pvab002. [DOI] [PubMed] [Google Scholar]
- 27.Manzoor BS, Walton SM, Sharp LK, Galanter WL, Lee TA, Nutescu EA. High number of newly initiated direct oral anticoagulant users switch to alternate anticoagulant therapy. J Thromb Thrombolysis. 2017;44(4):435–441. doi: 10.1007/s11239-017-1565-2. [DOI] [PubMed] [Google Scholar]
- 28.Xarelto. Package insert. Janssen Pharmaceuticals, Inc. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202439s021lbl.pdf. Published May 2016. Accessed 10 Dec 2020.
- 29.Jackson LR, 2nd, Kim S, Shrader P, et al. Early therapeutic persistence on dabigatran versus warfarin therapy in patients with atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. J Thromb Thrombolysis. 2018;46(4):435–439. doi: 10.1007/s11239-018-1715-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.