Abstract
The nuclear receptor farnesoid-X-receptor (FXR) plays an essential role in bile acid, glucose, and lipid homeostasis. In the last two decades, several diseases, such as obesity, type 2 diabetes, nonalcoholic fatty liver disease, cholestasis, and chronic inflammatory diseases of the liver and intestine, have been revealed to be associated with alterations in FXR functions. FXR has become a promising therapeutic drug target, particularly for enterohepatic diseases. Despite the large number of FXR modulators reported, only obeticholic acid (OCA) has been approved for primary biliary cholangitis (PBC) therapy as FXR modulator. In this review, we summarize the structure and function of FXR, the development of FXR modulators, and the structure–activity relationships of FXR modulators. Based on the structural analysis, we discuss potential strategies for developing future therapeutic FXR modulators to overcome current limitations, providing new perspectives for enterohepatic and metabolic diseases treatment.
Keywords: nuclear receptor, farnesoid X receptor, structure–activity relationships, FXR modulators, rational drug design, liver fibrosis diseases
Introduction
Nuclear receptors (NRs) are a large family of ligand-regulated transcription factors including receptors for steroid hormones, thyroid hormones, fatty acids and bile acids [1–3]. The human NR superfamily comprises 48 members that share highly conserved domains. NRs are involved in various physiological functions, ranging from development and differentiation to metabolic homeostasis [4]. Dysfunction of NRs leads to various diseases, such as cancer, diabetes, obesity, and liver disease. Therefore, the NR superfamily is one of the main therapeutic targets for human diseases. Indeed, NRs modulators account for more than 10% of all FDA-approved drugs [5]. The success of drug development targeting ligand-regulated NRs has heightened interest in finding NR modulators with remarkable selectivity and pharmacological properties.
The bile acid receptor farnesoid X receptor (FXR, NR1H4) is a member of the nuclear hormone receptor superfamily that is highly expressed in the liver and intestines and presents at lower levels in the kidney, adipose tissue, and adrenal gland [6–8]. FXR was first found as an orphan NR activated by farnesol derivatives, which are metabolic intermediates of the mevalonate pathway [9]. Subsequent studies have identified that bile acids such as chenodeoxycholic acid (CDCA) and cholic acid (CA) are endogenous ligands for FXR. Recent studies have determined that FXR functions as an enterohepatic regulator of bile acid homeostasis, lipid and glucose metabolism, and inflammation [10]. Thus, FXR has emerged as an important therapeutic target for various diseases, such as primary biliary cholangitis (PBC), type 2 diabetes mellitus (T2DM), and liver fibrosis. Here, we describe the structure and function of FXR and the development of FXR modulators. In addition, we reviewed the FXR modulators’ structure–activity relationships (SAR). Finally, we discuss potential strategies for developing future therapeutic FXR modulators for non-alcoholic steatohepatitis (NASH) and fibrosis diseases.
FXR structure and function
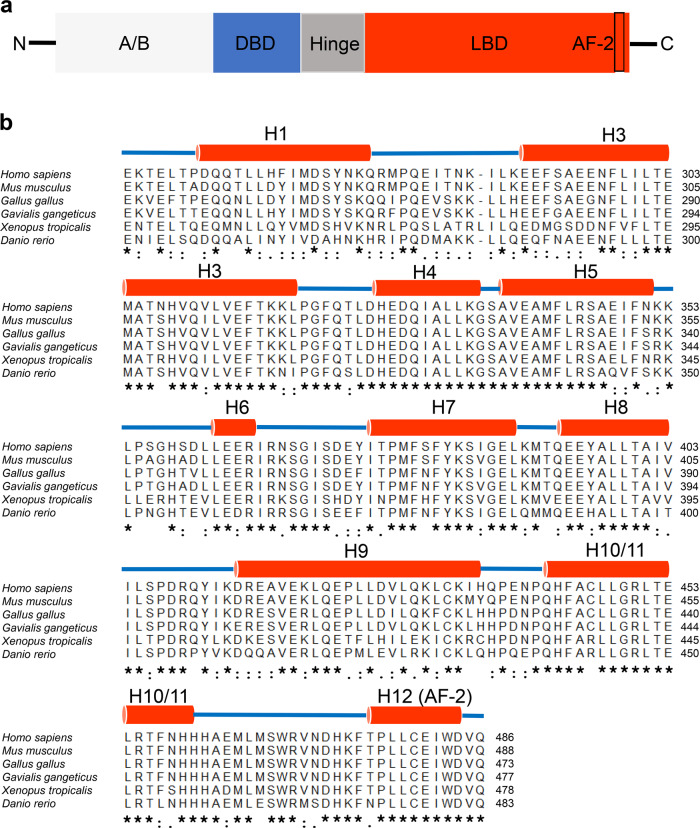
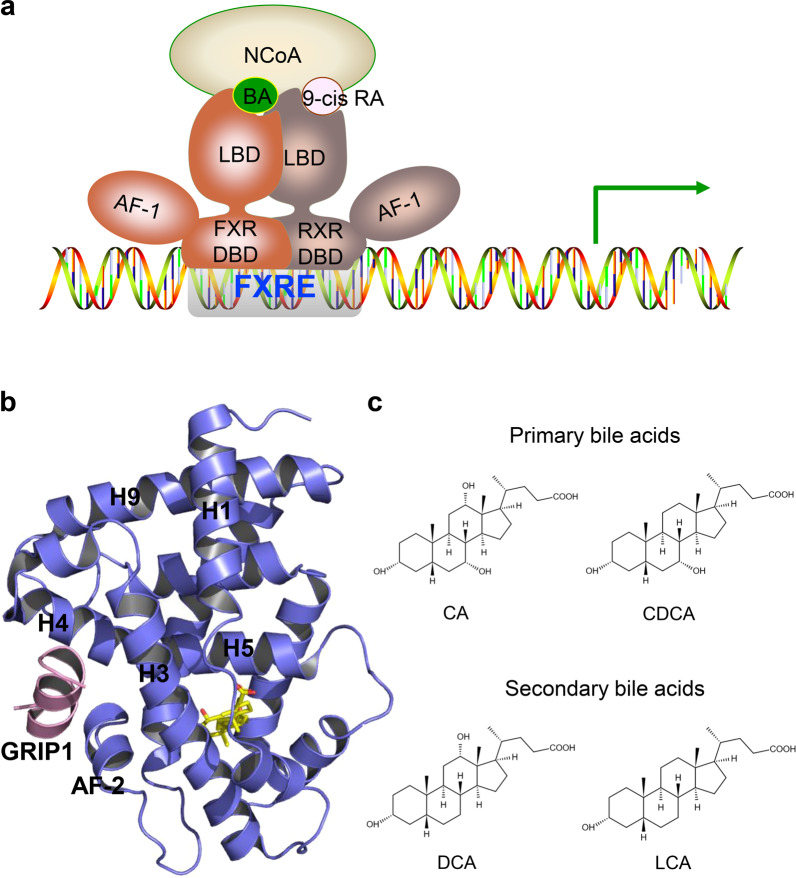
FXRα (NR1H4) and FXRβ (NR1H5) are two known FXR genes. The single FXRα gene is conserved from fish to humans [11] and encodes four transcript isoforms, FXRα1, FXRα2, FXRα3, and FXRα4, in humans and mice. FXRα1 and FXRα3 isoforms have four amino acids (MYTG) immediately adjacent to the DNA-binding domain (DBD) in a region referred to as the hinge domain (Fig. 1a), while FXRα3 and FXRα4 possess an extended N-terminus. FXRβ is a lanosterol sensor in rodents, rabbits, and dogs but constitutes a pseudogene in humans and primates [12, 13]. As members of the NR superfamily, the four FXRα proteins share highly conserved domains: the A/B region containing an N-terminal activation function-1 (AF1) domain, the central C region that has a DNA-binding domain (DBD), the C-terminal E region including a ligand-binding domain (LBD), and the D region hinge domain that links the DBD and the LBD (Fig. 1a). The four FXRα proteins shared the highest similarity in their DBD and LBD (Fig. 1b). The ligand binding to FXR, which forms a heterodimeric complex with the retinoid X receptor (RXR), triggers receptor conformational changes that regulate the recruitment of corepressors and coactivators, ultimately impacting the transcription of target genes (Fig. 2a) [14, 15]. The first FXR-LBD crystal structure was resolved in 2003 by X-ray crystallography [14, 16] revealing a three-layer helical sandwich arrangement that resembles most nuclear receptor structures. In particular, helix H12, containing the activation function-2 (AF-2) domain of the FXR-LBD, forms a hydrophobic cleft with helix H3 and helix H4, which stabilizes the binding between the coactivators and FXR through the hydrophobic interface with LXXLL motifs in coactivators (Fig. 2b) [14, 16].
Fig. 1. Domain organization and sequence alignment of FXR.
a Schematic diagram showing the functional domains of FXR. The DBD is blue, the hinge is gray and the LBD is red. The presence of AF-2 is indicated in the box. b Sequence alignment of the FXR ligand-binding domain among different species. The secondary structure elements are shown above the sequences. Identical residues are labeled with an asterisk. Partially conserved residues are labeled with a colon.
Fig. 2. Structural basis of ligand-regulated FXR activity.
a Upon ligand binding, FXR recruits transcriptional coactivators and binds to the FXRE of target genes as a heterodimer with RXR. b The structure of OCA bound with rFXR LBD in carton representation (PDB: 1OSV). The rFXR LBD is colored slate blue, and the SRC2 peptide is pink. The bound OCA is shown in stick representation with carbon and oxygen atoms depicted in yellow and red, respectively. c Representative structures of endogenous bile acids.
FXR is highly expressed in the mammalian liver and intestine, functions as a guardian of bile acid homeostasis, and has been identified as an important pharmacological target in the treatment of intrahepatic cholestasis, such as primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) [17–19]. In addition, FXR modulators have attracted attention owing to their roles in regulating glucose and lipid metabolism, therefore providing new strategies for treating T2DM, obesity, and liver fibrosis [20–22].
Numerous FXR modulators have been developed to treat liver disorders related to BA and lipid accumulation, and obeticholic acid (OCA) has been approved by the FDA and EMEA for the therapy of ursodeoxycholic acid (UDCA)-resistant patients with PBC. However, OCA has been reported to increase the risk of liver injury and result in pruritus [23, 24]. In addition, there is still no effective medical therapy for nonalcoholic fatty liver disease (NAFLD) thus far. This is an analysis of FXR modulators structural mechanisms and SAR to provide new insights into FXR drug design for treating NAFLD-like fibrosis diseases.
Structural basis for the activity of FXR modulators
Steroidal agonists
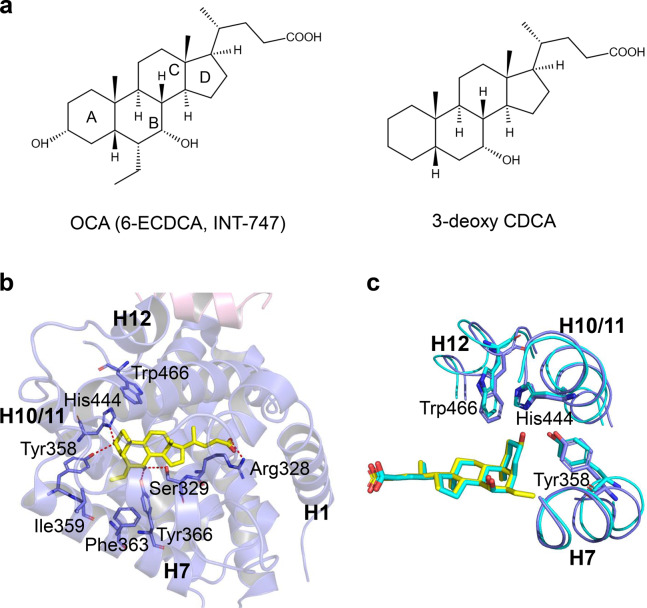
Primary bile acids, CA and CDCA, secondary bile acids, deoxycholic acid (DCA), and lithocholic acid (LCA), and taurine or glycine conjugates were identified as FXR endogenous agonists. The potency to activate FXR was CDCA > DCA > LCA > CA (Fig. 2c) [8]. To obtain highly potent FXR agonists, a panel of semisynthetic bile acid derivatives was generated from CDCA chemical modification. First, a series of 6α-alkyl-substituted CDCA analogs were synthesized. Among them, the 6α-ethyl-chenodeoxycholic acid (6-ECDCA, INT-747, OCA) was considered the most potent FXR agonist, with an EC50 of 99 nM in the FRET assay (Fig. 3a) [25]. The structure of the rFXR LBD in complex with OCA shows that the A ring of OCA faces helix H11 and helix H12, while the D ring faces helix H3 and helix H5 (Fig. 3b). The 7α-OH of OCA forms H-bonds with the side chains of Ser329 on helix H5 and Tyr366 on helix H7. 3α-OH forms an H-bond with the side-chain nitrogen of His444 on helix H11 stabilizing the π-cation interactions between the side chains of His444 on helix H11 and Trp466 on helix H12. This activation of the π-cation receptor trigger enables helix H12 to adopt an active conformation to recruit coactivators. In addition, the carboxylate oxygens of OCA establish an H-bond with the guanidino group of Arg328 on helix H5. Furthermore, the 6α-ethyl group of OCA forms hydrophobic interactions with the side chains of Ile359, Phe363, and Tyr366 on helix H7, leading to a higher ligand-binding affinity (Fig. 3b, c) [16, 25]. Although 3-deoxy-CDCA cannot form an H bond with His444, its A ring can still stabilize the π-cation interaction between His444 and Trp466 to activate FXR, indicating that 3α-OH is not necessary to activate FXR (Fig. 3c) [16]. However, the correctly positioned ring A is a molecular switch that mediates the activation of FXR. Additionally, the 3-deoxy-6-ethyl derivative showed comparable potency to 6-ECDCA in the cell-based reporter assay and AlphaScreen coactivator recruitment assay [26].
Fig. 3. Structural basis for the interaction of FXR with OCA.
a Chemical structures of OCA and 3-deoxy CDCA. b Overall structure of OCA bound to rFXR and the key interactions. FXR and OCA are colored slate blue and yellow, respectively. PDB: 1OSV. c Interactions of OCA (yellow) and 3-deoxy CDCA with the activation switch of FXR. PDB codes: 1OSV (rFXR/OCA), and 1OT7 (rFXR/3-deoxy CDCA).
Notably, OCA has been approved to treat UDCA-resistant patients in PBC. It represents the first FXR ligand that progressed in Phase III clinical trials in NASH patients [27, 28]. However, pruritus, a common side effect of OCA, occurred in 51% of subjects receiving the active ingredient and 18% receiving placebo. Other side effects, such as gallstones and acute cholecystitis, were more frequent in the OCA group than in the placebo group (3% and <1%, respectively). Treatment with OCA was also associated with an increase in low-density lipoprotein (LDL) cholesterol, consistent with the phase 2 FLINT study [28].
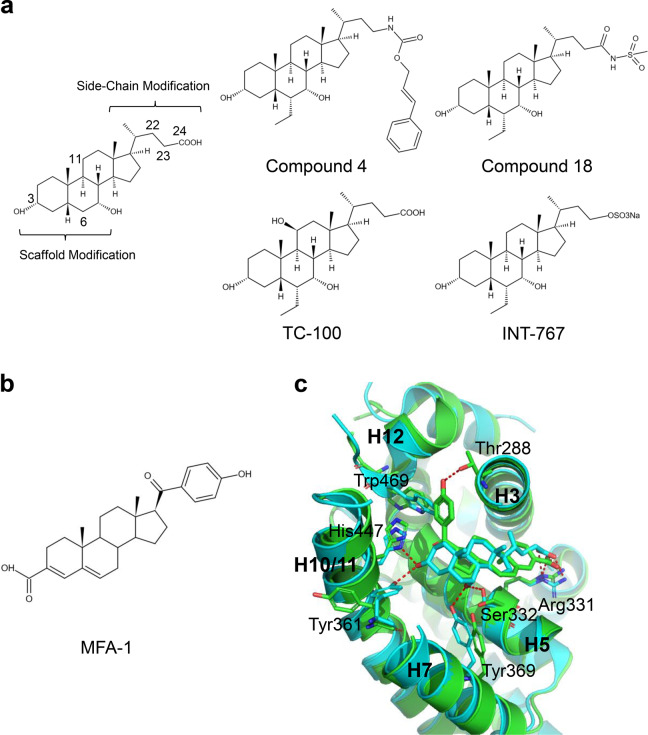
In 2004, Pellicciari et al. synthesized a series of CDCA derivatives with scaffold and side-chain modifications, which confirmed that 6α-alkyl substituents had more potency and efficacy [29]. The CDCA side chain was further modified by carbamate moieties to explore the existence of the FXR “back door” pocket [30]. In FXR AlphaScreen coactivator recruitment assays, compared with CDCA, Compound 4, with an EC50 of 0.15 μM and efficacy of 290%, showed very potent agonist activity (Fig. 4a). Compound 4 showed full agonist activity on FXR at 1 μM, comparable to that of 6-ECDCA. In contrast, CDCA proved to be only a partial agonist at FXR even at a concentration of 20 μM in HepG2 cells by FXR transactivation assay. In addition, Compound 4 has more potency than 6-ECDCA in regulating FXR target genes such as small heterodimer partner (SHP), bile salt export pump (BSEP), organic solute transporter beta (Ostβ), and cholesterol 7α-hydroxylase (Cyp7α1) in HepG2 cells [31]. Furthermore, Xiao et al. reported that Compound 18 (Fig. 4a), and the amidation of the carboxylic group of 6-ECDCA, endowed with high FXR potency, good pharmacokinetic properties, and pharmacological efficacy compared with 6-ECDCA [32]. The introduction of a hydroxyl group to the C ring of 6-ECDCA, especially at the C-11β position, generated a potent and selective FXR agonist TC-100 (Fig. 4a), with remarkable physicochemical and pharmacological profiles. In addition, docking studies highlighted that TC-100 could occupy the binding pocket of FXR. Moreover, the C11β hydroxyl group makes an additional hydrogen bond with the carbonyl group of Leu284 [33]. However, 7-deoxy-CDCA (LCA) decreases the affinity of FXR, and the 7β-epimer of CDCA (UDCA) cannot activate FXR [34, 35].
Fig. 4. Structural basis for the interaction of FXR with CDCA derivatives.
a Chemical structures of CDCA derivatives. b Chemical structure of MFA-1. c Superposition of the structures of FXR-CDCA (PDB: 4QE6; cyan) and FXR-MFA-1 (PDB: 3BEJ; green).
Intercept Pharmaceuticals reported a 23-sulfate derivative of 6-ECDCA (INT-747, OCA) named INT-767 (Fig. 4a) [36]. The potency of INT-767 (EC50 = 7 ± 1.5 nM) for FXR was approximately 10-fold higher than that of INT-747 (76 ± 4.3 nM) in the AlphaScreen coactivator recruitment assay. Moreover, FXR can be activated by INT-767 in cell-based transactivation assays. Compared with INT-747, INT-767 has a higher potency in modulating the FXR target genes SHP, BSEP, Ostβ, and Cyp7α1 in HepG2 cells. In diabetic mice, INT-767 decreased total cholesterol and triglyceride levels. In addition, INT-767 treatment significantly attenuates liver damage and the proinflammatory response in a rat model of NASH [37].
In conclusion, scientists have focused on bile acid scaffold modifications since bile acids were identified as FXR endogenous agonists. CDCA is a recognized privileged molecule among bile acids; its above-mentioned derivatives were synthesized to improve the potency, efficacy, and metabolic stability relative to CDCA. The SAR of CDCA derivatives can be summarized as follows: First, the 6α-alkyl group of the CDCA scaffold is an important group that can increase the ligand-binding potency to FXR. Second, the hydroxyl group at the 3α-position is unnecessary for the CDCA derivatives to activate FXR. Furthermore, the hydroxyl group at the 7α-position is critical for the binding affinity of CDCA derivatives to FXR. Finally, the side chain tolerates significant structural variations in the length and nature of the end group.
MFA-1 with a steroid ring was identified as a new FXR agonist by a high-throughput screen based on a homogeneous time-resolved fluorescence (HTRF) assay (Fig. 4b). MFA-1 has an EC50 of 16.9 nM in the coactivator recruitment assay. Compared with the structure of FXR in complex with CDCA, it is clear that MFA-1 binds in a flipped orientation relative to bile acids. This binding mode appears to be driven by the presence of a carboxylate on MFA-1 to make a salt-bridge interaction with Arg331 (Fig. 4c). In addition, receptor activation by MFA-1 differs due to bile acids in that it relies on direct interactions between the ligand and residues in helix H11 and helix H12. It only indirectly involves a protonated His447 that is part of the activation trigger (Fig. 4c) [38].
Nonsteroidal agonists
GW4064 derivatives
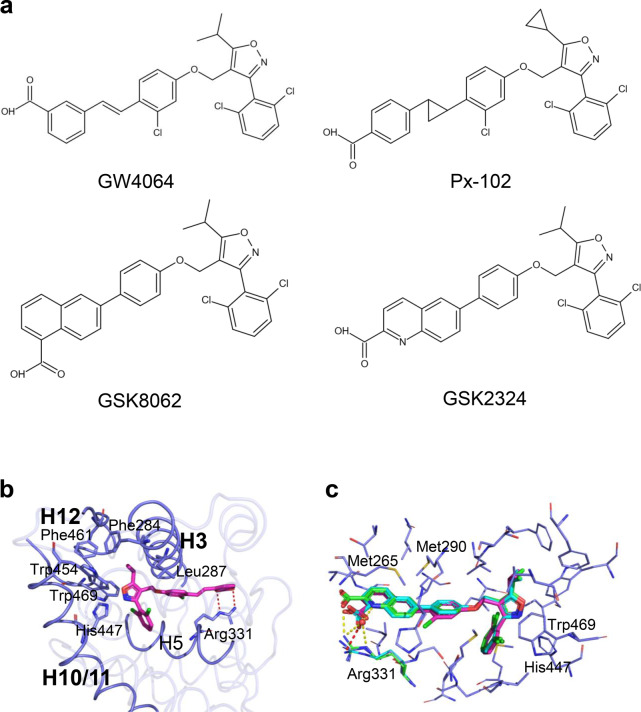
GW4064 is a landmark discovery in the field of nonsteroidal FXR agonists. GW4064 was initially published in 2000 as a potent and selective FXR full agonist (Fig. 5a) [39]. In the FXR-GW4064 cocrystal structure [40], the carboxylate oxygens of GW4064 form an H-bond with the guanidino group of Arg331 on helix H5, similar to the binding mode of the carboxylic acids of the bile acid. The isopropyl group stabilizes the hydrophobic core of the receptor by forming hydrophobic interactions with Phe284, Leu287, Trp454, and Phe461 of FXR. Furthermore, the 3-phenyl isoxazole moiety rests up against Trp454 and His447 on helix H10, while the isoxazole establishes an edge-to-face stacking interaction with Trp469 on helix H12 (Fig. 5b). These interactions lead to an active conformation of FXR capable of recruiting coactivators. However, its poor bioavailability, in vivo half-life, stilbene photolability, and potential toxicity limit further GW4064 application.
Fig. 5. Structural basis for the interaction of FXR with GW4064 and its derivatives.
a Chemical structures of GW4064 and its derivatives. b The structure of FXR is bound with GW4064. PDB: 3DCT; FXR is in slate blue, and GW4064 is in magenta. c Overlay of GW4064 (magenta), GSK8062 (cyan), and GSK2324 (green). The hydrogen bonds are shown as red dashes (GSK8062) and yellow dashes (GSK2324). PDB codes are the following: 3DCT (FXR/GW4064), 3DCU (FXR/GSK8062), and 3P89 (FXR/GSK2324).
A series of FXR ligands based on the modifications of GW4064 have been reported. Px-102 (PX20606, Fig. 5a) and its eutomer Px-104 (GS-9674), with a trans-cyclopropyl, showed similar FXR affinities to GW4064 but improved photolability and cell toxicity [41, 42]. GlaxoSmithKline scientists converted stilbene to naphthalene or quinoline to improve GW4064 drug properties. First, the GW4064 analog GSK8062 was generated (Fig. 5a, c), as an equipotent FXR full agonist with reduced GW4064-induced toxicity but poor oral bioavailability in rodents [40]. Subsequently, a series of heteroaryl bicyclic naphthalene replacements were prepared. For example, GSK2324, an equipotent FXR agonist of GSK8062 (Fig. 5a, c), showed the ability to improve GSK8062 pharmacological and pharmacokinetic properties [43]. Detailed accounts of more chemical features and pharmacological properties have been thoroughly summarized in recent reviews [44].
Fexaramine derivatives
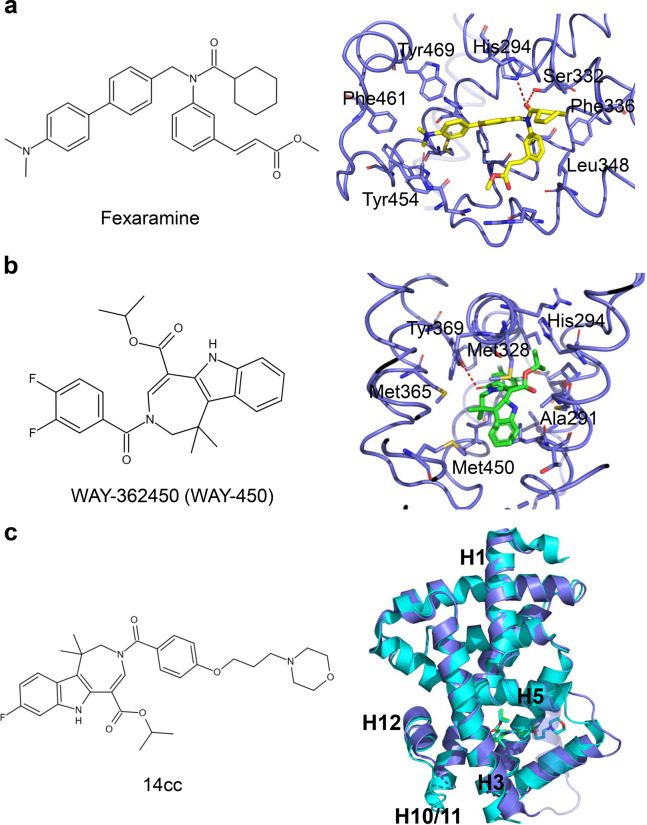
Fexaramine was discovered as a nonsteroidal FXR agonist structurally distinct from GW4064 (Fig. 6a) [14]. Although it shows high potency to FXR in cell-based reporter assays (EC50 = 25 nM) and FRET-based coactivator steroid receptor coactivator 1 (SRC1) binding assays (EC50 = 255 nM), fexaramine is an intestinal-restricted FXR agonist with limited direct FXR activation in the liver [45]. To further improve the pharmacological properties of fexaramine, many derivatives have been developed to prevent or treat alcoholic liver diseases, such as steatosis, cirrhosis, and NASH [46–48].
Fig. 6. Structural basis for the interaction of FXR with fexaramine and WAY-362450.
a Key interactions of FXR with fexaramine. Red dashes represent hydrogen bond interactions. PDB: 1OSH. b Key interactions of FXR with WAY-362450. Red dashes represent hydrogen bond interaction. PDB: 3FLI. c Overlay of 14cc (PDB: 3L1B; FXR and 14cc are in cyan) and WAY-362450 (PDB: 3FLI; FXR is in slate blue and WAY-362450 is in green).
WAY-362450 derivatives
WAY-362450 (WAY-450. XL335) was identified as a potent and selective FXR agonist (EC50 of 4 nM and 149% efficacy) in 2009 (Fig. 6b) [49]. WAY-362450 can protect against NASH in mice by attenuating liver inflammation and fibrosis [50]. A series of analogs, such as pyrrole [2,3-d] azepines and tetrahydroazepinoindoles, have been developed to improve aqueous solubility and retain its bioactivity [51, 52]. Compound 14cc reveals a full FXR agonist activity. Although the X-ray crystal structure of 14cc shows that the overall structure and the position of helix H12 are similar to those of WAY-362450, the helix H2 region is disordered, and the loop between helix H5 and helix H6 is also shifted to accommodate the morpholine linker (Fig. 6c) [52].
Structural basis for the activity of FXR partial agonists
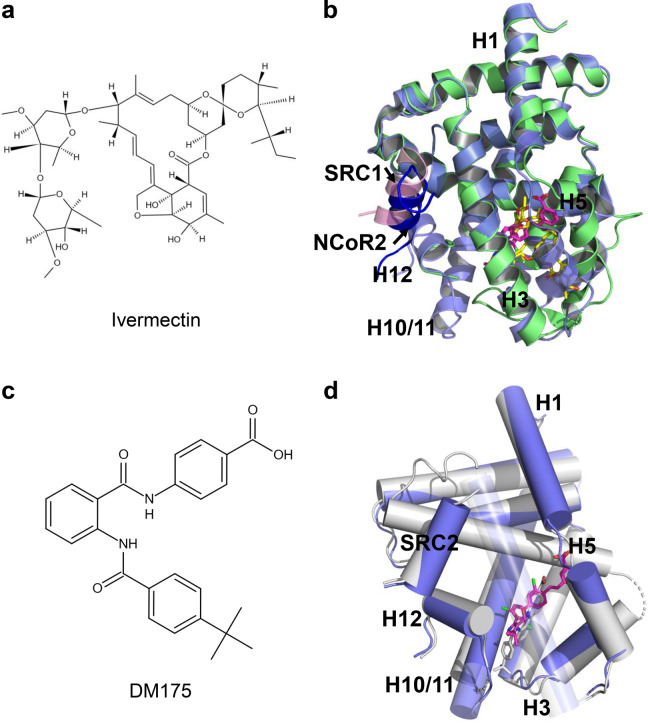
As a result of drug repositioning screening, the antiparasitic drug ivermectin was revealed as a novel FXR ligand with unique characteristics (Fig. 7a). Aside from the ability to promote the recruitment of coactivator motifs by FXR, ivermectin also induced the recruitment of the second corepressor motif nuclear receptor corepressor (NCoR-2) by FXR, a structural feature distinct from FXR full agonists. The unique binding of NCoR-2 by FXR was further validated by the crystal structure of the ternary complex of FXR LBD, ivermectin, and NCoR-2 (Fig. 7b). In the treatment of wild-type mice but not FXR-null mice, ivermectin decreased serum glucose and cholesterol levels, suggesting that ivermectin regulated metabolism by targeting FXR. Ivermectin treatment also improved hyperglycemia and hyperlipidemia in diabetic KK-Ay mice [53].
Fig. 7. Structural basis of FXR partial agonism.
a Chemical structure of ivermectin. b Superposition of the structure of FXR-ivermectin (PDB: 4WVD; FXR is in green and ivermectin is in yellow) and FXR-GW4064 (PDB: 3DCT; FXR is in slate blue and GW4064 is in magenta). SRC1 is colored in pink, and the NCoR-2 in blue. c Chemical structure of DM175. d Overlay of DM175 (PDB: 4QE8; FXR and DM175 are in gray) and GW4064 (PDB: 3DCT; FXR is in slate blue and GW4064 is in magenta).
Merk et al. developed the modulator DM175, which partially activates FXR in vitro and in mice (Fig. 7c). More importantly, they elucidated the molecular mechanism of FXR partial activation by crystallography- and NMR-based structural biology. FXR full agonists stabilize the formation of an extended helix H11 and the helix H11–H12 loop upon binding, which repositions helix H12 and enables coactivator recruitment (Fig. 7d). Partial agonism, in contrast, is conferred by a kink in helix H11 that destabilizes the helix H11–H12 loop and thus changes the orientation of helix H12. Finally, partial agonists induce conformational states capable of recruiting corepressors and coactivators, leading to an equilibrium of coactivator and corepressor binding [54].
Structural basis for the activity of FXR antagonists
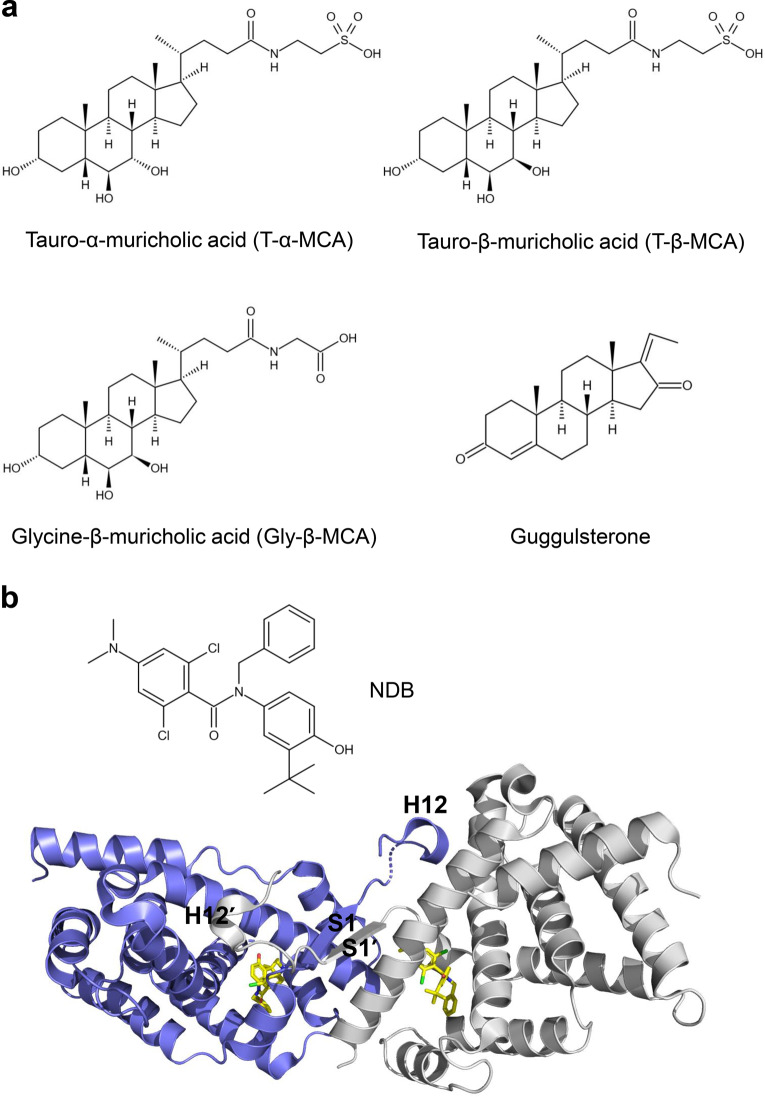
In addition to FXR agonists, many FXR antagonists have also been developed, mainly focused on BA-like and aromatic scaffolds (Fig. 8) [55]. Tauro-α-muricholic acid (T-α-MCA) and tauro-β-muricholic acid (T-β-MCA) are less hydrophobic bile acids that are functional antagonists to CDCA and GW4064-mediated activation of FXR. T-β-MCA-treated mice reduced the expression of ileal fibroblast growth Factor 15 (Fgf15) and Shp induced by taurocholic acid (TCA) [56]. Glycine-β-muricholic acid (Gly-β-MCA), a derivative of T-β-MCA, is a selective high-affinity and stable FXR inhibitor that can be administered orally, decreasing fasting serum insulin levels and reversible weight loss in HFD obese mice [57]. Guggulsterone, isolated from the guggul tree (Commiphora mukul), was identified as the first natural FXR antagonist. Guggulsterone inhibited FXR transactivation and coactivator recruitment induced by the agonist [58–60].
Fig. 8. FXR antagonists.
a Chemical structures of FXR antagonists. b Overall structure of the FXR LBD-NDB homodimer (PDB: 4OIV). The two monomers are in blue and gray, respectively.
In 2015, Xu et al. reported that the small molecule NDB functioned as a selective antagonist of FXR. SPR- and fluorescence quenching-based assays demonstrated that NDB binds to FXR. The antagonistic ability of NDB against FXR was revealed by AlphaScreen-based assay and cell-based reporter assay. NDB promoted the formation of FXR homodimerization, which caused severe conformational changes of helix H12 to occupy the coactivator binding site, leading to reduced coactivator binding (Fig. 8b). In addition, antagonist-induced FXR homodimerization also inhibited FXR/RXR heterodimer formation and decreased FXR target gene expression [61]. There are still many other FXR antagonists that have been analyzed in recent reviews [20, 62, 63].
FXR ligands in clinical studies
To date, there are no approved drugs for the treatment of NASH. In recent years, FXR agonists were considered promising therapeutic agents for NASH. OCA was the first FXR ligand to progress to phase 3 clinical trials on NASH. However, side effects such as pruritus and dyslipidemia associated with OCA raise concerns about its long-term safety. Several selective FXR agonists in clinical trials are summarized in Table 1.
Table 1.
FXR ligands in clinical studies.
| Agonists | PDB ID | Clinical trial phase | NCT number | Indication | Company |
|---|---|---|---|---|---|
| Obeticholic acid (INT-747) | 1OSV |
FDA approved Phase 2 Phase 3 |
PBC BAD NASH |
Intercept Pharmaceuticals | |
| EDP-305 | N/a | Phase 2 |
PBC NASH |
Enanta Pharmaceuticals | |
| MET409 | N/a | Phase 2 | NCT04702490 | NASH, T2DM | Metacrine |
| TERN-101 (LY-2562175) | N/a | Phase 2 | NCT04328077 | NASH | Terns Pharmaceuticals |
| Cilofexor (GS-9674) | N/a |
Phase 3 Phase 2 |
PSC NASH NASH |
Gilead Sciences | |
| Tropifexor (LJN452) | 7D42 |
Phase 2 Phase 2 Phase 2 |
PBC NASH BAD |
Novartis |
EDP-305 is a selective steroidal FXR agonist under development for treating NASH. The 12-week phase 2 study showed that EDP-305 reduced alanine aminotransferase (ALT) levels and MRI-based proton-density fat fraction (PDFF) in a longer-term trial assessing NASH patients with liver histology. An ongoing 72-week phase 2b trial is currently testing 1.5 and 2 mg doses to optimize further the balance between efficacy and tolerability [64].
MET409, a nonsteroidal FXR agonist, is now conducting a Phase 2a study evaluating MET409 (50 mg) alone or in combination with empagliflozin (10 mg) in patients with T2DM and NASH [65]. TERN-101, previously named LY2562175, is also a potent nonsteroidal FXR agonist [66]. The phase 1 studies results indicated that TERN-101 was well tolerated overall in healthy volunteer population, and no pruritus was reported. The safety, tolerability, efficacy, and PK of TERN-101 in tablet formulation is being assessed in a phase 2a study, with 12 weeks of dosing in noncirrhotic NASH patients [67].
Cilofexor (previously known as GS-9674), a synthetic derivative of GW4064, is reported as a nonsteroidal FXR agonist [44]. In a phase 2 study, cilofexor for 24 weeks was well tolerated and provided significant reductions in hepatic steatosis, liver biochemistry, and serum bile acids in patients with NASH [68]. In addition, the phase 2b trial suggested that the combination of cilofexor and firsocostat for 48 weeks improves key measures of NASH activity, including ballooning, inflammation, and steatosis, and may have an antifibrotic effect [69].
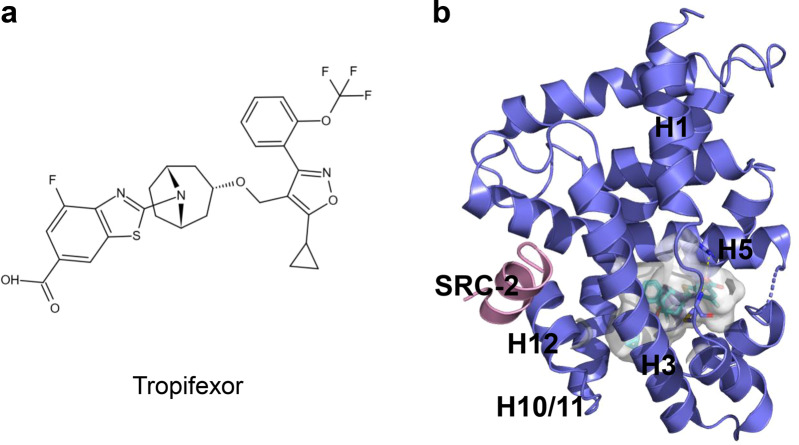
Tropifexor, also known as LJN452, is another highly potent nonsteroidal FXR agonist (Fig. 9a) [70]. The results from a phase 1 study indicate that tropifexor is well tolerated, with no drug-induced pruritus, and only mild and transient elevations in serum ALT, has a pharmacokinetic profile suitable for once-daily dosing and shows dose-dependent target engagement without altering plasma lipids in healthy volunteers [71]. A phase 2 study is ongoing to assess the efficacy, safety, and tolerability of oral tropifexor & licogliflozin combination therapy and each monotherapy for NASH and liver fibrosis patients’ treatment. The structural mechanism of tropifexor binding to FXR-LBD was revealed recently (Fig. 9b) [72].
Fig. 9. The structure of tropifexor binding to FXR-LBD.
a Chemical structure of tropifexor. b Cocrystal structure of FXR LBD (slate blue) with tropifexor (cyan). PDB: 7D42.
Structural mechanisms of FXR dimerization
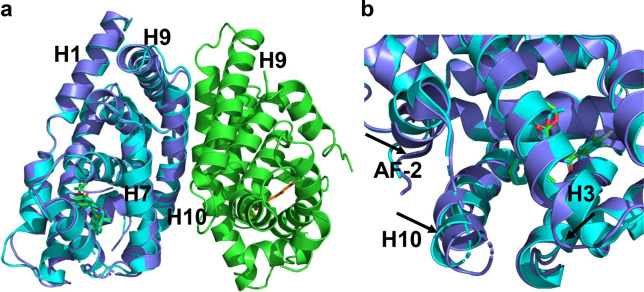
Recently, the crystal structures of the liganded FXR/RXR heterodimer in complex with coactivator peptides have been determined (Fig. 10a) [73, 74]. Structural analysis revealed an allosteric mechanism through which RXR binding stabilizes FXR active conformation, leading to enhanced FXR transactivation (Fig. 10b), suggesting that targeting RXR may become an alternative strategy in regulating FXR-mediated functions. Overall, the FXR homodimer and FXR/RXR heterodimer structures provide frameworks to design FXR modulators in treating FXR-related diseases.
Fig. 10. Structural basis of FXR/RXR heterodimerization.
a Superposition of the FXR/RXR heterodimer structure (PDB:5Z12; FXR is colored slate blue, and RXR is green). WAY-362450 and 9-cis-RA are shown in stick representation depicted in green and orange, respectively.) with the FXR–WAY-362450 structure (PDB:3FLI; cyan). b Heterodimerization with RXR alters the conformation of the FXR coregulator-binding site. The alignment reveals that shifts of AF2, H3, and H9 are induced by heterodimerization with RXR, which are indicated by arrows.
Future directions
FXR is increasingly recognized as an essential pharmacological target. FXR ligands have many beneficial effects treating NAFLD and/or NASH by decreasing hepatic lipogenesis, steatosis, and insulin resistance while also inhibiting inflammatory and fibrogenic responses in NASH patients [75–77]. However, the development of FXR ligands for treating liver diseases is challenging.
Following ligand binding, the function of FXR is mediated through the selective recruitment or release of specific coregulators, including both coactivators and corepressors [78, 79]. Indeed, the distinctive functional profiles of NRs in response to the binding of various ligands are largely determined by the selective usage of transcriptional coregulators [80]. Thus, small molecules called selective modulators with only a subset of FXR functions that selectively modulate the recruitment of coregulators are of great value for clinical purposes. As such, the benefits and side effects arising from the cross interaction of a variety of coregulators with FXR can be optimized by designing new selective modulators more suitable for clinical purposes by targeting individual coregulators and signaling pathways.
FXR is mainly expressed in the enterohepatic system and other tissues, including the adrenal gland, kidney, adipose tissue, stomach, macrophages, and bone marrow cells. Therefore, the therapeutic benefits of FXR modulation may increase by restricting FXR activity to specific tissues. Since the liver plays a key role in controlling metabolic homeostasis, excessive activation of FXR in the liver may interfere with the metabolic homeostasis of such important metabolites [81]. Intestinal-specific overexpression of constitutively active FXR reduces liver toxicity, bile acid pool size, and inflammatory infiltrates in mouse models of obstructive extrahepatic cholestasis and intrahepatic cholestasis. After that, given that intestinal FXR is key in regulating BA homeostasis, intestinal-specific FXR modulators were proposed to treat various enterohepatic diseases [82–84]. Tissue-specific FXR modulators may be developed by refining the physicochemical properties and pharmacokinetic profile of FXR ligands. Fexaramine and its derivatives, TC-100, have been identified as intestinal-specific FXR modulators that activate FXR signals in the intestine. Compared with conventional FXR ligands, tissue-selective FXR modulators seem to provide a strategy to increase the therapeutic index with reduced side effects, and develop predictive models for the biodistribution of FXR ligands becomes increasingly imperative [62, 81].
Although OCA has been shown to improve the histological features of NASH, the improvement of liver fibrosis still faces many problems [85, 86]. Peroxisome proliferator-activated receptors (PPARs, α, β, and γ) are fatty acid-activated NRs with a wide range of physiological actions. Importantly, PPARs have also been identified as promising pharmacological targets for NASH treatment [87–89]. Elafibranor, a dual PPARα/δ modulator, was also prepared for a phase III clinical trial [90]. Recently, the dual PPARα/γ agonist saroglitazar has received approval from the Indian Medicines Agency to treat NASH [91]. In addition, other anti-NASH drug targets have also been reported [92, 93]. Several studies have already confirmed the effectiveness of combination strategies [62, 94]. Thus, combining FXR modulators with other therapeutic agents may achieve more satisfactory clinical benefits.
Supplementary information
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31770814) and Project “111” sponsored by the State Bureau of Foreign Experts and Ministry of Education of China (BP2018017).
Competing interests
The authors declare no competing interest.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-021-00849-4.
References
- 1.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–95. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burris TP, Solt LA, Wang Y, Crumbley C, Banerjee S, Griffett K, et al. Nuclear receptors and their selective pharmacologic modulators. Pharmacol Rev. 2013;65:710–78. doi: 10.1124/pr.112.006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin L, Li Y. Structural and functional insights into nuclear receptor signaling. Adv Drug Deliv Rev. 2010;62:1218–26. doi: 10.1016/j.addr.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–6. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 6.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–5. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 7.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–8. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–53. doi: 10.1016/S1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 9.Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–93. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 10.Han CY. Update on FXR biology: promising therapeutic target? Int J Mol Sci. 2018;19:2069. doi: 10.3390/ijms19072069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maglich JM, Caravella JA, Lambert MH, Willson TM, Moore JT, Ramamurthy L. The first completed genome sequence from a teleost fish (Fugu rubripes) adds significant diversity to the nuclear receptor superfamily. Nucleic Acids Res. 2003;31:4051–8. doi: 10.1093/nar/gkg444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otte K, Kranz H, Kober I, Thompson P, Hoefer M, Haubold B, et al. Identification of farnesoid X receptor beta as a novel mammalian nuclear receptor sensing lanosterol. Mol Cell Biol. 2003;23:864–72. doi: 10.1128/MCB.23.3.864-872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modica S, Gadaleta RM, Moschetta A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl Recept Signal. 2010;8:e005. doi: 10.1621/nrs.08005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downes M, Verdecia MA, Roecker AJ, Hughes R, Hogenesch JB, Kast-Woelbern HR, et al. A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol Cell. 2003;11:1079–92. doi: 10.1016/S1097-2765(03)00104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–91. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 16.Mi LZ, Devarakonda S, Harp JM, Han Q, Pellicciari R, Willson TM, et al. Structural basis for bile acid binding and activation of the nuclear receptor FXR. Mol Cell. 2003;11:1093–100. doi: 10.1016/S1097-2765(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 17.Halilbasic E, Fuchs C, Traussnigg S, Trauner M. Farnesoid X Receptor agonists and other bile acid signaling strategies for treatment of liver disease. Dig Dis. 2016;34:580–8. doi: 10.1159/000445268. [DOI] [PubMed] [Google Scholar]
- 18.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–24. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci. 2006;31:572–80. doi: 10.1016/j.tibs.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 20.De Marino S, Festa C, Sepe V, Zampella A. Chemistry and pharmacology of GPBAR1 and FXR selective agonists, dual agonists, and antagonists. Handb Exp Pharmacol. 2019;256:137–65. doi: 10.1007/164_2019_237. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y. Recent progress on bile acid receptor modulators for treatment of metabolic diseases. J Med Chem. 2016;59:6553–79. doi: 10.1021/acs.jmedchem.5b00342. [DOI] [PubMed] [Google Scholar]
- 22.Carr RM, Reid AE. FXR agonists as therapeutic agents for non-alcoholic fatty liver disease. Curr Atheroscler Rep. 2015;17:500. doi: 10.1007/s11883-015-0500-2. [DOI] [PubMed] [Google Scholar]
- 23.Ali AH, Carey EJ, Lindor KD. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med. 2015;3:5. doi: 10.3978/j.issn.2305-5839.2014.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiorucci S, Biagioli M, Sepe V, Zampella A, Distrutti E. Bile acid modulators for the treatment of nonalcoholic steatohepatitis (NASH) Expert Opin Investig Drugs. 2020;29:623–32. doi: 10.1080/13543784.2020.1763302. [DOI] [PubMed] [Google Scholar]
- 25.Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, et al. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–72. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 26.Sepe V, Festa C, Renga B, Carino A, Cipriani S, Finamore C, et al. Insights on FXR selective modulation. Speculation on bile acid chemical space in the discovery of potent and selective agonists. Sci Rep. 2016;6:19008. doi: 10.1038/srep19008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mason A, Luketic V, Lindor K. Farnesoid-X receptor agonists: A new class of drugs for the treatment of PBC An international study evaluating the addition of INT-747 to ursodeoxycholic acid. J Hepatol. 2010;52:S1–2. doi: 10.1016/S0168-8278(10)60004-9. [DOI] [Google Scholar]
- 28.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellicciari R, Costantino G, Camaioni E, Sadeghpour BM, Entrena A, Willson TM, et al. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Med Chem. 2004;47:4559–69. doi: 10.1021/jm049904b. [DOI] [PubMed] [Google Scholar]
- 30.Pellicciari R, Gioiello A, Costantino G, Sadeghpour BM, Rizzo G, Meyer U, et al. Back door modulation of the farnesoid X receptor: design, synthesis, and biological evaluation of a series of side chain modified chenodeoxycholic acid derivatives. J Med Chem. 2006;49:4208–15. doi: 10.1021/jm060294k. [DOI] [PubMed] [Google Scholar]
- 31.Gioiello A, Macchiarulo A, Carotti A, Filipponi P, Costantino G, Rizzo G, et al. Extending SAR of bile acids as FXR ligands: discovery of 23-N-(carbocinnamyloxy)-3α,7α-dihydroxy-6α-ethyl-24-nor-5β-cholan-23-amine. Bioorg Med Chem. 2011;19:2650–8. doi: 10.1016/j.bmc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Xiao H, Li P, Li X, He H, Wang J, Guo F, et al. Synthesis and biological evaluation of a series of bile acid derivatives as FXR agonists for treatment of NASH. ACS Med Chem Lett. 2017;8:1246–51. doi: 10.1021/acsmedchemlett.7b00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pellicciari R, Passeri D, De Franco F, Mostarda S, Filipponi P, Colliva C, et al. Discovery of 3α,7α,11β-Trihydroxy-6α-ethyl-5β-cholan-24-oic Acid (TC-100), a novel bile acid as potent and highly selective FXR agonist for enterohepatic disorders. J Med Chem. 2016;59:9201–14. doi: 10.1021/acs.jmedchem.6b01126. [DOI] [PubMed] [Google Scholar]
- 34.Fujino T, Une M, Imanaka T, Inoue K, Nishimaki-Mogami T. Structure-activity relationship of bile acids and bile acid analogs in regard to FXR activation. J Lipid Res. 2004;45:132–8. doi: 10.1194/jlr.M300215-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Pellicciari R, Costantino G, Fiorucci S. Farnesoid X receptor: from structure to potential clinical applications. J Med Chem. 2005;48:5383–403. doi: 10.1021/jm0582221. [DOI] [PubMed] [Google Scholar]
- 36.Rizzo G, Passeri D, De Franco F, Ciaccioli G, Donadio L, Rizzo G, et al. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol Pharmacol. 2010;78:617–30. doi: 10.1124/mol.110.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu YB, Liu XY, Zhan W. Farnesoid X receptor agonist INT-767 attenuates liver steatosis and inflammation in rat model of nonalcoholic steatohepatitis. Drug Des Devel Ther. 2018;12:2213–21.. doi: 10.2147/DDDT.S170518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soisson SM, Parthasarathy G, Adams AD, Sahoo S, Sitlani A, Sparrow C, et al. Identification of a potent synthetic FXR agonist with an unexpected mode of binding and activation. Proc Natl Acad Sci USA. 2008;105:5337–42. doi: 10.1073/pnas.0710981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD, et al. Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem. 2000;43:2971–4. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- 40.Akwabi-Ameyaw A, Bass JY, Caldwell RD, Caravella JA, Chen L, Creech KL, et al. Conformationally constrained farnesoid X receptor (FXR) agonists: Naphthoic acid-based analogs of GW 4064. Bioorg Med Chem Lett. 2008;18:4339–43. doi: 10.1016/j.bmcl.2008.06.073. [DOI] [PubMed] [Google Scholar]
- 41.Abel U, Schlüter T, Schulz A, Hambruch E, Steeneck C, Hornberger M, et al. Synthesis and pharmacological validation of a novel series of non-steroidal FXR agonists. Bioorg Med Chem Lett. 2010;20:4911–7. doi: 10.1016/j.bmcl.2010.06.084. [DOI] [PubMed] [Google Scholar]
- 42.Kinzel O, Steeneck C, Schlüter T, Schulz A, Gege C, Hahn U, et al. Novel substituted isoxazole FXR agonists with cyclopropyl, hydroxycyclobutyl and hydroxyazetidinyl linkers: Understanding and improving key determinants of pharmacological properties. Bioorg Med Chem Lett. 2016;26:3746–53. doi: 10.1016/j.bmcl.2016.05.070. [DOI] [PubMed] [Google Scholar]
- 43.Bass JY, Caravella JA, Chen L, Creech KL, Deaton DN, Madauss KP, et al. Conformationally constrained farnesoid X receptor (FXR) agonists: heteroaryl replacements of the naphthalene. Bioorg Med Chem Lett. 2011;21:1206–13. doi: 10.1016/j.bmcl.2010.12.089. [DOI] [PubMed] [Google Scholar]
- 44.Gege C, Hambruch E, Hambruch N, Kinzel O, Kremoser C. Nonsteroidal FXR ligands: current status and clinical applications. Handb Exp Pharmacol. 2019;256:167–205. doi: 10.1007/164_2019_232. [DOI] [PubMed] [Google Scholar]
- 45.Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159–65. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Zhao Z, Zhou J, Guo Y, Wang G, Hao H, et al. A novel intestinal-restricted FXR agonist. Bioorg Med Chem Lett. 2017;27:3386–90.. doi: 10.1016/j.bmcl.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Evans RM, Downes M, Atkins A, Fang S, Suh JM, Baiga TJ, et al, inventors. Preparation of fexaramine analogs and methods for treating or preventing metabolic disorders or intestinal inflammation. WO 2015138969. 2015.
- 48.Evans RM, Downes M, Baiga TJ, Keana JFW, inventors. Fxr agonists and methods for making and using. WO 2015138986. 2015.
- 49.Flatt B, Martin R, Wang TL, Mahaney P, Murphy B, Gu XH, et al. Discovery of XL335 (WAY-362450), a highly potent, selective, and orally active agonist of the farnesoid X receptor (FXR) J Med Chem. 2009;52:904–7. doi: 10.1021/jm8014124. [DOI] [PubMed] [Google Scholar]
- 50.Zhang S, Wang J, Liu Q, Harnish DC. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J Hepatol. 2009;51:380–8. doi: 10.1016/j.jhep.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 51.Mehlmann JF, Crawley ML, Lundquist JTT, Unwalla RJ, Harnish DC, Evans MJ, et al. Pyrrole[2,3-d]azepino compounds as agonists of the farnesoid X receptor (FXR) Bioorg Med Chem Lett. 2009;19:5289–92. doi: 10.1016/j.bmcl.2009.07.148. [DOI] [PubMed] [Google Scholar]
- 52.Lundquist JT, Harnish DC, Kim CY, Mehlmann JF, Unwalla RJ, Phipps KM, et al. Improvement of physiochemical properties of the tetrahydroazepinoindole series of farnesoid X receptor (FXR) agonists: beneficial modulation of lipids in primates. J Med Chem. 2010;53:1774–87. doi: 10.1021/jm901650u. [DOI] [PubMed] [Google Scholar]
- 53.Jin L, Feng X, Rong H, Pan Z, Inaba Y, Qiu L, et al. The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism. Nat Commun. 2013;4:1937. doi: 10.1038/ncomms2924. [DOI] [PubMed] [Google Scholar]
- 54.Merk D, Sreeramulu S, Kudlinzki D, Saxena K, Linhard V, Gande SL, et al. Molecular tuning of farnesoid X receptor partial agonism. Nat Commun. 2019;10:2915. doi: 10.1038/s41467-019-10853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sepe V, Distrutti E, Fiorucci S, Zampella A. Farnesoid X receptor modulators 2014-present: a patent review. Expert Opin Ther Pat. 2018;28:351–64. doi: 10.1080/13543776.2018.1459569. [DOI] [PubMed] [Google Scholar]
- 56.Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–35. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Jiang C, Xie C, Lv Y, Li J, Krausz KW, Shi J, et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun. 2015;6:10166. doi: 10.1038/ncomms10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, et al. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science. 2002;296:1703–6. doi: 10.1126/science.1072891. [DOI] [PubMed] [Google Scholar]
- 59.Wu J, Xia C, Meier J, Li S, Hu X, Lala DS. The hypolipidemic natural product guggulsterone acts as an antagonist of the bile acid receptor. Mol Endocrinol. 2002;16:1590–7. doi: 10.1210/mend.16.7.0894. [DOI] [PubMed] [Google Scholar]
- 60.Cui J, Huang L, Zhao A, Lew JL, Yu J, Sahoo S, et al. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J Biol Chem. 2003;278:10214–20. doi: 10.1074/jbc.M209323200. [DOI] [PubMed] [Google Scholar]
- 61.Xu X, Xu X, Liu P, Zhu ZY, Chen J, Fu HA, et al. Structural basis for small molecule NDB (N-Benzyl-N-(3-(tert-butyl)-4-hydroxyphenyl)-2,6-dichloro-4-(dimethylamino) Benzamide) as a selective antagonist of farnesoid X receptor α (FXRα) in stabilizing the homodimerization of the receptor. J Biol Chem. 2015;290:19888–99. doi: 10.1074/jbc.M114.630475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, He Q, Wang G, Xu X, Hao H. FXR modulators for enterohepatic and metabolic diseases. Expert Opin Ther Pat. 2018;28:765–82. doi: 10.1080/13543776.2018.1527906. [DOI] [PubMed] [Google Scholar]
- 63.Jiang L, Zhang H, Xiao D, Wei H, Chen Y. Farnesoid X receptor (FXR): Structures and ligands. Comput Struct Biotechnol J. 2021;19:2148–59. doi: 10.1016/j.csbj.2021.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ratziu V, Rinella ME, Neuschwander-Tetri BA, Lawitz E, Denham D, Kayali Z, et al. EDP-305 in patients with NASH: a phase II double-blind placebo-controlled dose-ranging study. J Hepatol. 2021;S0168-8278:02155–3. doi: 10.1016/j.jhep.2021.10.018. [DOI] [PubMed] [Google Scholar]
- 65.Harrison SA, Bashir MR, Lee KJ, Shim-Lopez J, Lee J, Wagner B, et al. A structurally optimized FXR agonist, MET409, reduced liver fat content over 12 weeks in patients with non-alcoholic steatohepatitis. J Hepatol. 2021;75:25–33. doi: 10.1016/j.jhep.2021.01.047. [DOI] [PubMed] [Google Scholar]
- 66.Genin MJ, Bueno AB, Agejas Francisco J, Manninen PR, Bocchinfuso WP, Montrose-Rafizadeh C, et al. Discovery of 6-(4-{[5-Cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl]methoxy}piperidin-1-yl)-1-methyl-1H-indole-3-carboxylic acid: a novel FXR agonist for the treatment of dyslipidemia. J Med Chem. 2015;58:9768–72. doi: 10.1021/acs.jmedchem.5b01161. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Crittenden DB, Eng C, Zhang Q, Guo P, Chung D, et al. Safety, pharmacokinetics, pharmacodynamics, and formulation of liver-distributed farnesoid X-receptor agonist TERN-101 in healthy volunteers. Clin Pharmacol Drug Dev. 2021;10:1198–208. doi: 10.1002/cpdd.960. [DOI] [PubMed] [Google Scholar]
- 68.Patel K, Harrison SA, Elkhashab M, Trotter JF, Herring R, Rojter SE, et al. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology. 2020;72:58–71. doi: 10.1002/hep.31205. [DOI] [PubMed] [Google Scholar]
- 69.Loomba R, Noureddin M, Kowdley KV, Kohli A, Sheikh A, Neff G, et al. Combination therapies including cilofexor and firsocostat for bridging fibrosis and cirrhosis attributable to NASH. Hepatology. 2021;73:625–43. doi: 10.1002/hep.31622. [DOI] [PubMed] [Google Scholar]
- 70.Tully DC, Rucker PV, Chianelli D, Williams J, Vidal A, Alper PB, et al. Discovery of tropifexor (LJN452), a highly potent non-bile acid FXR agonist for the treatment of cholestatic liver diseases and nonalcoholic steatohepatitis (NASH) J Med Chem. 2017;60:9960–73. doi: 10.1021/acs.jmedchem.7b00907. [DOI] [PubMed] [Google Scholar]
- 71.Badman MK, Chen J, Desai S, Vaidya S, Neelakantham S, Zhang J, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel non-bile acid FXR agonist tropifexor (LJN452) in healthy volunteers. Clin Pharmacol Drug Dev. 2020;9:395–410. doi: 10.1002/cpdd.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang L, Xiao D, Li Y, Dai S, Qu L, Chen X, et al. Structural basis of tropifexor as a potent and selective agonist of farnesoid X receptor. Biochem Biophys Res Commun. 2021;534:1047–52. doi: 10.1016/j.bbrc.2020.10.039. [DOI] [PubMed] [Google Scholar]
- 73.Zheng W, Lu Y, Tian S, Ma F, Wei Y, Xu S, et al. Structural insights into the heterodimeric complex of the nuclear receptors FXR and RXR. J Biol Chem. 2018;293:12535–41. doi: 10.1074/jbc.RA118.004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang N, Zou Q, Xu J, Zhang J, Liu J. Ligand binding and heterodimerization with retinoid X receptor α (RXRα) induce farnesoid X receptor (FXR) conformational changes affecting coactivator binding. J Biol Chem. 2018;293:18180–91. doi: 10.1074/jbc.RA118.004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu Y, Liu H, Zhang M, Guo GL. Fatty liver diseases, bile acids, and FXR. Acta Pharm Sin B. 2016;6:409–12.. doi: 10.1016/j.apsb.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim SG, Kim BK, Kim K, Fang S. Bile acid nuclear receptor farnesoid X receptor: therapeutic target for nonalcoholic fatty liver disease. Endocrinol Metab. 2016;31:500–4. doi: 10.3803/EnM.2016.31.4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shah RA, Alkhouri N, Kowdley KV. Emerging drugs for the treatment of non-alcoholic steatohepatitis: a focused review of farnesoid X receptor agonists. Expert Opin Emerg Drugs. 2020;25:251–60. doi: 10.1080/14728214.2020.1796968. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y, Castellani LW, Sinal CJ, Gonzalez FJ, Edwards PA. Peroxisome proliferator-activated receptor-gamma coactivator 1alpha (PGC-1alpha) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Genes Dev. 2004;18:157–69. doi: 10.1101/gad.1138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fujino T, Sato Y, Une M, Kanayasu-Toyoda T, Yamaguchi T, Shudo K, et al. In vitro farnesoid X receptor ligand sensor assay using surface plasmon resonance and based on ligand-induced coactivator association. J Steroid Biochem Mol Biol. 2003;87:247–52. doi: 10.1016/j.jsbmb.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 80.Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–64. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- 81.Massafra V, Pellicciari R, Gioiello A, van Mil SWC. Progress and challenges of selective farnesoid X receptor modulation. Pharmacol Ther. 2018;191:162–77. doi: 10.1016/j.pharmthera.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 82.van Zutphen T, Bertolini A, de Vries HD, Bloks VW, de Boer JF, Jonker JW, et al. Potential of intestine-selective FXR modulation for treatment of metabolic disease. Handb Exp Pharmacol. 2019;256:207–34. doi: 10.1007/164_2019_233. [DOI] [PubMed] [Google Scholar]
- 83.Yin Y, Wang M, Gu W, Chen L. Intestine-specific FXR agonists as potential therapeutic agents for colorectal cancer. Biochem Pharmacol. 2021;186:114430. doi: 10.1016/j.bcp.2021.114430. [DOI] [PubMed] [Google Scholar]
- 84.Modica S, Petruzzelli M, Bellafante E, Murzilli S, Salvatore L, Celli N, et al. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology. 2012;142:355–65.e1-4. doi: 10.1053/j.gastro.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 85.Ratziu V, Sanyal AJ, Loomba R, Rinella M, Harrison S, Anstee QM, et al. REGENERATE: design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp Clin Trials. 2019;84:105803. doi: 10.1016/j.cct.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 86.Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–96. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 87.Tanaka N, Aoyama T, Kimura S, Gonzalez FJ. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol Ther. 2017;179:142–57. doi: 10.1016/j.pharmthera.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boeckmans J, Natale A, Rombaut M, Buyl K, Rogiers V, De Kock J, et al. Anti-NASH drug development hitches a lift on PPAR agonism. Cells. 2019;9:37. doi: 10.3390/cells9010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cave MC, Clair HB, Hardesty JE, Falkner KC, Feng W, Clark BJ, et al. Nuclear receptors and nonalcoholic fatty liver disease. Biochim Biophys Acta. 2016;1859:1083–99. doi: 10.1016/j.bbagrm.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ratziu V. Novel pharmacotherapy options for NASH. Dig Dis Sci. 2016;61:1398–405. doi: 10.1007/s10620-016-4128-z. [DOI] [PubMed] [Google Scholar]
- 91.Jain MR, Giri SR, Bhoi B, Trivedi C, Rath A, Rathod R, et al. Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver Int. 2018;38:1084–94. doi: 10.1111/liv.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2018;53:362–76. doi: 10.1007/s00535-017-1415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharma M, Premkumar M, Kulkarni AV, Kumar P, Reddy DN, Rao NP. Drugs for non-alcoholic steatohepatitis (NASH): quest for the holy grail. J Clin Transl Hepatol. 2021;9:40–50. doi: 10.14218/JCTH.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fiorucci S, Biagioli M, Distrutti E. Future trends in the treatment of non-alcoholic steatohepatitis. Pharmacol Res. 2018;134:289–98. doi: 10.1016/j.phrs.2018.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.