Summary
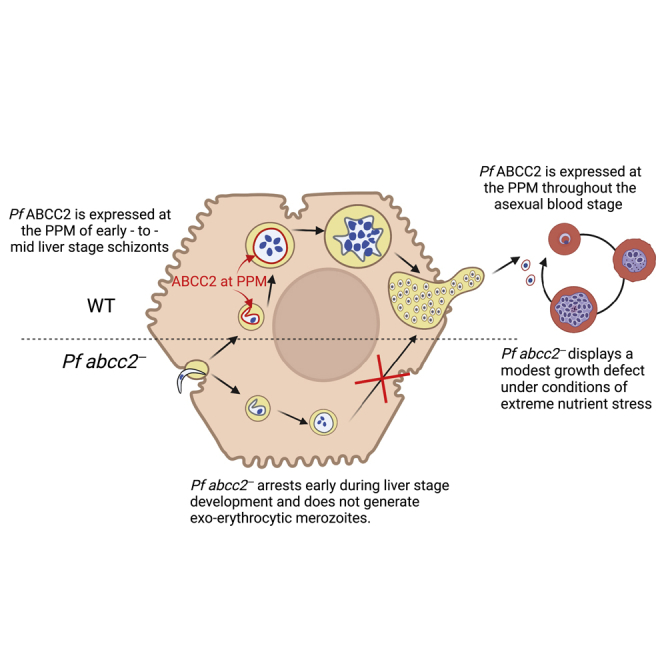
Plasmodium sporozoites invade hepatocytes and transform into liver stages within a parasitophorous vacuole (PV). The parasites then grow and replicate their genome to form exoerythrocytic merozoites that infect red blood cells. We report that the human malaria parasite Plasmodium falciparum (Pf) expresses a C-type ATP-binding cassette transporter, Pf ABCC2, which marks the transition from invasive sporozoite to intrahepatocytic early liver stage. Using a humanized mouse infection model, we show that Pf ABCC2 localizes to the parasite plasma membrane in early and mid-liver stage parasites but is not detectable in late liver stages. Pf abcc2— sporozoites invade hepatocytes, form a PV, and transform into liver stage trophozoites but cannot transition to exoerythrocytic schizogony and fail to transition to blood stage infection. Thus, Pf ABCC2 is an expression marker for early phases of parasite liver infection and plays an essential role in the successful initiation of liver stage replication.
Subject areas: Biological sciences, Parasitology, Cell biology
Graphical abstract
Highlights
-
•
Pf ABCC2 expression marks the transition from sporozoite to early liver stage
-
•
Pf ABCC2 localizes to the early and mid-liver stage plasma membrane
-
•
Pf ABCC2 is critical for initiation of exoerythrocytic schizogony
-
•
Pf abcc2– liver stages fail to transition to blood stage infection
Biological sciences; Parasitology; Cell biology
Introduction
Malaria caused by Plasmodium parasites continues to be a great global health concern. There were 240 million malaria cases with an estimated 627,000 deaths in 2020, which is a significant increase when compared to the previous years (World Health Organization, 2021). Among Plasmodium parasites that infect humans, Plasmodium falciparum (Pf) causes most morbidity and mortality (World Health Organization, 2021). Transmission of Pf to the human host occurs when motile sporozoites are released from the salivary glands and injected into the skin via the bite of an infected female Anopheles mosquito (Vaughan and Kappe, 2017; Loubens et al., 2020; Arredondo et al., 2021). Once sporozoites enter the bloodstream, they are transported to the liver and enter the parenchyma to target hepatocytes. Successful infection of hepatocytes requires the formation of a membrane-bound parasitophorous vacuole (PV), in which the invasive sporozoite dedifferentiates and transforms into a liver stage trophozoite (Arredondo et al., 2021). The liver stage trophozoite then undergoes an extensive growth and replication process called exoerythrocytic schizogony that involves cell mass expansion and numerous rounds of genome and organellar replication. A mature late liver stage schizont ultimately differentiates into tens of thousands of exoerythrocytic merozoites, which initiate the symptomatic erythrocytic cycle of infection. A relatively small number of sporozoites are injected into the skin, and an even smaller number of these sporozoites reach the liver via the circulation, rendering the liver stage a critical population bottleneck (Medica and Sinnis, 2005) and an attractive target for prophylactic drug and vaccine development (Kreutzfeld et al., 2017; Vaughan et al., 2017; Draper et al., 2018; Duffy and Gorres, 2020). However, there is an insufficient understanding of Plasmodium liver stage biology and most of our knowledge is derived from studies using the rodent malaria models Plasmodium berghei (Pb) and Plasmodium yoelii (Py). Thus, Pf liver stage development remains largely unexplored because the development of robust liver infection models for human parasites has been challenging.
In the recent years, significant progress has been made in identifying Pf proteins required for sporozoite traversal and invasion into hepatocytes (Arredondo et al., 2021). However, there are no known protein markers for early Pf liver stage differentiation and development, which should not be expressed in sporozoites but are expressed following sporozoite invasion of hepatocytes. Pioneering studies on sporozoite biology in the rodent malaria species Pb identified transcripts that are Upregulated in Infective Sporozoites (UIS) by subtractive cDNA hybridization (Kappe et al., 2001). These genes are highly transcribed in salivary gland sporozoites but remain translationally repressed such that the majority of protein expression is initiated during or just after productive hepatocyte invasion (Lindner et al., 2019). Two such UIS proteins in rodent malaria parasite species are UIS3 (Mueller et al., 2005b; Tarun et al., 2007) and UIS4 (Mueller et al., 2005a), which localize to the PV membrane. Gene-deficient parasites of both UIS3 and UIS4 in Py and Pb undergo partial arrest early in liver stage development (Mueller et al., 2005a, 2005b; Jobe et al., 2007; Tarun et al., 2007). However, the orthologous UIS3 gene in Pf has been refractory to gene deletion, and therefore the role of Pf UIS3 in liver stage development remains elusive. The Pf gene syntenic to Pb and Py UIS4, known as Pf ETRAMP10.3, is expressed in salivary gland sporozoites (MacKellar et al., 2010; Lindner et al., 2019; Marin-mogollon et al., 2019), late liver stages, and gametocytes (MacKellar et al., 2010; Marin-mogollon et al., 2019). Pf ETRAMP10.3 is therefore not useful as a marker for early Pf liver stage infection. Pf ETRAMP10.3 has also been refractory to gene deletion (MacKellar et al., 2010). Additional UIS proteins, including members of the 6-Cys family of proteins, Pf P36 and P52 (Ishino et al., 2005; van Dijk et al., 2005; Labaied et al., 2007; Vanbuskirk et al., 2009), are expressed in salivary gland sporozoites but not in early liver stages, and their deletion prevents the parasite from forming a PV. Thus, no unique markers of early Pf liver stage development are available yet.
The Pf C-type ATP-binding complex (ABC) transporter proteins, Pf ABCC1 and Pf ABCC2, were reported to be expressed in Pf replicating exoerythrocytic schizonts grown in cultured primary human hepatocytes (Rijpma et al., 2016). Gene deletion studies showed that Pf abcc1— liver stages developed normally in primary hepatocyte cultures, but Pf abcc2— liver stages suffered a developmental arrest and did not form mature exoerythrocytic schizonts (Rijpma et al., 2016). Thus, we explored whether Pf ABCC2 could serve as a marker for early Pf liver stage infection. Pf ABCC2 belongs to the ABC family of transporters, which constitute a highly conserved protein superfamily, that utilize the energy from ATP hydrolysis to export a wide range of substrates across cell membranes (Hollenstein et al., 2007; Upton et al., 2011; Wilkens, 2015). Proteins belonging to the ABC transporter family C (ABCC) are also commonly referred to as multidrug resistance-associated proteins (MRPs). MRP transporters are known to export organic anions such as glutathione (GSH) conjugates, glucuronide conjugates, and sulfate conjugates across biological membranes (Jones and George, 2007). The genomes of human malaria causing Plasmodium species, Pf, and Plasmodium vivax (Pv) contain two genes belonging to the ABCC subfamily, ABCC1 and ABCC2, whereas the rodent malaria species, Py and Pb, contain only one ABCC gene (Koenderink et al., 2010).
In this study, we report that Pf ABCC2 is not expressed in sporozoites but expression initiates in early liver stages, where the protein localizes to the parasite plasma membrane. Using a humanized mouse model of Pf liver stage infection, we show that Pf ABCC2 is critical for successful initiation of liver stage parasite growth and replication. Thus, Pf ABCC2 can serve as a protein that marks the successful transition to liver stage infection, and it likely plays a role in a critical exchange between the early liver stage cytoplasm and PV lumen.
Results
Pf ABCC2 localizes to the parasite plasma membrane in blood stage parasites and in early- to mid-phases of liver stage development
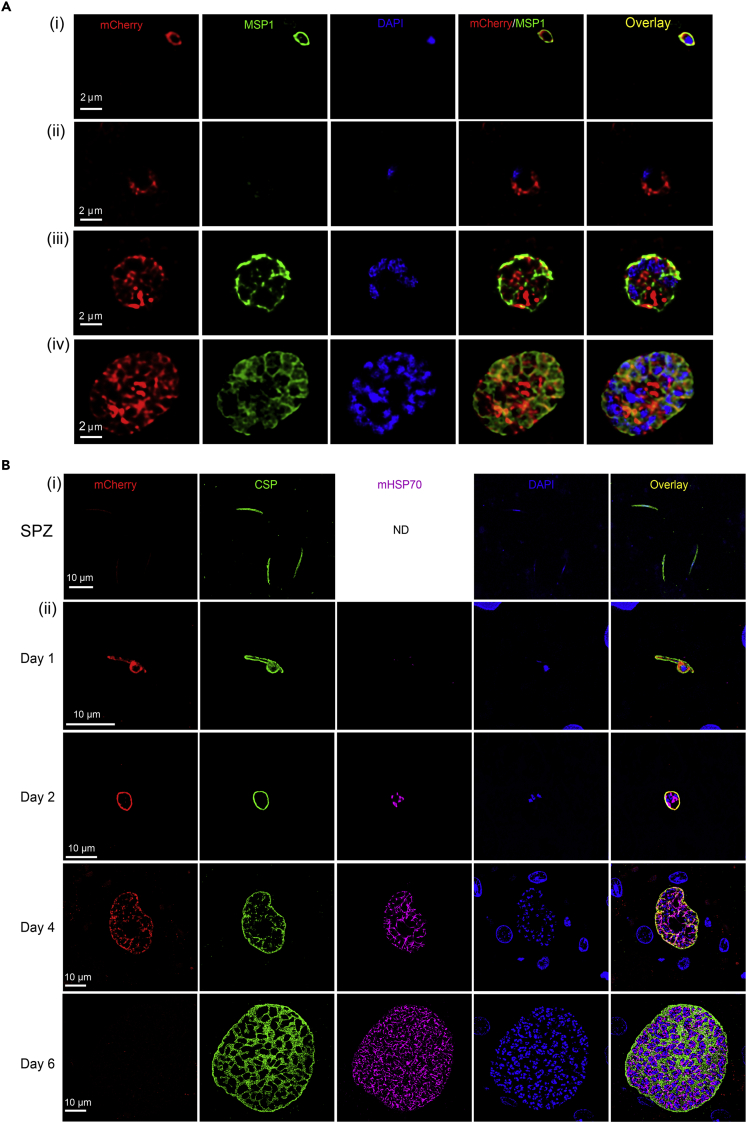
To evaluate the spatiotemporal localization of Pf ABCC2 throughout the parasite life cycle, we generated a transgenic Pf NF54 ABCC2 line using CRISPR/Cas9 gene editing tools (Goswami et al., 2020) that express ABCC2 with a C-terminal mCherry fusion tag (Figure S1A). Pf ABCC2mCherry clones were isolated by limiting dilution cloning. We then investigated blood stage expression of Pf ABCC2 via immunofluorescence assay (IFA) using antibodies against mCherry and MSP1 to detect Pf ABCC2 and mark the parasite plasma membrane (PPM), respectively. Pf ABCC2 expression was detected in intraerythrocytic parasite stages, including trophozoite and schizont stages (Figure 1A). Pf ABCC2 was co-localized with MSP1, indicating that it is likely positioned in the PPM. In early schizonts and segmenting schizonts, Pf ABCC2 was visible on plasma membrane invaginations (Figure 1A). Interestingly, Pf ABCC2 was also observed on the plasma membrane of free merozoites (Figure 1A). No differences in asexual blood stage growth kinetics under standard culture conditions were seen when comparing Pf NF54 and Pf ABCC2mCherry strains (Figures S1B). Therefore, fusion of the C-terminal mCherry tag to the Pf ABCC2 protein did not affect asexual blood stage development.
Figure 1.
Pf ABCC2 localizes to the parasite plasma membrane in Pf blood and liver stages
(A) Pf ABCC2mCherry blood stage parasites, (i) merozoite, (ii) ring, (iii) trophozoite, and (iv) schizont, were analyzed by IFA using antibodies against mCherry (red) and MSP1 (green). DNA is stained by DAPI. Scale bar size is 2 μm. Pf ABCC2mCherry localization to the parasite plasma membrane (PPM) was observed in all stages of the intraerythrocytic development and free merozoites as indicated by co-localization with the PPM marker MSP1.
(B) (i) IFA of Pf ABCC2mCherry salivary gland sporozoites dissected on day 15 of mosquito infection, using antibodies against mCherry (red) and CSP (green). DNA is stained with DAPI. Scale bar size is 10 μm. No Pf ABCC2mCherry expression could be detected in salivary sporozoites. (ii) FRG NOD huHep mice were infected with one million sporozoites of Pf ABCC2mCherry and livers were harvested 1-, 2-, 4-, and 6-days post sporozoite infection. Tissue sections were used for IFAs with antibodies against mCherry to localize ABCC2 (red), the PPM (CSP, green), and the parasite mitochondria (mHSP70, pink). DNA was stained with DAPI. Scale bar size is 10 μm. Pf ABCC2mCherry expression was detected as early as day 1 and persisted until day 4 postinfection. Expression of ABCC2 co-localized with the PPM marker CSP. No Pf ABCC2mCherry expression was detected on day 6.
Pf ABCC2mCherry parasites also showed normal mosquito stage development as counts for oocysts/midgut, oocyst prevalence, and salivary gland sporozoites/mosquito were within the range of what is observed in wildtype Pf NF54 infections (Figure S1C). We analyzed the expression of Pf ABCC2 in developing Pf ABCC2mCherry midgut oocysts on day 8 and on mature salivary gland sporozoites on day 15 after an infectious blood meal, both by live microscopy and by IFAs with antibodies against the mCherry tag. No Pf ABCC2mCherry expression was detected in midgut oocysts (Figure S1D) and salivary gland sporozoites (Figures 1B(i), S1E).
We next analyzed the expression dynamics of Pf ABCC2 throughout Pf liver stage development. Salivary gland sporozoites of the Pf ABCC2mCherry strain were used to infect FRG NOD huHep mice that harbor human hepatocytes (Azuma et al., 2007) as previously described (Goswami et al., 2020), and livers were harvested on 1–6 days after infection. Infected liver tissue sections were analyzed by IFA using antibodies against mCherry, CSP, and mitochondrial HSP70 (mHSP70) to detect expression of Pf ABCC2 and visualize the PPM and the parasite mitochondria, respectively. Pf ABCC2mCherry co-localized with CSP to the PPM as early as day 1 of intrahepatocytic parasite development, a time point when sporozoites undergo transformation into trophozoites. Expression continued on day 2 early liver stages and on day 4 liver stage schizonts (Figure 1B(ii)). Interestingly, Pf ABCC2 expression appeared to cease in late liver stage schizonts on day 6 after infection (Figure 1B(ii)). No Pf ABCC2mCherry expression was observed within the liver stage cytoplasm or mitochondria. These results indicate that Pf ABCC2 is not expressed in sporozoites, but expression is initiated in early liver stage development and continues into mid-liver stage schizogony. However, protein expression does not persist in late liver stage schizonts.
Pf ABCC2 is critical for the initiation of exoerythrocytic schizogony
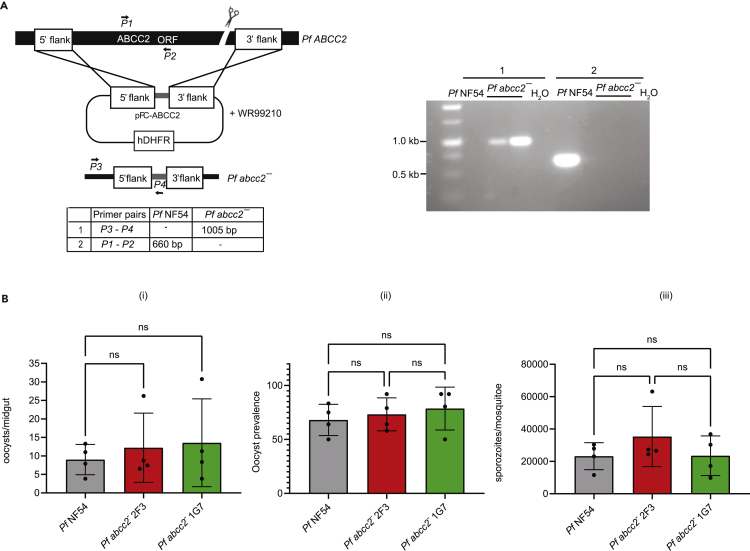
To investigate the role of Pf ABCC2 in liver stage development, we generated Pf abcc2– gene deletion parasites in the Pf NF54 strain via CRISPR/Cas9-mediated gene editing as previously described (Goswami et al., 2020). Successful recombination was confirmed by PCR genotyping and recombinant parasite populations were cloned by limiting dilution (Figure 2A). Pf abcc2– clones 2F3 and IG7 were used for further phenotypic analysis. Pf abcc2– gametocytes and wildtype Pf NF54 gametocytes were fed to Anopheles stephensi mosquitoes in standard membrane feeding assays. Quantitative analysis of mosquito infection showed that the number of oocysts/midgut, oocyst prevalence, and number of salivary gland sporozoites/mosquito were comparable between Pf abcc2– and Pf NF54 parasites (Figure 2B).
Figure 2.
Pf ABCC2 gene knockout and analysis of mosquito stage development
(A) The schematic depicts the generation of Pf abcc2– knockout parasites using CRISPR/Cas9-mediated gene editing. Primers used to verify the gene deletion are indicated and the sizes of the PCR amplicons are shown in base pairs. Agarose gel electrophoresis shows the PCR products corresponding to the gene deletion of Pf abcc2– in clones 2F3 and 1G7.
(B) Mature gametocyte cultures of Pf NF54 wild-type parasites (black) and Pf abcc2– clones 2F3 (red) and 1G7 (green) were fed to female Anophelesstephensi mosquitoes in a standard membrane feeding assay. Mosquito midguts were dissected on day 8 for oocyst enumeration and salivary glands were dissected on day 15 post membrane feeding assay for sporozoite enumeration. Graphs comparing (i) the counts for oocyst/midgut (ii) oocyst prevalence (iii) counts for salivary gland sporozoites/mosquito for Pf NF54 (black) and Pf abcc2– clones 2F3 (red) and 1G7 (green). Data are represented as mean ± SD, n = 4 biological replicates. Statistical analysis was carried out using two-way ANOVA using Tukey’s multiple comparison test. “ns” not significant. p > 0.05 is taken as not significant.
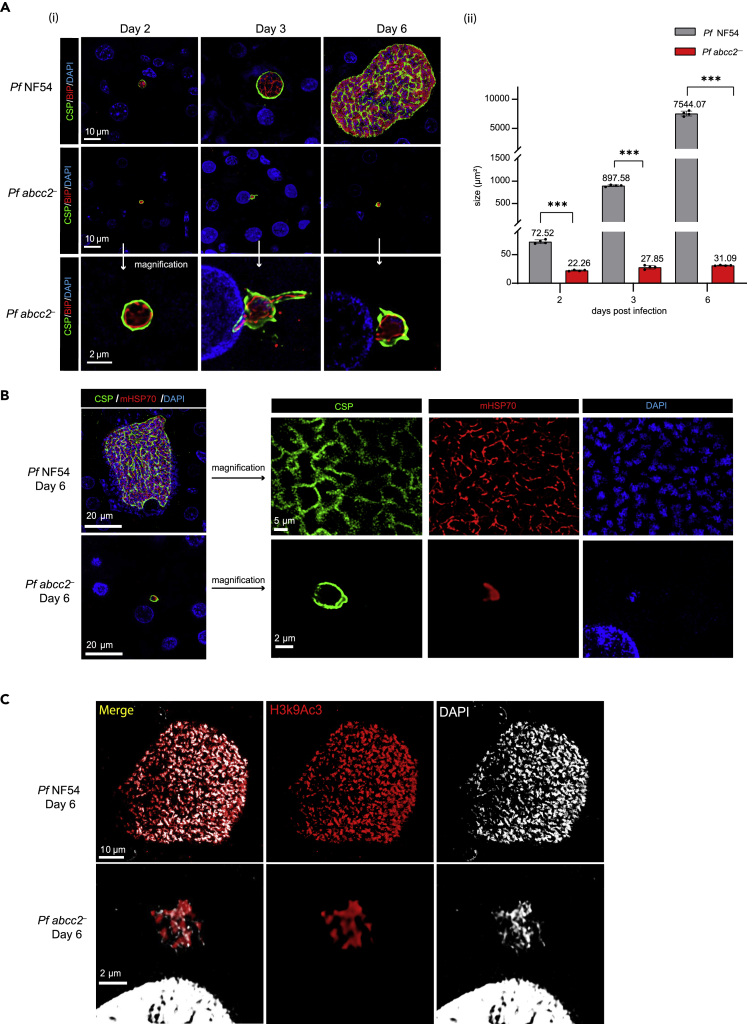
To evaluate the role of Pf ABCC2 in liver stage development, one million Pf abcc2– and Pf NF54 sporozoites were injected intravenously (i.v.) into FRG huHep mice. Infected livers were harvested on days 2, 3, and 6 postinfection, and liver stage development was compared between Pf abcc2– and Pf NF54 parasites by IFA. Antibodies against CSP, BiP, EXP1, and mHSP70 were used, which mark the PPM, the ER, the PV membrane (PVM), and mitochondria of the parasite, respectively (Figures 3 and S2). Pf abcc2– sporozoites were able to infect hepatocytes and transform into intrahepatocytic trophozoites as seen on day 2 postinfection (Figure 3A). PVM formation appeared normal in Pf abcc2– liver stages (Figure S2A). However, Pf abcc2– parasites were arrested in early liver stage development (Figures 3A(i), (ii)). A large fraction of Pf abcc2– liver stages were arrested while undergoing the developmental transition from sporozoites into trophozoites (Figure S2B). On day 2 postinfection, approximately 82% Pf abcc2– liver stages were observed in this stage. This was reduced to 45% on days 3 and 6 with a concomitant increase in the number of fully rounded liver stage trophozoites, indicating that a certain percentage of Pf abcc2– parasites undergo further differentiation. No difference in the numbers of infected hepatocytes was observed between Pf abcc2– parasites and Pf NF54 (Figure S2C). There was a slight increase in the size of Pf abcc2– liver stages from day 2 to day 3 (23 μm2–30 μm2), but this difference was not statistically significant (Figure 3A(ii)). There was no additional increase in the size of Pf abcc2– liver stages from day 3 to day 6. In contrast, Pf NF54 liver stage trophozoites (day 2) grew and differentiated normally as exoerythrocytic schizonts with a large increase in liver stage size from day 2 to day 6 (70 μm2–8000 μm2).
Figure 3.
Analysis of Pf abcc2– liver stage development
Tissue sections were prepared from FRG huHep mice that were infected with one million sporozoites of either Pf NF54 or Pf abcc2– on days 2, 3, and 6 post-sporozoite infection and analyzed by IFA.
(A) (i) Top panels: Liver stage development was compared between Pf NF54 and Pf abcc2– on days 2, 3, and 6 using antibodies against CSP (green) to mark the PPM and the ER marker BiP (red). DNA is stained with DAPI (blue). Scale bar size is 10 μm. Pf abcc2– parasites arrest very early in liver stage development. Bottom Panel: Magnified images of Pf abcc2– on days 2, 3, and 6 from the middle panel. Scale bar is 2 μm (ii) Comparison of the size of liver stage parasites (based on area at the parasite’s largest circumference) between Pf NF54 and Pf abcc2– on days 2, 3, and 6 post infection. Pf NF54 wildtype liver stage schizonts are significantly larger than Pf abcc2– on days 2, 3, and 6, with a massive difference apparent on day 6. Data are represented as mean ± SD. Each datapoint refers to the mean size of at least 20 parasites per lobe and four lobes were analyzed per mouse for each time point. Statistical analysis was carried out using two-way ANOVA using Tukey’s multiple comparison test. ∗∗∗p < 0.001, p > 0.05 is taken as ns.
(B) IFA comparing the cell growth and organellar development between Pf NF54 and Pf abcc2– on day 6 post sporozoite infection using antibodies against the PPM (CSP, green) and parasite mitochondria (mHSP70, red). DNA is stained with DAPI (blue). Scale bar size is 20 μm. The right panels represent a higher magnification image. Scale bar is 5 μm for Pf NF54 and 2 μm for Pf abcc2– .
(C) IFA comparing genome replication between Pf NF54 or Pf abcc2– on day 6 post sporozoite infection using antibodies against acetylated histone (H3K9Ac3, red). DNA is stained with DAPI (white). Scale bar size is 10 μm for Pf NF54 and 2 μm for Pf abcc2–.
At the time point of growth arrest, numerous Pf abcc2– liver stages had initiated exoerythrocytic schizogony, indicated by branching of the ER (Figure 3A) and mitochondria (Figure 3B). Although Pf abcc2– liver stages on day 6 exhibited some segregated DNA centers as visualized by immunostaining with an antibody against the acetylated histone marker, H3K9Ac3 (Figure 3C), genome replication appeared very limited. In contrast, Pf NF54 parasites on day 6 had undergone extensive organelle and genome replication (Figures 3A–3C).
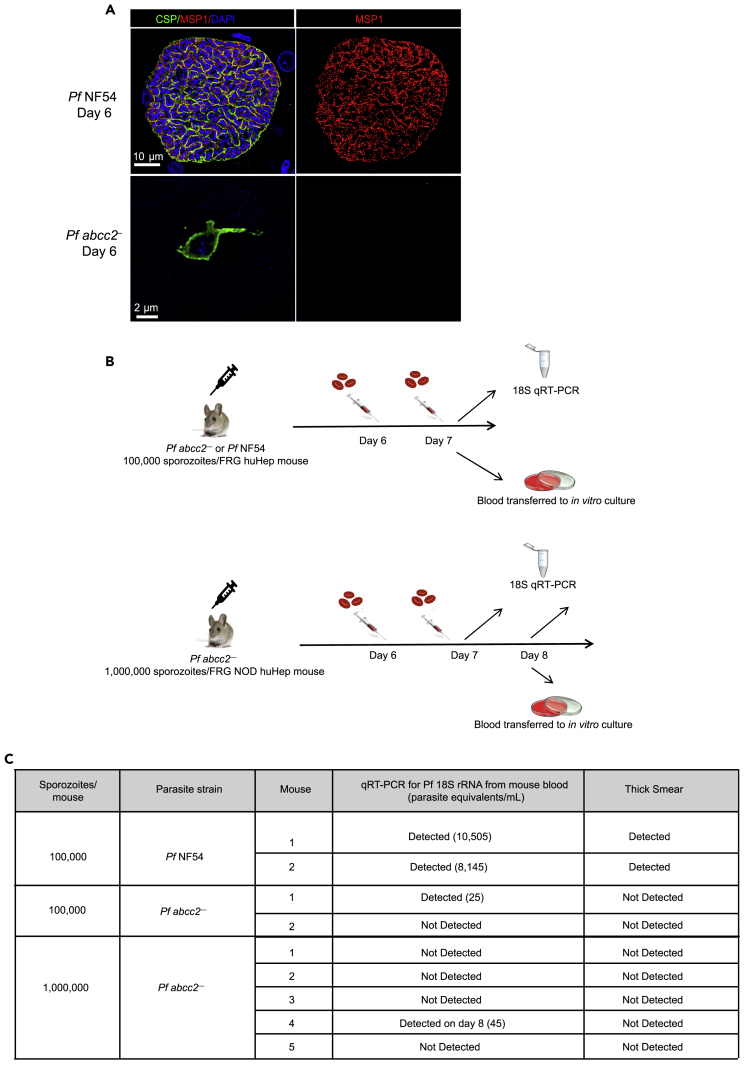
Pf abcc2– liver stages fail to transition to blood stage infection
Pf abcc2– liver stages arrest early in the developmental transition of the intrahepatocytic sporozoite to the trophozoite stage and to some degree in early exoerythrocytic schizogony, and lack significant genome and organelle replication (Figure 3). To elucidate whether Pf abcc2– liver stages express any markers for late liver stage differentiation and exoerythrocytic merozoite formation, IFAs were performed on liver sections of Pf NF54 and Pf abcc2–-infected mice at day 6 postinfection, using antibodies against CSP; this marks the PPM, and MSP1, which labels the PPM and additionally acts as a marker for exoerythrocytic merozoite formation (Goswami et al., 2020). Day 6 Pf abcc2– liver stages did not express MSP1 (Figure 4A). However, histological analysis of liver stage infection is not sufficiently sensitive to detect rare liver stage forms that could complete exoerythrocytic schizogony, form exoerythrocytic merozoites, and transition to blood stage infection. We have previously shown that the FRG huHep liver-chimeric mouse model constitutes a powerful in vivo model to assess Pf liver stage exoerythrocytic merozoite transition to blood stage infection (liver-to-blood stage transition) (Vaughan et al., 2012; Foquet et al., 2018; Goswami et al., 2020). Thus, to evaluate whether Pf abcc2– liver stage growth arrest completely abrogates the formation of any infectious exoerythrocytic merozoites, we conducted liver-to-blood stage transition experiments in FRG huHep mice. We injected 100,000 Pf NF54 sporozoites or 100,000 Pf abcc2– sporozoites i.v. into two FRG huHep mice each; in addition, we dose escalated to one million sporozoites of Pf abcc2– into five additional FRG NOD huHep mice each (Figure 4B). To detect the transition of exoerythrocytic merozoites into the blood, human red blood cells (RBCs) were injected on days 6 and 7 post-sporozoite infection as described previously (Vaughan et al., 2012; Foquet et al., 2018). On day 7, all mice that had been administered 100,000 sporozoites (either Pf NF54 or Pf abcc2–) were euthanized and exsanguinated. We next employed a highly sensitive Pf 18S rRNA qRT-PCR assay that has been used as a diagnostic tool in controlled human malaria infection (CHMI) studies (Seilie et al., 2019). This assay can detect a single ring stage parasite in 50 μL of whole blood (20 parasite equivalents/1 mL of blood), which corresponds to 7400 Pf 18S rRNA copies/mL of blood (Walther et al., 2005; Hodgson et al., 2015). Fifty μL of whole blood from infected FRG huHep mice was analyzed by qRT PCR and remaining blood was transferred to in vitro blood stage culture with human RBCs. Blood samples of both mice infected with 100,000 Pf NF54 sporozoites were positive by qRT-PCR on day 7 (Figure 4C). Furthermore, parasites could be detected by Giemsa-stained thin blood smear within 1–5 days of in vitro culture. In one of two mice infected with 100,000 Pf abcc2– sporozoites, 25 parasite equivalents/mL were detected by qRT-PCR, which is close to the limit of detection of the assay (20 parasites/mL) (Figure 4C). However, no parasites could be detected in Giemsa-stained thick or thin smears from in vitro culture of blood transitioned from Pf abcc2–-infected mice for up to two weeks of culture. The five FRG NOD huHep mice infected with one million Pf abcc2– sporozoites were analyzed by qRT-PCR on days 7 and 8 postinfection. On day 8, all mice were euthanized, exsanguinated, and blood was transferred to in vitro culture. No parasite 18S rRNA was detected from any of the five mice on day 7. In one of five mice, 44 parasite equivalents/mL of blood was detected by qRT-PCR on day 8, whereas the remaining four mice remained negative (Figure 4C). No parasites could be detected by thick or thin Giemsa-stained blood smears from any of the cultured blood after four weeks of culture. Samples from in vitro culture were also analyzed by qRT-PCR every week for four weeks and no parasite nucleic acid could be detected. These results show that the severe early liver stage growth defect of Pf abcc2– that we observed in infected liver tissue completely abrogated the formation of infectious exoerythrocytic merozoites in an in vivo infection model.
Figure 4.
Pf abcc2– liver stages do not form infectious exoerythrocytic merozoites
(A) Tissue sections were prepared from FRG huHep mice infected with one million sporozoites of either Pf NF54 or Pf abcc2–. On day 6 post sporozoite infection, liver stages were analyzed by IFA for expression of the PPM markers MSP1 (red) and CSP (green). DNA is stained with DAPI (blue). Scale bar size is 10 μm for Pf NF54 and 2 μm for Pf abcc2–.
(B) Schematics showing the experimental design of liver stage to blood stage transition experiments in humanized liver mice. Upper Panel: 100,000 sporozoites from Pf NF54 and Pf abcc2– were injected into two FRG huHep mice, respectively. Mice were repopulated with human RBCs as depicted and 50 μL blood samples were collected from all mice for parasite 18S rRNA qRT-PCR samples on day 7. Two mice from each group infected with either Pf NF54 or Pf abcc2– were exsanguinated on day 7, and blood was transferred to in vitro culture and grown in culture for three weeks. Lower Panel: One million sporozoites from Pf abcc2– were injected into five FRG NOD huHep mice each. Mice were repopulated with human RBCs as depicted and 50 μL blood samples were collected from all mice for parasite 18S rRNA qRT-PCR samples on day 7 and 8. All five mice were exsanguinated on day 8, and blood was transferred to in vitro culture and grown in culture for six weeks.
(C) qPCR and blood smear analysis of transition experiments. Both mice infected with Pf NF54 were positive for Pf 18S rRNA by qRT-PCR and parasites could be detected by thick blood smears from the blood transferred to in vitro culture from the two mice. In one of two mice infected with Pf abcc2, 25 parasite equivalents/mL could be detected by qRT-PCR. The assay has a threshold detection cutoff of 20 parasite equivalents/mL. However, no parasites were detected by thick smear in the blood transitioned to in vitro culture for up to three weeks. One in five mice infected with one million sporozoites of Pf abcc2– was positive for Pf 18S rRNA on day 8 (44 parasite equivalents/mL). However, no parasites were detected in thick blood smears after six weeks of in vitro culture.
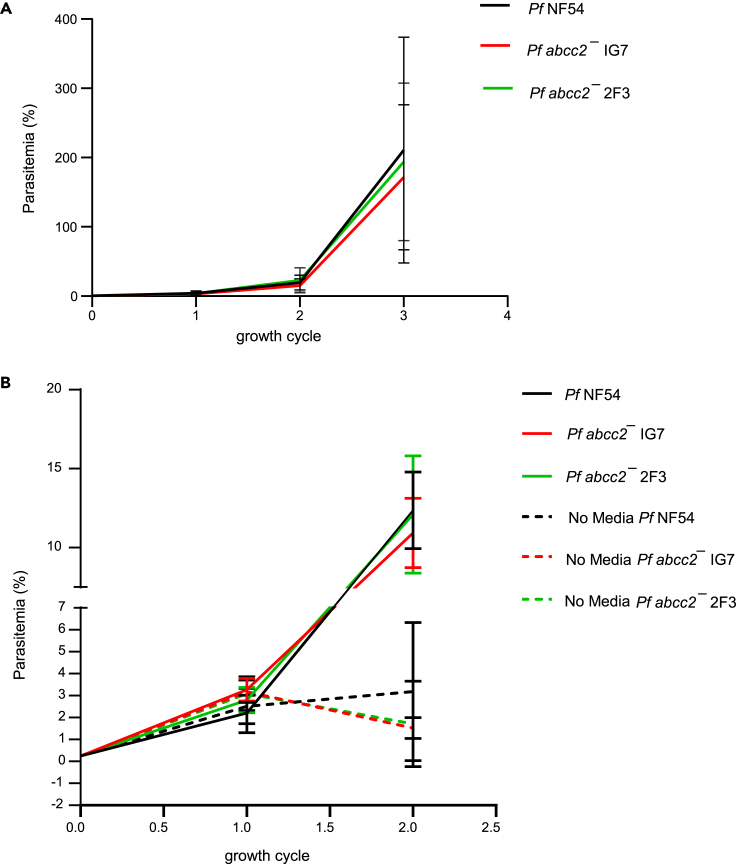
Pf ABCC2 is dispensable for parasite growth under optimal culture conditions
To thoroughly analyze the potential role of Pf ABCC2 in asexual blood stage replication under different culture conditions, the parasitemias of Pf abcc2– clones 2F3 and 1G7 were compared to Pf NF54 parasites over three replication cycles with media supplemented with 5% serum and 0.25% Albumax. No significant difference in growth was observed between Pf abcc2– and Pf NF54 parasites, indicating that under optimal growth conditions, Pf ABCC2 is dispensable for asexual blood stage growth and replication (Figure 5A). To investigate whether Pf ABCC2 plays a role in parasite survival under conditions of nutrient and oxidative stress, we performed growth assays in which we did not change media over a period of two replication cycles and compared the parasitemia between Pf abcc2– and Pf NF54. Parasites were set up on day 0 at 0.25% parasitemia with media supplemented with 0.25% Albumax and 5% human serum. Pf abcc2– and Pf NF54 parasites grown under conditions of daily media change were used as controls. There was no significant difference in parasite growth between Pf abcc2– and Pf NF54 in the control group that received daily media change (Figure 5B). However, in the group that did not receive daily media change, there was a 2-fold reduction in parasitemia for the Pf abcc2– parasites as compared to Pf NF54 parasites after the second replication cycle, but this difference was not statistically significant.
Figure 5.
Pf ABCC2 is dispensable for intraerythrocytic growth under optimal media conditions but might play a role under conditions of stress
(A) Asexual blood stage parasitemia is compared between Pf NF54 (black line) and Pf abcc2– clones 1G7 (red line) and 2F3 (green line) over three replication cycles under optimal media conditions. Data are represented as mean ± SD, n = 3 biological replicates.
(B) Asexual blood stage parasitemia is compared between Pf NF54 (solid black line) and Pf abcc2– clones 1G7 (solid red line) and 2F3 (solid green line) under the conditions of daily media change and between Pf NF54 (dashed black line) and Pf abcc2– clones 1G7 (dashed red line) and 2F3 (dashed green line) under conditions of no media change for two replication cycles. Data are represented as mean ± SD, n = 3 biological replicates. Statistical analysis was carried out using two-way ANOVA.
To evaluate whether lack of ABCC2 results in a disadvantage when grown in competition with wild-type parasites, Pf abcc2– and Pf NF54 parasites were mixed in equal ratios and cultured together over nine consecutive replication cycles (Figure S3). Aliquots were removed every week during the competition experiment, extracted, and analyzed by qRT-PCR. No differences in relative abundance of Pf abcc2– and Pf NF54 parasites were detected, indicating that the wild-type parasites did not outcompete Pf abcc2– parasites.
Discussion
Infectious Plasmodium salivary gland sporozoites are transmitted via mosquito bite and these sporozoites home to the liver and invade hepatocytes. Sporozoites prepare for hepatocyte infection by expression of proteins required for invasion, and by transcribing translationally repressed mRNAs that encode proteins which are involved in the establishment and modification of their intrahepatocytic niche (Lindner et al., 2019). During invasion, the parasite utilizes the hepatocyte plasma membrane to form a PV, which serves as the replication compartment for the nascent liver stage. Within the PV, the crescent-shaped intracellular sporozoite dedifferentiates into a spherical trophozoite, which is accompanied by extensive cellular remodeling and includes the dissolution of the invasive organelles and the inner membrane complex (Vaughan and Kappe, 2017). This prepares the early liver stage for entry into exoerythrocytic schizogony, which marks a transition point after which mobilization of host hepatocyte resources, which fuel the parasite’s enormous cell expansion and genome replication becomes essential. Some molecular determinants of hepatocyte invasion, PV formation, and PV modification have been elucidated mostly in rodent malaria models (Vaughan and Kappe, 2017; Arredondo et al., 2018). However, proteins that are critical for the initiation of exoerythrocytic schizogony remain mostly unknown, particularly for human malaria parasites.
Here, we studied Pf ABCC2 expression and function using endogenously tagged protein, gene knockout, and a liver-humanized mouse model of infection. Pf ABCC2 was not expressed in Pf sporozoite stages. Our findings are corroborated by recent Pf salivary gland proteome data where no peptides for Pf ABCC2 could be detected in midgut or salivary gland sporozoites by mass spectrometry (Lindner et al., 2019). We observed that Pf ABCC2 expression is initiated during the early phases of liver stage development, as early as day 1 pos tinfection and persists until mid-stage exoerythrocytic schizogony. We also show that this member of the ABCC family of transmembrane transporters localizes to the liver stage plasma membrane. Pf ABCC2 gene deletion resulted in a severe early liver stage developmental defect, as Pf abcc2– liver stages were arrested in development either in the transformation of sporozoites into trophozoites or at the onset of exoerythrocytic schizogony, which correlated with the onset of Pf ABCC2 protein expression. Although Pf ABCC2 was expressed in asexual stages, including trophozoites and schizonts, it was dispensable for blood stage growth under optimum in vitro culture conditions. Interestingly, in contrast to the lack of Pf ABCC2 expression in sporozoites, we did detect its expression in erythrocytic merozoites.
A previous study of Pf ABCC2 using in vitro hepatocyte infections showed that it was expressed in replicating mid-to-late exoerythrocytic schizonts and gene-deficient-Pf ABCC2 parasites aborted growth in mid-to-late liver stage development (Rijpma et al., 2016). In contrast, our findings reported here, indicate that Pf ABCC2 is expressed in early-to-mid liver stages, and Pf abcc2– liver stages abort development at the transformation of sporozoites into trophozoites and at the trophozoite-to-schizont transition point. These differences in findings might be attributed to differences between an in vitro (primary human hepatocytes) used in the previous study (Rijpma et al., 2016) and the in vivo (liver humanized mice) model of liver stage development used here.
Using liver-humanized mice repopulated with human RBCs, we have demonstrated here that Pf abcc2– liver stages were unable to transition from the liver stage to the blood stage. No culturable asexual parasites could be recovered from humanized mice challenged with one million Pf abcc2– sporozoites, even after prolonged culture with RBCs. Nonetheless, in two of seven challenged mice, we could detect Pf 18S rRNA by a highly sensitive qRT-PCR diagnostic assay. Because Pf abcc2– liver stages persist in the liver at least until day 6, we speculate that the 18s rRNA signal detected in blood is likely originating from parasite nucleic acid (18S rRNA or genomic 18S rDNA) released from dying early liver stage-infected hepatocytes rather than from viable exoerythrocytic merozoites.
Multiple studies over the last decade have shown the efficacy of whole sporozoite-based vaccines against malaria (38–41). Targeted gene deletions enable generation of designed genetically attenuated parasites (GAP) that can arrest either early in liver stage development (replication-deficient) or late in liver stage development (replication-competent) (42–47). Pf abcc2- parasites undergo further liver stage development than what has been reported for the PfGAP3KO, a replication-deficient strain which carries deletions in the SAP1, P52, and P36 genes and that has been tested in humans (Kublin et al., 2017). Thus, it might be of interest to evaluate the Pf abcc2- parasites as a replication-deficient immunogen in humans.
Two hallmarks of liver stage development are the radical cellular transformation of intracellular sporozoites into trophozoites, followed by massive cell growth and genome replication during exoerythrocytic schizogony (Graewe et al., 2012; Vaughan and Kappe, 2017). High amounts of toxic oxidative by-products likely accumulate from the intense cellular reorganization and the metabolic turnover that might occur during these distinct development phases. These toxic metabolites would need to be actively exported from the parasite cytoplasm. Various Plasmodium membrane transporters have been identified that function at the parasite-host interface in the asexual stages to maintain redox homeostasis within the parasite (Koenderink et al., 2010; Martin, 2019). Because all eukaryotic ABC transporters are exporters, it is reasonable to hypothesize that the liver stage PPM-localized Pf ABCC2 actively exports toxic metabolites from the liver stage cytoplasm into the PV lumen. There is evidence that several members of the human ABCC transporter family play a critical role in maintaining redox homeostasis in the liver by secreting organic anions from the liver into bile (Liang and Aszalos, 2006). Mammalian ABCC transporters are known to export organic anions such as glutathione conjugates, glucuronide conjugates, and sulfate conjugates across biological membranes (Rebbeor et al., 2002; Cole and Deeley, 2006; Hollenstein et al., 2007; Jones and George, 2007; Zimmermann et al., 2008). Pf encodes two ABCC transporters, ABCC1 and ABCC2 (González-Pons et al., 2009; Koenderink et al., 2010). Although there are no known substrates for Pf ABCC2 studied here, there is evidence that Pf ABCC1 in the asexual stages, exports reduced glutathione (GSH) conjugates from the parasite cytoplasm into the host RBC (Raj et al., 2009). Based on the structural homology in the nucleotide binding domains of Pf ABCC1 and ABCC2, it is likely that GSH conjugates are also substrates for Pf ABCC2.
Polymorphisms and mutations in the Pf ABCC loci have been associated with drug resistance and changes in parasite fitness (Jones and George, 2007; Seeger and van Veen, 2009; Koenderink et al., 2010). Multiple studies have reported a role of Pf ABCC1 in antimalarial drug resistance (Mu et al., 2003; Dahlström et al., 2009a, 2009b; Raj et al., 2009), and gene disruption of Pf ABCC1 has been associated with reduced parasite fitness (Raj et al., 2009). Increased expression of Pf ABCC2 has been reported to alter sensitivity to antmalarial drugs (Mok et al., 2014). Recently it was reported that polymorphisms and mutations in the Pf ABCC2 allele might be associated with reduced parasite fitness in artemisinin-resistant Pf strains (Li et al., 2019). In this study we did not observe any loss of fitness in Pf abcc2– parasites under optimal cell culture conditions and when both Pf abcc2– and Pf NF54 wild-type strains were cultured together over prolonged periods of time. However, we observed a modest growth phenotype in Pf abcc2– parasites under conditions of severe nutrient and oxidative stress caused by the non-removal of spent media. This growth reduction might be caused by increased accumulation of toxic metabolites in the Pf abcc2– compared to Pf NF54 parasites, which in turn is caused by a lack of export of these metabolites out of the Pf abcc2– parasite cytoplasm. It has been previously reported that GSH-mediated heme detoxification in infected RBCs is crucial for parasite fitness (Meierjohann et al., 2002), and this regulation might be compromised in the Pf abcc2–. Nonetheless, we cannot completely rule out the possibility that Pf ABCC1 compensates for lack of Pf ABCC2 in the blood stages, and therefore we observed only modest blood stage growth defects in the Pf abcc2– parasites.
In conclusion, we have provided evidence that Pf ABCC2 marks the transition of invasive sporozoites to early intrahepatocytic Pf liver stage development. Pf ABCC2 is critical for successful parasite transition to exoerythrocytic schizogony, and its disruption results in complete loss of exoerythrocytic merozoite formation and blood stage infection in the liver-humanized mouse model.
Limitations of the study
The data shown in this manuscript does not address the possibility of a delay in release of Pf abcc2– parasites from the liver to blood or delayed growth of blood stage parasites originating from the liver. There are technical limitations for performing such an experiment. It requires the frequent administration of immunocompromised FRG huHep mice with clodronate liposomes and cyclophosphamide to deplete the innate immune cells such as macrophages and neutrophils, respectively. This treatment is required to prevent elimination of human RBCs by these innate immune cells in the spleen and ensures sustained repopulation of mouse blood with human RBCs. These repeated handlings increase the risk of mortality in the already immunocompromised FRG huHep mice and makes these mice much more susceptible to bacterial infections.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-P.falciparum CSP | BEI Resources | BEI-MRA-183A clone 2A10 |
| Anti-P.vivax BiP (immunoglobulin-binding protein) | Stefan H.I. Kappe | polyclonal |
| Anti-P. yoeli ACP (Acyl Carrier Protein) | Stefan H.I. Kappe | clone 40001 |
| Anti-P. vivax mitochondrial HSP70 | Stefan H.I. Kappe | Rabbit polyclonal |
| Anti- P.falciparum MSP-1 | European Malaria Reagent Repository | clone 12.10 |
| Anti-Pf Exp1 | Gift from Klaus Lingelbach | Rabbit polyclonal sera |
| Anti-mCherry | Thermo Fischer | Cat# M11217 clone 16D7; RRID: AB_2536611 |
| Donkey anti-mouse 488 | Invitrogen | Cat # A21202; RRID: AB_141607 |
| Donkey anti-mouse 594 | Invitrogen | Cat # A21203; RRID: AB_141633 |
| Donkey anti-mouse 647 | Invitrogen | Cat # A31571; RRID: AB_162542 |
| Donkey anti-rabbit 488 | Invitrogen | Cat # A21206; RRID: AB_2535792 |
| Donkey anti-rabbit 594 | Invitrogen | Cat # A21207; RRID: AB_141637 |
| Donkey anti-rabbit 647 | Invitrogen | Cat # A31573; RRID: AB_2536183 |
| Biological samples | ||
| P.falciparum NF54 | Walter Reed Army Institute of Research (WRAIR) | MBR-0004, Lot Number A004 produced at Seattle Biomed from WRAIR Lot Number 0148 |
| P.falciparum abcc2– | This Manuscript | N/A |
| P.falciparum ABCC2mCherry | This Manuscript | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| RPMI 1640 media containing hypoxanthine, sodium bicarbonate, and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid | InvitrogenTM (custom made) | Project No: PB11096 |
| Human Serum | Valley Biomedical | Cat # HS1004 |
| AlbuMAX™ II Lipid-Rich BSA | Thermo Fischer | Cat # 11021029 |
| Gentamicin (10 mg/mL) | InvitrogenTM | Cat # 15710064 |
| O+ human blood | Valley Biomedical | Cat # 10020 |
| WR99210 | Jacobus Pharmaceuticals | N/A |
| NucliSENS lysis buffer | bioMérieux, Marcy-l’Étoile, France | Cat # 280134 |
| Paraformaldehyde, 16% w/v aq. soln., methanol free | Alfa Aesar | Cat # 43368-9M |
| Schneider’s Drosophila Medium | GibcoTM | Cat # 21720024 |
| 4-,6- diamidino-2-phenylindole (DAPI) | Thermo Fischer | Cat # D1306 |
| ProLong™ Gold Antifade Mountant | InvitrogenTM | Cat # P36930 |
| 4-Aminobenzoic acid (p-aminobenzoic acid or pABA) | Aldrich | Cat #100336 |
| Heparin (sodium salt from porcine intentinal mucosa) | Sigma-Aldrich | Cat # H5515-100KU |
| Potassium permanganate | ||
| Methanol | VWR | Cat # BDH1135-4LG |
| Fluriso™ (Isoflurane, USP) | Vet One | Cat # 502,017 |
| 1 kb Ladder | Promega | Cat # G5711 |
| Experimental models: Organisms/strains | ||
| Mouse: FRG® KO on C57Bl/6—Human Repopulated, 70%+ | Yecuris™ | Cat # 10-0006 |
| Mouse: FRG® KO on NOD—Human Repopulated, 70%+ | Yecuris™ | Cat # 10-0013 |
| Oligonucleotides (restrictions sites are underlined and overhangs are in italics) | ||
| Primers for Pf abcc2¯ generation (restrictions sites are underlined and overhangs are in italics): | ||
| ABCC2_5′flank_F 5′-TATACTCGAGATATTGAGCATCTGTTTAT ACATTG-3′ |
IDT | N/A |
| ABCC2_5′flank_R 5′-CCAAGCTAGCTATAGGCGCGCCTAAAAA CGGAATCTTTCTAACTTTACT-3′ |
IDT | N/A |
| ABCC2_3′flank_F 5′-AGGCGCGCCTATAGCTAGCTTGGGAAAA ACAATTAAATTG-3′ |
IDT | N/A |
| ABCC2_3′flank_R 5′-TATACTCGAGTTGTTAAAAAAGTTACCTG AATTGTTC-3′ |
IDT | N/A |
| ABCC2_guide1 F 5′-tattGGCCCTTTCCCTCTTCACTA-3′ | IDT | N/A |
| ABCC2_guide1 R 5′-aaacTAGTGAAGAGGGAAAGGGCC-3′ | IDT | N/A |
| Genotyping_P1 5′-GGTATCGTAGGAAAATCAGG-3′ | IDT | N/A |
| Genotyping_P2 5′-GTATACAACTTCTCCCTTTGCG-3′ | IDT | N/A |
| Genotyping_P3 5′-AGGCGCGCCTATACCCGGGTTGG-3′ | IDT | N/A |
| Genotyping_P4 5′-GCATGAATGGTTCATGC-3′ | IDT | N/A |
| Primers for Pf ABCC2mcherry generation (restrictions sites are underlined and overhangs are in italics): | IDT | N/A |
| 5′mCherry ABCC2_R 5′-tcttcttctccttttgaaacATTTA ATTGTTTTTCTTGAAGCAAGTTAGCCAACTCTG TTTGTGTTTTAACATCTTTATAACTAC-3′ |
IDT | N/A |
| 3′mCherryABCC2_F 5′-ATTGCGGCCGCGAGTGAGTTTATGT ATATAATGTGTATATC-3′ |
IDT | N/A |
| 3′mCherry ABCC2_R5′ AAACGAATTCGTTAAAAAAGTTACCT GAATTGTTC 3′ |
IDT | N/A |
| ABCC2_guide3 F 5′-tattTTGAAGCAAGTTAGCTAATT-3′ | IDT | N/A |
| ABCC2_guide3 R 5′-aaacAATTAGCTAACTTGCTTCAA-3′ | IDT | N/A |
| ABCC2_ORF_F_genotyping_P4 5′-GGTATCGTAGGAAAAT CAGG-3′ |
IDT | N/A |
| mCherry_rev_genotyping_P5 5′-CCACCAGTACTATGTCTTCC-3′ | IDT | N/A |
| Primers for blood stage competition assay genotyping primers: Pf abcc2¯ primers: Linker_F 5′-CGCGCCTATAGCTAGCTTG-3′ ABCC_3UTR_R 5′-CCCTTTCCAATATAGGCGTTC-3′ Pf NF54 primers ABCC_ORF_F 5′GCATCACATGCACCCTTATG-3′ ABCC_ORF_R 5′AGAAGGTTAGCGCCAGAACA-3′ |
IDT | N/A |
| Primer for screening Pf serial dilutions: 18SF (5′-AACCTGGTTGA TCCAGTAGTCATATG-3′) |
IDT | N/A |
| Primer for screening Pf serial dilutions: 18SR (5′-CCAAAAATTG GCCTTGCATTGTTAT-3′) |
IDT | N/A |
| Recombinant DNA | ||
| Plasmid: p-ABCC2KO | This manuscript (modified from Goswami et al., 2020) | N/A |
| Plasmid: pFC-ABCC2-mCherry | This manuscript (modified from Goswami et al., 2020) | N/A |
| Software and algorithms | ||
| Adobe Illustrator | Adobe | Adobe Illustrator 2020 https://www.adobe.com/products/illustrator.html |
| GraphPad Prism | GraphPad Prism Software | GraphPad Prism 9.2.0.332 https://www.graphpad.com/scientific-software/prism/ RRID: SCR_002798 |
| Lightning software | Leica Microsystems | https://www.leica-microsystems.com/products/confocal-microscopes/p/leica-tcs-sp8/ |
| Geneious® | Biomatters Ltd. | Geneious® 11.1.4 https://www.geneious.com/resources/ |
| Biorender.com | https://biorender.com/ | |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Stefan H.I. Kappe (Stefan.Kappe@seattlechildrens.org).
Materials availability
Plasmids and transgenic Plasmodium falciparum strains generated in the study are available upon request from the Lead contact with a material transfer agreement.
Experimental model and subject details
Animals
Study approval
This study was carried out in accordance with the recommendations of the NIH Office of Laboratory Animal Welfare standards (OLAW welfare assurance #D16-00119). Mice were maintained and bred under specific pathogen-free conditions at the Center for Global Infectious Disease Research, Seattle Children’s Research Institute. The protocol was approved by the Center for Infectious Disease Research Institutional Animal Care and Use Committee (IACUC) under protocol 00480.
Mice
Female FRG and FRG NOD KO huHep mice, over 4 months of age were purchased from Yecuris Inc. In our previous studies, we have shown that both male and female genders of FRG and FRG NOD KO huHep mice can be used for Plasmodium sporozoite infection (Vaughan et al., 2015; Sack et al., 2017) and female mice were used in this study as also previously reported (Vaughan et al., 2012; Schäfer et al., 2020). Serum albumin levels of all mice used exceeded 4 mg/mL, corresponding to >70% human hepatocyte repopulation. Mice were maintained on drinking water containing 3% Dextrose and were cycled on 8 mg/L NTBC once a month for 4 days to maintain hepatocyte chimerism.
In vitro culturing of Pf parasite lines
Pf WT NF54, Pf abcc2– and Pf ABCC2mcherry were cultured in custom-made RPMI 1640 media containing hypoxanthine, sodium bicarbonate, and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Invitrogen, Life Technologies, Grand Island, NY) supplemented with 5% human serum (Valley Biomedical) and 5% albumax (Invitrogen, Life Technologies, Grand Island, NY) and gentamycin (Invitrogen), in presence of O+ blood (Valley Biomedical) at 3–5% hematocrit, in an atmosphere of 5% CO2, 5% O2, and 90% N2.
Method details
Generation of Pf abcc2– and Pf ABCC2mCherry parasites using CRISPR-Cas9-mediated gene editing
All oligonucleotides used for generating transgenic parasite strains are mentioned in detail in the STAR Methods. The CRISPR-Cas9 vector and gene targeting strategy have been previously described (Goswami et al., 2020). The p-ABCC2KO plasmid was generated by cloning the 5′ and-3′ homology arms flanking the Pf ABCC2 gene into the pFC vector followed by cloning the guide RNA sequence. 5% sorbitol synchronized ring stage Pf NF54 parasites at 5–8% parasitemia were electroporated with 100 μg of p-ABCC2KO plasmid at 0.31 kV and 950 μF using a BioRad Gene Pulser (BioRad, Hercules, CA). Positive selection with 8 nM WR99210 (WR; Jacobus Pharmaceuticals, Princeton, NJ) was administered 24 h after the transfection and kept for five days. Drug resistant parasites were rescued after 21 days. These parasites were screened by PCR using primers P1 and P2 that bind inside the open reading frame and give a PCR amplicon only for the wildtype locus of Pf ABCC2 and primers P3 and P4 that give a PCR amplicon only in the recombinant allele.
Pf ABCC2mCherry parasites were generated via CRISPR/Cas9-mediated gene editing as described above. pFC-ABCC2-mCherry plasmid was generated using the pFC-mCherry vector backbone, into which the 5′ homology arm amplified from within the open reading frame and carrying non-synonymous mutations in the guide sequence, 3′ homology arm and guide RNA were cloned. Pf NF54 parasites were transfected with 100 μg of the pFC-ABCC2-mCherry plasmid in the pFC plasmid backbone. Transfected parasites were positively selected as mentioned above, recombinant parasites were screened up PCR using primers P5 and P3 and cloned by limiting dilution.
Parasite cloning by limiting dilution
Pf abcc2–and Pf ABCC2mCherryparasites were cloned out of the mixed parasite pool by limiting dilution in 96-well flat bottomed plates (Mikolajczak et al., 2014). Cultures were serially diluted and then plated at a density of 0.3 parasites per well in a 100μL volume at 2% hematocrit. Cultures were fed once a week and fresh blood was added every three – four days at 0.3% hct. The wells were screened for presence of Plasmodium 18S RNA parasites on day 18 using primers 18SF (5′-AACCTGGTTGATCCAGTAGTCATATG-3′) and 18SR (5′-CCAAAAATTGGCCTTGCATTGTTAT-3′). The positive wells were expanded and screened for recombination using the PCR strategies mentioned earlier.
Asexual growth kinetics assays
Under optimal media conditions between Pf NF54 wildtype and Pf abcc2–
Ring stage parasites of Pf NF54 WT and Pf abcc2– were set up at 0.5% parasitemia and 3% hematocrit in media containing 5% Albumax and 0.25% human serum. Fresh media was repleted daily. Growth was monitored over three replication cycles by making thin blood smeared every other day. Cultures were cut back by 3 – 5 folds after the second replication cycle to prevent parasite cultures from crashing. The parasitemia after the third replication cycle was calculated by multiplying the parasitemia based on blood smear with the diluted fold change.
Under conditions of no media change Pf NF54 wildtype and Pf abcc2–
Ring stage parasites of Pf NF54 WT and Pf abcc2– were set up at 0.25% parasitemia and 3% hematocrit in media containing 0.25% Albumax and 5% human serum. No fresh media was given throughout the duration of the experiment of two replication cycles or 4 days, but flasks were gassed daily. Pf NF54 wildtype and Pf abcc2– grown under the same media change and with daily media change were used as controls. Growth was monitored over two replication cycles by making thin blood smear every other day.
Between Pf NF54 wildtype and Pf ABCC2mCherry
Ring stage parasites of Pf NF54 WT and Pf ABCC2mCherry were set up at 0.25% parasitemia and 3% hematocrit in media containing 0.25% Albumax and 5% human serum. Fresh media was repleted daily. Growth was monitored over three replication cycles by making thin blood smeared every other day. The parasitemia after the third replication cycle was calculated by multiplying the parasitemia based on blood smear with the diluted fold change.
Measurement of competitive asexual blood stage growth between Pf NF54 and Pf abcc2– parasites
To compare competitive asexual blood stage replication and growth between the Pf NF54 and Pf abcc2– parasites, synchronized parasites at ring stages were mixed in equal ratio at 0.5% parasitemia each for Pf NF54 and Pf abcc2–. These mixed parasites were cultured in vitro, and bulk parasite samples were collected every other day for nine replication cycles. Bulk parasites were utilized to extract genomic DNA and set up quantitative PCR using primers specific to Pf NF54 wildtype or Pfabcc2–.
Gametocyte culture and mosquito infections
Pf WT NF54, Pf abcc2– and Pf ABCC2mcherry cultures were grown in gametocyte media containing RPMI 1640 media containing hypoxanthine, sodium bicarbonate, and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Invitrogen, Life Technologies, Grand Island, NY) supplemented with 10% human serum (Valley Biomedical) in presence of O+ blood (Valley Biomedical) at 4% hematocrit, in an atmosphere of 5% CO2, 5% O2, and 90% N2. Gametocyte cultures were initiated at 4% hematocrit and 0.8–1% parasitemia (mixed stages) and maintained for up to 15 days with daily medium changes. On days 15–17 post set up, stage V gametocytemia was evaluated by Giemsas-stained thin blood smears. Mature gametocyte cultures were spun down at 800 g for 2 min at 37°C to pellet infected RBCs. A volume of pre-warmed 100% human serum equal to the packed RBC volume was added to resuspend the RBC pellet. The resuspended pellet was diluted to a gametocytemia of 0.4–0.5% with pre-warmed feeding media containing 50% human serum and 50% human RBC and immediately fed to non–blood fed adult female Anopheles stephensi mosquitoes 3 to 7 days after hatching by membrane feeding assay. Mosquitoes were allowed to feed through Parafilm for up to 20 min. Following blood feeding, mosquitoes were maintained for up to 19 days at 27°C, 75% humidity, and provided with 8% dextrose solution in PABA water. Oocyst prevalence was checked on day 7–9 post feed by microdissecting approximately 12 midguts per cage. Sporozoite numbers were detected by dissecting and grinding salivary glands in Schneider′s Insect Medium (Sigma) on days 14–18 post feed.
Phenotypic analysis of Pf abcc2– parasites in FRG-huHep mice
Pf NF54 and Pf abcc2– sporozoites were isolated from salivary glands of infected Anopheles stephensi mosquitoes. For analysis of Pf abcc2– liver stage, 1 × 106 Pf NF54 and 1 × 106 Pf abcc2– sporozoites were injected intravenously (retro-orbital) into three FRG NOD huhep mice per group. Livers were harvested on days 2, 3 and 6 and used for IFA. To evaluate for blood stage transition of Pf abcc2– in FRG huhep mice, 1 × 105 Pf NF54 and Pf abcc2– sporozoites were injected intravenously into two FRG huHep mice per group. On day 6, 400 μL of 70% RBCs were injected intravenously and on day 7, 50μL of blood from each mouse was sent for qPCR analysis to detect parasite 18S RNA. On day 7, two mice per group were euthanized, blood was collected by cardiac puncture, washed three times in asexual media, a volume of human RBCs equal to the packed RBC volume was added and cultured in vitro. Fresh media was replaced daily, and cultures were analyzed every 2–3 days by thick smear for presence of parasites for upto 6 weeks. Samples for 18S rRNA qRT-PCR were analyzed every 5–7 days.
To evaluate for blood stage transition of Pf abcc2– in FRG NOD huhep mice, 1 × 106 Pf abcc2–sporozoites were injected intravenously into 5 FRG NOD huHep mice. On day 6, 400μL of 70% RBCs were injected intravenously and on day 7 and 8, 50μL of blood from each mouse was analyzed by qRT-PCR analysis to detect parasite 18s RNA. On day 8, all mice were euthanized, blood was collected by cardiac puncture, washed three times and transferred to in vitro culture as described above. Fresh media was replaced daily, and cultures were analyzed every 2–3 days by thick smear for presence of parasites for upto 6 weeks. Samples for 18S rRNA qRT-PCR were analyzed every 5–7 days.
Plasmodium 18S rRNA qRT-PCR quantification of parasite load
For quantification of Plasmodium 18S rRNA from mouse blood, 50 μL of whole blood was added to 2 mL of NucliSENS lysis buffer (bioMérieux, Marcy-l’Étoile, France) and frozen immediately at −80°C.
One mL of lysate was subsequently processed using the Abbott m2000sp using mSample RNA preparation kit (Abbott, Niles, IL). The eluate was tested by qRT-PCR for the pan-Plasmodium 18S rRNA target. The qRT-PCR reaction was performed using 35 μL SensiFAST™ Probe Lo-ROX One-Step Kit (Bioline, Taunton, MA) and 15 μL of extracted eluate. Plasmodium 18S rRNA primers/probes (LCG BioSearch Technologies, Novato, CA) were as follows: Forward primer PanDDT1043F19 (0.2 μM): 5′-AAAGTTAAGGGAGTGAAGA-3′; Reverse primer PanDDT1197R22 (0.2 μM): 5′-AAGACTTTGATTTCTCATAAGG-3′; Probe (0.1 μM): 5'-[CAL Fluor Orange 560]- ACCGTCGTAATCTTAACCATAAACTA[T(Black Hole Quencher-1)]GCCGACTAG-3'[Spacer C3]). Cycling conditions were RT (10 min) at 48°C, denaturation (2 min) at 95°C and 45 PCR cycles of 95°C (5 s) and 50°C (35 s).
Immunofluorescence assay of liver stage parasites
FRG huHep mice were injected with one million sporozoites of Pf NF54, Pf abcc2-mCherry or Pf abcc2– sporozoites. Livers were harvested on different days post infection, fixed in 4% (vol/vol) paraformaldehyde (PFA, Alfa Aesar) in 1X PBS for 18–24 h in a shaker at room temperature, followed by two washes in 1X PBS for 2 h. 50 μm thick sections were made using a Vibratome apparatus (Ted Pella, Redding, CA). For IFA, liver sections were permeabilized in 1X Tris-buffered saline (TBS) containing 3% (vol/ vol) H2O2 and 0.25% (vol/vol) Triton X-100 for 2 h at room temperature with gentle agitation. Sections were then blocked in 1xTBS containing 5% (vol/vol) dried milk (TBS-M) for at least 1 h and incubated with primary antibody in TBS-M at 4°C overnight with gentle agitation. After three, 10-minute-long washes in 1x TBS, fluorescent secondary antibodies and 4-,6- diamidino-2-phenylindole (DAPI) at 1 mg/mL were added in TBS-M for 2 h at room temperature in a similar manner as above. After three, 5-minute-long washes in 1x TBS, the section was incubated in 0.06% (wt/vol) KMnO4 for 45 s to quench the background fluorescence. Sections were mounted in polylysine-coated slides overlaid with ProLong™ Gold Antifade Mountant (Molecular Probes, CA) and a coverslip. Primary antibodies used included Pf CSP clone 2A10 (1:500), P. yoelii BiP polyclonal (1:1000), P. yoelii ACP (1:500), P. vivax mHSP70 (1:1000) and Pf MSP-1 clone 12.10 (1:500, European Malaria Reagent Repository), mCherry clone 16D7 (1:500, Thermo Scientific), Pf Exp1 rabbit polyclonal sera (1:200, provided by Klaus Lingelbach). Secondary antibodies include donkey anti-mouse 488, donkey anti-mouse 594, donkey anti-mouse 647, donkey anti-rabbit 488, donkey anti-rabbit 594, donkey anti-rabbit 647 and donkey anti-rat 594. All secondary antibodies were used at a dilution of 1:1000.
Immunofluorescence assay of blood stage parasites
Thin blood smears of Pf ABCC2mCherry parasites were fixed in 100% ice-cold methanol processed for 3 min followed by two washes in 1X PBS at room temperature. Slides were marked with blocked in 2% bovine serum albumin (BSA) for 1 h at room temperature, followed by incubation with primary antibody in 2% BSA overnight at 4°C. Slides were washed three times in 1X PBS, 8 min per wash, followed by incubation with secondary antibodies (1:1000) and DAPI (1 mg/mL) in 2% BSA for 1 h. Slides were washed three times in 1X PBS, 8 min per wash and mounted in ProLong™ Gold Antifade Mountant (Molecular Probes, CA) and a coverslip. Primary antibodies used included Pf MSP-1 clone 12.10 (1:500, European Malaria Reagent Repository) and α-mCherry clone 16D7 (1:500, Thermo Scientific). Secondary antibodies include donkey anti-mouse 488 and donkey anti-rat 594. All secondary antibodies were used at a dilution of 1:1000.
Immunofluorescence assay of parasite mosquito stages
Mosquito midguts were dissected in Schneider’s insect medium (Gibco™) and fixed with 4% (vol/ vol) PFA in 1X PBS for 1 h at room temperature in a microcentrifuge tube. Midguts were washed with 1X PBS and then permeabilized and blocked with 1X PBS containing 3% BSA and 0.25% Triton X-100 for 2 h at room temperature. Midguts were washed with 1X PBS, and primary antibody (were diluted in 1X PBS/3% BSA and incubated with the midguts at 4°C overnight with end-over-end rotation. The primary antibody used was α-mCherry clone 16D7 (1:500, Thermo Scientific). Following three washes with 1X PBS, fluorescent secondary antibodies were diluted with 1x PBS plus 3% BSA and incubated with midguts for 2 h with end-over-end rotation at room temperature. Midguts were washed three times with 1X PBS, stained with 4-,6- diamidino-2-phenylindole (DAPI) at 1 mg/mL in 1X PBS for 5 to 10 min at room temperature, and washed once more with 1x PBS. Midguts were mounted on a polylysine-coated microscope slide and overlaid with ProLong™ Gold Antifade Mountant (Molecular Probes, CA) and a coverslip. Secondary antibodies include donkey anti-mouse 488 and donkey anti-rat 594.
Salivary gland sporozoites (3 μL) were spotted onto a polylysine-coated microscope slide glass slide (with 12 wells) and allowed to dry overnight. Sporozoites were fixed in 4% PFA for 20 min, followed by 3 washes in 1X PBS. Blocking and permeabilization were carried out together in 2% BSA with 0.02% Triton X-100 for 2 h. Wells were incubated with primary antibody diluted in 2% BSA in PBS overnight at 4°C. Wells were washed three times with 1X PBS, followed by incubation with secondary antibody in 2% BSA in PBS and DAPI for 2 h. Wells were washed three times, a drop of ProLong™ Gold Antifade Mountant was added on each well and a coverslip was carefully placed on the slide. Primary antibodies used included Pf CSP clone 2A10 (1:500) and mCherry clone 16D7 (1:500, Thermo Scientific). Secondary antibodies include donkey anti-mouse 488 and donkey anti-rat 594.
Microscopy
All images were acquired using the Stellaris 8 confocal microscope (Leica Microsystems) with 63x water objective and processed using the Lightning software (Leica Microsystems). For quantification of parasite liver stage size, IFA using the CSP antibody was used to measure the area of liver stage parasites. The parasite was assumed to be elliptical in shape and therefore area was calculated from its longest (a) and shortest (b) circumferential diameter (πab).
Quantification and statistical analysis
Quantification was represented by the mean ± standard deviation. Calculations and statistical tests indicated in the figure legends were performed using GraphPad Prism Software. The statistical tests used are one-way and two-way ANOVA and unpaired t-test, as indicated. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. A p value > 0.05 was considered not significant.
Acknowledgments
We thank the insectary team at the Center for Global Infectious Disease Research for providing Anopheles stephensi mosquitoes. This work was funded by the National Institutes of Health (R01 AI125706). Graphical Abstract was created with BioRender.com.
Author contributions
S.H.I.K., A.V., and D.G., conceived the work and designed experiments. D.G., S.K., W.B., J.A., M.H., and N.C. carried out laboratory work and collected and analyzed the data. C.P., A.M.S., and S.C.M. created and analyzed the qRT-PCR data and contributed to discussion. D.G., A.V., and S.H.I.K. wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: May 20, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104224.
Supplemental information
Data and code availability
-
-
All data reported in this paper will be shared by the lead contact upon request.
-
-
This paper does not report original code.
-
-
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Arredondo S.A., Swearingen K.E., Martinson T., Steel R., Dankwa D.A., Harupa A., Camargo N., Betz W., Vigdorovich V., Oliver B.G., et al. The micronemal plasmodium proteins P36 and P52 act in concert to establish the replication-permissive compartment within infected hepatocytes. Front. Cell. Infect. Microbiol. 2018;8:413. doi: 10.3389/fcimb.2018.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo S.A., Schepis A., Reynolds L., Kappe S.H.I. Parasitology secretory organelle function in the plasmodium sporozoite. Trends Parasitol. 2021:1–13. doi: 10.1016/j.pt.2021.01.008. [DOI] [PubMed] [Google Scholar]
- Azuma H., Paulk N., Ranade A., Dorrell C., Al-dhalimy M., Ellis E., Strom S., Kay M.A., Finegold M., Grompe M. Robust expansion of human hepatocytes in Fah−/− /Rag2−/− / Il2rg −/− mice. Nat. Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S.P.C., Deeley R.G. Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol. Sci. 2006;27 doi: 10.1016/j.tips.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Dahlström S., Ferreira P.E., Veiga M.I., Sedighi N., Wiklund L., Mårtensson A., Färnert A., Sisowath C., Osório L., Darban H., et al. Plasmodium falciparum multidrug resistance protein 1 and artemisinin-based combination therapy in Africa. J. Infect. Dis. 2009;200:1456–1464. doi: 10.1086/606009. [DOI] [PubMed] [Google Scholar]
- Dahlström S., Veiga M.I., Mårtensson A., Björkman A., Gil J.P. Polymorphism in Pfmrp1 (Plasmodium falciparum multidrug resistance protein 1) amino acid 1466 associated with resistance to sulfadoxine-pyrimethamine treatment. Antimicrob. Agents Chemother. 2009;53:2553–2556. doi: 10.1128/AAC.00091-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper S.J., Sack B.K., King C.R., Nielsen C.M., Rayner J.C., Higgins M.K., Long C.A., Seder R.A. Malaria vaccines: recent advances and new horizons. Cell Host Microbe. 2018;24:43–56. doi: 10.1016/j.chom.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy P.E., Gorres J.P. Malaria vaccines since 2000: progress, priorities, products. NPJ Vaccines. 2020;5:1–9. doi: 10.1038/s41541-020-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foquet L., Schafer C., Minkah N.K., Alanine D.G.W., Flannery E.L., Steel R.W.J., Sack B.K., Camargo N., Fishbaugher M., Betz W., et al. Plasmodium falciparum liver stage infection and transition to stable blood stage infection in liver-humanized and blood-humanized FRGN KO mice enables testing of blood stage inhibitory antibodies (reticulocyte-binding protein homolog 5) in vivo. Front. Immunol. 2018;9:1–8. doi: 10.3389/fimmu.2018.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Pons M., Szeto A.C., González-Méndez R., Serrano A.E. Identification and bioinformatic characterization of a multidrug resistance associated protein (ABCC) gene in Plasmodium berghei. Malar. J. 2009;8:1–13. doi: 10.1186/1475-2875-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami D., Betz W., Locham N.K., Parthiban C., Brager C., Schäfer C., Camargo N., Nguyen T., Kennedy S.Y., Murphy S.C., et al. A replication-competent late liver stage-attenuated human malaria parasite. JCI Insight. 2020;5 doi: 10.1172/jci.insight.135589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graewe S., Stanway R.R., Rennenberg A., Heussler V.T. Chronicle of a death foretold: plasmodium liver stage parasites decide on the fate of the host cell. FEMS Microbiol. Rev. 2012;36:111–130. doi: 10.1111/j.1574-6976.2011.00297.x. [DOI] [PubMed] [Google Scholar]
- Hodgson S.H., Douglas A.D., Edwards N.J., Kimani D., Elias S.C., Chang M., Daza G., Seilie A.M., Magiri C., Muia A., et al. Increased sample volume and use of quantitative reverse-transcription PCR can improve prediction of liver-to-blood inoculum size in controlled human malaria infection studies. Malar. J. 2015;14:1–9. doi: 10.1186/s12936-015-0541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenstein K., Dawson R.J., Locher K.P. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 2007;17:412–418. doi: 10.1016/j.sbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Ishino T., Chinzei Y., Yuda M. Two proteins with 6-cys motifs are required for malarial parasites to commit to infection of the hepatocyte. Mol. Microbiol. 2005;58:1264–1275. doi: 10.1111/j.1365-2958.2005.04801.x. [DOI] [PubMed] [Google Scholar]
- Jobe O., Lumsden J., Mueller A., Williams J., Silva-Rivera H., Kappe S.H.I., Schwenk R.J., Matuschewski K., Krzych U. Genetically attenuated Plasmodium berghei liver stages induce sterile protracted protection that is mediated by major histocompatibility complex class I–dependent interferon-γ–producing CD8 + T cells. J. Infect. Dis. 2007;196:599–607. doi: 10.1086/519743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P.M., George A.M. Multidrug resistance in parasites : ABC transporters , P-glycoproteins and molecular modelling. Int. J. Parasitol. 2007;35:555–566. doi: 10.1016/j.ijpara.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Kappe S.H., Gardner M.J., Brown S.M., Ross J., Matuschewski K., Ribeiro J.M., Adams J.H., Quackenbush J., Cho J., Carucci D.J., et al. Exploring the transcriptome of the malaria sporozoite stage. Proc. Natl. Acad. Sci. U S A. 2001;98:9895–9900. doi: 10.1073/pnas.171185198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenderink J.B., Kavishe R.A., Rijpma S.R., Russel F.G.M. The ABCs of multidrug resistance in malaria. Trends Parasitol. 2010;26:440–446. doi: 10.1016/j.pt.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Kreutzfeld O., Müller K., Matuschewski K. Engineering of genetically arrested parasites ( GAPs ) for a precision malaria. Vaccine. 2017;7:1–13. doi: 10.3389/fcimb.2017.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kublin J.G., Mikolajczak S.A., Sack B.K., Fishbaugher M.E., Seilie A., Shelton L., VonGoedert T., Firat M., Magee S., Fritzen E., et al. Complete attenuation of genetically engineered Plasmodium falciparum sporozoites in human subjects. Sci. Transl. Med. 2017;9:1–12. doi: 10.1126/scitranslmed.aad9099. [DOI] [PubMed] [Google Scholar]
- Labaied M., Harupa A., Dumpit R.F., Coppens I., Mikolajczak S.A., Kappe S.H.I. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect. Immun. 2007;75:3758–3768. doi: 10.1128/IAI.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Kumar S., Mcdew-white M., Haile M., Cheeseman I.H., Emrich S., Button-Simons K., Nosten F., Kappe S.H.I., Ferdig M.T., et al. Genetic mapping of fitness determinants across the malaria parasite Plasmodium falciparum life cycle. PLoS Genet. 2019;15:e1008453. doi: 10.1371/journal.pgen.1008453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Aszalos A. Multidrug transporters as drug targets. Curr. Drug Targets. 2006:911–921. doi: 10.2174/138945006778019264. [DOI] [PubMed] [Google Scholar]
- Lindner S.E., Swearingen K.E., Shears M.J., Walker M.P., Vrana E.N., Hart K.J., Minns A.M., Sinnis P., Moritz R.L., Kappe S.H.I. Transcriptomics and proteomics reveal two waves of translational repression during the maturation of malaria parasite sporozoites. Nat. Commun. 2019;10:1–13. doi: 10.1038/s41467-019-12936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubens M., Vincensini L., Fernandes P., Briquet S., Marinach C., Silvie O. Plasmodium sporozoites on the move: switching from cell traversal to productive invasion of hepatocytes. Mol. Microbiol. 2020:1–12. doi: 10.1111/mmi.14645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKellar D.C., O’Neill M.T., Aly A.S.I., Sacci J.B., Cowman A.F., Kappe S.H.I. Plasmodium falciparum PF10_0164 (ETRAMP10.3) is an essential parasitophorous vacuole and exported protein in blood stages. Eukaryot. Cell. 2010;9:784–794. doi: 10.1128/EC.00336-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-mogollon C., Salman A.M., Koolen K.M.J., Bolscher J.M. A P . falciparum NF54 reporter line expressing mCherry-luciferase in gametocytes , sporozoites , and liver-stages. Front. Cell. Infect. Microbiol. 2019;9:1–13. doi: 10.3389/fcimb.2019.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R.E. The transportome of the malaria parasite. Biol. Rev. 2019;8 doi: 10.1111/brv.12565. [DOI] [PubMed] [Google Scholar]
- Medica D.L., Sinnis P. Quantitative dynamics of plasmodium yoelii sporozoite transmission by infected anopheline Mosquitoes. Infect Immun. 2005;73:4363–4369. doi: 10.1128/IAI.73.7.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meierjohann S., Walter R.D., Müller S. Regulation of intracellular glutathione levels in erythrocytes infected with chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum. Biochem. J. 2002;368:761–768. doi: 10.1042/BJ20020962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczak S.A., Lakshmanan V., Fishbaugher M., Camargo N., Harupa A., Kaushansky A., Douglass A.N., Baldwin M., Healer J., O’Neill M., et al. A next-generation genetically attenuated Plasmodium falciparum parasite created by triple gene deletion. Mol. Ther. J. Am. Soc. Gene Ther. 2014;22:1707–1715. doi: 10.1038/mt.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok S., Liong K.Y., Lim E.H., Huang X., Zhu L., Preiser P.R., Bozdech Z. Structural polymorphism in the promoter of pfmrp2 confers Plasmodium falciparum tolerance to quinoline drugs. Mol. Microbiol. 2014;91:918–934. doi: 10.1111/mmi.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J., Ferdig M.T., Feng X., Joy D.A., Duan J., Furuya T., Subramanian G., Aravind L., Cooper R.A., Wootton J.C., et al. Multiple transporters associated with malaria parasite responses to chloroquine and quinine. Mol. Microbiol. 2003;49:977–989. doi: 10.1046/j.1365-2958.2003.03627.x. [DOI] [PubMed] [Google Scholar]
- Mueller A.-K., Camargo N., Kaiser K., Andorfer C., Frevert U., Matuschewski K., Kappe S.H.I. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc. Natl. Acad. Sci. U.S A. 2005;102:3022–3027. doi: 10.1073/pnas.0408442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A., Labaied M., Kappe S.H.I., Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433:164–167. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- Raj D.K., Mu J., Jiang H., Kabat J., Singh S., Sullivan M., Fay M.P., Mccutchan T.F., Su X. Disruption of a plasmodium falciparum multidrug resistance-associated protein ( PfMRP ) alters its fitness and transport of antimalarial drugs and glutathione. J. Biol. Chem. 2009;284:7687–7696. doi: 10.1074/jbc.M806944200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeor J.F., Connolly G.C., Ballatori N. Inhibition of Mrp2- and Ycf1p-mediated transport by reducing agents : evidence for GSH transport on rat Mrp2. Biochim. Biophys. Acta. 2002;1559:171–178. doi: 10.1016/s0005-2736(01)00454-0. [DOI] [PubMed] [Google Scholar]
- Rijpma S.R., Van Der Velden M., Gonzalez-Pons M., Annoura T., van Schaijk B.C.L., van Gemert G.-J., van den Heuvel J.M.W., Ramesar J., Chevalley-Maurel S.C., et al. Multidrug ABC transporters are essential for hepatic development of Plasmodium sporozoites. Cell Microbiol. 2016;18:369–383. doi: 10.1186/s40945-017-0033-9.Using. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack B.K., Mikolajczak S.A., Fishbaugher M., Vaughan A.M., Flannery E.L., Nguyen T., Betz W., Jane Navarro M., Foquet L., Steel R.W.J., et al. Humoral protection against mosquito bite-transmitted Plasmodium falciparum infection in humanized mice. NPJ Vaccines. 2017;2:1–10. doi: 10.1038/s41541-017-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer C., Roobsoong W., Kangwanrangsan N., Bardelli M., Rawlinson T.A., Dambrauskas N., Trakhimets O., Parthiban C., Goswami D., Reynolds L.M., et al. A humanized mouse model for plasmodium vivax to test interventions that block liver stage to blood stage transition and blood stage infection. iScience. 2020;23 doi: 10.1016/j.isci.2020.101381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M.A., van Veen H.W. Molecular basis of multidrug transport by ABC transporters. Biochim. Biophys. Acta. 2009;1794:725–737. doi: 10.1016/j.bbapap.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Seilie A.M., Chang M., Hanron A.E., Billman Z.P., Stone B.C., Zhou K., Olsen T.M., Daza G., Ortega J., Cruz K.R., et al. Beyond blood smears: qualification of plasmodium 18s rrna as a biomarker for controlled human malaria infections. Am. J. Trop. Med. Hyg. 2019;100:1466–1476. doi: 10.4269/ajtmh.19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarun A.S., Dumpit R.F., Camargo N., Labaied M., Liu P., Takagi A., Wang R., Kappe S.H.I. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8 + T cells. J. Infect. Dis. 2007;196:608–616. doi: 10.1086/519742. [DOI] [PubMed] [Google Scholar]
- Upton D.C., Gregory B.L., Arya R., Unniraman S. ABC transporters: a riddle wrapped in a mystery inside an enigma. Immunol. Res. 2011;49:14–24. doi: 10.1007/s12026-010-8190-x. [DOI] [PubMed] [Google Scholar]
- van Dijk M.R., Douradinha B., Franke-Fayard B., Heussler V., van Dooren M.W., van Schaijk B., van Gemert G.-J., Sauerwein R.W., Mota M.M., Waters A.P., et al. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc. Natl. Acad. Sci. U S A. 2005;102:12194–12199. doi: 10.1073/pnas.0500925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbuskirk K.M., O'Neill M.T., De la Vega P., Maier A.G., Krzych U., Williams J., Dowler M.G., Sacci J.B., Kangwanrangsan N., Tsuboi T., et al. Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc. Natl. Acad. Sci. U S A. 2009;106:13004–13009. doi: 10.1073/pnas.0906387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan A.M., Mikolajczak S.A., Wilson E.M., Grompe M., Kaushansky A., Camargo N., Bial J., Ploss A., Kappe S.H.I. Technical advance Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J. Clin. Invest. 2012;122 doi: 10.1172/JCI62684.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan A.M., Pinapati R.S., Cheeseman I.H., Camargo N., Fishbaugher M., Checkley L.A., Nair S., Hutyra C.A., Nosten F.H., Anderson T.J.C., et al. Plasmodium falciparum genetic crosses in a humanized mouse model. Nat. Methods. 2015;12:631–633. doi: 10.1038/nmeth.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan A.M., Kappe S.H.I., Vaughan A.M., Genetically S.H.I.K. Expert Review of Vaccines Genetically attenuated malaria parasites as vaccines. Expert Rev. Vaccin. 2017;16:765–767. doi: 10.1080/14760584.2017.1341835. [DOI] [PubMed] [Google Scholar]
- Vaughan A.M., Kappe S.H.I. Malaria Parasite Liver Infection and Exoerythrocytic Biology. Cold Spring Harbor Laboratory Med. 2017;7 doi: 10.1101/cshperspect.a025486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther M., Dunachie S., Keating S., Vuola J.M., Berthoud T., Schmidt A., Maier C., Andrews L., Andersen R.F., Gilbert S., et al. Safety, immunogenicity and efficacy of a pre-erythrocytic malaria candidate vaccine, ICC-1132 formulated in Seppic ISA 720. Vaccine. 2005;23:857–864. doi: 10.1016/j.vaccine.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Wilkens S. Structure and mechanism of ABC transporters. F1000Prime Rep. 2015;7:1–9. doi: 10.12703/P7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, W . World Health Organization; 2021. World Malaria Report 2021. [Google Scholar]
- Zimmermann C., Van De Wetering K., Van De Steeg E., Wagenaar E., Vens C., Schinkel A.H. Species-dependent transport and modulation properties of human and mouse multidrug resistance protein 2 (MRP2/Mrp2, ABCC2/Abcc2) Drug Metab. Dispos. 2008;36:631–640. doi: 10.1124/dmd.107.019620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
-
All data reported in this paper will be shared by the lead contact upon request.
-
-
This paper does not report original code.
-
-
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.