Abstract
Objective
Neurosurgical guidelines have resulted in improved clinical outcomes and more optimized care for many complex neurosurgical pathologies. As momentum in global neurosurgical efforts has grown, there is little understanding about the application of these guidelines in low- and middle-income countries.
Methods
A 29-question survey was developed to assess the application of specific recommendations from neurosurgical brain and spinal cord injury guidelines. Surveys were distributed to an international cohort of neurosurgeons and neurotrauma stakeholders.
Results
A total of 82 of 222 (36.9%) neurotrauma providers responded to the survey. The majority of respondents practiced in low- and middle-income countries settings (49/82, 59.8%). There was a significantly greater mean traumatic brain injury volume in low-income countries (56% ± 13.5) and middle-income countries (46.5% ± 21.3) compared with high-income countries (27.9% ± 13.2), P < 0.001. Decompressive hemicraniectomy was estimated to occur in 61.5% (±30.8) of cases of medically refractory intracranial pressure with the lowest occurrence in the African region (44% ± 37.5). The use of prehospital cervical immobilization varied significantly by income status, with 36% (±35.6) of cases in low-income countries, 52.4% (±35.5) of cases in middle-income countries, and 95.2% (±10) in high-income countries, P < 0.001. Mean arterial pressure elevation greater than 85 mm Hg to improve spinal cord perfusion was estimated to occur in 71.7% of cases overall with lowest occurrence in Eastern Mediterranean region (55.6% ± 24).
Conclusions
While some disparities in guideline implementation are inevitably related to the availability of clinical resources, other differences could be more quickly improved with accessibility of current evidence-based guidelines and development of local data.
Key words: Evidence-based guidelines, Global neurosurgery, Low- and middle-income countries, Neurotrauma, Spinal cord injury, Traumatic brain injury
Abbreviations and Acronyms: AMR-US/Can, Region of the Americas (US and Canada); CT, Computed tomography; HIC, High-income country; ICP, Intracranial pressure; LIC, Low-income country; LMICs, Low- and middle-income countries; MAP, Mean arterial pressure; MIC, Middle-income country; TBI, Traumatic brain injury; TSI, Traumatic spinal injury; WHO, World Health Organization
Introduction
In recent decades, the neurosurgical community has devoted considerable time and effort to the creation of high-quality, evidence-based clinical practice guidelines.1 The implementation of these guidelines has resulted in improved clinical outcomes2,3 and a greater degree of optimized care for many complex neurosurgical pathologies.3, 4, 5, 6 Simultaneously, there has been a growing movement to address the substantial global burden of untreated neurosurgical disease via international neurosurgical education. Along with these efforts has come a greater understanding of the significant worldwide incidence of neurosurgical disease.7, 8, 9 This growing literature thoroughly demonstrates that neurological trauma represents a leading cause of morbidity and mortality worldwide. There are approximately 13.8 million neurosurgical conditions requiring surgery each year and 6.1 million estimated to be related to traumatic brain injury (TBI) with another 400,000 estimated to be related to traumatic spinal injury (TSI).10 Notably, the majority of these injuries occur in low- and middle-income countries (LMICs), where neurosurgical expertise is often less accessible.11
As we gain a greater understanding of the burden of traumatic brain and spinal injury in LMICs, the question is begged as to whether neurosurgical guidelines have been successfully disseminated and implemented in these regions. Moreover, some authors have questioned the relevance of these clinical practice guidelines, which are largely generated from studies based in high-income countries (HICs), for use in limited resource settings in which availability of imaging and critical care equipment is more sparse and thus must be triaged differently.12 As a result, it is possible that factors such as resource availability, surgical training, language barrier, and access to scientific literature result in a fundamental disconnect between these evidence based recommendations and their real-world application in LMICs where the majority of trauma occurs. In beginning to address some of these questions we performed a global survey of neurosurgical providers to obtain their perspective on the frequency with which several guideline-based recommendations are currently employed for traumatic brain and spinal injury.
Methods
Survey Design
This study was approved by the Harvard Medical School institutional review board (IRB18-1544). Using the EQUATOR (Enhancing the QUAlity and Transparency Of health Research) checklist,13 we developed a 29-question survey to assess the quality and availability of local health care infrastructure for the management of traumatic brain and cervical spine injury. Survey variables were designed to collect data on participant demographics, geographic location, and local trauma workload. Additionally, focused questions were constructed based upon specific recommendations from the Guidelines for the Management of Severe Traumatic Brain Injury, fourth edition5 and the Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injury, second edition.6 A nonexhaustive list of 6 specific recommendations from both guideline documents were chosen to concisely cover a variety of guideline topics and broadly assess frequency and availability of evidence-based perioperative and surgical management of traumatic brain and cervical spine injury (Table 1). Survey respondents were asked to estimate the proportion of traumatic brain and spine injury cases in which the guideline parameters were followed. The full details of the survey have been published previously.14
Table 1.
Summary Table of Guideline Level Recommendations Included in the Global Neurotrauma Survey with Accompanying Levels of Evidence
| Guideline Category | Level of Evidence | Recommendation |
|---|---|---|
| Guidelines for the Management of Severe Traumatic Brain Injury, 4th edition | ||
| Seizure prophylaxis | Level IIA | Phenytoin is recommended to decrease the incidence of early post-traumatic seizure (within 7 days of injury) when the overall benefit is thought to outweigh the complications associated with such treatment. |
| CSF drainage | Level III | Use of CSF drainage to lower ICP in patients with an initial GCS <6 during the first 12 hours after injury may be considered. |
| Hyperosmolar therapy | Not supported∗ | Mannitol is effective for control of raised ICP at doses of 0.25 g/kg to 1 g/kg body weight. Arterial hypotension (systolic blood pressure <90 mm Hg) should be avoided. |
| Steroids | Level I | The use of steroids is not recommended for improving outcome or reducing ICP. In patients with severe TBI, high-dose methylprednisolone was associated with increased mortality and is contraindicated. |
| Anesthetics, analgesics, sedatives | Level IIB | High dose barbiturate administration is recommended to control elevated ICP refractory to maximum standard medical and surgical treatment. |
| Decompressive craniectomy | Level IIA | A large frontotemporoparietal decompressive craniectomy is recommended for reduced mortality and improved neurologic outcomes in patients with severe TBI. |
| Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injuries, 2nd edition | ||
| Immobilization | Level II | Spinal immobilization of all trauma patients with a cervical spine or spinal cord injury or with a mechanism of injury having the potential to cause cervical spine injury is recommended. |
| Radiographic assessment | Level I | In the awake, symptomatic patient, high-quality CT imaging of the cervical spine is recommended. |
| Radiographic assessment | Level I | If high-quality CT imaging is not available, a 3-view cervical spine series (AP, lateral, and odontoid) is recommended. |
| Pharmacology management | Level I | Administration of methylprednisolone for treatment of acute spinal cord injury is not recommended. |
| Cardiopulmonary management | Level III | Maintain mean arterial BP 85 to 90 mm Hg after spinal cord injury is recommended. |
| Cervical spine fractures† | Level II and III | Surgical fixation versus medical management is recommended based on specific fracture type and ligamentous integrity |
CSF, cerebrospinal fluid; ICP, intracranial pressure; GCS, Glasgow Coma Scale; TBI, traumatic brain injury; CT, computed tomography; AP, anteroposterior; BP, blood pressure.
In the 4th edition of the guidelines for severe traumatic brain injury, the evidence supporting the prior recommendations regarding mannitol for hyperosmolar therapy, previously categorized as Level III, were found to not meet current standards for Level III evidence. As such the previous recommendations were continued into the 4th edition with the notation that they were not supported by adequate evidence based on updated criteria.
The Guidelines for Acute Cervical Spine and Spinal Cord Injury provide detailed surgical and non-surgical recommendations for a variety of specific cervical spine fracture types. Application of these recommendations were broadly summarized in our survey by asking how often mechanically unstable cervical spine fractures underwent surgical fixation.
Identification of Participants
The perspective of neurotrauma providers was sought to obtain first-hand perceptions on the quality and availability of local neurotrauma care.15 Efforts were made to include respondents who were content experts in global neurosurgical development and TBI and who were representative of all World Health Organization (WHO) geographic regions. To achieve this, a participant list was initiated by including all registered attendees of the 2019 International Conference on Recent Advances in Neurotraumatology (ICRAN) meeting.16 In addition, all members registered with the open access Global Neurosurgery forum were included.17 Lastly, neurosurgeons from any WHO region that was disproportionately under-represented by this aggregate list were sought out by searching the American Association of Neurological Surgeons membership directory by country until equal representation was achieved from all WHO regions. In total, 222 participants received email invitations to participate in the study. The survey was administered electronically via Google Forms (Google; Mountain View, California, USA) on January 5, 2019. The survey was administered in English. Responses were collected from January 1 to January 31, 2019. A modified Dillman technique18 with weekly reminder emails were sent out during this period to all invited participants who had not yet responded to the survey. Following this, data extraction was performed for statistical analysis.
Data Analysis
Descriptive statistics were used to summarize all demographic variables. Analysis of variance was used to determine statistically significant differences between means for categorical predictors—WHO region and World Bank Income classification. In cases in which significantly different means were identified pairwise t test with Bonferroni correction was used to further delineate statistically significant relationships. All statistical testing was 2-sided and P values less than 0.05 were considered significant. The statistical analysis was conducted using R statistical software, version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). Heat matrices were constructed using Tableau Public (Version 2019.3.0) software (Tableau, Seattle, Washington, USA).
Results
Demographics
A total of 222 neurotrauma care providers were invited to participate in the survey. Of these, 82 responded with a total response rate of 36.9%. Most respondents identified themselves as faculty neurosurgeons (62/82, 75.6%) with the remainder identifying as neurosurgical trainees (14/82, 17.1%) or non-neurosurgical physicians (6/82, 7.3%), which included critical care physicians and general surgeons. There was a total of 47 countries represented among the respondents, with equal representation from all WHO geographic regions. Additionally, all World Bank Income strata were represented with the majority of respondents residing and practicing in low- and middle-income country settings (49/82, 59.8%) (Table 2).
Table 2.
Demographics of Survey Respondents and Nonrespondents in Terms of WHO Region, World Bank Income Classification and Profession
| Respondents, n (%) | Nonrespondents, n (%) | Total, n (%) | P Value∗ | |
|---|---|---|---|---|
| WHO region | ||||
| AMR-US/Can | 12 (14.6) | 13 (9.3) | 25 (100) | NS |
| AMR-L | 12 (14.6) | 18 (12.9) | 30 (100) | NS |
| EUR | 16 (19.5) | 44 (31.4) | 60 (100) | NS |
| EMR | 9 (11) | 23 (16.4) | 32 (100) | NS |
| AFR | 10 (12.2) | 16 (11.4) | 26 (100) | NS |
| SEAR | 11 (13.4) | 11 (7.9) | 22 (100) | NS |
| WPR | 12 (14.6) | 15 (10.7) | 27 (100) | NS |
| World Bank Income Classification | ||||
| HIC | 33 (40.2) | 58 (41.4) | 91 (100) | NS |
| MIC | 34 (41.4) | 67 (47.9) | 101 (100) | NS |
| LIC | 15 (18.3) | 17 (12.1) | 32 (100) | NS |
| Profession | ||||
| Faculty (Neurosurgery) | 75.6 (62) | |||
| Trainee (Neurosurgery) | 17.1 (14) | |||
| Other | 7.3 (6) |
Professional details not available for nonrespondents.
WHO, World Health Organization; AMR-US/Can, region of the Americas (US and Canada); NS, not significant; AMR-L, region of the Americas (Latin America); European region; EMR, Eastern Mediterranean region; AFR, African region; EUR, SEAR, Southeast Asia region; WPR, Western Pacific region; HIC, high-income country; MIC, middle-income country; LIC, low-income country.
P values derived from Fisher Exact test comparing proportion of respondent vs non-respondent by region and income level. A P value less than 0.05 was considered significant.
Severe TBI
The mean reported proportion of total operative volume due to TBI was 40.7% (±20.2) overall. There was a significantly greater mean TBI volume in low-income countries (LICs) (56% ± 13.5) and middle-income countries (MICs) (46.5% ± 21.3) compared with HICs (27.9% ± 13.2), P < 0.001. Additionally, there was a greater mean reported TBI volume in Africa (52% ± 14), Latin America (48.3% ± 19.9), the Eastern Mediterranean region (51.1% ± 22.6), and Southeast Asia (49.1% ± 18.7) relative to the United States and Canada (23.3% ± 11.5), P < 0.05. The mean estimated TBI volumes in Europe and the Western Pacific regions were 31.3% (±17.8) and 38.3% (±19.9), respectively, and were not significantly different from the United States and Canada (AMR-US/Can).
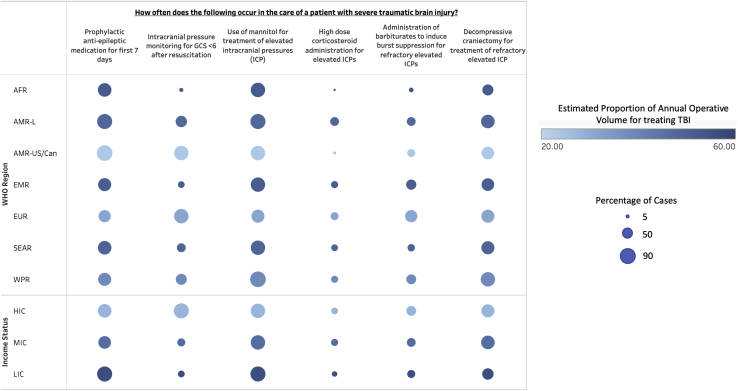
On average, 69% (±33.3) of patients are estimated to receive prophylactic antiepileptic medications for 7 days after TBI with no significant differences by world region or income status. Intracranial pressure (ICP) monitoring is estimated to occur in 45.6% (±40.6) of cases, with significantly lower usage in LICs (16% ± 28.5) and MICs (25.3% ± 33.1) relative to HICs (80% ± 25). Notably, Africa is the region with the lowest use of ICP monitoring (6% ± 9.7) and was significantly lower than AMR-US/Can (86.7% ± 13), P < 0.001. Mannitol is used to treat elevated ICP in 75.4% (±30) of cases worldwide without significant variation by region or income.
High-dose corticosteroid usage for elevated ICP is not recommended, based on Level 1 evidence, and is estimated to occur in just 16.1% (±28.9) of cases with the lowest usage being in Africa (2% ± 6.3) and the greatest usage being in Latin America (26.7% ± 37.5). Administration of barbiturates for refractory ICPs occurred in 31% (±32.4) of cases overall. The lowest use was in Africa, with 8% (±10.3), and the greatest use was in Europe, with 53.8% (±32.4). Decompressive hemicraniectomy was estimated to occur in 61.5% (±30.8) of cases of medically refractory ICP with the lowest occurrence in Africa 44% (±37.5) and the highest in the Western Pacific region (75% ± 24.3). There was no statistically significant variation by region or income for high dose corticosteroid usage, administration of barbiturates, or decompressive hemicraniectomy for refractory ICPs (Figure 1 and Table 3).
Figure 1.
Heat map summarizing survey responses for implementation of selected recommendations from The Guidelines for Severe Traumatic Brain Injury, 4th edition, separated by world region and income status. Size of circle indicates the estimated percentage of cases in which the recommendation is implemented. Color density indicates average operative volume attributable to traumatic brain injury for that region or income level. WHO, World Health Organization; AFR, African region; AMR-L, region of the Americas (Latin America); AMR-US/Can, region of the Americas (US and Canada); EMR, Eastern Mediterranean region; EUR, European region; SEAR, Southeast Asia region; WPR, Western Pacific region; HIC, high-income country; MIC, middle-income country; LIC, low-income country.
Table 3.
Average Estimated Proportion of Cases Attributable to Traumatic Brain Injury by Global Region and Income Classification
| Average Estimated TBI Volume | Prophylactic Antiepileptic Medication for First 7 Days |
Intracranial Pressure Monitoring for GCS <6 After Resuscitation |
Use of Mannitol for Treatment of Elevated ICP |
High-Dose Corticosteroid Administration for Elevated ICPs |
Administration of Barbiturates to Induce Burst Suppression for Refractory Elevated IICs |
Decompressive Craniectomy for Treatment of Refractory Elevated ICP |
|
|---|---|---|---|---|---|---|---|
| Level IIA | Level III | Not Supported | Level I | Level IIB | Level IIA | ||
| WHO region | |||||||
| AFR | 52 (14)∗ | 70 (39.2) | 6 (9.7)† | 76 (24.6) | 2 (6.3) | 8 (10.3) | 44 (37.5) |
| AMR-L | 48.3 (19.9)‡ | 81.7 (28.9) | 46.7 (42.1) | 81.7 (26.2) | 26.7 (37.5) | 28.3 (31.3) | 66.7 (34.5) |
| AMR-US/Can | 23.3 (11.5) | 86.7 (13) | 76.7 (22.3) | 75 (35.3) | 3.3 (7.7) | 23.3 (23.9) | 56.7 (28.1) |
| EMR | 51.1 (22.6)‡ | 62.2 (23.3) | 15.6 (26)∗ | 73.3 (22.4) | 17.8 (27.3) | 37.8 (40.6) | 57.8 (25.4) |
| EUR | 31.3 (17.8) | 52.5 (41.2) | 76.3 (34.4) | 61.3 (38.3) | 23.8 (32.8) | 53.8 (32.4) | 63.8 (28.5) |
| SEAR | 49.1 (18.7)‡ | 69.1 (30.2) | 27.3 (36.1)‡ | 76.4 (29.4) | 16.4 (25) | 20 (25.3) | 61.8 (35.2) |
| WPR | 38.3 (19.9) | 65 (37.3) | 45 (40.1) | 88.3 (23.3) | 18.3 (38.6) | 35 (37.3) | 75 (24.3) |
| Income status | |||||||
| HIC | 27.9 (13.2) | 71.5 (34.3) | 80 (25) | 76.4 (32.6) | 15.2 (29.6) | 37 (34.3) | 61.2 (28.7) |
| MIC | 46.5 (21.3)† | 60.6 (35) | 25.3 (33.1)† | 72.9 (29.1) | 19.4 (29.3) | 28.8 (31.2) | 67.1 (29.5) |
| LIC | 56 (13.5)† | 82.7 (21.2) | 16 (28.5)† | 78.7 (27.7) | 10.7 (27.1) | 22.7 (30.1) | 49.3 (36.1) |
| Total | 40.7 (20.2) | 69 (33.3) | 45.6 (40.6) | 75.4 (30) | 16.1 (28.9) | 31 (32.4) | 61.5 (30.8) |
Listing of studied TBI Guideline recommendations along with estimated proportion of clinically appropriate scenarios in which the recommendation is followed.
TBI, traumatic brain injury; GCS, Glasgow Coma Scale; ICP, intracranial pressure; WHO, World Health Organization; AFR, African region; AMR-L, region of the Americas (Latin America); AMR-US/Can, region of the Americas (US and Canada); EMR, Eastern Mediterranean region; EUR, European region; SEAR, Southeast Asia region; WPR, Western Pacific region; HIC, high-income country; MIC, middle-income country; LIC, lower-income country.
P < 0.01.
P < 0.001.
P < 0.05.
Acute Cervical Spine and Spinal Cord Injury
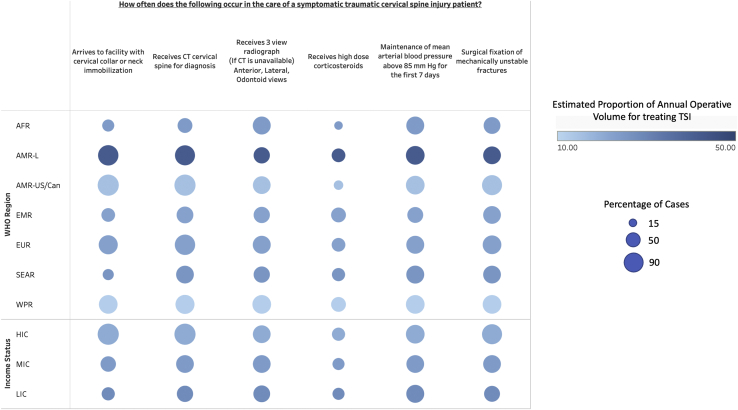
The overall mean estimated proportion of annual operations due to TSI was 23.2% (±17.3). There was a greater reported volume in LICs (28% ± 12.6) and MICs (24.1% ± 19.6) relative to HICs (20% ± 16.6), although these differences were not statistically significant, P = 0.31. Latin America was the region with the greatest annual TSI volume (41.7% ± 23.3) and was significantly greater than the US and Canada (15% ± 9), P < 0.01. Western Pacific region was the region with the lowest annual volume, (11.7% ± 10.3) and was significantly lower than the United States and Canada, P < 0.001. The reported volumes for Africa (24% ± 12.6), Eastern Mediterranean region (22.2% ± 12), Europe (22.5% ± 19.9), and Southeast Asia (25.5 ± 12.9) were not significantly different from each other or the United States and Canada.
The use of prehospital cervical immobilization varied significantly by income status, with 36% (±35.6) of cases in LICs, 52.4% (±35.5) of cases in MICs, and 95.2% (±10) in HICs, P < 0.001. Additionally, there was significantly lower cervical immobilization in Africa (32% ± 36.8), the Eastern Mediterranean region (40% ± 24.5), and Southeast Asia region (27.3% ± 27.2) compared with AMR-US/Can (96.7% ± 7.8), P < 0.001. The availability of computed tomography (CT) for evaluation of cervical spine injuries was dependent upon income classification with use in 98.2% (±7.7) of HIC cases, 68.2% (±32.7) of MIC cases, and 57.3% (±36.1) of LIC cases, P < 0.001. Africa had a significantly lower availability of CT (46% ± 32.7) relative to AMR-US/Can (98.3% ± 5.8). In the absence of the availability of CT imaging, a 3-view cervical spine film is recommended for evaluation of cervical injuries in symptomatic patients based on Level 1 evidence. This is estimated to occur in 66.6% (±36.3) of cases without significant variation by region or income.
Additionally, Level 1 evidence indicates that high-dose corticosteroids should not be given for acute cervical spinal cord injuries.19 This is estimated to occur in 36.1% (±33.3) of cases overall with the lowest usage in Africa (16% ± 20.7) and the highest in Eastern Mediterranean region (48.9% ± 31.8). There was no significant variation by income or region for high-dose corticosteroid usage. Mean arterial pressure (MAP) elevation greater than 85 mm Hg to improve spinal cord perfusion was estimated to occur in 71.7% of cases overall with lowest occurrence in Eastern Mediterranean region (55.6% ± 24) and highest in both AMR-US/Can (78.3% ± 18) and Western Pacific region (78.3% ± 23.3). There was no significant variation in MAP management by region or income status. Lastly, although there was no significant variation by region, the availability of surgical fixation for mechanically unstable cervical spine fractures was significantly dependent upon income status with treatment occurring in 54.7% (±26.7) of LIC cases, P < 0.01, 68.2% (±30.4) of MIC cases, P < 0.05, and 86.7% (±24.8) of HIC cases (Figure 2 and Table 4).
Figure 2.
Heat map summarizing survey responses for implementation of selected recommendations from The Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injury, 2nd edition, separated by world region and income status. Size of circle indicates the estimated percentage of cases in which the recommendation is implemented. Color density indicates average operative volume attributable to traumatic brain injury for that region or income level. WHO, World Health Organization; AFR, African region; AMR-L, region of the Americas (Latin America); AMR-US/Can, region of the Americas (US and Canada); EMR, Eastern Mediterranean region; EUR, European region; SEAR, Southeast Asia region; WPR, Western Pacific region; HIC, high-income country; MIC, middle-income country; LIC, low-income country.
Table 4.
Average Estimated Proportion of Cases Attributable to Traumatic Spinal Cord Injury by Global Region and Income Classification
| Average Estimated TSI Volume | Arrives To Facility with Cervical Collar or Neck Immobilization |
Receives CT Cervical Spine for Diagnosis |
Receives 3-View Radiograph (if CT Is Unavailable) - Anterior, Lateral, Odontoid Views |
Receives High-Dose Corticosteroids |
Maintenance 0f Mean Arterial Blood Pressure Above 85 Mm Hg for the First 7 Days |
Surgical Fixation of Mechanically Unstable Fractures |
|
|---|---|---|---|---|---|---|---|
| Level II | Level I | Level I | Level I | Level III | Levels II and III | ||
| WHO region | |||||||
| AFR | 24 (12.6) | 32 (36.8)∗ | 46 (32.7)∗ | 70 (31.6) | 16 (20.7) | 70 (34.3) | 62 (22) |
| AMR-L | 41.7 (23.3)† | 90 (18.1) | 86.7 (19.7) | 60 (43.5) | 38.3 (37.6) | 75 (28.4) | 68.3 (35.6) |
| AMR-US/Can | 15 (9) | 96.7 (7.8) | 98.3 (5.8) | 66.7 (40.3) | 20 (28.3) | 78.3 (18) | 88.3 23.3) |
| EMR | 22.2 (12) | 40 (24.5)∗ | 62.2 (40.6) | 60 (37.4) | 48.9 (31.8) | 55.6 (24) | 68.9 (36.2) |
| EUR | 22.5 (19.91) | 81.3 (32.2) | 91.3 (21.9) | 71.3 (337.9) | 41.3 (30.5) | 70 (38.6) | 75 (29.7) |
| SEAR | 25.5 (12.9) | 27.3 (27.2)∗ | 69.1 (36.2) | 58.2 (31.6) | 38.2 (34) | 70.9 (27.4) | 69.1 (30.2) |
| WPR | 11.7 (10.3)∗ | 78.3 (27.6) | 80 (28.3) | 76.7 (33.9) | 48.3 (39.5) | 78.3 (23.3) | 76.7 (30.6) |
| Income status | |||||||
| HIC | 20 (16.6) | 95.2 (10) | 98.2 (7.7) | 69.1 (40) | 38.2 (35.1) | 78.2 (26.6) | 86.7 (24.8) |
| MIC | 24.1 (19.6) | 52.4 (35.5)∗ | 68.2 (32.7)∗ | 65.9 (32.9) | 34.7 (31.6) | 65.9 (31.3) | 68.2 (30.4)‡ |
| LIC | 28 (12.6) | 36 (35.6)∗ | 57.3 (36.1)∗ | 62.7 (36.9) | 34.7 (35) | 70.7 (26) | 54.7 (26.7)† |
| Total | 23.2 (17.3) | 66.6 (36.9) | 78.3 (31.1) | 66.6 (36.3) | 36.1 (33.3) | 71.7 (28.8) | 73.2 (29.9) |
Listing of studied TSI Guideline recommendations along with estimated proportion of clinically appropriate scenarios in which the recommendation is followed.
TSI, traumatic spinal injury; CT, computed tomography; WHO, World Health Organization; AFR, African region; AMR-L, region of the Americas (Latin America); AMR-US/Can, region of the Americas (US and Canada); EMR, Eastern Mediterranean region; EUR, European region; SEAR, Southeast Asia region; WPR, Western Pacific region; HIC, high-income country; MIC, middle-income country; LIC, lower-income country.
P < 0.001.
P < 0.01.
P < 0.05.
Discussion
Estimated Burden of Traumatic Brain and Spinal Injury
The findings of our survey indicate that traumatic brain and spinal injury represent approximately 41% and 23%, respectively, of annual neurosurgical operative volumes worldwide, with greater proportions being reported from neurosurgical providers in LMICs. In a recent meta-analysis, Dewan et al.9 estimated that approximately 69 million individuals suffer from TBI each year, with Southeast Asia being a region of greatest incidence. Similarly, in a different survey of neurosurgical providers, 51% of respondents reported TBI as one of the most commonly seen neurosurgical conditions worldwide.20 Our data corroborate these findings regarding the importance of TBI as a global cause of morbidity and mortality, particularly in resource-limited settings.
Evaluating the dissemination and implementation of neurosurgical practice guidelines is a complex topic of research with multiple potential confounders and difficulty in establishing direct causality. Previous studies have attempted to evaluate neurosurgical guideline impact directly via patient outcomes2,21 and indirectly via bibliometric analyses.22 While evaluating the success of neurosurgical guideline implementation remains a growing area of academic interest, the existing literature focuses primarily on HIC settings, thus creating an opportunity to begin asking similar questions in LMICs where the majority of traumatic brain and spine injuries have been shown to occur.
Improving Guideline-Based Care
Our findings indicate that there is already relatively good compliance with many TBI and TSI guideline recommendations, including use of prophylactic antiepileptic drugs, mannitol for refractory ICPs, and MAP management for acute cervical spinal cord injury. However, there were also several recommendations in which guideline adherence was significantly limited based upon region and income status. For instance, prehospital cervical immobilization was significantly rarer in LMICs. Similarly, availability of high-quality CT imaging was directly impacted by income status. A potential commonality among these recommendations is that they are resource- and equipment-dependent. In the case of cervical immobilization, collars are a depletable resource that can be prohibitively expensive, and in the case of imaging, CT and magnetic resonance imaging scanners along with the energy infrastructure and technological expertise required to support these investigations are often beyond all but the larger tertiary-care facilities in LMICs.23 It is worth noting that, despite these financial gaps, the use of 3-view plain film for cervical injury was similar across all regions and income levels, demonstrating flexibility in the guideline recommendations when all ideal technologies are not available.
Interestingly, there was a wide degree of variation regarding usage of ICP monitoring by region and income status. However, this remains a topic of heavy debate within the literature24, 25, 26 and in the guidelines is categorized as “may be considered” based upon Level III evidence.5 Additionally, the perioperative management of these devices requires a high degree of nursing expertise and bedside sterility, the absence of which may push the risks beyond the benefits in many LMIC hospitals. Similarly, the availability of surgical fixation for mechanically unstable cervical spine fractures was also significantly lower in low- and middle-income settings. While many surgical tools and resources can be sterilized and reused and are thus sustainable, spinal implants are yet another exhaustible resource which may represent a significant limiting factor in providing guideline-based care.
While some aspects of clinical care are equipment dependent, others are primarily information dependent. It is notable that administration of high-dose corticosteroids for acute cervical spine injury is still estimated to occur in more than one-third of cases worldwide and in nearly one-half of all cases in the Eastern Mediterranean region, despite recommendations against this based upon Level I evidence. Hurlbert and Moulton27 demonstrated the simple power of information availability when they found a complete practice reversal regarding the administration of methylprednisolone for acute cervical spinal cord injury before and after28 guideline publication. The fact that reported methylprednisolone usage remains so high globally and in particular regions indicates potentially important gaps in dissemination of guideline information to LMICs.
Mechanisms for this lack of information availability could include language barriers and low access to proprietary medical literature. In one survey of Southeast Asian neurosurgical trainees, only 13% reported having neurosurgical resources in their primary language, and just 53% had access to online journals via their training program.29 While addressing workforce and equipment deficiencies in global neurosurgical care represents a significant logistical and financial dilemma, improving access to information could be a cost-effective and relatively expedient opportunity to immediately improve the availability of evidence-based neurosurgical care worldwide. In recent decades, the organized neurosurgical community has generated many high-quality guideline documents with excellent summary data.1 The strategic translation of these documents into key languages along with subsidized open-access of these articles could dramatically increase their readership among the LMIC neurosurgical community.
Finally, it is worth briefly addressing the fact that much of the data supplying these, and most other, neurosurgical guidelines are based largely from studies in HICs rather than LMICs. Indeed, Tropeano et al.30 recently demonstrated that Africa and Southeast Asia collectively generate approximate 2% of recently published TBI literature despite being the site of 38% of estimated annual traumatic brain injuries. Because of this fact, questions have been raised in the literature as to the applicability of neurosurgical guidelines in LMICs.12,31, 32, 33 Toward this point, Kolias et al.34 raise the argument that data from randomized trials demonstrating the benefits of decompressive hemicraniectomy are predicated upon the availability adjunctive therapies such as critical care and ICP monitoring, which are notably absent in many LMICs. They propose that the absence of these adjunctive measures renders the results of these trials to have limited generalizability outside of HICs. Similarly, there are examples in which the pathologic mechanisms in LMICs are fundamentally different than those in HICs.35 For example, lower-velocity motor bikes are a highly cited cause of TBI in LMICs compared with the high-velocity deceleration injuries common to motor vehicle accidents in HICs.36 Similarly, many infectious diseases resulting in neurologic manifestations are uniquely endemic to many LMICs and notably absent in HICs.7
In response to these arguments, efforts are being made to develop international trauma registries and high-quality data collection based in LMICs that will begin to allow for context-specific guidelines derived from local research.37, 38, 39 In the meantime, evidence-based guidelines based on the most up-to-date science on treatment of TBI and SCI, as well as other neurosurgical pathologies, should be considered the evidentiary benchmark from which necessary context-specific adaptations should be made. Important efforts have been made to make these adaptations in a structured and rigorous way. For example, Rubiano et al.40 have recently published the BOOTStraP protocol which uses consensus from regional experts to adapt the best evidence-based treatment algorithms to the capabilities of low resource emergency facilities. Such efforts towards “Resource-Based Guidelines” are an important adjunct to the dissemination of the already available high-quality neurosurgical evidence-based guidelines.
Limitations
Our study is limited in that the data inherently lacks objectivity based upon the survey methodology. Importantly, percentages likely reflect the population treated at the respondent's institution rather than the region as a whole, for instance it is unlikely that 44% of patients with refractory ICPs truly receive decompressive hemicraniectomy in Africa and thus, in some cases, our values may represent an overestimation. However, objective data on this topic are currently unavailable and will take considerable time and resources to gather. Our findings, although limited to estimates, do make intuitive sense, and corroborate with similar published reports.20,41,42 These data are a first step at addressing this important topic of research.
It is also noteworthy that comparison of practice patterns between HIC and LMIC settings is prone to bias and complicated by a host of systemic factors that go beyond the perspective of a clinical neurosurgeon, such as national level trauma referral systems, prehospital care, and equipment supply chains. Thus, our survey, which is limited to the clinical perspective, may lack the strategic viewpoint of a ministry of health or other high-level administrative position that has further insight into these contributing factors. Additionally, our survey questions focused on specific clinical parameters and did not ask for providers' perceptions of the availability or utility of guidelines in limited resource settings. While answering this question was beyond the scope of our current study, it has been discussed among centers in HIC settings. For instance, Volovici et al.43 studied this question by evaluating use and perceptions of TBI guidelines among 65 European trauma centers. Of those centers that did not implement guideline care, the most commonly cited reasons were lack of time to consult guidelines, lack of knowledge or resources to implement recommendations, and feeling that each clinical scenario is unique and thus not amenable to universal protocols. Structured qualitative evaluation of this question in LMICs will be an important next step in increasing the dissemination of implementation of guideline care in these settings.
Conclusions
Our study suggests that significant global regional and economic disparities exist in the implementation of guideline-based care for severe traumatic brain and spinal cord injury. While some disparities are inevitably related to the availability of clinical resources, which should be addressed over time, other differences in adherence could be mitigated more quickly by improved dissemination and accessibility of evidence-based guideline recommendations and structured adaptation of guidelines to local capabilities.
CRediT authorship contribution statement
Jacob R. Lepard: Conceptualization, Investigation, Formal analysis, Writing – original draft. Saniya Mediratta: Writing – original draft, Writing – review & editing. Andres M. Rubiano: Supervision, Writing – review & editing. Kee B. Park: Supervision, Writing – review & editing.
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Shank C.D., Lepard J.R., Walters B.C., Hadley M.N. Towards evidence-based guidelines in neurological surgery. https://doi.org/10.1093/neuros/nyy414 [e-pub ahead of print]. Neurosurgery. [DOI] [PubMed]
- 2.Tarapore P.E., Tarapore P.E., Vassar M.J., et al. Establishing a TBI program of care—benchmarking outcomes after institutional adoption of evidence-based guidelines. https://doi.org/10.1089/neu.2015.4114 [e-pub ahead of print]. J Neurotrauma. [DOI] [PubMed]
- 3.Kesinger M.R., Nagy L.R., Sequeira D.J., Charry J.D., Puyana J.C., Rubiano A.M. A standardized trauma care protocol decreased in-hospital mortality of patients with severe traumatic brain injury at a teaching hospital in a middle-income country. Injury. 2014;45:1350–1354. doi: 10.1016/j.injury.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 4.Olson J.J., Kalkanis S.N., Ryken T.C. Evidence-based clinical practice parameter guidelines for the treatment of adults with diffuse low grade glioma: introduction and methods. J Neurooncol. 2015;125:449–456. doi: 10.1007/s11060-015-1847-5. [DOI] [PubMed] [Google Scholar]
- 5.Carney N., Totten A.M., O’Reilly C., et al. Guidelines for the management of severe traumatic brain injury, Fourth Edition. Neurosurgery. 2017;80:6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 6.Hadley M.N., Walters B.C. Introduction to the guidelines for the management of acute cervical spine and spinal cord injuries. Neurosurgery. 2013;72(suppl 2):5–16. doi: 10.1227/NEU.0b013e3182773549. [DOI] [PubMed] [Google Scholar]
- 7.Robertson F.C., Lepard J.R., Mekary R.A., et al. Epidemiology of central nervous system infectious diseases: a meta-analysis and systematic review with implications for neurosurgeons worldwide. https://doi.org/10.3171/2017.10.JNS17359 [e-pub ahead of print]. J Neurosurg. [DOI] [PubMed]
- 8.Dewan M.C., Rattani A., Mekary R., et al. Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. https://doi.org/10.3171/2017.10.JNS17439 [e-pub ahead of print]. J Neurosurg. [DOI] [PubMed]
- 9.Dewan M.C., Rattani A., Gupta S., et al. Estimating the global incidence of traumatic brain injury. https://doi.org/10.3171/2017.10.JNS17352 [e-pub ahead of print]. J Neurosurg. [DOI] [PubMed]
- 10.Dewan M.C., Rattani A., Fieggen G., et al. Global neurosurgery: the current capacity and deficit in the provision of essential neurosurgical care. Executive Summary of the Global Neurosurgery Initiative at the Program in Global Surgery and Social Change. https://doi.org/10.3171/2017.11.JNS171500 [e-pub ahead of print]. J Neurosurg. [DOI] [PubMed]
- 11.Corley J., Lepard J., Barthélemy E., Ashby J.L., Park K.B. Essential neurosurgical workforce needed to address neurotrauma in low- and middle-income countries. World Neurosurg. 2019;123:295–299. doi: 10.1016/j.wneu.2018.12.042. [DOI] [PubMed] [Google Scholar]
- 12.Patel A., Vieira M.M.C., Abraham J., et al. Quality of the development of traumatic brain injury clinical practice guidelines: a systematic review. PLoS One. 2016;11:e0161554. doi: 10.1371/journal.pone.0161554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley K., Clark B., Brown V., Sitzia J. Good practice in the conduct and reporting of survey research. Int J Qual Health Care. 2003;15:261–266. doi: 10.1093/intqhc/mzg031. [DOI] [PubMed] [Google Scholar]
- 14.Mediratta S., Lepard J.R., Barthélemy E.J., Corley J., Park K.B. Barriers to neurotrauma care in low- to middle-income countries: an international survey of neurotrauma providers. J Neurosurg. 2021:1–10. doi: 10.3171/2021.9.JNS21916. [DOI] [PubMed] [Google Scholar]
- 15.Shrime M.G., Bickler S.W., Alkire B.C., Mock C. Global burden of surgical disease: an estimation from the provider perspective. Lancet Glob Health. 2015;3(suppl 2):S8–S9. doi: 10.1016/S2214-109X(14)70384-5. [DOI] [PubMed] [Google Scholar]
- 16.Iaccarino C., Kolias A., Adelson P.D., et al. Consensus statement from the international consensus meeting on post-traumatic cranioplasty. Acta Neurochir (Wien) 2021;163:423–440. doi: 10.1007/s00701-020-04663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Global Neurosurgery Committee. https://globalneurosurgery.org/ Available at.
- 18.Lepard J., Shank C., Agee B., Hadley M., Walters B. Neurosurgical resident research education: a survey of United States residency program directors. https://doi.org/10.3171/2019.7.JNS19632 [e-pub ahead of print]. J Neurosurg. [DOI] [PubMed]
- 19.Walters B.C., Hadley M.N., Hurlbert R.J., et al. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery. 2013;60(suppl 1):82–91. doi: 10.1227/01.neu.0000430319.32247.7f. [DOI] [PubMed] [Google Scholar]
- 20.Dewan M.C., Rattani A., Baticulon R.E., et al. Operative and consultative proportions of neurosurgical disease worldwide: estimation from the surgeon perspective. https://doi.org/10.3171/2017.10.JNS17347 [e-pub ahead of print]. J Neurosurg. [DOI] [PubMed]
- 21.Asaithambi G., Chaudhry S.A., Hassan A.E., Rodriguez G.J., Suri M.F.K., Qureshi A.I. Adherence to guidelines by emergency medical services during transport of stroke patients receiving intravenous thrombolytic infusion. J Stroke Cerebrovasc Dis. 2013;22:e42–45. doi: 10.1016/j.jstrokecerebrovasdis.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Lepard J.R., Walters B.C. A bibliometric analysis of neurosurgical practice guidelines. Neurosurgery. 2020;86:605–614. doi: 10.1093/neuros/nyz240. [DOI] [PubMed] [Google Scholar]
- 23.Obungoloch J., Harper J.R., Consevage S., et al. Design of a sustainable prepolarizing magnetic resonance imaging system for infant hydrocephalus. MAGMA. 2018;31:665–676. doi: 10.1007/s10334-018-0683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chesnut R.M., Temkin N., Carney N., et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367:2471–2481. doi: 10.1056/NEJMoa1207363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melhem S., Shutter L., Kaynar A. A trial of intracranial pressure monitoring in traumatic brain injury. Crit Care. 2014;18:302. doi: 10.1186/cc13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan Q., Wu X., Sun Y., et al. Impact of intracranial pressure monitoring on mortality in patients with traumatic brain injury: a systematic review and meta-analysis. J Neurosurg. 2015;122:574–587. doi: 10.3171/2014.10.JNS1460. [DOI] [PubMed] [Google Scholar]
- 27.Hurlbert R.J., Moulton R. Why do you prescribe methylprednisolone for acute spinal cord injury? A Canadian perspective and a position statement. Can J Neurol Sci. 2002;29:236–239. doi: 10.1017/s0317167100002006. [DOI] [PubMed] [Google Scholar]
- 28.Hurlbert R.J., Hamilton M.G. Methylprednisolone for acute spinal cord injury: 5-year practice reversal. Can J Neurol Sci. 2008;35:41–45. doi: 10.1017/s031716710000754x. [DOI] [PubMed] [Google Scholar]
- 29.Lepard J.R., Corley J., Sankey E.W., et al. Training neurosurgeons in Myanmar and surrounding countries: the resident perspective. World Neurosurg. 2020;139:75–82. doi: 10.1016/j.wneu.2020.03.114. [DOI] [PubMed] [Google Scholar]
- 30.Tropeano M.P., Spaggiari R., Ileyassoff H., et al. A comparison of publication to TBI burden ratio of low- and middle-income countries versus high-income countries: how can we improve worldwide care of TBI? Neurosurg Focus. 2019;47:E5. doi: 10.3171/2019.8.FOCUS19507. [DOI] [PubMed] [Google Scholar]
- 31.Dulf D., Coman M.A., Tadevosyan A., Chikhladze N., Cebanu S., Peek-Asa C. A 3-country assessment of traumatic brain injury practices and capacity. World Neurosurg. 2021;146:e517–e526. doi: 10.1016/j.wneu.2020.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan T., Khan M. Paradigm shift: from standard-driven protocols to resource-driven guidelines for neurotrauma management in low- and middle-income countries. J Neurosci Rural Pract. 2020;11:5–6. doi: 10.1055/s-0040-1701558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubiano A.M., Carney N., Chesnut R., Puyana J.C. Global neurotrauma research challenges and opportunities. Nature. 2015;527:S193–S197. doi: 10.1038/nature16035. [DOI] [PubMed] [Google Scholar]
- 34.Kolias A.G., Rubiano A.M., Figaji A., Servadei F., Hutchinson P.J. Traumatic brain injury: global collaboration for a global challenge. Lancet Neurol. 2019;18:136–137. doi: 10.1016/S1474-4422(18)30494-0. [DOI] [PubMed] [Google Scholar]
- 35.Clark D., Joannides A., Ibrahim Abdallah O., et al. Management and outcomes following emergency surgery for traumatic brain injury—a multi-centre, international, prospective cohort study (the Global Neurotrauma Outcomes Study) Int J Surg Protoc. 2020;20:1–7. doi: 10.1016/j.isjp.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lepard J.R., Spagiari R., Corley J., et al. Differences in outcomes of mandatory motorcycle helmet legislation by country income level: a systematic review and meta-analysis. PLoS Med. 2021;18:e1003795. doi: 10.1371/journal.pmed.1003795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maas A.I.R., Menon D.K., Adelson P.D., et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 38.Whiffin C.J., Smith B.G., Esene I.N., et al. Neurosurgeons’ experiences of conducting and disseminating clinical research in low-income and middle-income countries: a reflexive thematic analysis. BMJ Open. 2021;11:e051806. doi: 10.1136/bmjopen-2021-051806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griswold D.P., Khan A.A., Chao T.E., et al. Neurosurgical randomized trials in low- and middle-income countries. Neurosurgery. 2020;87:476–483. doi: 10.1093/neuros/nyaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubiano A.M., Vera D.S., Montenegro J.H., et al. Recommendations of the Colombian consensus committee for the management of traumatic brain injury in prehospital, emergency department, surgery, and intensive care (beyond one option for treatment of traumatic brain injury: a stratified protocol [BOOTStraP]) J Neurosci Rural Pract. 2020;11:7–22. doi: 10.1055/s-0040-1701370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohan M., Layard Horsfall H., Solla D.J.F., et al. Decompressive craniotomy: an international survey of practice. Acta Neurochir (Wien) 2021;163:1415–1422. doi: 10.1007/s00701-021-04783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolias A.G., Viaroli E., Rubiano A.M., et al. The current status of decompressive craniectomy in traumatic brain injury. Curr Trauma Rep. 2018;4:326–332. doi: 10.1007/s40719-018-0147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volovici V., Ercole A., Citerio G., et al. Variation in guideline implementation and adherence regarding severe traumatic brain injury treatment: a CENTER-TBI survey study in Europe. World Neurosurg. 2019;125:e515–e520. doi: 10.1016/j.wneu.2019.01.116. [DOI] [PubMed] [Google Scholar]