Abstract
Objective
The purpose of this study was to compare the levels of 8-oxoG and 8-oxodG in urine of patients with cervical carcinoma and healthy women to evaluate their influences on cervical carcinoma.
Methods
In this study, urine samples were collected from 70 patients with cervical carcinoma, 24 patients with one-year follow-up, and 100 healthy women. The contents of 8-oxodG and 8-oxoG in urine were assayed by ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS).
Results
The levels of 8-oxoG and 8-oxodG were higher in patients with cervical carcinoma (P < 0.000), while AUC of 8-oxoG and 8-oxodG was higher than 0.7. Specifically, the high-risk HPV (HR-HPV) positive group had higher 8-oxoG levels (P < 0.000), but there was no difference in 8-oxodG levels. Yet, 8-oxoG level was associated with lymphatic metastasis, lymph-vascular space infiltration (LVSI) and stromal infiltration, while 8-oxodG level was affected by the differentiation degree and stromal infiltration. According to statistics, the distinct cut-off index of lymphatic metastasis was 7.282 nmol/mmol creatinine. After operation, the concentrations of 8-oxoG and 8-oxodG dropped significantly (8-oxoG P < 0.000, 8-oxodG P = 0.004). Except for chemotherapy group, the urinary 8-oxoG dose of all treatment groups and 8-oxodG dose of chemo-radiotherapy group declined obviously.
Conclusions
8-oxoG may be a potential biomarker for cervical carcinoma.
Keywords: 8-oxoG, Oxidative damage, Cervical carcinoma, UHPLC- MS/MS, Urine, Biomarker
8-oxoG, oxidative damage, cervical carcinoma, UHPLC- MS/MS, urine, biomarker.
1. Introduction
Cervical carcinoma is one of the main cancers endangering women's health. Although the introduction and application of human papillomavirus vaccine (HPV) has reduced global burden of this disease, it is still a major public health problem in many countries and regions, especially in developing countries with low vaccine coverage [1]. In China, the incidence rate of cervical carcinoma is still gradually increasing, and patients tend to be younger. The diagnostic criteria of cervical carcinoma mainly rely on biopsy (colposcope), which is an invasive examination. Early diagnosis and treatment of cervical carcinoma is an important consideration for treatment effect and prognosis. Therefore, it is a new and effective biomarker for the treatment of cervical carcinoma.
Reactive oxygen species (ROS) are chemical reaction molecules that are produced in cell metabolism and cell exposure to harmful environments. Excessive ROS may damage important cellular components and lead to defects in DNA repair mechanism and antioxidant defense system, thus changing cell homeostasis [2]. ROS have influences on cell proliferation, the imbalance of cell growth and tumorigenesis, which also play important roles in growth factors, mitosis, and development of cancers [3, 4, 5]. However, nucleic acids are often transformed into various modified bases when exposed to oxygen free radicals. At the same time, more than 20 different oxidation products of purine and pyrimidine in nucleic acid are assayed [6]. Among these oxidation products, there are 2 kinds of guanosine, including 2′-deoxy-7,8-dihydro-8-oxo-(8-oxodG) and 8-oxo-7,8-dihydroguanosine (8-oxoG). 8-oxodG is the oxidation product of guanine nucleotides in DNA, while 8-oxoG is the product of RNA. The oxidation products of these two guanine nucleotides have essential parts in gene translation and intracellular environmental stability, which is related to mutation potential [7]. During transcription and translation, 8-oxoG and 8-oxodG can pair with adenine or cytosine, resulting in abnormal gene expression [8].
8-oxoG and 8-oxodG are excreted into the urine without change, and their concentrations are not affected by circadian rhythm. Therefore, this study used it as a biomarker of oxidative stress in a non-invasive manner [9, 10]. A variety of cancer studies have shown that oxidative stress was involved in the occurrence and development of tumors, in which compounds could be regarded as tumor biomarkers [11, 12, 13]. So far, there were few studies on the effects of 8-oxoG and 8-oxodG on cervical carcinoma. In this study, we compared the levels of 8-oxoG and 8-oxodG in the urine of patients with cervical carcinoma and healthy women to evaluate their effects on cervical carcinoma.
2. Materials and Methods
Urine sample collection: Our initial plan was to study 100 patients with cervical carcinoma and 100 healthy women. However, 23 patients were rejected and 36 patients failed (unable to collect before surgery). Finally, we collected urine samples from 70 patients with cervical carcinoma, and did not exclude any healthy women. According to requirements of the Research Ethics Committee (approval registration No.: 2020-zz-020), urine samples were collected with the informed consent of the First Affiliated Hospital of Wenzhou Medical University. In the cervical carcinoma group, urine samples were collected from women aged 26–70 who underwent radical hysterectomy from 2013 to 2016. All surgical specimens were confirmed by pathology. Urine samples were collected from all patients before operation, and 24 cases were followed up one year after operation. The control group was healthy women (23–75 years old) who underwent physical examination in the physical examination department of our hospital. Furthermore, within 3 months before samples were collected, health standards included no malignant tumor, no hypertension, no diabetes, no systemic diseases, no smoking, no excessive drinking and no hormone therapy. And the following patients with cervical carcinoma should be excluded, including patients with other malignant tumors of reproductive organs, preoperative history of radiotherapy or chemotherapy, and taking hormone drugs 3 months before sample collection. Besides, smokers and drunkards were excluded from this study. Smokers were defined as people who buy tobacco at least once a year, while drunkards are defined as people who drink more than one bottle of beer a day. The urine samples were immediately frozen at -20 °C to maintain its stability.
Urine sample preparation: This study used the stable isotope labeled 8-oxodG (8-oxodG-IS) and 8-oxoG (8-oxoG-IS) as internal standards (Fisher Scientific (USA)). In short, frozen urine was cultured at 37 °C for 5 min, thawed at 7500 g and centrifuged at 4 °C for 5 min. Our staff mixed 200 μL supernatant and 200 μL working solution (including 70% methanol and 30% 10 mm ammonium acetate (PH = 3.7)), and added 10 μL 480 pg/μL 8-oxodG-IS and 8-oxoG-IS to the mixing system. Next, under the condition of vortex motion for 2 min, our personnel cultured the mixture at 37 °C for 10 min and centrifuged at 12000 g at 4 °C for 15 min. Finally, 8-oxodG and 8-oxoG in the supernatant were analyzed by UHPLC- MS/MS or stored at -80 °C.
The amount of creatinine in each urine sample was quantified by a chemical analyzer (BECKMAN COULTER AU5800 SERIES, USA) to relatively standardize the levels of 8-oxodG and 8-oxoG, which was due to changes in urine volume, especially changes in glomerular function. Frozen urine samples were thawed at 7500 g at 4 °C and centrifuged for 5 min, and the supernatant was detected by BECKMAN analyzer.
Ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC- MS/MS): The concentrations of oxidized guanine nucleoside 8-oxodG and 8-oxoG in urine were determined by improved UHPLC and MS. The conditions and optimization parameters of UHPLC were described in detail elsewhere [14]. In short, we analyzed all samples by using UHPLC (Agilent 1290 Infinity) and C18 column (Agilent, 3 μm, 3.00 × 100 mm) at a flow rate of 0.4 mL/min at 35 °C. Mobile phase A was 0.1% formic acid mixed with 5 mm ammonium acetate, and mobile phase B was methanol mixed with 0.1% formic acid. In order to reduce liquid loss, we maintained a constant temperature (4 °C) in the sample chamber and abandoned early and late elution components as quality control.
Agilent triple quadrupole mass spectrometer (Agilent, 6490, USA) was equipped with jet stream ESI ionization source and iFunnel technology (120 V high voltage RF, 50 V low voltage RF, 30 psi nitrogen pressure atomizer and 2000 V ESI pin voltage). Multiple reaction mode (MRM) was used for detection and quantitative analysis, and the data collection mode was set to positive ion detection. Moreover, the drying temperature and flow rate of gas were measured at 200 °C and 16 L/min, while those of sheath gas were 400 °C and 12 L/min respectively.
Statistical analysis: Repeat each step three times. All data were analyzed by SPSS software (SPSS Statistics 19.0 IBM Co., Armonk, NY, USA). All data were expressed as mean ± standard deviation. Independent sample T-test and paired sample T-test were used for the comparison between two groups, while one-way ANOVA was used for the analysis among three groups, P < 0.05 was statistically significant. When the three components were different, LSD test was applied for further multiple comparison.
3. Results
-
1.
The levels of 8-oxoG and 8-oxodG were higher in patients with cervical carcinoma
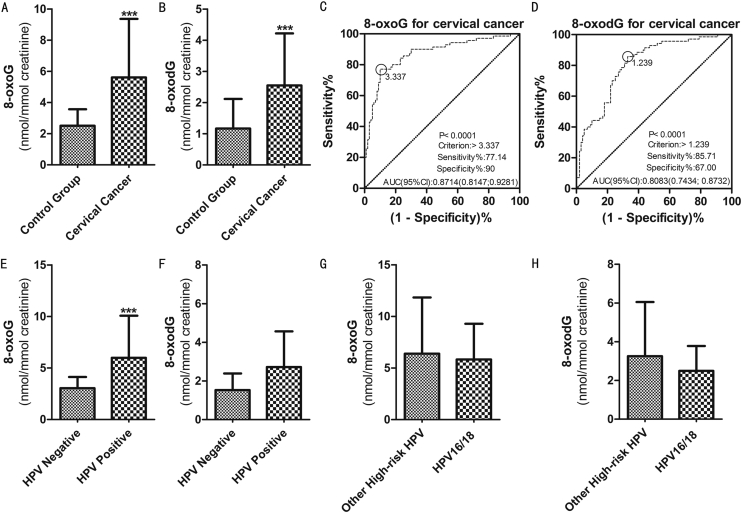
There was no difference in age between patients with cervical carcinoma and control group (P = 0.07). We measured the urinary doses of 8-oxoG and 8-oxodG in both groups, which helped to determine that the concentration of 8-oxoG was higher than that of 8-oxodG. In the control group and cervical carcinoma group, 8-oxoG values were 2.507 ± 1.060 and 5.610 ± 3.753 nmol/mmol creatinine respectively, while 8-oxodG values were 1.168 ± .950 and 2.548 ± 1.673 nmol/mmol creatinine respectively. The levels of 8-oxoG and 8-oxodG in patients with cervical carcinoma were higher than those in the healthy group (P < 0.000) (Figure 1AB). This study analyzed the potential role of 8-oxoG and 8-oxodG in the diagnosis of cervical carcinoma by ROC curve. The significant statistical index of area under curve (AUC) was 8-oxodG 0.8083 (0.7434; 0.8732) (p < 0.001), while 8-oxoG 0.8714 (0.8147; 0.9281) (p < 0.001). The AUC of 8-oxoG and 8-oxodG were higher than 0.7; the sensitivity and specificity were shown in Figure 1CD; the sensitivity of 8-oxoG was 77.14%, the specificity was 90%, and the critical value was 3.337; the sensitivity of 8-oxodG was 85.71%, the specificity was 67%, and the cut-off value was 1.239.
-
2.
The level of 8-oxoG increased in high-risk HPV positive group
Figure 1.
The concentrations of 8-oxodG and 8-oxoG in the study groups by UHPLC-MS/MS. (A) Comparison of 8-oxoG between control group and cervical carcinoma group. (B) Comparison of 8-oxodG between control group and cervical carcinoma group. (C) ROC curve of 8-oxoG in cervical carcinoma group and control group. (D) ROC curve of 8-oxodG in cervical carcinoma group and control group. (E) Comparison of 8-oxoG between HPV negative group and HPV positive group. (F) Comparison of 8-oxodG between HPV negative group and HPV positive group. (G) Comparison of 8-oxoG between HPV 16/18 group and other high-risk HPV groups. (H) Comparison of 8-oxodG between HPV 16/18 group and other high-risk HPV groups. Data were expressed as mean ± SD. ∗P < 0.05, ∗∗∗P < 0.000 were considered to be statistically significant.
Among patients with cervical carcinoma, 50 patients were tested for HPV. Some did not receive HPV testing due to vaginal bleeding, and some had pathological diagnosis before operation. Among these 50 patients, 41 were infected with high-risk HPV, and 9 were not. In the high-risk HPV positive group, the level of 8-oxoG was higher (as shown in Figure 1E, P < 0.000), while there was no difference in the level of 8-oxodG (as shown in Figure 1F, P = 0.067). Subsequently, we divided these 41 patients into two branches according to HPV genotype, including HPV 16/18 group and high-risk HPV group. There were 29 cases in HPV 16/18 group and 12 cases in high-risk group. According to statistics, there was little difference between 8-oxoG and 8-oxodG (as shown in Figure 1GH).
-
3.
8-oxoG level was associated with lymphatic metastasis, lymph-vascular space infiltration (LVSI) and stromal infiltration, while 8-oxodG level was affected by the differentiation degree and stromal infiltration.
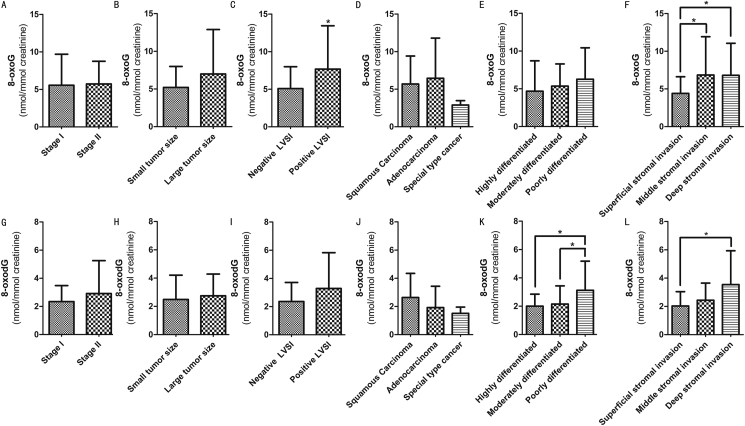
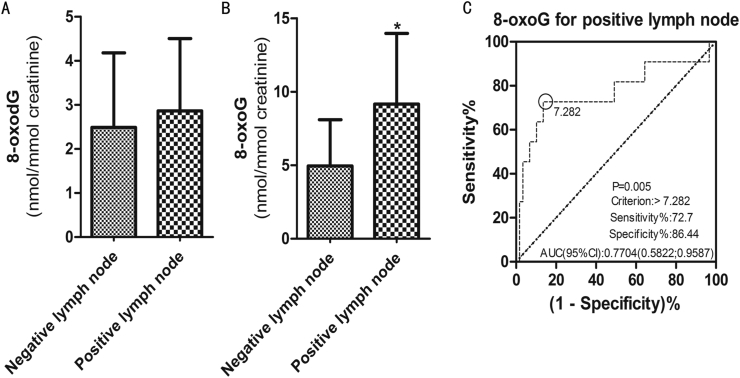
In this study, all patients with cervical carcinoma who underwent the radical hysterectomy were in the early stage (stage I-II), including 45 cases in stage I and 25 cases in stage II. There was no difference between 8-oxoG and 8-oxodG in these two stages. According to the postoperative pathology, we divided patients into several groups according to tumor size, lymphatic infiltration, histopathological type, differentiation degree, stromal infiltration and lymphatic metastasis. In addition, 63 patients were histologically diagnosed as squamous cell carcinoma, 4 as adenocarcinoma and 3 as special tumor types, including adenosarcoma, neuroendocrine tumor and carcinosarcoma. The demarcation line of tumor size was 4 cm. It turned out that there was no difference between two molecules in histopathological type and tumor size. The worse the differentiation degree, the higher the 8-oxoG and 8-oxodG values (P = 0.034 in 8-oxodG). As shown in Figure 2, deep stromal infiltration caused the increased levels of 8-oxoG and 8-oxodG (P = 0.024 & P = 0.004). As shown in Figure 3B, the dose of 8-oxoG was higher in some patients with lymph-vascular space infiltration and lymphatic metastasis (P = 0.021 & P = 0.016). We also analyzed the potential diagnostic effect of 8-oxoG on cervical carcinoma by ROC curve, especially in patients with lymphatic metastasis. The significant statistical index of cut-off value was 7.282 nmol/mmol creatinine (Figure 3C).
-
4.
The concentrations of 8-oxoG and 8-oxodG decreased after operation, which was not affected by postoperative treatment.
Figure 2.
Different clinical characteristics of 8-oxoG and 8-oxodG in cervical carcinoma group. (A) Comparison of 8-oxoG between Stage I and Stage II. (B) Comparison of 8-oxoG between small tumor group and large tumor group. (C) 8-oxoG of lymph-vascular space infiltration between negative group and positive group. (D) Expression of 8-oxoG in different histopathological types. (E) Expression of 8-oxoG in different differentiation degrees. (F) Expression of 8-oxoG in different stromal infiltration groups. (G) Comparison of 8-oxodG between Stage I and Stage II. (H) Comparison of 8-oxodG between small tumor group and large tumor group. (I) 8-oxodG of lymph-vascular space infiltration between negative group and positive group. (J) Expression of 8-oxodG in different histopathological types. (K) Expression of 8-oxodG in different differentiation degrees. (L) Expression of 8-oxodG in different stromal infiltration groups. Data were expressed as mean ± SD. ∗P < 0.05 was considered to be statistically significant.
Figure 3.
Effects of 8-oxoG and 8-oxodG on lymphatic metastasis in cervical carcinoma group. (A) Comparison of 8-oxodG on lymphatic metastasis between negative group and positive group. (B) Comparison of 8-oxoG on lymphatic metastasis between negative group and positive group. (C) ROC curve of 8-oxoG on lymphatic metastasis between negative group and positive group. The cut-off value was 7.282. Data were expressed as mean ± SD. ∗P < 0.05 was considered to be statistically significant.
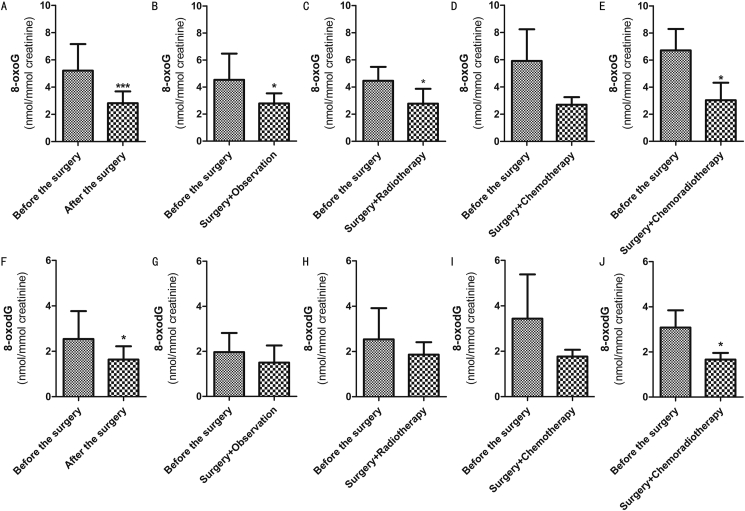
We collected urine samples from patients with cervical carcinoma one year after operation and analyzed the changes of 8-oxoG and 8-oxodG levels after operation. Besides, we obtained a total of 24 samples diagnosed with squamous cell carcinoma, which had no local or distant recurrence. The preoperative 8-oxoG value was 5.206 ± 1.948 nmol/mmol creatinine, which decreased to 2.820 ± .864 nmol/mmol creatinine one year after operation. The preoperative 8-oxodG value was 2.539 ± 1.231 nmol/mmol creatinine, which reduced to 1.638 ± .583 nmol/mmol creatinine after operation. All in all, the concentrations of 8-oxoG and 8-oxodG dropped significantly after operation (8-oxoG P < 0.000, 8-oxodG P = 0.004), as shown in Figure 4AF. Since chemo-radiotherapy helped to improve the level of oxidative stress, we continued to compare the effects of different postoperative treatments on the levels of 8-oxoG and 8-oxodG. According to postoperative histopathology and NCCN guidelines, it was recommended to closely observe 11 patients after operation, 4 patients received radiotherapy, 5 patients took chemo-radiotherapy simultaneously, and 4 patients received cisplatin chemotherapy. The test revealed that the levels of 8-oxoG and 8-oxodG in all treatment groups showed a downward trend. Specifically, the level of 8-oxoG declined significantly in all groups except the chemotherapy group, but the level of 8-oxodG decreased markedly only in the chemo-radiotherapy group (Figure 4). This situation showed that in patients with good postoperative control (no recurrence or metastasis), the values of 8-oxoG and 8-oxodG remained unchanged and were not affected by postoperative treatment.
Figure 4.
Changes of 8-oxoG and 8-oxodG in cervical carcinoma group according to different postoperative treatment methods. (A) 8-oxoG in 24 patients. (B) 8-oxoG in observation group. (C) 8-oxoG in radiotherapy group. (D) 8-oxoG in chemotherapy group. (E) 8-oxoG in chemo-radiotherapy group. (F) 8-oxodG in 24 patients. (G) 8-oxodG in observation group. (H) 8-oxodG in radiotherapy group. (I) 8-oxodG in chemotherapy group. (J) 8-oxodG in chemo-radiotherapy group. Data were expressed as mean ± SD. ∗P < 0.05, ∗∗∗P < 0.000 were considered to be statistically significant.
4. Discussion
Although cervical carcinoma is considered to be the most preventable malignant tumor among all cancers, the number of female patients with cervical carcinoma increases significantly every year in China. Thus, new and efficient biomarkers and better medical strategies are expected to reduce the mortality of cervical carcinoma.
Studies have shown that oxidative stress played a crucial role in the occurrence and development of several human cancers [2]. 8-oxoG and 8-oxodG were widely regarded as markers of oxidative stress. However, the levels of 8-oxoG and 8-oxodG in patients with cervical carcinoma were significantly higher than those in healthy women. Recent studies have shown that 8-oxodG increased significantly in different stages from SILs (squamous intraepithelial lesion, divided into low-grade and high-grade) to cervical carcinoma, which implied that 8-oxodG had an essential part in the occurrence and development of cervical carcinoma [15, 16, 17]. This may be a mechanism to promote tumorigenesis, or it may be a result of excessive tumor release. Previous studies have shown that 8-oxoG and 8-oxodG tended to pair with adenine or cytosine during transcription and translation, leading to GC:TA translocation. Translocation of key proteins (including cell proliferation, apoptosis, migration, angiogenesis and resistance to treatment) may induce changes in their biological characteristics and promote the occurrence and development of carcinogenesis [8]. Once the tumor is produced, oxidative stress cascades due to the release of cancer cells by oxidants themselves, resulting in a vicious circle [18]. For example, in order to enable cancer cells to survive in normal and starvation environments, autophagy of HeLa cells stimulates ROS production through NADPH oxidase, which helps to activate JAK2/STAT3 pathway and up-regulate the transcription of growth factors [19].
Base excision repair is one of the most important DNA repair measures, which has an important impact on maintaining the integrity of the genome structure. In mammalian cells, OGG1 detection and removal of 8-oxodG is the rate-limiting step of base excision repair [20]. Over-expression of OGG1 caused accelerated clearance of 8-oxodG in HeLa cells [21]. Targeting OGG1 inhibitor TH5487 can inhibit the activity of OGG1 and increase the concentration of 8-oxodG [22]. In previous studies on OGG1 knockout mice, 8-oxodG levels increased [23, 24, 25]. With the extension of the observation period, 8-oxodG was more prone to pulmonary adenomatosis or carcinoma [25]. MUTYH cooperated with OGG1 to promote its function to eliminate 8-oxodG in vivo. And more MUTYH rs3219489 C alleles increased the risk of cervical carcinoma [26]. The dysfunction of DNA repair combined with damaged cellular proteins, resulting in increased proliferation and genome instability. Therefore, host cells tended to accumulate more and more damaged genetic materials that could not be recovered. In addition, previous studies have shown that these wrong genetic information may appear more frequently in RNA than in DNA, including two reasons. One was that RNA molecules could not protect hydrogen bonds due to single strand, and the other was that RNA excision repair system could not operate [27, 28, 29]. In this study, the concentration of 8-oxoG was usually higher than that of 8-oxodG, and 8-oxoG presented significant differences in all groups. According to ROC curve analysis, the AUC of 8-oxoG and 8-oxodG was more than 0.7, and the AUC of 8-oxoG was even greater than 0.85. Thus, 8-oxoG may be a potential biomarker for cervical carcinoma.
It is generally believed that the high-risk HPV infection on the cervix may last for many years or decades, which is an important determinant of the development of cervical carcinoma [30]. The contradiction between high infection on HPV and low incidence rate of cervical carcinoma in women suggests that the occurrence of malignant tumors requires additional environmental factors and alterations of somatic cell gene. HPV infection can lead to the genital meatus inflammation, which can easily up-regulate the production and release of ROS [31]. Because ROS can damage DNA and destroy the double strand of DNA, it is considered to be a basic procedure of viral DNA integration into host genome, resulting in the over-expression of oncogenes including E6 and E7. E6 and E7 have high affinity for p53 and pRB and can enter S-phase block without blocking G1 phase block, resulting in efficient immortalization of keratinocytes [32]. Moreover, artificially induced chronic oxidative stress in human cervical carcinoma cells can destroy oxidized DNA and increase the integration rate of HPV [33], which means that oxidative stress is a stimulant of HPV. Studies have shown that E6/E7 mRNA expression was rare in transient infection, but over-expressed in persistent infection [34]. In our study, 8-oxoG in HPV positive group was significantly increased compared with HPV negative group, indicating that there was a stronger oxidative stress in tumor cells of HPV patients.
At present, it is well known that the main cause of death of cervical carcinoma is its recurrence and metastasis. The positive feature of pelvic lymph node is one of the three high risk factors for recurrence and metastasis. For patients with positive pelvic lymph nodes in cervical carcinoma, the 5-year survival rate after radical hysterectomy was 64%–68.2%, which will be affected by the location and number of positive lymph nodes [35]. Patients with positive lymph-vascular space infiltration and deep 1/3 stromal infiltration (two intermediate risk factors) have higher recurrence and mortality [36]. However, in this study, 8-oxoG was associated with lymphatic metastasis, lymph-vascular space infiltration and stromal infiltration, while 8-oxodG was relevant to differentiation degree and stromal infiltration. ROC curve was used to determine the critical value of preoperative metastasis prediction. ROC curve analysis revealed that the AUC of 8-oxoG in positive pelvic lymph nodes was greater than 0.7, which could be used as a potential carcinoma biomarker to predict prognosis. More importantly, the concentrations of 8-oxoG and 8-oxodG decreased after operation. Chemo-radiotherapy was the main adjutant treatment for cervical carcinoma after operation. Previous studies have shown that radiotherapy and chemotherapy could increase the level of oxidative stress in vivo [37, 38]. However, we found that the biomarker of oxidative stress one year after operation was not affected by postoperative treatment, which made it a potential biomarker to evaluate the treatment response of cervical carcinoma.
So far, few studies have explored the connection between the oxidation products of guanine nucleotide and patients with cervical carcinoma, let alone 8-oxoG in cervical carcinoma patients. In this study, the levels of 8-oxoG and 8-oxodG in patients with cervical carcinoma were higher and lower after operation, and were not affected by postoperative treatment. 8-oxoG was associated with lymphatic metastasis, lymph-vascular space infiltration and stromal infiltration, while 8-oxodG was relevant to differentiation degree and stromal infiltration. After a literature review of recently published data and works, we concluded that through LC-MS/MS, the reference values of 8-oxodG in healthy people were 0.81–3.13 nmol/mmol creatinine and 1.36–4.61 nmol/mmol creatinine of 8-oxoG [39]. The values of the control group were consistent with that, and the levels of 8-oxoG in patients with cervical carcinoma exceeded this range. The concentration of 8-oxoG in the general group was higher than that of 8-oxodG, and there was significant difference in the concentration of 8-oxoG in comparison of multiple groups. Thus, 8-oxoG may be more suitable as a potential biomarker for diagnosis, prognosis, and treatment response of cervical carcinoma. However, this study has some limitations, that is, we do not conduct external validation, and our sample is too small to conduct internal validation. This study is a clinical observation, not a mechanism study. In summary, in clinical treatment, a large number of studies are required to improve the effects of 8-oxoG as a biomarker of cervical carcinoma. It is suggested to further study the exact mechanism between guanine base oxidation products and HPV infection in patients with cervical carcinoma, expand the sample size and center scale, and increase more collection time points to evaluate the potential value of 8-oxoG as a biomarker of cervical carcinoma in clinical treatment, so as to be used for risk prediction, treatment strategy selection and response evaluation.
Declarations
Author contribution statement
Rong-Rong Lin: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Xiang-Yu Li: Performed the experiments; Analyzed and interpreted the data.
Qing-Hua Weng: Performed the experiments.
Xing-Xing Zhou: Contributed reagents, materials, analysis tools or data.
Fei-Yun Zheng; Jian-Ping Cai: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank the staff of Beijing Hospital and Beijing Institute of Geriatrics of the Ministry of Health for their selfless help.
Contributor Information
Fei-Yun Zheng, Email: zfy5710@163.com.
Jian-Ping Cai, Email: caijp61@vip.sina.com.
References
- 1.Arbyn M., Weiderpass E., Bruni L., et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global Health. 2020;8:e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kruk J., Aboul-Enein H.Y. Reactive oxygen and nitrogen species in carcinogenesis: implications of oxidative stress on the progression and development of several cancer types. Mini Rev. Med. Chem. 2017;17:904–919. doi: 10.2174/1389557517666170228115324. [DOI] [PubMed] [Google Scholar]
- 3.Reczek C.R., Chandel N.S. ROS promotes cancer cell survival through calcium signaling. Cancer Cell. 2018;33:949–951. doi: 10.1016/j.ccell.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Ahn J.H., Cho M.G., Sohn S., Lee J.H. Inhibition of PP2A activity by H2O2 during mitosis disrupts nuclear envelope reassembly and alters nuclear shape. Exp. Mol. Med. 2019;51:64. doi: 10.1038/s12276-019-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basu Baul T.S., Longkumer I., Duthie A., et al. Triphenylstannyl((arylimino)methyl)benzoates with selective potency that induce G1 and G2/M cell cycle arrest and trigger apoptosis via ROS in human cervical cancer cells. Dalton Trans. 2018;47:1993–2008. doi: 10.1039/c7dt04037g. [DOI] [PubMed] [Google Scholar]
- 6.Barciszewski J., Barciszewska M.Z., Siboska G., et al. Some unusual nucleic acid bases are products of hydroxyl radical oxidation of DNA and RNA. Mol. Biol. Rep. 1999;26:231–238. doi: 10.1023/a:1007058602594. [DOI] [PubMed] [Google Scholar]
- 7.Sekiguchi M. Molecular devices for high fidelity of DNA replication and gene expression. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2006;82:278–296. doi: 10.2183/pjab.82.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maki H. Origins of spontaneous mutations: specificity and directionality of base-substitution, frameshift, and sequence-substitution mutageneses. Annu. Rev. Genet. 2002;36:279–303. doi: 10.1146/annurev.genet.36.042602.094806. [DOI] [PubMed] [Google Scholar]
- 9.Fraga C.G., Shigenaga M.K., Park J.W., et al. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc. Natl. Acad. Sci. U. S. A. 1990;87:4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olinski R., Rozalski R., Gackowski D., et al. Urinary measurement of 8-OxodG, 8-OxoGua, and 5HMUra: a noninvasive assessment of oxidative damage to DNA. Antioxidants Redox Signal. 2006;8:1011–1019. doi: 10.1089/ars.2006.8.1011. [DOI] [PubMed] [Google Scholar]
- 11.Zitka O., Krizkova S., Krejcova L., et al. Microfluidic tool based on the antibody-modified paramagnetic particles for detection of 8-hydroxy-2'-deoxyguanosine in urine of prostate cancer patients. Electrophoresis. 2011;32:3207–3220. doi: 10.1002/elps.201100430. [DOI] [PubMed] [Google Scholar]
- 12.Loft S., Olsen A., Moller P., et al. Association between 8-oxo-7,8-dihydro-2'-deoxyguanosine excretion and risk of postmenopausal breast cancer: nested case-control study. Cancer Epidemiol. Biomarkers Prev. 2013;22:1289–1296. doi: 10.1158/1055-9965.EPI-13-0229. [DOI] [PubMed] [Google Scholar]
- 13.Brancato B., Munnia A., Cellai F., et al. 8-Oxo-7,8-dihydro-2'-deoxyguanosine and other lesions along the coding strand of the exon 5 of the tumour suppressor gene P53 in a breast cancer case-control study. DNA Res. 2016;23:395–402. doi: 10.1093/dnares/dsw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao Y.H., Xu L.N., Weng Q.H., et al. The ratio of plasma and urinary 8-oxo-Gsn could Be a novel evaluation index for patients with chronic kidney disease. Oxid. Med. Cell. Longev. 2018;2018:4237812. doi: 10.1155/2018/4237812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sgambato A., Zannoni G.F., Faraglia B., et al. Decreased expression of the CDK inhibitor p27Kip1 and increased oxidative DNA damage in the multistep process of cervical carcinogenesis. Gynecol. Oncol. 2004;92:776–783. doi: 10.1016/j.ygyno.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Jelic M., Mandic A., Kladar N., et al. Lipid peroxidation, antioxidative defense and level of 8-hydroxy-2-deoxyguanosine in cervical cancer patients. J. Med. Biochem. 2018;37:336–345. doi: 10.1515/jomb-2017-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zahra K., Patel S., Dey T., et al. A study of oxidative stress in cervical cancer- an institutional study. Biochem. Biophys. Rep. 2021;25:100881. doi: 10.1016/j.bbrep.2020.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monkkonen T., Debnath J. Inflammatory signaling cascades and autophagy in cancer. Autophagy. 2018;14:190–198. doi: 10.1080/15548627.2017.1345412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon S., Woo S.U., Kang J.H., et al. STAT3 transcriptional factor activated by reactive oxygen species induces IL6 in starvation-induced autophagy of cancer cells. Autophagy. 2010;6:1125–1138. doi: 10.4161/auto.6.8.13547. [DOI] [PubMed] [Google Scholar]
- 20.Kuznetsov N.A., Koval V.V., Zharkov D.O., et al. Kinetics of substrate recognition and cleavage by human 8-oxoguanine-DNA glycosylase. Nucleic Acids Res. 2005;33:3919–3931. doi: 10.1093/nar/gki694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebraud E., Pinna G., Siberchicot C., et al. Chromatin recruitment of OGG1 requires cohesin and mediator and is essential for efficient 8-oxoG removal. Nucleic Acids Res. 2020;48:9082–9097. doi: 10.1093/nar/gkaa611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Visnes T., Cazares-Korner A., Hao W., et al. Small-molecule inhibitor of OGG1 suppresses proinflammatory gene expression and inflammation. Science. 2018;362:834–839. doi: 10.1126/science.aar8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minowa O., Arai T., Hirano M., et al. Mmh/Ogg1 gene inactivation results in accumulation of 8-hydroxyguanine in mice. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4156–4161. doi: 10.1073/pnas.050404497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klungland A., Rosewell I., Hollenbach S., et al. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakumi K., Tominaga Y., Furuichi M., et al. Ogg1 knockout-associated lung tumorigenesis and its suppression by Mth1 gene disruption. Cancer Res. 2003;63:902–905. [PubMed] [Google Scholar]
- 26.Chen H., Wang H., Liu J., et al. Association of the MUTYH Gln324His (CAG/CAC) variant with cervical carcinoma and HR-HPV infection in a Chinese population. Medicine (Baltim.) 2019;98 doi: 10.1097/MD.0000000000015359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poulsen H.E., Specht E., Broedbaek K., et al. RNA modifications by oxidation: a novel disease mechanism? Free Radic. Biol. Med. 2012;52:1353–1361. doi: 10.1016/j.freeradbiomed.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Nunomura A., Tamaoki T., Tanaka K., et al. Intraneuronal amyloid beta accumulation and oxidative damage to nucleic acids in Alzheimer disease. Neurobiol. Dis. 2010;37:731–737. doi: 10.1016/j.nbd.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong Q., Lin C.L. Oxidative damage to RNA: mechanisms, consequences, and diseases. Cell. Mol. Life Sci. 2010;67:1817–1829. doi: 10.1007/s00018-010-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Sanjose S., Quint W.G., Alemany L., et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 31.Ohnishi S., Ma N., Thanan R., et al. DNA damage in inflammation-related carcinogenesis and cancer stem cells. Oxid. Med. Cell. Longev. 2013;2013:387014. doi: 10.1155/2013/387014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szymonowicz K.A., Chen J. Biological and clinical aspects of HPV-related cancers. Cancer Biol. Med. 2020;17:864–878. doi: 10.20892/j.issn.2095-3941.2020.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Wongworawat Y., Filippova M., Williams V.M., et al. Chronic oxidative stress increases the integration frequency of foreign DNA and human papillomavirus 16 in human keratinocytes. Am J Cancer Res. 2016;6:764–780. [PMC free article] [PubMed] [Google Scholar]
- 34.Burd E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003;16:1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakuragi N. Up-to-date management of lymph node metastasis and the role of tailored lymphadenectomy in cervical cancer. Int. J. Clin. Oncol. 2007;12:165–175. doi: 10.1007/s10147-007-0661-2. [DOI] [PubMed] [Google Scholar]
- 36.Cao L., Wen H., Feng Z., et al. Role of adjuvant therapy after radical hysterectomy in intermediate-risk, early-stage cervical cancer. Int. J. Gynecol. Cancer. 2021;31:52–58. doi: 10.1136/ijgc-2020-001974. [DOI] [PubMed] [Google Scholar]
- 37.Kim W., Lee S., Seo D., et al. Cellular stress responses in radiotherapy. Cells. 2019;8 doi: 10.3390/cells8091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cauli O. Oxidative stress and cognitive alterations induced by cancer chemotherapy drugs: a scoping review. Antioxidants (Basel) 2021;10 doi: 10.3390/antiox10071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chao M.R., Evans M.D., Hu C.W., et al. Biomarkers of nucleic acid oxidation - a summary state-of-the-art. Redox Biol. 2021;42:101872. doi: 10.1016/j.redox.2021.101872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.