Abstract
Scar formation can lead to glaucoma filtration surgery (GFS) failure, wherein transforming growth factor (TGF)-β is the core regulator. To reducing scar formation, this paper presents our study on the design of hydrogels to deactivate TGF-β1. We hypothesized that excess TGF-β1 can be removed from aqueous humor through the addition of oxidized hyaluronic acid (O-HA) hydrogels that are seeded with decorin (O-HA + D). Immunohistochemistry and enzyme-linked immunosorbent assay (ELISA) were performed to demonstrate the adsorption properties of O-HA + D hydrogel, thus reducing the TGF-β1 concentration in aqueous humor. In the light that collagen contraction in human Tenon's capsule fibroblasts (HTFs) and the angiogenesis of human umbilical vein endothelial cells (HUVECs) can be activated by TGF-β1 and β2, we performed the quantitative analysis of polymerase chain reaction to determine the effect of O-HA + D on the type I collagen, fibronectin, and angiogenesis. Our results illustrate that O-HA + D can inhibit the increase of α-SMA expression in HTF induced by TGF-β1 and that O-HA + D can inhibit the production of collagen I and fibronectin in HTF treated with TGF-β1. Furthermore, we performed in vivo studies by employing a rabbit model, where rabbits were treated with hydrogels post GFS. Our results demonstrate that, as compared with other groups, the rabbits treated with O-HA + D had the greatest reduction in inflammatory cells with reduced level of collagen in wounds. Taken together, the present study paves the way toward the treatment of post-glaucoma fibrosis following surgery.
Keywords: Glaucoma filtration surgery, TGF-β1, Decorin, anti-scarring therapy, Hydrogel
Highlights
-
•
Scar formation can lead to glaucoma filtration surgery (GFS) failure, where the transforming growth factor (TGF)-β is the core regulator.
-
•
Excess TGF-β1 can be absorbed and deactivated from aqueous humor by using hyaluronic acid (HA) hydrogels seeded with decorin (termed as O-HA + D).
-
•
Their anti-scarring capability with the injected O-HA + D hydrogel was significantly improved in both the in vitro and in vivo studies.
1. Introduction
Glaucoma is an eye disease characterized by increased intraocular pressure, optic nerve damage, and vision loss. It is the second most common blindness disease in the world [1]. At present, the most common treatment for glaucoma is trabeculectomy to reduce intraocular pressure and delay nerve damage [2]. The proliferation of fibroblasts at the filtration mouth, as well as fibrosis of the conjunctiva and sclera, are the main reasons underlying trabeculectomy failure [3]; the failure rate was reported to be 15%–25% [4].
TGF-β is a core regulator of factors involved in the formation of bleb scarring, and elevated levels of TGF-β1 and β2 have been detected in the filtering bleb of patients after glaucoma filtration surgery (GFS) [[5], [6], [7], [8], [9]]. TGF-β induces a variety of inflammatory cytokines to promote fibrosis, thereby stimulating the formation of new blood vessels [10]. TGF-β binds to the TGF-β receptor on the surface of Tenon's capsule fibroblasts and has strong chemotaxis on inflammatory cells, thus enhancing the inflammatory response. Also, the bound TGF-β can promote fibroblast proliferation and activation, enhance the expression of connective tissue growth factor (CTGF), increase the expression of type I and type II collagen in the extracellular matrix (ECM) [11], inhibit ECM degradation, and promotes its deposition [12]. Upon the GFS, fibrous vascular connective tissue is formed for healing at the surgery site, meanwhile resulting in the scarring of the filtering bleb and degrading the quality of surgery [[13], [14]].
For preventing postsurgical scarring, current therapeutic strategies or methods mainly focus on targeting inflammation and TGF-β downstream genes. Among them, the common methods include those directly inhibiting fibroblast proliferation and/or intervening at the level of signaling proteins/molecules downstream of TGF-β. Antifibrosis, such as Mitomycin-C (MMC) [16] and 5-fluorouracil [17], are commonly used to inhibite inflammation and fibroblast activity. Meanwhile, it is noticed that antifibrosis can also result in complications such as postoperative corneal toxicity, endophthalmitis and late follicular leakage [18]. Liposomes [19], chitosan [20,21], cyclodextrin [22], phenylalanine hydrogel [23], and polyester [24] have been used as controlled drug-release devices to improve the continuous drug release, which, however, are still challenged by maintaining effective drug concentrations over an appropriate period. In addition, inhibitors of AR12286 and S58 have also been reported to be able to reduce fibrosis by down-regulating TGF-β receptor signaling pathways. Notably, the postoperative scar formation typically takes a time period more than 2 weeks and during this period, cells continuously produce TGF-β in the form of autocrine or paracrine signaling via the exposure to eye fluid pressure and fluid shear stress [25]. As a result, the aforementioned strategies suffer the limitation in inhibiting the fibrosis process and for improvement, direct inhibition of TGF-β may be a more effective strategy.
Decorin is a small-molecule proteoglycan mainly found in connective tissues and collagen fibrils. It has a variety of biological activities and regulates and controls tissue morphogenesis, cell proliferation, and collagen fiber formation [26]. The anti-fibrotic effect of decorin has been reported and demonstrated in experimental glaucoma filtration surgery [27]. Decorin has also been reported to control collagen deposition, ultimately guide fiber arrangement and prevent scar tissue formation [28,29]. Interestingly, decorin was also found to be a natural antagonist of TGF-β1 and able bind to TGF-β1 and block its activity [30,31], thus providing a means to regulate the effect of TGF-β [32,33]. This is because not only can decorin bind to TGF-β by forming a complex that interferes with the binding of TGF-β to its receptor and inhibits the signal transduction effect of TGF-β, but also the decorin–TGF-β complex can reduce the content of TGF-β by recognizing decorin receptors and activating endocytosis.
Spacers made from biodegradable materials are implanted beneath the conjunctiva during trabeculectomy. The implanted biodegradable spacer is to keep the subconjunctival space open and increase the success rate of GFS. For this purpose, hyaluronic acid (HA, conjugate base hyaluronate), also called hyaluronan, is one of promising candidates. Sodium hyaluronate is a biodegradable linear polysaccharide with anti-inflammatory and anti-fibrotic properties. High-molecular-weight sodium hyaluronate (4.6 × 105–2.8 × 106) inhibits phagocytosis [34]. Sodium hyaluronate can also inhibit the induced release of inflammatory factors, such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β, while reducing the percentages of CD4 (+), CXCR3 (+), CD40 (+), and CD44 (+) cells in the conjunctiva [35]. Also, HA has been widely used in tissue engineering, wound healing, and cancer studies [[36], [37], [38], [39], [40], [41]].
Based on the above discussion, it is rational to develop methods to deplete excess TGF-β from aqueous humor, thus providing an effective means to inhibit the scar formation after GFS. The paper presents our study on the development of such a method, wherein the excess TGF-β1 is reactivated by using hyaluronic acid (HA) hydrogels that are seeded with decorin. By both in vitro and in vivo studies, we illustrated the hydrogels with decorin can recognize and thus deactivate TGF-β1; prevent the contact and adhesion between the iris and trabecula; reduce scar formation; and reduce inflammation.
2. Experimental section
2.1. Materials
Materials, growth factors and cells used in the present study were purchased from varying suppliers. HA with an average molecular weight of Da was purchased from Bloomage Freda Biopharm Co., Ltd. (Harrogate, UK); sodium periodate (NaIO4), ethylene glycol, adipic acid dihydrazide (ADH), paraformaldehyde (PFA), and sucrose from Sigma-Aldrich Co. (St. Louis, MO, USA); trizol from Thermo Fisher Scientific (Waltham, MA, USA); DEPC from Biotopped (Beijing, People's Republic of China); and neutral balsam and 50 kDa cellulose dialysis from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, People's Republic of China). TGF-β1 and IL-6 were from MedChemExpress (Shanghai, People's Republic of China); and human Tenon's capsule fibroblasts (HTFs) and human umbilical vein endothelial cells (HUVECs) from the Institute of Biochemistry and Cell Biology and the Chinese Academy of Sciences (Shanghai, People's Republic of China). Dulbecco's Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were from Thermo Fisher Scientific; hematoxylin and eosin stain from Solarbio (Beijing, People's Republic of China); TGF-β1 polyclonal antibody from Boster Biological Technology Co., Ltd. (Wuhan, People's Republic of China); and IL-6 polyclonal antibody from Affinity Biosciences Co., Ltd. (Beijing, People's Republic of China).
2.2. Animal models for in vivo studies
All animal experimental procedures and husbandry were approved by the Experimental Animal Welfare Ethics Committee of the Harbin Medical University via the protocol of #2019046. Nine (9) New Zealand rabbits, 3- to 5-months old and weighing 1.5–2.0 kg, were purchased and acclimatized for 1 week prior to the experiments. These rabbits were then randomly divided into three groups, each with 3 rabbits, for surgery with different post-treatments, i.e. the ones without injection, injected with O-HA, injected with O-HA + D, named as trabeculectomy group, O-HA group, and O-HA + D group. For each group of 3 rabbits, three eyes (the right one of each rabbit) were used for surgery, where standard trabeculectomy was performed; while the other eyes were used for control. On Day 1, 4, and day 7 postsurgery, the three eyes with surgery were selected from each group for aqueous humor sampling. The rabbits were euthanized and sacrificed for histological examination on Day 7 pos surgery.
2.3. Preparation of O-HA
Oxidized HA (O-HA) was synthesized as described before [42,43]. Briefly, a mixture of HA (1% [w/v'], 40 mL) and sodium periodate (0.25 M, 800 μL) was dissolved in double-distilled water in a dark ice bath for 2.5 h. The reaction was stopped by the addition of ethylene glycol (160 μL) for 30 min. The formed O-HA was immediately precipitated after adding anhydrous alcohol in an equal volume. Then, the O-HA was obtained via centrifugation at 2000 rpm for 20 min and re-solubilized in MQ H2O, then subjected to dialysis (50 kDa cutoff) against MQ H2O for 3 days. Eventually, the dialyzed solution was lyophilized, yielding a white fluffy product.
2.4. Preparation of the O-HA + D hydrogel
A mixture solution of O-HA (4,5 and 6% [w/v], in MQ H2O) and decorin (2, 5 and 10 μg/mL, in MQ H2O) were separately dissolved in PBS (pH of 7.4, at 4 °C) and then gently mixed with an 8% (w/v) concentration of adipic acid dihydrazide (ADH) to form O-HA + D hydrogels, respectively.
2.5. Fourier-transform infrared spectroscopy (FTIR)
To confirm the structure of O-HA and O-HA + D, FTIR was performed by using a Shimadzu FTS400 spectrometer (Shimadzu Corporation, Kyoto, Japan).
2.6. Characterization of rheological behavior of O-HA solutions
O-HA was prepared into aqueous solutions with different concentrations. The rheological or dynamic viscoelastic behavior of those solutions was examined by a using a Bohlin Gemini II rheometer (Malvern Instruments, Malvern, UK) with a parallel plate geometry (40 mm in diameter). The gap between the top and bottom plates was set at 500 μm for all measurements. The elastic (G′) and loss (G″) moduli were characterized by the strain-controlled frequency sweep with a range of 0.01–10 Hz at room temperature.
2.7. Degradation properties of O-HA + D hydrogel
The degradation study of O-HA + D hydrogel was carried out in phosphate buffered saline (PBS) at 37 °C. In brief, 0.3 mL of O-HA + D solution was solidified in a 3 mL syringe for 15 min. The O-HA + D hydrogel was transferred to a six-well plate, and 3 mL of PBS was added to each well and replaced once a day. At the set time points, the O-HA + D hydrogel was removed and blotted gently with filter paper to remove surface water. The hydrogels were freeze-dried to obtain dry weight (Wd). The degradation percentage was calculated by , where Wi is the initial weight of hydrogel.
2.8. Cell culture
HTF cells were grown in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin in a humidified incubator at 37 °C with 5% CO2. Cells were maintained in the logarithmic growth phase. Cells from generations 5–10 were used for the experiments and for each experiment, the cells were of the same line and from the same generation. There was HTFs/mL cultured in a six-well plate. When the cells reached 80% confluence, the cells were starved in serum-free DMEM for 12 h prior to experiments. They were cultured in growth medium with TGF-β1 (5 ng/mL) or with different concentrations of decorin (2, 5 and 10 μg/mL) for 24 h. For in vitro experiments, the cultured HTFs were exposed to O-HA + D hydrogel (100 μL, with or without 10 μg/mL decorin) for 24 h.
HUVECs were grown in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin in a humidified incubator at 37 °C with 5% CO2. There was 1 × 105 HUVECs/mL cultured in a six-well plate. When the cells reached 80% confluence, the cells were starved in serum-free DMEM for 12 h before the experiments. They were cultured in growth medium with TGF-β1 (10 ng/mL) or with different concentrations of decorin for 48 h. For in vitro experiments, the cultured HUVECs were exposed to O-HA + D hydrogel (100 μL, with or without 6 μg/mL decorin) for 48 h.
2.9. Real-time PCR
Real-time (RT) polymerase chain reaction (PCR) was performed on a 7500 real-time PCR system (Applied Biosystems; Thermo Fisher Scientific) with the Fats Start Universal SYBR Green Master (Rox; Hoffman-La Roche Ltd., Basel, Switzerland) with the following cycling conditions: 5 °C for 2 min, 95 °C for 10 min, 95 °C for 15 s, 60 °C for 1 min, and then step 3 initiated for 40 cycles. The primer sequences for reverse transcription polymerase chain reaction (RT-PCR) are shown in Table S1. Gene expression was normalized with the GAPDH expression level. RT-PCR analyses were performed in triplicate for 3–5 independent repeats.
2.10. Immunohistochemistry
The hydrogel was incubated in TGF-β1(5 ng/mL, PBS) or IL-6 (5 ng/mL, PBS) at 37 °C for 1 h and shaken slightly. The gel samples were fixed with 4% paraformaldehyde for 15 min and washed three times with 1 × PBS for 10 min each time. The samples were blocked with 0.5% bovine serum albumin (BSA) for 30 min. Afterward, the samples were incubated with TGF- β1 (1:100) antibodies or IL-6 (1:200) antibodies overnight and subsequently washed with 1 × PBS for 15 min for each wash. The samples were then incubated with the secondary antibody at 37 °C in the dark for 30 min and subsequently washed again thrice for 10 min for each wash. All imaging was performed on an Olympus Fluoview FV1000 confocal microscope (Olympus Corporation, Tokyo, Japan).
2.11. Filtration surgery in rabbits
IOP was measured before and after surgery with Schiötz Tonometer. All surgeries were performed under a coaxial illumination stereo microscope with a magnification of 10x (Zeiss, Germany). The experimental rabbits were generally anesthetized with sodium barbital (30 mg/kg) via ear vein, and the right eye was topical anesthetized with 1% acaine. The subconjunctival tissue separated into a 6 × 7 mm space to expose the sclera, and a scleral flap with a thickness of 1/2 and a size of 3 × 3 mm was made under the conjunctival flap. The corneoscleral tissue of 1 × 2 mm was removed, and a peripheral iridectomy were then performed. The two corners of the scleral flap were sutured to the scleral bed with 10–0 nylon thread. The conjunctival flap was closed with interrupted sutures by 10–0 nylon thread. Before the final stitch of the conjunctival flap, inject hydrogel (0.1 mL with or without 10 μg/mL decorin) into the subconjunctival space with a 1 mL syringe. Levofloxacin eye ointment was applied after surgery. Afterward, the hydrogel was refilled to the subconjunctival space on the fourth day of surgery. One week after the experiment, the animals were sacrificed by injecting an overdose of sodium barbital into the rabbits, the eyeballs were immediately enucleated, and the surgical tissue was removed and preserved in 4% paraformaldehyde for fixation.
2.12. ELISA
TGF-β1 cytokine levels in aqueous humor were detected by using the commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA). The optical density of each well was subsequently read using a multimode plate reader (Tecan infinite M Mano, Tecan, Switzerland) at a wavelength of 450 nm.
2.13. Hematoxylin and eosin
Tissue samples were cleaned with 1 × PBS and fixed with 4% paraformaldehyde for 24 h and then dehydrated and embedded in paraffin. Serial sections (5 μm thick) were cut, dehydrated, and stained with hematoxylin and eosin stain for light microscopy examination.
2.14. Statistical analysis
All statistical analyses were performed using GraphPad Prism 5. The results were presented as the means ± standard deviation. Comparisons between two treatments were made using Student's t-test (two-tailed, unequal variance) and comparisons between multiple treatments were made using analysis of variance (ANOVA), ‘∗‘, ‘∗∗‘, and ‘∗∗∗’ indicates p value < 0.05, 0.01, and 0.001 respectively.
3. Results and discussion
Given its good biocompatibility, as well as its anti-inflammatory [44], anti-infection [45], and anti-edema [46] properties, HA has been used for various biomedical applications [[36], [37], [38], [39], [40], [41]]. HA hydrogel can be injected at designated sites and has minimally invasive effects; as such, HA hydrogel can be used for in situ injection. For this reason, we chose to use HA as base materials in this study [47]. Decorin-grafted HA (O-HA + D) was injected into the surgical site of the trabeculectomy with the rationale illustrated in Fig. 1, where O-HA + D adsorbs TGF-β, forms a complex, recognizes the decorin receptor, and activates endocytosis to reduce the amount of TGF-β in aqueous humor. In addition to their potential role as collectors of anti-scarring agents, they can also mechanically separate the conjunctiva from the scleral surface for a period of time, thereby preventing adhesions between the two surfaces [48,49].
Fig. 1.
Schematic of the preparation, trabeculectomy treatment, and adsorption mechanism of O-HA + D.
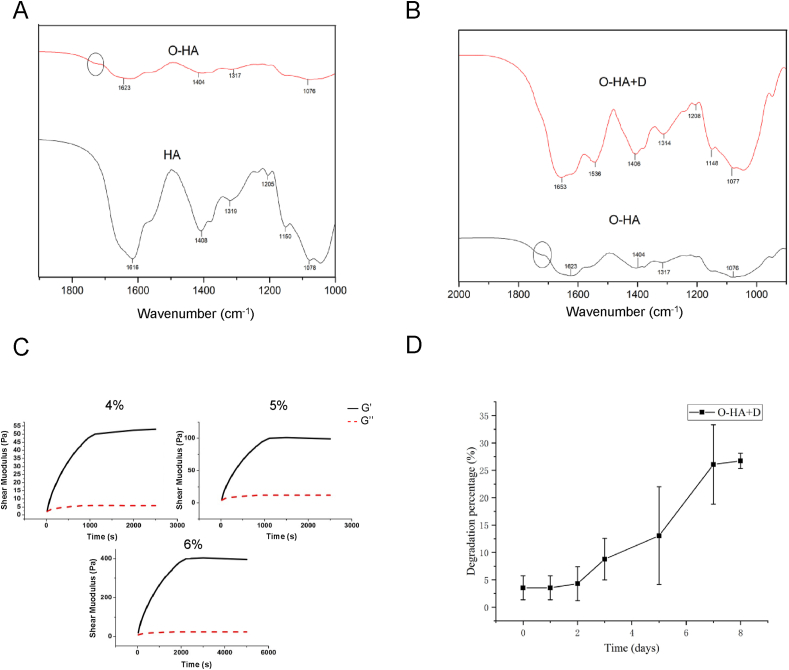
We first synthesized oxidized HA (O-HA) by reacting HA and sodium periodate (NaIO4) following a previously reported method [50]. We confirmed the presence of aldehyde groups using FTIR spectroscopy (Fig. 2A). We found that there was a newly formed peak at 1700 cm−1 which was associated with the C O stretch of O-HA. The freeze–dried hydrogels of O-HA + D were also confirmed by FTIR (Fig. 2B). The peak at 1700 cm−1 appeared to weaken due to aldehyde consumption, which formed amine bonds between O-HA and ADH or decorin. This suggests that all free aldehyde groups have been consumed. Since viscoelasticity is one key parameter for injectable hydrogel, we studied the viscoelastic behavior of the O-HA solutions with varying concentrations (Fig. 2C), with the results illustrating that the elastic modulus and setting time of the O-HA solution increased with the concentrations of O-HA. On this basis, we selected 5% HA and injected it into the surgical site after 15 min. We further showed the degradation kinetics of the O-HA + D hydrogels in a PBS solution (Fig. 2D). On Day 7, degradation percentages for O-HA + D hydrogel was 26 ± 7%, indicating that gel as a physical spacer had certain stability after injection.
Fig. 2.
Characterization of hydrogels. FTIR spectra of (A) hyaluronic acid, oxidated hyaluronic acid, and (B) and decorin-grafted oxidated hyaluronic acid. (C) The viscoelasticity measurements of oxidated hyaluronic acid. (D) Degradation profile of the decorin-grafted oxidated hyaluronic acid hydrogels.
Scarring of the filtering bleb following glaucoma surgery is a key factor that leads to its loss of function [13]. Scar formation of the filtering bleb is a complex biological reaction process involving a large number of growth factors, plasma proteins, and cytokines [51]. The wound healing process after conjunctival injury is mainly divided into three stages: inflammatory response stage, cell proliferation stage and recombinant plastic stage. Following filtration surgery, various cytokines and inflammatory mediators that are stimulated by the tissue trauma surrounding the surgical area during the inflammatory reaction period promote the proliferation of fibroblasts, which migrate to the wound [10]. Many cytokines are involved in these three stages, the central mediator being TGF-β. At the same time, fibroblasts are stimulated by various factors; collagen, elastin, laminin, and other ECM proteins are synthesized, deposited, and contracted. After these tissues are formed, a dense collagenous scar under the conjunctiva is formed, which promotes wound healing and leads to filtration surgery failure [52]. Fibroblasts are involved in the construction and remodeling of scar tissue [53]. The in vitro culture of Tenon capsule fibroblasts is often used to study scar models of glaucoma filtering surgery.
Type I collagen is a fibrillar collagen found in most connective tissues, including follicular tissue, and is encoded by COL1α1 and COL1α2, respectively. Fibronectin (FN) is required for collagen matrix assembly and is also present in fibrotic matrices. TGF-β promotes wound healing and scarring after GFS by recruiting fibroblasts, promoting fibroblast proliferation, and activating fibroblast-to-myofibroblast differentiation [54]. Alpha-smooth muscle actin (α-SMA) increases the contractile activity of myofibroblasts. Increased levels of α-SMA expression and deposition of ECM proteins including fibronectin (FN) and collagen are key features of the myofibroblast phenotype [[55], [56], [57]].
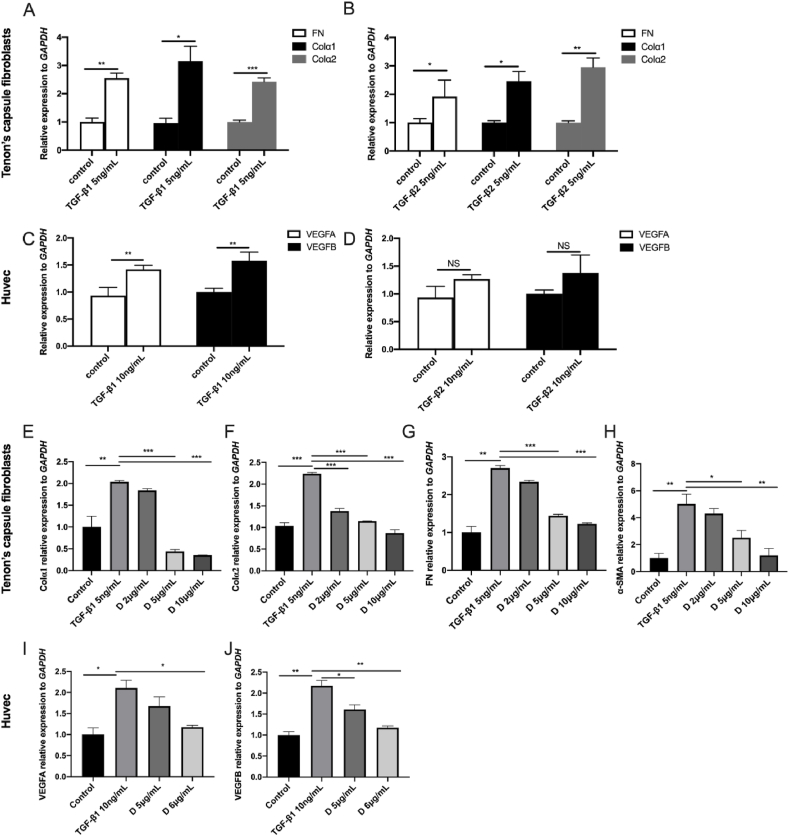
In this experiment, we treated HTFs with TGF-β1 and TGF-β2 (5 ng/mL), and assayed the mRNA expression of COL1α1, COL1α2, and FN expression (Fig. 3A and B). The expression of COL1α1, COL1α2, and FN genes were significantly higher than in the control group, indicating that TGF-β can promote the proliferation of HTFs, as well as HTF transformation into myofibroblasts, which then initiates the entire scar response. This is aligned with the findings from large number of experimental studies [[58], [59], [60]]. To further quantify the effect of decorin on the expression of COL1α1, COL1α2, FN, and α-SMA mRNA, we treated HTFs with different concentrations of decorin. In this experiment, five groups were compared to assess COL1α1, COL1α2, FN, and α-SMA expression (Fig. 3E–G). We included TGF-β1 (5 ng/mL) as a positive control. When HTFs were treated with different concentrations of decorin, COL1α1, FN, and α-SMA mRNA expression decreased at all doses, except at 2 μg/mL (Fig. 3E, G, and 3H). COL1α2 expression was significantly downregulated compared to the positive control (Fig. 3F).
Fig. 3.
TGF-β1- and TGF-β2-induced production of (A, B) fibrosis marker and (C, D) vascularization maker. Effect of decorin on (E) COL1α1, (F) COL1α2, (G) FN, (H) α-SMA, (I) VEGFA, and (J) VEGFB gene expression. The relative gene expression (mean ± standard deviation [SD]) was the average of three independent experiments conducted in triplicate. Asterisks indicate statistically significant different responses (∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001).
Cytokines can stimulate hematopoiesis and play an important role in the repair process following tissue injury. Vascular endothelial cell growth factor (VEGF) plays an important role in the formation of blood vessels and is closely related to wound healing. It not only stimulates the growth of endothelial cells, but also binds to the VEGF receptors on the surface of Tenon capsule fibroblasts to promote the proliferation and migration of target cells, which is conducive to the formation of scars [61,62]. Thus, we stimulated HUVECs by TGF-β1 and TGF-β2 (10 ng/mL) and assayed the mRNA expression of VEGFA and VEGFB (Fig. 3C and D). The results showed that the expression of VEGFA and VEGFB genes in HUVECs treated with TGF-β1 was significantly increased (Fig. 3C; P < 0.01), but there was no significant difference in TGF-β2 group (Fig. 3D). We used TGF-β1 (10 ng/mL) as a positive control and quantified the effect of decorin on the expression of VEGFA and VEGFB mRNA. The expression of VEGFA mRNA (Fig. 3I) was significantly downregulated (P < 0.05) after treating the cells with decorin (6 μg/mL). VEGFB mRNA expression decreased at all doses (Fig. 3J).
It has been shown that small proteoglycans containing leucine-rich repeats in their core proteins can form complexes with TGF-β. The high affinity site of TGF-β on the peptide Leu155-Val260, the KD value even reaches 7 × 10−9 M. Due to this high affinity, it is also proved that decorin can specifically bind to TGF-β [63].
Neutrophils and macrophages mainly secrete IL-6, TNF-α and other factors to aggravate inflammation [64]. Inflammatory factors can recruit and activate vascular endothelial cells and fibroblasts [65]. The adsorption capacity of O-HA + D hydrogel was determined using an immunofluorometric assay incubated in 1 μg/mL TGF-β1 or IL-6 solution for 1 h (Fig. 4A). The O-HA group hydrogel in TGF-β1 solution and the O-HA + D group hydrogel in IL-6 solution had no fluorescence, except for the slight fluorescence observed at the edge. The O-HA + D group hydrogel showed strong green fluorescence, indicating that the addition of decorin enabled hydrogel to adsorb TGF-β1. Following the preliminary experiments, we chose to graft 10 μg/mL of decorin with oxidized HA. TGF-β1 induced the production of fibrosis markers and vascularization makers inhibited by O-HA and O-HA + D hydrogel (Fig. 4B–G).
Fig. 4.
O-HA + D hydrogel specifically adsorbs TGF- β1 and reduces fibrosis and vascularization. Confocal fluorescence microscopy images of hydrogel incubated in TGF-β1 or IL-6 solution for 1 h (A). Effect of hydrogel on (B) FN, (C) COL1α1, (D) COL1α2, (E) α-SMA, (F) VEGFA, and (G) VEGFB gene expression. A total of three confocal images were taken for each group from three independent experiments. The relative gene expression (mean ± SD) was an average of three independent experiments conducted in triplicate. Asterisks indicate statistically significant different responses (∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001).
Intraocular pressure decreases after trabeculectomy, so we measured it after surgery to ensure the success of the filtration procedure. We measured IOP before (12.23 mmHg) and after surgery (8.54 mmHg) wtih a Schiötz tonometer. We performed clinical examinations on rabbit eyes after glaucoma surgery (Figure SI.1). We observed that the rabbits did not shed tears after glaucoma surgery Also, the cornea was clear, the conjunctiva was not hyperemia, and there was no edema at the surgical site. It is illustrated that the hydrogel has no local clinical side effects. The blebs were small, thick, flat, and vascularized in the trabeculectomy group (Figure SI·1B). Compared with the trabeculectomy group, the angiogenesis phenomenon of the O-HA group was reduced, demonstrating that O-HA hydrogel could alleviate the inflammatory response of scarring after glaucoma surgery. There are slightly bulged, and thinner blebs clearly visible in the O-HA (Figure SI·1C) and O-HA + D groups (Figure SI.1D). On Day 7 post surgery, the total area of functional filtration bleb in the O-HA + D group was significantly larger than in the trabeculectomy and the O-HA group. This suggests O-HA + D has an inhibitory effect on scarring after glaucoma surgery.
On Day 7 post surgery, all groups of rabbits were sacrificed by an injection of excess barbiturate on the ear vein. After the eyeball was removed, the white rabbits were dissected, and the hearts, liver, kidneys, cornea, and retina of the rabbits were removed to observe the toxic effects of hydrogel on the rabbits themselves. Similar to the trabeculectomy group, both O-HA and O-HA + D groups had no pathological changes in the heart, liver, kidneys, cornea, and retina and the tissue remained normal, demonstrating that both O-HA and O-HA + D are biocompatible (Fig. 5A, Figure SI.2). After GFS, aqueous humor flows from the anterior chamber into the subconjunctival hole and penetrates the conjunctival tissue. TGF-β [66,67], VEGF [68], platelet-derived growth factor (PDGF), IL-8, serum amyloid A [69], TNF-α [70], and plasminogen activator inhibitor-1 (PAI-1) [71] has been detected in the aqueous humor. Thus, the concentration of TGF-β1 in the aqueous humor was measured on Days 1, 4, and 7 (Fig. 5B). The concentration of TGF-β1 in aqueous humor increased significantly on Day 4, and both hydrogel groups demonstrated reduced concentrations in the aqueous humor. The volume of the injected hydrogel (100 μL) is much smaller than that of the filter bleb. However, the amount of TGF-β1 that can be adsorbed by this volume is limited and easily saturated. If a large amount is injected at one time, it may interfere with the filtering passage. At the same time, according to the degradation curve of the decorin-grafted oxidated hyaluronic acid hydrogels, the hydrogel is degraded by 7.29%–13.76% within 3–5 days. To effectively reduce this peak, we choose to refill on Day 4. On Day 7, only the O-HA + D group was able to effectively inhibit the concentration of TGF-β1 in the aqueous humor, suggesting that the gel could adsorb TGF-β1 so as to maintain it at a low concentration over the examined time period.
Fig. 5.
Bleb tissue post-glaucoma filtration surgery in four groups (control group, treatment groups [i.e., trabeculectomy without injection, injected with O-HA; injected with O-HA + D]). (A) Histological examination of the liver, kidney, and heart. (B) Determination of TGF-β1 in aqueous humor. (C) mRNA levels of CTGF, Collagen I, and VEGFA were determined by RT-PCR. (D) Histological examination of bleb tissue on day 7. Relative gene expression (mean ± SD) was an average of three independent experiments conducted in triplicate. Asterisks indicate statistically significant different responses (∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001). Scale bar = 50 μm.
TGF-β binds to the TGF-β receptor on the surface of Tenon's capsule fibroblasts, has a strong chemotactic effect on inflammatory cells, enhances the inflammatory response [72], promotes the proliferation and activation of fibroblasts, and enhances the downstream mediator of connective tissue growth factor (CTGF). CTGF expression increases the expression of type I and type II collagen in the ECM [11], inhibits ECM degradation, and promotes its deposition [12]. In our study, we examined the expression of Collagen I, CTGF, and VEGFA in bleb tissues isolated from rabbit eyes (Fig. 5C). RT-PCR results showed that compared with the trabeculectomy group, the mRNA levels of CTGF, Collagen I, and VEGFA in the O-HA group were significantly reduced (all P < 0.05). Similarly, O-HA + D treatment significantly reduced the expression of CTGF, Collagen I, and VEGFA induced by GFS (all P < 0.01).
We evaluated the effect of O-HA + D hydrogel injection on the subconjunctival fibrotic after GFS. Hematoxylin and eosin staining was conducted on Day 7 (Fig. 5D), which demonstrated reduced inflammatory cells in the subconjunctival region in rabbits from the O-HA and O-HA + D groups when compared to rabbits in the trabeculectomy group. In particular, the O-HA + D group had a more pronounced evacuated structure as compared to the O-HA group. The inhibition of collagen contraction demonstrated the effect of O-HA + D on the conjunctival fibroblasts.
4. Conclusions
Post glaucoma filtration surgery, scar formation regulated by TGF-β poses a big challenge for recovery. To overcome this challenge, we synthesized the oxidized hyaluronic acid seeded with decorin, or O-HA + D, to deactivate TGF-β1 as evaluated both in vitro and in vivo. We conclude that O-HA + D hydrogel prevents scar formation in the GFS rabbit model by adsorbing TGF-β1 and thereby inhibiting the expression of pro-fibrotic genes. Our results illustrate that O-HA + D hydrogel can recognize and deactivate TGF-β1, thus reducing the concentration of TGF-β1 in aqueous humor and the inflammatory response and further inhibiting the proliferation of fibrous tissue and the formation of new blood vessels. Also, our results illustrate that O-HA + D hydrogel can act as a physical spacer to inhibit wound healing and prevent channel obstruction. Together, our work paves the way toward the treatment of post-glaucoma fibrosis following surgery.
Credit author statement
Ruiqi Wang, Boyang Chen: Methodology, Investigation, Formal analysis, Data curation. Wei Yan, Yuping Wu: Validation. Cao Wang, Bosong Zhang, Fengzhen Liu, Hui Tian: Supervision, Visualization. Ruiqi Wang: Data curation, Writing – original draft. Haiying Wei, Xiongbiao Chen, and Weiming Tian: Writing- Reviewing and Editing. Haiying Wei and Weiming Tian: Conceptualization, Resources, Project administration and Funding acquisition.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work.
Acknowledgments
This research is supported by the National Natural Science Foundation of China (grants 81770923 and 51773050). English-language editing of this manuscript was provided by Journal Prep Services.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2022.100260.
Contributor Information
Haiying Wei, Email: imweihy@126.com.
Weiming Tian, Email: tianweiming@hit.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Tham Y.-C., et al. Ophthalmology. 2014;121(11):2081. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Liang Y.B., et al. J. Glaucoma. 2015;24(6):469. doi: 10.1097/IJG.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 3.Liang L., et al. Mol. Med. Rep. 2019;20(3):2929. doi: 10.3892/mmr.2019.10507. [DOI] [PubMed] [Google Scholar]
- 4.Wang W.-H., et al. Artif. Cell Nanomed. Biotechnol. 2018;46(4):831. doi: 10.1080/21691401.2017.1345926. [DOI] [PubMed] [Google Scholar]
- 5.Tripathi R.C., et al. Exp. Eye Res. 1994;59(6):723. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- 6.Cordeiro M.F. Clin. Sci. 2003;104(2):181. doi: 10.1042/CS20020150. [DOI] [PubMed] [Google Scholar]
- 7.Picht G., et al. Graefes Arch. Clin. Exp. Ophthalmol. 2001;239(3):199. doi: 10.1007/s004170000252. [DOI] [PubMed] [Google Scholar]
- 8.Min S.H., et al. Kor. J. Ophthalmol. 2006;20(3):162. doi: 10.3341/kjo.2006.20.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saika S., et al. Graefe’s Arch. Clin. Exp. Ophthalmol. 2001;239(3):234. doi: 10.1007/s004170100275. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka O., et al. BMC Ophthalmol. 2015;15(1):19. [Google Scholar]
- 11.Nigdelioglu R., et al. J. Biol. Chem. 2016;291(53):27239. doi: 10.1074/jbc.M116.756247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stahnke T., et al. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0172592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlunck G., et al. Exp. Eye Res. 2016;142:76. doi: 10.1016/j.exer.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Van Bergen T., et al. Clin. Ophthalmol. 2014;8:857. doi: 10.2147/OPTH.S48745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masoumpour M., et al. Open Ophthalmol. 2016;10:28. doi: 10.2174/1874364101610010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta V., et al. Int. J. Otorhinolaryngol. Head Neck Surg. 2017;4(1):176. [Google Scholar]
- 18.Cabourne E., et al. Cochrane Database Syst. Rev. 2015;(11) doi: 10.1002/14651858.CD006259.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.J. Drug Deliv. Sci. Technol. 2007;17(1):43. [Google Scholar]
- 20.Cho I.S., et al. Acta Biomater. 2016;39:124. doi: 10.1016/j.actbio.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Shao T., et al. Diagn. Pathol. 2011;6(1):1. doi: 10.1186/1746-1596-6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merritt S.R., et al. Exp. Eye Res. 2013;116:9. doi: 10.1016/j.exer.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao C.-C., et al. Regener. Biomater. 2020;7(1):99. doi: 10.1093/rb/rbz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun J., et al. J. Mater. Chem. B. 2017;5(31):6400. doi: 10.1039/c7tb01556a. [DOI] [PubMed] [Google Scholar]
- 25.Ng C.P., Swartz M.A. Comput. Methods Biomech. Biomed. Eng. 2005;8(S1):205. [Google Scholar]
- 26.Markmann A., et al. Matrix Biol. 2000;19(7):631. doi: 10.1016/s0945-053x(00)00097-4. [DOI] [PubMed] [Google Scholar]
- 27.Grisanti S., et al. Invest. Ophthalmol Vis. 2005;46(1):191. doi: 10.1167/iovs.04-0902. [DOI] [PubMed] [Google Scholar]
- 28.Chouhan G., et al. Biomaterials. 2019;210:41. doi: 10.1016/j.biomaterials.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Chakravarti S., et al. Invest. Ophthalmol. Vis. Sci. 2000;41(11):3365. [PMC free article] [PubMed] [Google Scholar]
- 30.Kolb M., et al. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280(6):L1327. doi: 10.1152/ajplung.2001.280.6.L1327. [DOI] [PubMed] [Google Scholar]
- 31.Jahanyar J., et al. J. Heart Lung Transplant. 2007;26(1):34. doi: 10.1016/j.healun.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Hausser H., et al. FEBS Lett. 1994;353(3):243. doi: 10.1016/0014-5793(94)01044-7. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J., et al. J. Biol. Chem. 2007:282. doi: 10.1074/jbc.M701997200. [DOI] [PubMed] [Google Scholar]
- 34.Forrester J.V., Balazs E.A. Immunology. 1980;40(3):435. [PMC free article] [PubMed] [Google Scholar]
- 35.Oh H.J., et al. J. Ocular Pharmacol. Therapeutics. 2014;30(7):533. doi: 10.1089/jop.2013.0050. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y.-f., et al. ACS Appl. Mater. Interfaces. 2017;9(11):9327. doi: 10.1021/acsami.6b15187. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y., et al. Oncogene. 2019;38(22):4297. doi: 10.1038/s41388-019-0719-4. [DOI] [PubMed] [Google Scholar]
- 38.Rajaram A., et al. J. Biomater. Sci. Polym. Ed. 2015;26(7):433. doi: 10.1080/09205063.2015.1016383. [DOI] [PubMed] [Google Scholar]
- 39.Rajaram A., et al. 3D Print. Addit. Manuf. 2014;1(4):194. [Google Scholar]
- 40.Little C.J., et al. J. Funct. Biomater. 2014;5(3):197. doi: 10.3390/jfb5030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang M.-D., et al. Front. Mater. Sci. 2013;7(3):269. [Google Scholar]
- 42.Ma X., et al. Fibers Polym. 2017;18(5):817. [Google Scholar]
- 43.Frana C.G., et al. Eur. Polym. J. 2019;121:109288. [Google Scholar]
- 44.Clark R.A., et al. JCB (J. Cell Biol.) 1996;134(4):1075. doi: 10.1083/jcb.134.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pirnazar P., et al. J. Periodontol. 1999;70(4):370. doi: 10.1902/jop.1999.70.4.370. [DOI] [PubMed] [Google Scholar]
- 46.Jackson G.W., James D.F. Biorheology. 1982;19(1–2):317. doi: 10.3233/bir-1982-191-234. [DOI] [PubMed] [Google Scholar]
- 47.Chae J.J., et al. Adv. Sci. 2021;8(2):2001908. doi: 10.1002/advs.202001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosentreter A., et al. Albrecht von Graæes Archiv. für Ophthalmol. 2010;248(9):1319. doi: 10.1007/s00417-010-1385-y. [DOI] [PubMed] [Google Scholar]
- 49.Song D.S., et al. other. 2019;(25):98. [Google Scholar]
- 50.Su W.-Y., et al. Acta Biomater. 2010;6(8):3044. doi: 10.1016/j.actbio.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 51.Chang M.R., et al. J. Ocular Pharmacol. Therapeutics Offic. J. Assoc. Ocular Pharmacol./ Therapeut. 1998;14(1):75. [Google Scholar]
- 52.Shi H., et al. Investig. Ophthalmol. Visual Sci. 2017;58(3):1478. doi: 10.1167/iovs.16-21163. [DOI] [PubMed] [Google Scholar]
- 53.Schreier T., et al. Res. Exp. Med. 1993;193(1):195. doi: 10.1007/BF02576227. [DOI] [PubMed] [Google Scholar]
- 54.Darby I.A., et al. Clin. Cosmet. Invest. Dermatol. 2014;7:301. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hinz B., et al. Mol. Biol. Cell. 2001;12(9):2730. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang F., et al. Investig. Ophthalmol. Visual Sci. 2019;60(7):2743. doi: 10.1167/iovs.18-26526. [DOI] [PubMed] [Google Scholar]
- 57.Zhu H., et al. Exp. Cell Res. 2020;387(2):111802. doi: 10.1016/j.yexcr.2019.111802. [DOI] [PubMed] [Google Scholar]
- 58.Tong J., et al. Investig. Ophthalmol. Visual Sci. 2014;55(4):2747. doi: 10.1167/iovs.13-13422. [DOI] [PubMed] [Google Scholar]
- 59.Chung E.J., et al. Biotechnol. Lett. 2014;36(6):1217. doi: 10.1007/s10529-014-1487-4. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Z., et al. Molecular Biology. 2012;46(4):563. [Google Scholar]
- 61.Chong R.S., et al. Investig. Ophthalmol. Visual Sci. 2017;58(9):3432. doi: 10.1167/iovs.17-21480. [DOI] [PubMed] [Google Scholar]
- 62.Van Bergen T., et al. Exp. Eye Res. 2011;93(5):689. doi: 10.1016/j.exer.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 63.Schönherr E., et al. Arch. Biochem. Biophys. 1998;355(2):241. doi: 10.1006/abbi.1998.0720. [DOI] [PubMed] [Google Scholar]
- 64.Boshtam M., et al. Inflammation. 2017;40(1):340. doi: 10.1007/s10753-016-0477-1. [DOI] [PubMed] [Google Scholar]
- 65.Liu Z., et al. Exp. Ther. Med. 2017;14(6):5833. doi: 10.3892/etm.2017.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takai Y., et al. Investig. Ophthalmol. Visual Sci. 2012;53(1):241. doi: 10.1167/iovs.11-8434. [DOI] [PubMed] [Google Scholar]
- 67.Tripathi R.C., et al. Exp. Eye Res. 1994;59(6):723. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- 68.Park H.-Y.L., et al. Archiv. Ophthalmol. 2012;130(6):685. doi: 10.1001/archophthalmol.2011.2799. [DOI] [PubMed] [Google Scholar]
- 69.Xiong Q., et al. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0088403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sawada H., et al. Investig. Ophthalmol. Visual Sci. 2010;51(2):903. doi: 10.1167/iovs.09-4247. [DOI] [PubMed] [Google Scholar]
- 71.Dan J., et al. Arch. Ophthalmol. 2005;123(2):220. doi: 10.1001/archopht.123.2.220. [DOI] [PubMed] [Google Scholar]
- 72.Qian L.W., et al. Wound Repair Regen. 2016;24(1):26. doi: 10.1111/wrr.12381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.