Abstract
Derivatization of azaarenes can create molecules of biological importance, but reductive functionalization of weakly reactive azaarenes remains a challenge. Here the authors show a dearomative, diastereoselective annulation of azaarenes, via ruthenium(II) reductive catalysis, proceeding with excellent selectivity, mild conditions, and broad substrate and functional group compatibility. Mechanistic studies reveal that the products are formed via hydride transfer-initiated β-aminomethylation and α-arylation of the pyridyl core in the azaarenes, and that paraformaldehyde serves as both the C1-building block and reductant precursor, and the use of Mg(OMe)2 base plays a critical role in determining the reaction chemo-selectivity by lowering the hydrogen transfer rate. The present work opens a door to further develop valuable reductive functionalization of unsaturated systems by taking profit of formaldehyde-endowed two functions.
Subject terms: Synthetic chemistry methodology, Homogeneous catalysis
Derivatization of azaarenes can create molecules of biological importance, but reductive functionalization of weakly reactive azaarenes remains a challenge. Here the authors show a dearomative, diastereoselective annulation of azaarenes, via ruthenium(II) reductive catalysis.
Introduction
Azaarenes constitute a class of ubiquitously distributed substances applied in numerous fields of science and technology1,2. The development of new strategies enabling efficient and selective transformation of weakly reactive azaarenes into functional frameworks is of important significance, as they not only pave the avenues to access novel functional products, but also enrich the synthetic connotation of the azaarenes. To date, except the well-established electrophilic substitution utilizing azaarenes as the nucleophiles under harsh conditions3,4, the recently emerged C–H bond activation/functionalization has offered many desirable ways for structural modification of the azaarenes5–7. In comparison with these aromaticity-retaining transformations, only a handful examples focused on dearomative coupling of active indole derivatives8–11, whereas dearomative functionalization of inert pyridine-fused azaarenes (e.g., quinolines, isoquinolines, naphthyridines, phenanthroline, etc.)12–14 has been scarcely explored.
In recent years, hydrogen transfer-mediated coupling reactions have emerged as appealing tools in the production of various functional products, since there is no need for high pressurized H2 and elaborate experimental setups. For instance, in addition to the well-known reductive amination applied for amine syntheses15,16, several groups such as Beller17,18, Kempe19,20, Kirchner21,22, Liu23–25, and others26–29 have applied borrowing-hydrogen strategy to alkylate amines and the α-site of carbonyl compounds with alcohols. Krische has demonstrated elegant contributions on the linkage of alcohols/carbonyls with unsaturated C–C bonds30–32. Bruneau et al. have achieved β-C(sp3)−H alkylation of N-alkyl cyclic amines33,34. The Li group has converted phenols into synthetically useful amines35,36. Our group has reported a reductive quinolyl β-C–H alkylation with a low-active heterogeneous cobalt catalyst37. Later, Donohoe et al. have demonstrated interesting examples on the β-functionalization of azaarenes38–40. Despite these important advances, the strategy incorporating a tandem coupling sequence into the reduction of azaarenes remains to date a challenge due to the difficulty in controlling the reaction selectivity: on one hand, the azaarenes tend to undergo direct hydrogenation to form non-coupled cyclic amines under catalytic reduction conditions, on the other hand, it is hard to selectively transfer hydrogen only to one specific sites among different substrates.
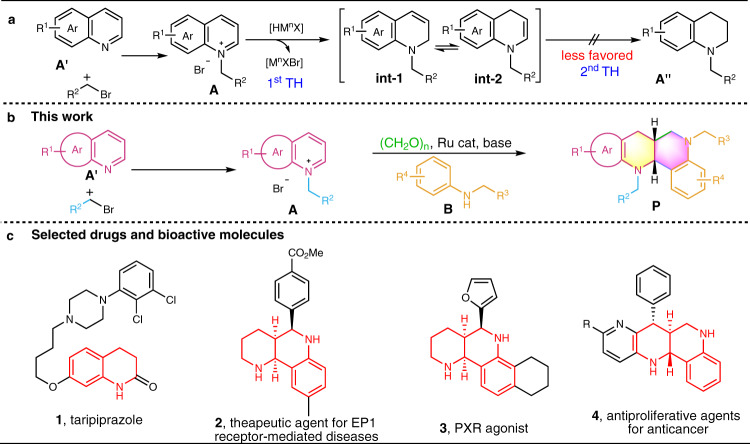
Here, we conceived that, through an initial N-alkylation of azaarenes A′ with bromoalkanes to form azaarenium salts41,42, a solution to achieve the desired synthetic purpose would be offered: (i) the combination of a suitable metal catalyst (M) and hydrogen donor (HD) forms reductive metal hydride species [HMnX] in-situ, which allows hydride transfer (TH) to the azaarenium salts A to generate allylic amine int-1 and its tautomer N-alkyl enamine int-2 (Fig. 1a). Such an enamine (int-2) has higher β-reactivity in trapping electrophiles than its −NH counterpart and lowers the formation of non-coupled cyclic amine A″. (ii) It is relatively difficult to reduce electron-rich enamine int-2 to the undesired cyclic amine A″.
Fig. 1. Diastereoselective construction of functional polycyclic N-heterocycles by hydride transfer-initiated intermolecular annulation of the azaarenes.
a The formation of N-alkyl enamine int-2. b ruthenium-catalyzed dearomative annulation reaction of azaarenium salts A with aniline derivatives B and paraformaldehyde. c Selected drugs and bioactive molecules.
Based on the above idea, we here report a dearomative annulation reaction of azaarenium salts A with aniline derivatives B and paraformaldehyde (Fig. 1b) under ruthenium(II) reductive catalysis, which offers a general way for diastereoselective construction of fused syn-N-heterocycles P featuring promising structural motifs of teterahydroquinoline and hexahydro-1,6-naphthyridine that are frequently found in natural alkaloids43,44 and biomedical molecules45–47, as exemplified by the leading anesthesia drug taripiprazole 1, active composition 2 used for treating EP1 receptor-mediated diseases45, PXR agonist 346, and anticancer agents 4 (Fig. 1c)47.
Results
Investigation of reaction conditions
We commenced our studies by performing the reaction of N-benzyl quinolinium bromide A1, N-ethylaniline B1, paraformaldehyde, and base in MeOH at 65 °C for 18 h by employing [RuCl2 (p-cymene)]2 as the catalyst. Among various bases and acids tested, Mg(OMe)2 exhibited the best chemo-selectivity since there is no formation of by-product N-benzyl tetrahydroquinoline A1″ (Table 1, entries 1, 4 and Supplementary Table 1 in the Supplementary Information (SI)). The absence of catalyst or base failed to yield product P1 (entries 5, 6), showing that both of them are indispensable for the product formation. Then, we screened several other metal catalysts applied frequently in hydrogen transfer reactions (see Supplementary Table 1 in SI). The results showed that Ir(I) or Ir(III) catalysts were also applicable, but the base metal catalysts (Co, Fe, Mn, and Ni) were totally ineffective for the transformation (entries 7, 8 and Supplementary Table 1). Here, we chose the cost-effective [Ru(p-cymene)Cl2]2 as the preferred catalyst to further evaluate the solvents and temperatures, it showed that methanol and 55 °C were more preferable (entries 9, 10). Decrease of the base or (CH2O)n amount diminished the product yields (entries 11, 12). Thus, the optimal yield of product P1 was obtained when the reaction in methanol was performed at 55 °C for 18 h by using the combination of [Ru(p-cymene)Cl2]2 and Mg(OMe)2 (entry 10). Interestingly, the use of Mg(OMe)2 base always resulted in excellent selectivity in affording product P1 (entries 3, and 7, 12).
Table 1.
The optimization of reaction conditionsa.
| Entry | Catalyst | Base | P1 (%)b | A1″ (%)b |
|---|---|---|---|---|
| 1 | [Ru(p-cymene)Cl2]2 | K2CO3 | <10 | 8 |
| 2 | [Ru(p-cymene)Cl2]2 | t-BuOK | < 10 | 42 |
| 3 | [Ru(p-cymene)Cl2]2 | Mg(OMe)2 | 90 | 0 |
| 4 | [Ru(p-cymene)Cl2]2 | MeOK | < 10 | 53 |
| 5 | - | Mg(OMe)2 | 0 | 0 |
| 6 | [Ru(p-cymene)Cl2]2 | – | 0 | 0 |
| 7 | [Cp*IrCl2]2 | Mg(OMe)2 | 85 | trace |
| 8 | [IrCl(COD)]2 | Mg(OMe)2 | 74 | trace |
| 9 | [Ru(p-cymene)Cl2]2 | Mg(OMe)2 | (0, 0, 61)c | (0, 0, 0)c |
| 10 | [Ru(p-cymene)Cl2]2 | Mg(OMe)2 | (58, 91, 86)d | (0, 0, 0)d |
| 11 | [Ru(p-cymene)Cl2]2 | Mg(OMe)2 | (33, 25)e | (0, 0)e |
| 12 | [Ru(p-cymene)Cl2]2 | Mg(OMe)2 | (42, 30)f | (0, 0)f |
Cp*: 1,2,3,4,5-pentamethylcyclopentadiene, cod: 1,5-cyclooctadiene, DMF: N,N-dimethylformamide.
aUnless otherwise stated, the reaction in MeOH (1 mL) was performed with A1 (0.2 mmol), B1 (0.2 mmol), cat. (1 mol%), base (0.75 eq), (CH2O)n (10 eq) at 65 °C for 18 h under N2 protection.
bNMR yield by using anisole as the internal standard.
cYields are with respect to the use of DMF, 1,4-dioxane, ethanol as the solvent, respectively.
dYields are with respect to the temperature at 45 °C, 55 °C, 75 °C, respectively.
eYields are with respect to 0.5 and 0.3 eq of Mg(OMe)2, respectively.
fYields are with respect to 8 and 5 eq (CH2O)n, respectively.
Substrate scope
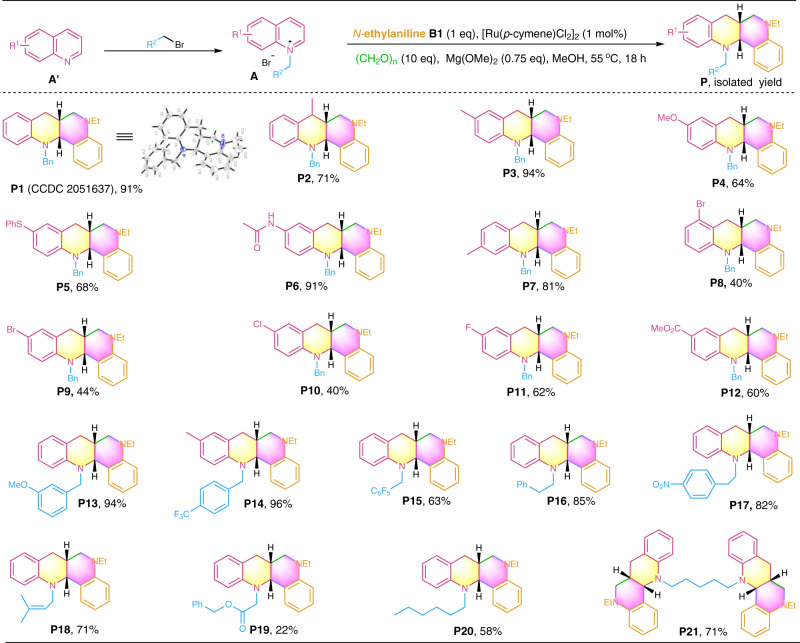
With the availability of the optimal reaction conditions (Table 1, entry 10), we then assessed the substrate scope of the newly developed synthetic protocol. As shown in Fig. 2, various quinolinium salts A (A1–A21, see Supplementary Fig. 1 in SI for their structures) in combination with N-ethylaniline B1 and paraformaldehyde were evaluated. Gratifyingly, all the reactions underwent smooth reductive annulation and furnished the desired fused N-heterocycles in reasonable to excellent isolated yields with excellent syn-diastereoselectivity (P1–P21, d.r. > 20:1). The structure of compound P1 was confirmed by X-ray crystallography diffraction and NOESY spectrum (Supplementary Fig. 3–5 and Supplementary Table 2 in SI). The application of 1,5-dibromopentane for di N-alkylation generated the intramolecular alkyl-linked product P21 in a good yield. Noteworthy, a variety of functionalities (e.g., −Me, −OMe, −SPh, amido, −F, −Cl, −Br, ester, −CF3, −NO2, alkenyl, and alkyl) on the quinolinium salts were well tolerated, and their electronic properties affected the product formation to some extent. Interestingly, no reduction of the nitro and alkenyl groups was observed (P17 and P18), and the halo-substrates also did not undergo hydrodehalogenation, indicating that the reaction proceeds in a chemoselective manner. In general, quinolines bearing an electron-donating group (P2–P7, and P13–P14) afforded relatively higher product yields than those having an electron-withdrawing group (P8–P10, and P12), presumably because the electron-rich quinolinium salts can result in more reactive enamine intermediates that are beneficial to the electrophilic coupling process (Fig. 1a). The retention of these functionalities offers the potential for post-functionalization of the obtained products.
Fig. 2. Diastereoselective construction of fused N-heterocycles P1 − P21 by variation of quinolines.
Reactions were conducted on a 0.2 mmol scale under the standard conditions. Isolated yields are reported.
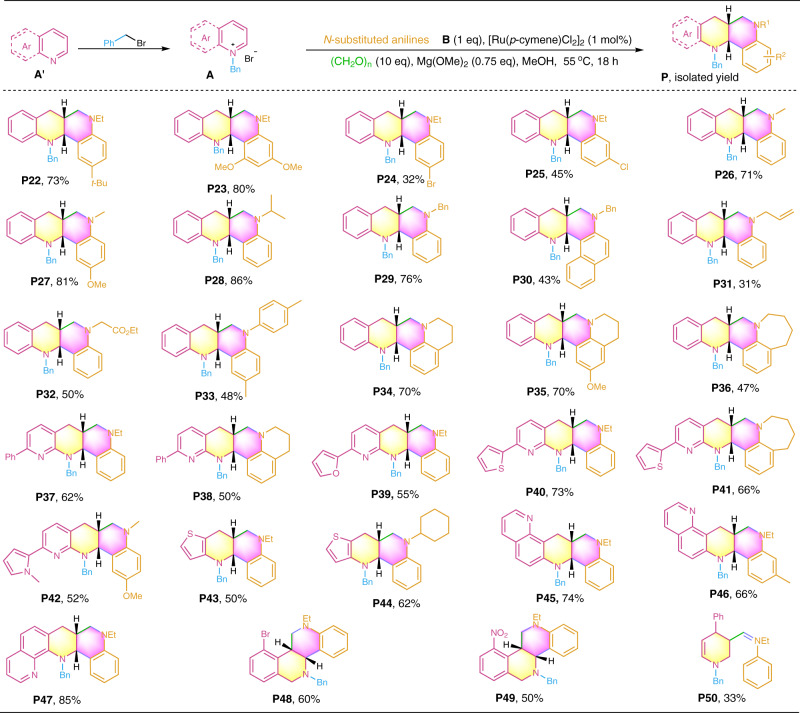
Next, we turned our attention to the synthesis of structurally diversified products by variation of both azaarenes A′ and anilines B. First, a series of N-alkyl anilines (B2–B18, see Supplementary Fig. 2 in SI for their structures) in combination with quinolinium salt A1 were tested. As illustrated in Fig. 3, all the reactions efficiently afforded the desired product in moderate to excellent isolated yields with exclusive syn-selectivity (P22–P36, d.r. > 20:1). The electronic properties of the substituents on the benzene ring of the anilines significantly affected the product formation. Especially, anilines containing electron-donating groups (P22–P23, P27 and P35) gave much higher yields than those with electron-withdrawing groups (P24–P25). This observation is attributed to electron-rich anilines favoring the electrophilic coupling process during the formation of the products. In addition to N-alkyl anilines, diarylamine B13 also served as an effective coupling partner, affording the N-aryl product P33 in a moderate yield. As expected, primary anilines were not applicable for the transformation, as they easily reacted with formaldehyde to form aminals. Interestingly, tetrahydroquinolines (B14 and B15) and 2,3,4,5-tetrahydro-1H-benzo[b]azepine (B16), two specific aniline derivatives, also underwent efficient multicomponent annulation to afford the polycyclic products (P34–P35, P38 and P41). In addition to quinolines, other azaarenes, such as 1,8-naphthyridines (A22–A25), thieno[3,2-b]pyridine A26, 1,7-phenanthroline A27, 1,10-phenanthroline A28, and 5-substituted isoquinolines (A29 and A30) were also amenable to the transformation, delivering the desired products in an efficient manner (P37–P49, d.r. > 20:1), these examples demonstrate the capability of the developed chemistry in the functionalization of pyridine-containing azaarenes including the N-bidentate ligands (P37–P42, P47). Unfortunately, more challenging pyridine derivative failed to yield the desired product, only β-aminoalkyl product (P50) was obtained.
Fig. 3. Diastereoselective access to fused N-heterocycles P22–P50 by variation of both azaarenes and anilines.
Reactions were conducted on a 0.2 mmol scale under the standard conditions. Isolated yields are reported.
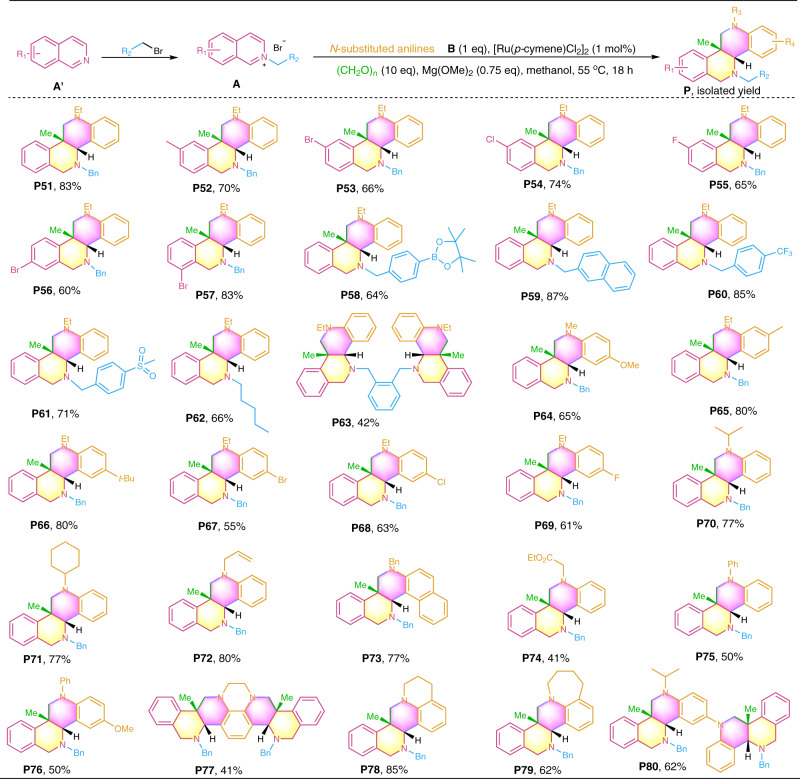
Noteworthy, 5-substituted isoquinolines afforded the desired annulation products (Fig. 3, P48 and P49), whereas 5-nonsubstituted isoquinolines generated products P by installing an additional methyl group at the β-site of the N-heteroaryl reactants, and all the products are produced with exclusive syn-diastereoselectivity (d.r. > 20:1, Supplementary Fig. 6). As shown in Fig. 4, N-benzyl isoquinolinium salts were firstly employed to couple with paraformaldehyde and N-ethylaniline B1. All the reactions gave rise to the desired annulation products in moderate to excellent yields upon isolation (P51–P63). Then, the transformation of secondary anilines including the N-alkyl and N-aryl ones was evaluated. Gratifyingly, all these anilines smoothly coupled with N-benzyl quinolium salt A1 and paraformaldehyde, delivering the annulation products in reasonable to high yields (P64–P76). Similar to the results described in Figs. 2 and 3, various functionalities on both isoquinolium salts and anilines are well tolerated (−Bn, −Et, −Me, −F, −Cl, −Br, boronic ester, −SO2Me, −n-hexyl, −OMe, −CF3, −CO2Me, alkenyl, cyclohexyl, and i-propyl). The substituents on the aryl ring of the isoquinoline salts have little influence on the product formation, whereas the substituents of the anilines significantly affected the product yields. Generally, aniline bearing an electron-donating group afforded higher yields (e.g., P64–P66 and P70–P73) than those of anilines with an electron-withdrawing group (e.g., P67–P69 and P74), suggesting that the reaction involves an electrophilic coupling process. Benzocyclic amines (1,2,3,4-tetrahydroquinoxaline, 1,2,3,4-tetrahydroquinoline, and 2,3,4,5-tetrahydro-1H-benzo[b]azepine) and N1-isopropyl-N4-phenylbenzene-1,4-diamine also served as effective coupling partners, affording the polycyclic products in moderate to high yields (P77–P80). These examples show the practicality of the developed chemistry in the construction of structurally complex polycyclic N-heterocycles.
Fig. 4. Diastereoselective access to fused N-heterocycles P51–P80 by employing various isoquinolinium salts.
Reactions were conducted on a 0.2 mmol scale under the standard conditions. Isolated yields are reported.
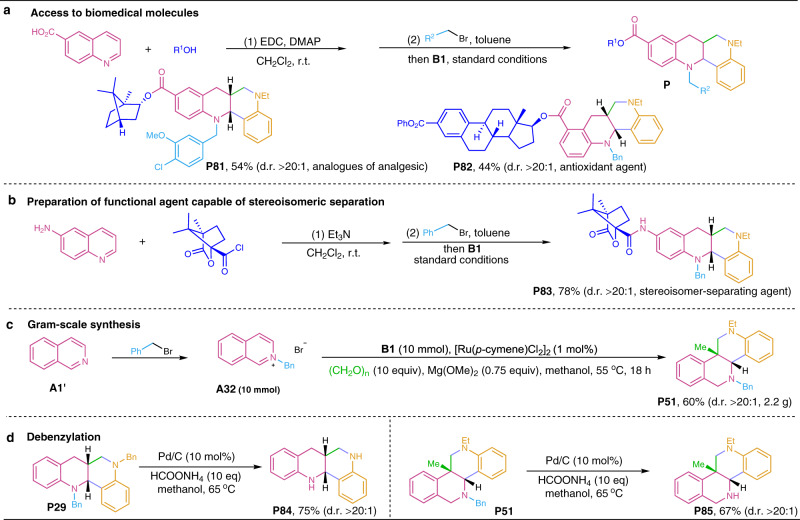
Synthetic applications
Further, we explored the synthetic applications of the developed chemistry. As shown in Fig. 5a, 6-ester quinolinium salts, arising from initial esterification of 6-carboxylic quinoline and subsequent pretreatment with benzyl bromide, efficiently reacted with aniline B1 and paraformaldehyde to afford products P81 and P82 (d.r. > 20:1), which are the analogs of analgesic48 and the agents used for antioxidation and antiproliferation49, respectively. Through successive amidation and formation of N-benzyl heteroarenium salt, 6-amino quinoline was efficiently transformed in combination with aniline B1 into camphanic amide P83 (d.r. > 20:1, Fig. 5b), an agent capable of stereoisomeric separation50. Further, the gram-scale synthesis of product P51 (d.r. > 20:1) was successfully achieved by scaling up the reactants to 10 mmol, which still gave a desirable yield (Fig. 5c). Interestingly, representative compounds P29 and P51 underwent efficient debenzylation to afford N-unmasked products P84 (d.r. > 20:1) and P85 (d.r. > 20:1) in the presence of a Pd/HCOONH4 system in methanol (Fig. 5d), which demonstrates the practicality of the developed chemistry in further preparation of fused heterocycles containing a useful −NH motif.
Fig. 5. Synthetic applications of the developed chemistry.
a, b Structural modification of biomedical molecules. c Gram-scale synthesis. d Debenzylation of P29 and P51.
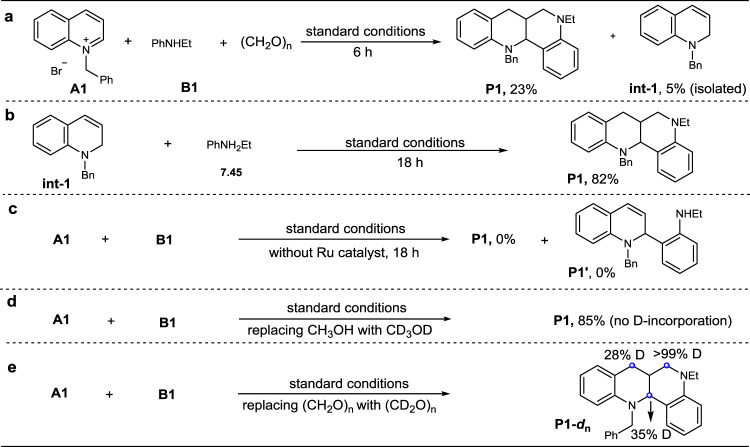
Mechanistic investigations
To gain mechanistic insights into the reaction, we conducted several control experiments (Fig. 6). First, the model reaction was interrupted after 6 hours to analyze the product system. Except for the formation of product P1 in 23% yield, a dihydroquinoline int-1 was isolated in 5% yield (Fig. 6a). Subjection of compound int-1 (Fig. 6a and Supplementary Fig. 8) with aniline B1 under the standard conditions resulted in product P1 in high isolated yield (Fig. 6b), showing that int-1 is a key reaction intermediate. However, removal of Ru-catalyst from the standard conditions failed to produce P1 and the α-arylated product P1′ (Fig. 6c), revealing that the reaction initiates with Ru-catalyzed hydrogen transfer, instead of nucleophilic arylation of substrate A1 with aniline B1. Further, the model reaction using deuterated methanol solvent yielded product P1 without any D-incorporation (Fig. 6d). In sharp contrast, the same reaction by replacing paraformaldehyde with the fully deuterated one gave product P1-dn with 35% and 28% D-ratios at the α and γ-sites and more than 99% D-ratio at the newly formed aminomethyl group (Fig. 6e and Supplementary Fig. 10). These two crucial experiments show that the formaldehyde serves as both the source of the reductant and C1-building block for the formation of the newly formed β-methylene group, and the initial reduction of A1 to give either int-1 or int-2 is reversible (Fig. 1a). In parallel, we conducted the control experiments in terms of the generation of product P51 (Supplementary Fig. 7 in SI). The results also support that dihydroquinoline int-6 (Supplementary Fig. 7b) and β-methyl dihydroquinoline int-9 (Supplementary Fig 9) are the reaction intermediates, and formaldehyde serves as the reductant source and C1-building block in the construction of the product (Supplementary Fig. 7d, e and Supplementary Fig. 11).
Fig. 6. Control experiments for mechanistic studies.
a Intermediate Detection. b Intermediate verification. c Ruling out possible reaction step. d, e Deuterium-labeling experiments to verify hydrogen donor and C1 source.
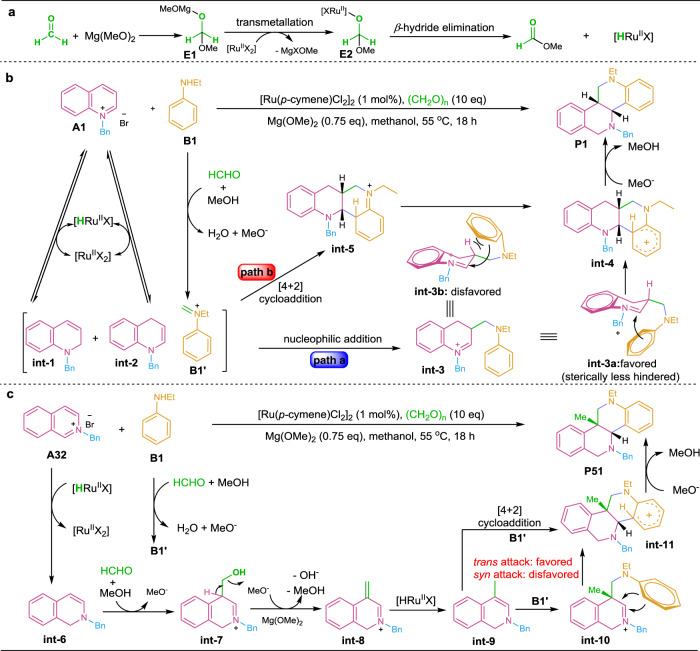
Based on the above findings, the plausible pathways toward the formation products P1 and P51 are depicted in Fig. 7. Initially, the metal hydride species [RuIIHX] is generated via Mg(OMe)2 addition to formaldehyde (E1) followed by transmetallation (E2) with [RuIIX2] and β-hydride elimination and release of formate ester (detected by GC-MS analysis, Supplementary Fig. 12). Then, the hydride transfer from [RuIIHX] to quinolinium salt A1 forms dihydroquinoline int-1 and its enamine tautomer int-2 along with regeneration of the catalyst precursor [RuIIX2]. Meanwhile, the condensation between aniline B1 and formaldehyde affords iminium B1′. Then, the β-nucleophilic addition of int-2 to B1′ gives the β-aminoalkyl iminium int-3. Further, the electron-rich benzene ring of int-3 attacks the iminium motif from both the same (int-3b) and opposite (int-3a) sides of the H-atom at the β-site. In comparison, the form of int-3a (opposite side) is more favorable due to the less steric hindrance, thus affording product P1 with syn-selectivity after deprotonation of the coupling adduct int-4 (path a of Fig. 7b, namely electrophilic aryl C–H aminoalkylation). Alternatively, the [4 + 2] cycloaddition of int-2 and B1′ via endo or exo π-π stacking also rationalizes the formation of int-4 and product P1 (path b of Fig. 7b, via int-5 and int −4). Similarly, the generation of product P51 from isoquinoline is shown in Fig. 6c. The hydride transfer from [RuIIHX] to isoquinolinium salt A32 initially forms enamine int-6 (Supplementary Fig. 7a, b and Fig. 8). Then, the β-capture of formaldehyde by int-6 followed by based-facilitated dehydration of int-7 and hydride transfer to alkenyl iminium salt int-8 forms β-methyl enamine int-9 (Supplementary Fig. 7a and Fig. 9). Subsequently, the β-capture of B1′ by int-9 followed by intramolecular attack of the electron-rich phenyl ring to the iminium motif of int-10 from the sterically less hindered back side of the methyl group, or the [4 + 2] cycloaddition of int-9 and B1′ via π-π stacking gives intermediate int-11. Finally, the deprotonation of int-11 generates product P51 with syn-diastereoselectivity (Fig. 7c).
Fig. 7. Plausible reaction mechanism.
a The production of metal hydride species. b Possible pathways for the formation product P1. c Possible pathway for the formation product P51.
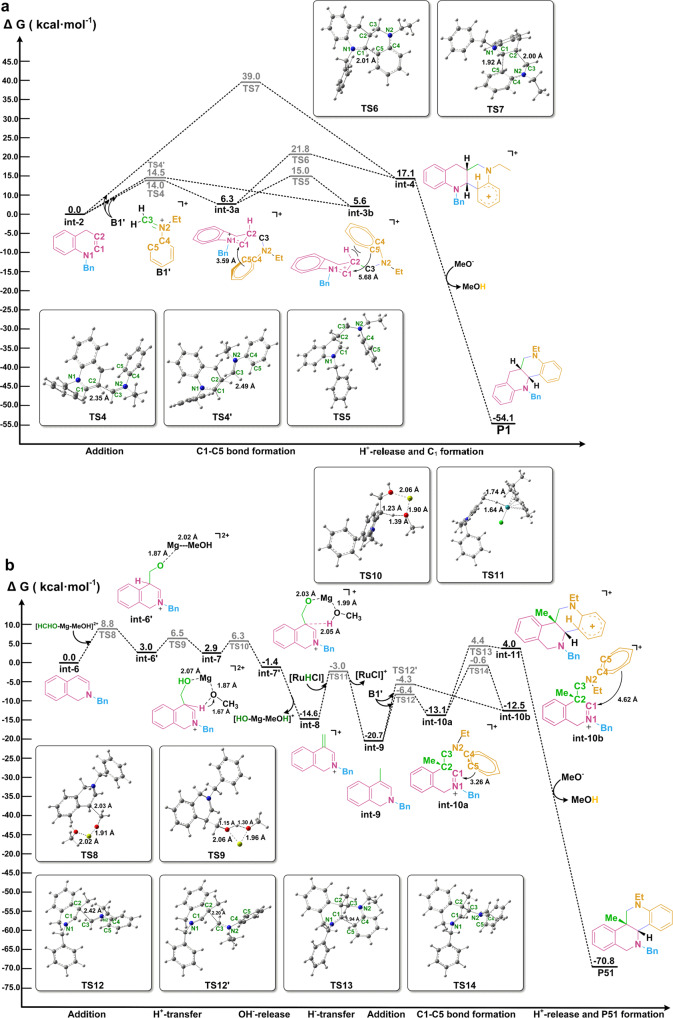
To better reveal the selective formation of products P1 and P51, computational study was performed using the density functional theory and the relevant data was listed in the Supplementary Dataset file. First, the participation of Mg(OMe)2, MeOK, and t-BuOK as the bases in the generation of [RuIIHCl] was calculated. The energy for the transmetallation step with Mg(OMe)2 (int-22 → int-23, ΔG = 10.1 kcal mol−1 in supplementary Fig. 13) is significantly higher than the other two bases (int-26 → int-27, ΔG = −3.6 kcal mol−1 for t-BuOK in supplementary Fig. 14; int-30 → int-23, ΔG = −0.6 kcal mol−1 for MeOK in supplementary Fig. 15). The results reveal that the Mg2+ ion can better stabilize adduct E1 (Fig. 7a) and make the dissociation of -MgOMe as well as the transmetallation process more difficult, thus resulting in a slow forming rate of [RuIIHCl]. Correspondingly, a slow generation of enamine int-2 via hydride transfer from [RuIIHCl] to azaarenium salt A1 is beneficial to the capture of int-2 by B1′, and effectively suppresses the formation of undesired N-benzyl tetrahydroquinoline A1″ (Table 1).
Next, the free energy profile for the conversion of int-2 and B1′ to P1 is depicted in Fig. 8a and supplementary Fig. 16. The formation of int-3a and int-3b via β-nucleophilic addition of int-2 to B1′ has the energy barriers of 14.0 kcal mol−1 (TS4) and 14.5 kcal mol−1 (TS4′), respectively, representing endothermic processes (ΔG = 6.3 kcal·mol−1 and ΔG = 5.6 kcal·mol−1). The only difference between int-3a and int-3b is the dihedral of C2-C3-N2-C4 (int-3a, −76.0° vs int-3b, 106.9°), and the conversion of int-3a to int-3b is rather easy with only an energy barrier of 8.7 kcal·mol−1. However, int-3b is not a favorable intermediate, as it has high stereoscopic hindrance of the N-ethyl group and pyridyl β-H as well as the long distance (~5.7 Å) between the pyridyl α-carbon (C1) and aniline ortho-carbon (C5). Therefore, int-3a becomes a favorable intermediate, and the attack of electron-rich aryl group to electron-deficient iminium motif generates int-4 by intramolecular C1–C5 bond formation with an energy barrier of 15.5 kcal mol−1 (TS6). Finally, the formation of product P1, via deprotonative aromatization of int-4, is a thermodynamically favorable process from the energetic point of view (ΔG = −54.1 kcal mol−1). In terms of the [4 + 2] cycloaddition of B1′ and int-2, the manner of endo π-π stacking encountered commonly in the Diels-Alder reactions has a significant energy barrier of 39.0 kcal mol−1 (TS7). So, this pathway is disfavored. Meanwhile, the calculation results show that the manner of exo π-π stacking is also difficult to take place due to the higher steric hindrance and long interaction distance. Based on the computational studies, path a shown in Fig. 7b is believed to be a favorable way in generating product P1.
Fig. 8. DFT studies (free energies in kcal mol−1).
a Potential energy surfaces for the process from int-2 to P1. b Potential energy surfaces for the process from int-6 to P51.
Further, the calculation results for the generation of product P51 are depicted in Fig. 8b and supplementary Fig. 17. The formation of requisite intermediate int-9 involves four main steps: (i) the β-addition of int-6 to HCHO (int-6 → int-6′), (ii) proton transfer from the methanol (int-6′ → int-7), (iii) Mg(OMe)2-induced proton abstraction and dissociation of OH- (int-7 → int-7′ → int-8), and (iv) hydride transfer (int-8 → int-9). Noteworthy, from step (i) to (iii), the formation of int-8 clearly proceeds under the assistance of Mg2+, and these steps can easily take place with a maximum energy barrier of 8.8 kcal·mol−1 (TS8). Next, the formation of int-9 by hydride transfer from [RuIIHCl] complex to int-8 proceeds with an energy barrier of 11.6 kcal·mol−1 (TS11), which is exothermic process (ΔG = − 6.1 kcal mol−1). Once int-9 is formed, the generation product P51 proceeds with a similar way of Fig. 8a: β-addition of int-9 to B1′, intramolecular cyclization via C1–C5 bond formation, and based-promoted deprotonation of int-11 to yield product P51. The calculation results show that the processes from int-9 to P51 have a slightly higher barrier than that of generating P1 in Fig. 8a (ΔG≠ = 17.5 kcal mol−1 for TS13 vs ΔG≠ = 15.5 kcal mol−1 for TS6).
In summary, by a strategy incorporating a tandem coupling sequence into the reduction of azaarenium salts, we have developed a intermolecular syn-diastereoselective annulation reaction by reductive ruthenium(II) catalysis. A variety of azaarenes were efficiently transformed in combination with a large variety of aniline derivatives into fused N-heterocycles by employing paraformaldehyde as both a crucial agent to generate active ruthenium(II)-hydride species and a C1-building block, proceeding with readily available feedstocks, excellent selectivity, mild conditions, and broad substrate and functional group compatibility. The present work has established a practical platform for the transformation of ubiquitously distributed but weakly reactive azaarenes into functional organic frameworks that are difficult accessible with the existing approaches, and further discovery of bioactive and drug-relevant molecules due to the promising potentials of the obtained compounds featuring the teterahydroquinolyl and hexahydro-1,6-naphthyridyl motifs. Mechanistic studies reveal that the products are formed via hydride transfer-initiated β-aminomethylation and α-arylation of the azaarenium salts, and the use of Mg(OMe)2 as a base plays a critical role in determining the reaction chemo-selectivity by lowering the hydrogen transfer rate. The work presented fills an important gap in the capabilities of utilizing azaarenes as the synthons to access fused N-heterocycles, and opens a door to further develop valuable reductive functionalization of inert unsaturated systems by taking profit of formaldehyde-endowed two functions.
Methods
Typical procedure I for the synthesis of product P1
Under N2 atmosphere, [Ru(p-cymene)Cl2]2 (1 mol %), 1-benzylquinolin-1-ium bromide A1 (0.2 mmol), N-ethylaniline B1 (0.2 mmol), Mg(OMe)2 (0.75 eq, 12.9 mg), (CH2O)n (10.0 eq, 60 mg) and methanol (1 mL) were introduced in a Schlenk tube, successively. Then the Schlenk tube was closed and the resulting mixture was stirred at 55 °C for 18 h. After cooling down to room temperature, the mixture was extracted with ethyl acetate, washed with 5% Na2CO3 solution, dried with anhydrous sodium sulfate, and then concentrated by removing the solvent under vacuum. Finally, the residue was purified by preparative TLC on silica to give 12-benzyl-5-ethyl-5,6,6a,7,12,12a-hexahydrodibenzo[b,h][1,6]naphthyridine P1.
See Supplementary methods for the structure and synthesis methods of the employed substrates, intermediates and utility. The analytical data and NMR spectra of all obtained compounds (P1–P85, int-1 and int-6) are presented within Supplementary Information. See Supplementary Figs. 18–197 for NMR spectroscopic data of all compounds.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This paper is dedicated to Professor Matthias Beller on the occasion of his 60th birthday. We thank the National Natural Science Foundation of China (21971071, 22163007), Natural Science Foundation of Guangdong Province (2021A1515010155), the Fundamental Research Funds for the Central Universities (2020ZYGXZR075), and Guizhou Province Science Foundation ([2020]1Y050) for financial support.
Author contributions
M.Z. conceived the idea, analyzed the data, directed the project, and wrote the manuscript. H.Z. and Y.W. carried out all the catalytic experiments. H.Z. drew the structures of all the obtained compounds and analyzed the single crystal structures. C.-G.C. performed the DFT calculations. Z.-D.T. and J.Y. synthesized the raw material. H.-F.J. and P.H.D. discussed the mechanistic aspects and revised the manuscript. All the authors have read the manuscript and agree with its content.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 2051637 (P1). The data can be obtained free of charge from The Cambridge Crystallographic Data Centre [http://www.ccdc.cam.ac.uk/data_request/cif]. The data supporting the findings of this study are available within the article and its Supplementary Information files. Any further relevant data are available from the authors on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: He Zhao, Yang Wu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-29985-z.
References
- 1.Taylor RD, MacCoss M, Lawson ADG. Rings in drugs. J. Med. Chem. 2014;57:5845–5859. doi: 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]
- 2.Bunz UHF, Freudenberg J. N‑heteroacenes and N-heteroarenes as N‑nanocarbon segments. Acc. Chem. Res. 2019;52:1575–1587. doi: 10.1021/acs.accounts.9b00160. [DOI] [PubMed] [Google Scholar]
- 3.Li G, et al. Ruthenium-catalyzed meta-selective C-H sulfonation of azoarenes with arylsulfonyl chlorides. Org. Chem. Front. 2017;4:1145–1148. doi: 10.1039/C7QO00004A. [DOI] [Google Scholar]
- 4.Moors SLC, Deraet X, Van Assche G, Geerlings P, De Proft F. Aromatic sulfonation with sulfur trioxide: mechanism and kinetic model. Chem. Sci. 2017;8:680–688. doi: 10.1039/C6SC03500K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandeepan P, et al. 3d Transition metals for C-H activation. Chem. Rev. 2019;119:2192–2452. doi: 10.1021/acs.chemrev.8b00507. [DOI] [PubMed] [Google Scholar]
- 6.Arockiam PB, Bruneau C, Dixneuf PH. Ruthenium(II)-catalyzed C-H bond activation and functionalization. Chem. Rev. 2012;112:5879–5918. doi: 10.1021/cr300153j. [DOI] [PubMed] [Google Scholar]
- 7.Proctor RSJ, Phipps RJ. Recent advances in Minisci-type reactions. Angew. Chem. Int. Ed. 2019;58:13666–13699. doi: 10.1002/anie.201900977. [DOI] [PubMed] [Google Scholar]
- 8.Zhou W-J, et al. Reductive dearomative arylcarboxylation of indoles with CO2 via visible-light photoredox catalysis. Nat. Commun. 2020;11:3263. doi: 10.1038/s41467-020-17085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai L, et al. Palladium/norbornene-catalyzed C-H alkylation/alkyne insertion/indole dearomatization domino reaction: assembly of spiroindolenine-containing pentacyclic frameworks. Angew. Chem. Int. Ed. 2018;57:5151–5155. doi: 10.1002/anie.201801894. [DOI] [PubMed] [Google Scholar]
- 10.Xia Z-L, Zheng C, Wang S-G, You S-L. Catalytic asymmetric dearomatization of indolyl dihydropyridines through an enamine isomerization/spirocyclization/transfer hydrogenation sequence. Angew. Chem. Int. Ed. 2018;57:2653–2656. doi: 10.1002/anie.201712435. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Zheng C, You SL. Iridium-catalyzed asymmetric allylic dearomatization by a desymmetrization strategy. Angew. Chem. Int. Ed. 2015;54:15093–15097. doi: 10.1002/anie.201708419. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Ibanez MA, Macia B, Pizzuti MG, Minnaard AJ, Feringa BL. Catalytic enantioselective addition of dialkylzinc reagents to N-acylpyridinium salts. Angew. Chem. Int. Ed. 2009;48:9339–9341. doi: 10.1002/anie.200904981. [DOI] [PubMed] [Google Scholar]
- 13.Kubota K, Watanabe Y, Hayama K, Ito H. Enantioselective synthesis of chiral piperidines via the stepwise dearomatization/borylation of pyridines. J. Am. Chem. Soc. 2016;138:4338–4341. doi: 10.1021/jacs.6b01375. [DOI] [PubMed] [Google Scholar]
- 14.Gribble MW, Jr., Guo S, Buchwald SL. Asymmetric Cu-catalyzed 1,4-dearomatization of pyridines and pyridazines without preactivation of the heterocycle or nucleophile. J. Am. Chem. Soc. 2018;140:5057–5060. doi: 10.1021/jacs.8b02568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afanasyev OI, Kuchuk E, Usanov DL, Chusov D. Reductive amination in the synthesis of pharmaceuticals. Chem. Rev. 2019;119:11857–11911. doi: 10.1021/acs.chemrev.9b00383. [DOI] [PubMed] [Google Scholar]
- 16.Irrgang T, Kempe R. Transition-metal-catalyzed reductive amination employing hydrogen. Chem. Rev. 2020;120:9583–9674. doi: 10.1021/acs.chemrev.0c00248. [DOI] [PubMed] [Google Scholar]
- 17.Elangovan S, et al. Efficient and selective N-alkylation of amines with alcohols catalysed by manganese pincer complexes. Nat. Commun. 2016;7:12641. doi: 10.1038/ncomms12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pena-Lopez M, Piehl P, Elangovan S, Neumann H, Beller M. Manganese-catalyzed hydrogen-autotransfer C-C bond formation: alpha-alkylation of ketones with primary alcohols. Angew. Chem. Int. Ed. 2016;55:14967–14971. doi: 10.1002/anie.201607072. [DOI] [PubMed] [Google Scholar]
- 19.Deibl N, Kempe R. General and mild cobalt-catalyzed C-alkylation of unactivated amides and esters with alcohols. J. Am. Chem. Soc. 2016;138:10786–10789. doi: 10.1021/jacs.6b06448. [DOI] [PubMed] [Google Scholar]
- 20.Kallmeier F, Fertig R, Irrgang T, Kempe R. Chromium-catalyzed alkylation of amines by alcohols. Angew. Chem. Int. Ed. 2020;59:11789–11793. doi: 10.1002/anie.202001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mastalir M, et al. Divergent coupling of alcohols and amines catalyzed by isoelectronic hydride Mn-I and Fe-II PNP pincer complexes. Chem. Eur. J. 2016;22:12316–12320. doi: 10.1002/chem.201603148. [DOI] [PubMed] [Google Scholar]
- 22.Mastalir M, et al. Air stable Iron(II) PNP pincer complexes as efficient catalysts for the selective alkylation of amines with alcohols. Adv. Synth. Catal. 2016;358:3824–3831. doi: 10.1002/adsc.201600689. [DOI] [Google Scholar]
- 23.Wang Y, Shao Z, Zhang K, Liu Q. Manganese-catalyzed dual-deoxygenative coupling of primary alcohols with 2-arylethanols. Angew. Chem. Int. Ed. 2018;57:15143–15147. doi: 10.1002/anie.201809333. [DOI] [PubMed] [Google Scholar]
- 24.Fu S, Shao Z, Wang Y, Liu Q. Manganese-catalyzed upgrading of ethanol into 1-butanol. J. Am. Chem. Soc. 2017;139:11941–11948. doi: 10.1021/jacs.7b05939. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, et al. Manganese-catalyzed selective upgrading of ethanol with methanol into isobutanol. ChemSusChem. 2019;12:3069–3072. doi: 10.1002/cssc.201802689. [DOI] [PubMed] [Google Scholar]
- 26.Huang F, Liu Z, Yu Z. C-Alkylation of ketones and related compounds by alcohols: transition-metal-catalyzed dehydrogenation. Angew. Chem. Int. Ed. 2016;55:862–875. doi: 10.1002/anie.201507521. [DOI] [PubMed] [Google Scholar]
- 27.Xu ZJ, Yu XL, Sang XX, Wang DW. BINAP-copper supported by hydrotalcite as an efficient catalyst for the borrowing hydrogen reaction and dehydrogenation cyclization under water or solvent-free conditions. Green. Chem. 2018;20:2571–2577. doi: 10.1039/C8GC00557E. [DOI] [Google Scholar]
- 28.Vellakkaran M, Singh K, Banerjee D. An efficient and selective nickel-catalyzed direct N-alkylation of anilines with alcohols. ACS Catal. 2017;7:8152–8158. doi: 10.1021/acscatal.7b02817. [DOI] [Google Scholar]
- 29.Wang DW, Zhao KY, Xu CY, Miao HY, Ding YQ. Synthesis, structures of benzoxazolyl iridium(III) complexes, and applications on C-C and C-N bond formation reactions under solvent-free conditions: catalytic activity enhanced by noncoordinating anion without silver effect. ACS Catal. 2014;4:3910–3918. doi: 10.1021/cs5009909. [DOI] [Google Scholar]
- 30.Spielmann K, Xiang M, Schwartz LA, Krische MJ. Direct conversion of primary alcohols to 1,2-amino alcohols: enantioselective iridium-catalyzed carbonyl reductive coupling of phthalimido-allene via hydrogen auto-transfer. J. Am. Chem. Soc. 2019;141:14136–14141. doi: 10.1021/jacs.9b08715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swyka RA, et al. Conversion of aldehydes to branched or linear ketones via regiodivergent rhodium-catalyzed vinyl bromide reductive coupling-redox isomerization mediated by formate. J. Am. Chem. Soc. 2019;141:6864–6868. doi: 10.1021/jacs.9b03113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doerksen RS, Meyer CC, Krische MJ. Feedstock reagents in metal-catalyzed carbonyl reductive coupling: minimizing preactivation for efficiency in target-oriented synthesis. Angew. Chem. Int. Ed. 2019;58:14055–14064. doi: 10.1002/anie.201905532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan K, et al. Iridium-catalyzed oxidant-free dehydrogenative C-H bond functionalization: selective preparation of N-arylpiperidines through tandem hydrogen transfers. Angew. Chem. Int. Ed. 2012;51:8876–8880. doi: 10.1002/anie.201204582. [DOI] [PubMed] [Google Scholar]
- 34.Sundararaju B, Achard M, Sharma GVM, Bruneau C. sp(3) C-H bond activation with ruthenium(II) catalysts and C(3)-alkylation of cyclic amines. J. Am. Chem. Soc. 2011;133:10340–10343. doi: 10.1021/ja203875d. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, et al. Formal direct cross-coupling of phenols with amines. Angew. Chem. Int. Ed. 2015;54:14487–14491. doi: 10.1002/anie.201506751. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z, Zeng H, Gong H, Wang H, Li C-J. Palladium-catalyzed reductive coupling of phenols with anilines and amines: efficient conversion of phenolic lignin model monomers and analogues to cyclohexylamines. Chem. Sci. 2015;6:4174–4178. doi: 10.1039/C5SC00941C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie F, et al. Direct reductive quinolyl beta-C-H alkylation by multispherical cavity carbon-supported cobalt oxide nanocatalysts. ACS Catal. 2017;7:4780–4785. doi: 10.1021/acscatal.7b01337. [DOI] [Google Scholar]
- 38.Grozavu A, et al. The reductive C3 functionalization of pyridinium and quinolinium salts through idium-catalysed interrupted transfer tydrogenation. Nat. Chem. 2019;11:242–247. doi: 10.1038/s41557-018-0178-5. [DOI] [PubMed] [Google Scholar]
- 39.Reeves BM, Hepburn HB, Grozavu A, Lindsay-Scott PJ, Donohoe TJ. Transition-metal-free reductive hydroxymethylation of isoquinolines. Angew. Chem. Int. Ed. 2019;58:15697–15701. doi: 10.1002/anie.201908857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grozavu A, Hepburn HB, Bailey EP, Lindsay-Scott PJ, Donohoe TJ. Rhodium catalysed C-3/5 methylation of pyridines using temporary tearomatisation. Chem. Sci. 2020;11:8595–8599. doi: 10.1039/D0SC02759F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang WX, Yu CB, Ji Y, Liu LJ, Zhou YG. Iridium-catalyzed asymmetric hydrogenation of heteroaromatics bearing a hydroxyl group, 3-hydroxypyridinium salts. ACS Catal. 2016;6:2368–2371. doi: 10.1021/acscatal.5b02625. [DOI] [Google Scholar]
- 42.Ye ZS, et al. Enantioselective iridium-catalyzed hydrogenation of 1-and 3-substituted isoquinolinium salts. Angew. Chem. Int. Ed. 2013;52:3685–3689. doi: 10.1002/anie.201208300. [DOI] [PubMed] [Google Scholar]
- 43.Muthukrishnan I, Sridharan V, Carlos Menendez J. Progress in the chemistry of tetrahydroquinolines. Chem. Rev. 2019;119:5057–5191. doi: 10.1021/acs.chemrev.8b00567. [DOI] [PubMed] [Google Scholar]
- 44.Sridharan V, Suryavanshi PA, Carlos Menendez J. Advances in the chemistry of tetrahydroquinolines. Chem. Rev. 2011;111:7157–7259. doi: 10.1021/cr100307m. [DOI] [PubMed] [Google Scholar]
- 45.Aguilar, N. et al. Substituted tricyclic compounds with activity two towards EP1 receptors. WO 2013/149996 A1 (2013).
- 46.Cerra B, et al. Exploiting chemical toolboxes for the expedited generation of tetracyclic quinolines as a novel class of PXR agonists. Acs. Med. Chem. Lett. 2019;10:677–681. doi: 10.1021/acsmedchemlett.8b00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin-Encinas E, et al. Synthesis of novel hybrid quinolino[4,3-b][1,5]naphthyridines and quinolino[4,3-b][1,5]naphthyridin-6(5H)-one derivatives and biological evaluation as topoisomerase I inhibitors and antiproliferatives. Eur. J. Med. Chem. 2020;195:112292. doi: 10.1016/j.ejmech.2020.112292. [DOI] [PubMed] [Google Scholar]
- 48.Lee WH, et al. Small molecule inhibitors of PSD95-nNOS protein-protein interactions as novel analgesics. Neuropharmacology. 2015;97:464–475. doi: 10.1016/j.neuropharm.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klisuric OR, et al. Structural analysis and biomedical potential of novel salicyloyloxy estrane derivatives synthesized by microwave irradiation. Struct. Chem. 2016;27:947–960. doi: 10.1007/s11224-015-0678-5. [DOI] [Google Scholar]
- 50.Licea-Perez H, et al. Camphanic acid chloride: a powerful derivatization reagent for stereoisomeric separation and its DMPK applications. Bioanalysis. 2015;7:3005–3017. doi: 10.4155/bio.15.219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 2051637 (P1). The data can be obtained free of charge from The Cambridge Crystallographic Data Centre [http://www.ccdc.cam.ac.uk/data_request/cif]. The data supporting the findings of this study are available within the article and its Supplementary Information files. Any further relevant data are available from the authors on request.