Summary
Optimal health and development from preconception to adulthood are crucial for human flourishing and the formation of human capital. The Nurturing Care Framework, as adapted to age 20 years, conceptualises the major influences during periods of development from preconception, through pregnancy, childhood, and adolescence that affect human capital. In addition to mortality in children younger than 5 years, stillbirths and deaths in 5–19-year-olds are important to consider. The global rate of mortality in individuals younger than 20 years has declined substantially since 2000, yet in 2019 an estimated 8·6 million deaths occurred between 28 weeks of gestation and 20 years of age, with more than half of deaths, including stillbirths, occurring before 28 days of age. The 1000 days from conception to 2 years of age are especially influential for human capital. The prevalence of low birthweight is high in sub-Saharan Africa and even higher in south Asia. Growth faltering, especially from birth to 2 years, occurs in most world regions, whereas overweight increases in many regions from the preprimary school period through adolescence. Analyses of cohort data show that growth trajectories in early years of life are strong determinants of nutritional outcomes in adulthood. The accrual of knowledge and skills is affected by health, nutrition, and home resources in early childhood and by educational opportunities in older children and adolescents. Linear growth in the first 2 years of life better predicts intelligence quotients in adults than increases in height in older children and adolescents. Learning-adjusted years of schooling range from about 4 years in sub-Saharan Africa to about 11 years in high-income countries. Human capital depends on children and adolescents surviving, thriving, and learning until adulthood.
This is the first in a Series of four papers about optimising child and adolescent health and development
Introduction
Children's optimal health and development are the ambition of families everywhere and are central to the formation of human capital. The World Bank's Human Capital Project uses the Human Capital Index, which includes measures of mortality, growth, and education, to assess how countries invest in the capabilities and economic potential of citizens.1 A broadened concep-tualisation of human capital should include health and wellbeing, the knowledge, and interpersonal and socioemotional skills needed to fulfil individual and societal potential. Human capital is formed through intergenerational factors and interactive biological–environmental–behavioural processes; it originates before conception and extends throughout childhood, adolescence, and beyond.2, 3
The Sustainable Development Goals (SDGs), agreed to by all UN member states, provide a pathway to human flourishing and the development of human capital.4 During the 25 years of the Millennium Development Goals (MDGs)5 era, from 1990 to 2015, child mortality was halved, including in many of the world's poorest countries, through societal progress and direct health interventions.6 Achieving the ambitious SDGs in the 15 year timeframe (2015–30) will require faster progress. The SDGs are broader than the MDGs, with an ambitious agenda. First, they go beyond survival to children thriving,4 including goals for nutrition, child development,7 and education;8 all important for human capital formation.3 Second, they adopt a life course approach, which extends beyond 5 years, to include school-age children and adolescents.2 Third, the SDGs are more ambitious than the MDGs and aim for wider transformation in health and educational systems and other changes in multiple sectors that affect health and wellbeing.4 Finally, the SDGs extend beyond a focus on health and education to include economic and environmental goals, along with the social responsibility necessary to ensure sustainability.
The four papers in this Series on optimising child and adolescent health and development aim to explore the determinants and building blocks of thriving, from preconception through fetal development up to 20 years of age. In this first Series paper, we consider conditions of survival, growth, disability, and education in world regions and evidence from longitudinal studies on the crucial periods in the life-cycle before adulthood that build the foundation for human capital. Subsequent Series papers consider the importance of inequities in the determinants of human capital,9 interventions that have proven to be of benefit,10 the important need to improve quality of health services,11 and the way forward to enhance global and national commitments for the health and development of children and adolescents.12
Key messages.
-
•
Human capital requires a foundation of health, knowledge, skills, and learning acquired from preconception to 20 years of age; healthy growth and development from conception to the second birthday are crucial
-
•
Mortality rates before 20 years of age are important measures of the survival component of human capital; of the 8·6 million deaths before 20 years of age in 2019, more than half were stillbirths or occurred in the first month after birth
-
•
Regional disparities are large with children in south Asia and sub-Saharan Africa having the worst chance to survive and thrive
-
•
The Nurturing Care Framework, as adapted to extend from preconception through childhood and adolescence, conceptualises influences on the formation of human capital and identifies crucial periods for interventions
Nurturing care and human capital
The interactive biological–environmental–behavioural processes that determine a child's health and development operate through time-dependent crucial and sensitive periods.13 Factors preceding conception and exposures in the prenatal and early postnatal periods lay the foundation for future health and wellbeing. Adversities can disrupt the course of development—operating through neurobiological processes, such as inflammation and dysregulation of the hypothalamic–pituitary axis—which undermines human capital accrual by increasing the vulnerability of children's health, development, and learning potential. However, human development operates as a dynamic system with mediators and moderators that can alter the life course by mitigating the negative consequences associated with adversities and promoting adaptive processes through protective factors.14 A conceptual framework to inform the accrual of human capital should incorporate the biological–environmental–behavioural life course processes, the time-dependent periods, and the dynamic changes resulting from mediating and moderating mechanisms to guide policies and programmes that enable children and adolescents to survive and thrive.
Nurturing care is an evidence-based, dynamic framework that was initially proposed to inform policies that would ensure that young children could reach their full developmental potential.7, 15 Nurturing care is activated through a stable environment and behaviours that ensure good health and nutrition, provides protection from threats, and ensures opportunities for learning through relationships that are emotionally supportive and responsive.15 As a dynamic framework, nurturing care is responsive to mechanisms that mitigate adversities, enhance resilience, and promote the wellbeing of marginalised groups.14
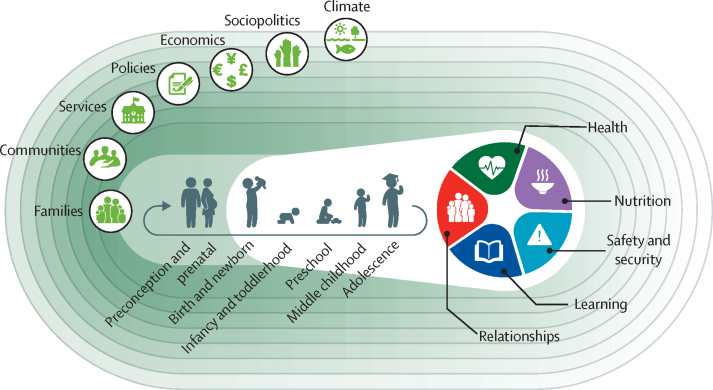
Nurturing care has been adapted to include deter-minants of human capital accrual, beginning with preparation for conception, and continuing through the provision of health care, nutrition, and learning opportunities in the context of responsive care throughout pregnancy, childhood, and adolescence, with support from the community and family-centred services and policies.16 Nurturing care is particularly important during the time-dependent crucial and sensitive periods when neural plasticity is high, and opportunities for mitigation of adversities are strong.17 The benefits of nurturing care continue throughout childhood and adolescence. With supportive parenting, ongoing learning, participation, relationship negotiation, and coping with adversities, children build the crucial components of human capital. In addition to gaining academic skills, children learn to modulate their emotions and behaviour, assume responsibilities for themselves and others, and become increasingly self-efficacious.7, 18 With increasing agency and autonomy, adolescents play more important roles in shaping relationships and developing learning and nutritional environments.18 Nurturing care is an evidence-based framework that integrates health and nutrition, the focus of this Series paper, along with responsive caregiving, learning, security, and safety to promote equity and build human capital from preconception through adolescence (figure 1).16 Families play a central role in the process, supported by a comprehensive, multisectoral system of services and opportunities that include nurturing care and support the development of human capital (appendix p 3).
Figure 1.
The Nurturing Care Framework from preconception to adolescence
Adapted from Black and colleagues.16
Surviving to age 20 years
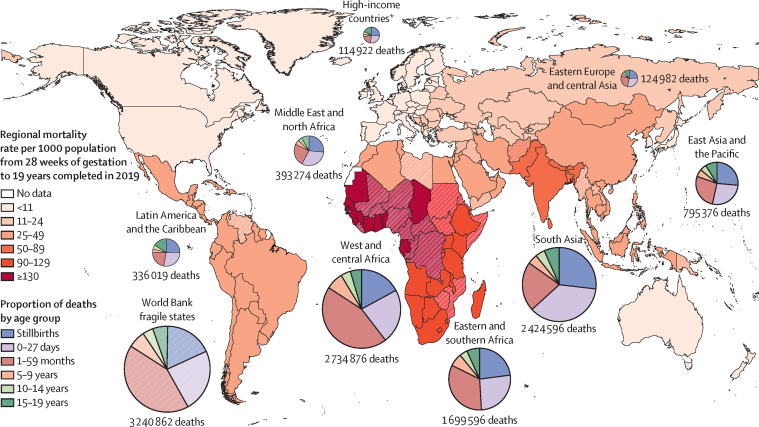
Globally, an estimated 8·62 million deaths occurred between 28 weeks of gestation and 20 years of age in 2019 (appendix p 4).19, 20, 21, 22, 23, 24, 25 Of these deaths, 1·97 million (22·8%) were stillbirths and 2·44 million (28·3%) were neonatal. 2·75 million (31·9%) individuals who died were 1–59-months-old, 506 000 (5·9%) were 5–9-years-old, 368 000 (4·3%) were 10–14-years-old, and 595 000 (6·9%) were 15–19-years-old (appendix pp 5–6). Globally, mortality rates before age 20 years declined substantially from 2000 to 2019, dropping from 111·43 deaths (90% uncertainty interval [UI] 108·96–112·95) per 1000 pregnancies reaching 28 weeks of gestation in 2000 to 62·02 deaths (60·63–65·60) in 2019 (appendix pp 6–8), a 2·98% (90% UI 2·75–3·19) average annual rate of reduction (AARR). The AARR for stillbirths, neonates, and other age groups are provided in the appendix (p 7).
Regional survival disparities remained large in 2019, with the highest mortality rate in west and central Africa (143·19 deaths [90% UI 133·10–160·59] per 1000 pregnancies reaching 28 weeks of gestation) compared with the lowest in high-income countries (HICs; 10·08 deaths [9·86–10·33] per 1000 pregnancies reaching 28 weeks of gestation; 14-times lower than west and central Africa; appendix pp 8–13; table 1). The regional differences were also reflected in the varying age distribution of deaths. For example, the number of neonatal deaths were 30 000 (25·9%) of 115 000 in HICs compared with 882 000 (36·4%) of 2·42 million deaths in south Asia (figure 2; appendix p 4). Fragile and conflict-affected countries have a large number of deaths (3·24 million [90% UI 3·04–3·64]); figure 2; appendix pp 4, 14).26, 27
Table 1.
Global and regional all-cause mortality rates by age in 2019
| Stillborn | 0–27 days | 1–59 months | 5–9 years | 10–14 years |
15–19 years |
Risk of death between 28 weeks of gestation and 19 years completed | |||
|---|---|---|---|---|---|---|---|---|---|
| Both | Females | Males | |||||||
| West and central Africa | 22·82 (19·78–27·67) | 30·86 (26·87–36·93) | 65·89 (57·44–78·24) | 12·39 (11·24–13·69) | 8·25 (6·34–11·42) | 11·13 (9·69–13·22) | 10·55 (9·24–12·51) | 11·69 (10·14–13·93) | 143·19 (133·10–160·59) |
| Eastern and southern Africa | 20·52 (18·69–23·61) | 23·87 (21·65–27·98) | 32·32 (29·06–37·94) | 7·33 (6·60–8·00) | 4·96 (4·19–7·34) | 9·27 (8·40–10·46) | 7·51 (6·80–8·46) | 11·02 (9·98–12·44) | 94·61 (90·49–104·55) |
| South Asia | 18·17 (17·58–22·12) | 25·09 (23·02–27·28) | 15·51 (13·99–17·04) | 3·10 (2·78–3·39) | 2·85 (2·09–4·14) | 4·69 (4·09–5·37) | 4·70 (4·10–5·37) | 4·67 (4·09–5·37) | 67·63 (65·38–73·42) |
| Middle East and north Africa | 10·43 (9·18–12·42) | 12·44 (10·86–14·89) | 9·79 (8·15–12·08) | 2·26 (2·02–2·49) | 2·25 (1·87–3·13) | 4·55 (4·24–4·92) | 3·15 (2·93–3·43) | 5·88 (5·49–6·35) | 41·05 (38·28–46·25) |
| Latin America and the Caribbean | 7·95 (7·40–8·75) | 9·06 (8·41–10·00) | 7·26 (6·60–8·08) | 1·26 (1·20–1·34) | 1·56 (1·44–1·76) | 5·01 (4·84–5·22) | 2·54 (2·45–2·66) | 7·40 (7·16–7·70) | 31·70 (30·70–33·39) |
| East Asia and the Pacific | 7·29 (6·71–8·09) | 7·55 (6·86–8·52) | 7·46 (6·69–8·36) | 1·55 (1·35–1·77) | 1·39 (1·06–2·02) | 2·51 (2·16–2·96) | 1·88 (1·60–2·23) | 3·08 (2·66–3·62) | 27·45 (26·21–29·50) |
| Eastern Europe and central Asia | 5·03 (4·68–5·56) | 6·02 (5·42–6·81) | 5·53 (4·94–6·55) | 1·01 (1·00–1·03) | 1·18 (1·14–1·23) | 2·52 (2·47–2·58) | 1·78 (1·74–1·83) | 3·23 (3·16–3·29) | 21·12 (20·38–22·54) |
| High-income countries* | 2·74 (2·60–2·91) | 2·69 (2·57–2·81) | 1·98 (1·88–2·08) | 0·45 (0·44–0·46) | 0·55 (0·51–0·59) | 1·71 (1·65–1·78) | 1·07 (1·03–1·11) | 2·32 (2·24–2·41) | 10·08 (9·86–10·33) |
| Global | 13·87 (13·55–15·43) | 17·48 (16·61–18·97) | 20·57 (18·93–22·26) | 3·83 (3·63–4·02) | 2·90 (2·67–3·57) | 4·89 (4·67–5·24) | 4·15 (3·94–4·46) | 5·59 (5·36–5·97) | 62·02 (60·63–65·60) |
Data from UN Inter-agency Group for Child Mortality Estimation.20, 21, 24 Mortality rates, and the corresponding 90% uncertainty intervals, are expressed in the unit of deaths per 1000 population at the beginning of each age group and who are subject to risk of dying in that group. The mortality rate for the 15–19 age group was disaggregated by sex using the sex ratio in central death rates from the UN WPP201976 and standard life table approaches.
High-income countries are listed in the appendix (pp 9–10).
Figure 2.
Regional mortality rates and age-specific proportion of deaths from 28 weeks of gestation to 19 years in 2019
Data from UN Inter-agency Group for Child Mortality Estimation.20, 21, 24 Data to reproduce this figure available in table 1 and the appendix (pp 4, 6). Fragile states are represented by broken colours and identified according to the World Bank definition.27 *High-income countries are listed in the appendix (pp 9–10).
Stillbirths are a major contributor to preventable mortality globally, with 1·97 million stillbirths (90% UI 1·92–2·19) in 2019 (figure 2; appendix pp 4, 14).20, 22 Globally, 832 000 (42·3%) stillbirths occurred during labour (intrapartum stillbirths; appendix p 15),20 but this proportion varied by world region (appendix pp 15–16). The rate of stillbirths was eight-times higher in west and central Africa (22·82 stillbirths [90% UI 19·78–27·67] per 1000 pregnancies) compared with HICs (2·74 stillbirths [2·60–2·91] per 1000 pregnancies; table 1).
The global neonatal mortality rate (NMR) reduced by nearly half from 30·35 (90% UI 29·62–31·17) per 1000 live births in 2000 to 17·48 (16·61–18·97) per 1000 live births in 2019 (appendix pp 5–6).19, 21 However, wide regional disparities remained in 2019. In west and central Africa, the NMR was 30·86 deaths (26·87–36·93) per 1000 live births, more than 11-times higher than in HIC (2·69 [2·57–2·81] per 1000 live births; table 1). Worldwide, the leading causes of neonatal mortality in 2019 were preterm birth complications, intrapartum-related complications, congenital anomalies, lower-respiratory infections, and sepsis (appendix p 15).25 Preterm birth complications were the predominant causes of neonatal death in all regions (appendix p 17). The proportion of neonates who died from infectious causes or intrapartum events was positively associated with regional NMRs.
There were 20·57 deaths (90% UI 18·93–22·26) before age 5 years per 1000 children who survived to 1 month of age in 2019, a 56·1% decline from the mortality rate in 2000 (46·84 [45·03–46·93]; appendix pp 5–6).19, 21 There were considerable disparities in mortality across regions in this age group in 2019: 1·98 deaths (1·88–2·08) per 1000 1-month-olds were reported in HICs, more than 33-times lower than the highest mortality rate reported in west and central Africa (65·89 [57·44–78·24]; table 1). Globally in 2019, the most common causes of death for children aged 1 to 59 months were lower-respiratory infections, diarrhoea, malaria, injuries, measles, congenital anomalies, and tuberculosis (appendix p 15).25 The most common direct causes of deaths in west and central Africa were malaria, diarrhoea, and lower-respiratory infections. By contrast in HICs, which reported the lowest mortality, the main causes were congenital anomalies, followed by injuries (appendix p 18).
Globally, mortality rates in 5–9-year-olds were 3·83 (90% UI 3·63–4·02) per 1000 children reaching 5 years of age, 2·90 (2·67–3·57) in children reaching 10 years of age, and 4·89 (4·67–5·24) in adolescents reaching 15 years of age in 2019 (table 1). West and central Africa consistently had the highest mortality rates, whereas HICs had the lowest. Compared with HICs, survival was 28-times lower in 5–9-years-olds and seven-times lower in 15–19-years-olds in west and central Africa (table 1). The age distribution of deaths by age also differed across regions (figure 2; appendix p 4). For example, the number of deaths in 5–9-year-olds were 13 135 (3·9%) of 336 019 deaths from 28 weeks of gestation and 20 years of age in Latin America and the Caribbean and 19 4267 (7·1%) of 2·74 million deaths in west and central Africa.
At the global level, the leading causes of death in 2019 were diarrhoea and malaria in 5–9-year-olds; malaria and neoplasms in 10–14-year-olds; self-harm and neoplasms in 15–19-year-old females; and road traffic injuries and interpersonal violence in 15–19-year-old males (appendix p 15).23 Regionally there are substantial differences in the causes of death that vary by the mortality rate and the presence of endemic diseases, such as malaria (appendix pp 19–22).
In fragile and conflict-affected countries,27 causes of death were very similar to west and central Africa, where many of these countries are located (figure 2; appendix p 23). These countries have a higher proportion of deaths due to collective violence compared with the global total.23
Thriving to age 20 years
Low birthweight
Low birthweight is a substantial global problem, associated with both short-term and long-term health consequences affecting human capital. Low birthweight is defined as birthweight less than 2500 g and can be due to either preterm birth (<37 completed weeks of gestation), growth restriction (measured as small for gestational age [SGA]; ie, less than the tenth percentile of weight for gestational age and sex compared with an international standard for fetal weight28), or both. In 2012, 10·7 million babies were estimated to have been born SGA and had low birthweight, with 11·2 million babies born SGA but with a birthweight of at least 2500 g.29 Infants who are preterm or have growth-restriction at birth have an increased risk of death (including those who are SGA but weigh ≥2500 g),19, 30 reduced linear growth,31 and increased risk of poor development.7
A 2019 estimate suggests that 20·47 million (90% uncertainty range 17·37–24·02) livebirths were low birthweight in 2015.32 About half of the world's low birthweight babies were born in south Asia (9·81 million [26·8%] of 37·15 million total births in south Asia had low birthweight). The number of low birthweight babies born in south Asia is almost double the number born in sub-Saharan Africa (5·00 million birth [4·35–6·15]; appendix p 24). Progress in reducing low birthweight rates is too slow to reach the global nutritional target of a 30% reduction by 2025.33 Between 2000 and 2015 the AARR was 1·2%, but an AARR of 2·7% was required to meet the 30% reduction target.
Regional differences in height and body-mass index (BMI) from birth to 20 years
Globally the prevalence of stunting in children younger than 5 years has declined from 203·6 million (33·1%) of 615·7 million in 2000 to 149·2 million (22·0%) of 677·9 million in 2020.34 However, regional differences persist with the highest rates occurring in south Asia.
The age patterns in height and BMI in children younger than 5 years were assessed with the latest national Demographic and Health Survey or Multiple Indicator Cluster Survey done since 2010 in low-income and middle-income countries (LMICs). Mean length or height and mean BMI were calculated for each month of age and these results were used to calculate regional means as the average of countries in the region weighted by the population of children younger than 5 years (appendix p 25). Additionally, local polynomial approximations were used to produce smoothed graphs of mean length (appendix pp 26–29) or height and BMI (appendix pp 30–33) by age and sex.35
For 5–19-year-olds (including all individuals up to their 20th birthday) the regional growth trajectories were derived from a pooled analysis of measured height and weight data from 2081 population-based surveys that included a total of 65 million participants.36 Data were pooled using Bayesian hierarchical models to estimate mean height and BMI by country, year, sex, and single year of age, including weighting by national populations to produce regional averages. The statistical model incorporated non-linear time trends and changes with age to represent the adolescent growth spurts.
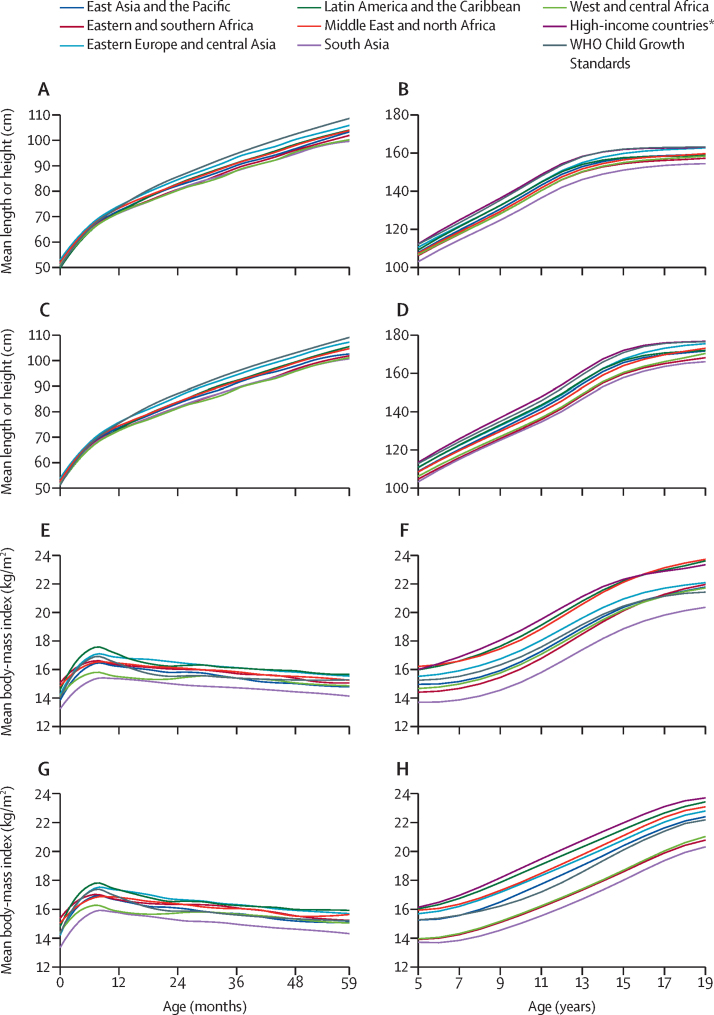
Patterns of linear growth from birth to the 20th birthday vary substantially by world region. Mean lengths or heights from birth to 5 years were compared with the international growth standard.37 For both females and males, the mean length in cm at birth was close to the standard (figure 3), but the mean length-for-age Z scores already showed evidence of growth faltering in early infancy (appendix pp 26–29). From later in infancy until 5 years there was substantial variation in the mean heights by region with the highest linear growth in eastern Europe and central Asia and the lowest in south Asia. Despite the different data sources and analytical methods for the two age ranges, the regional curves were very similar when they come together at 5 years. From 5 years to about 12 years the increases in mean height were largely parallel in all regions, continuing a pattern of growth from the starting points (figure 3). During the pubertal growth period from 13 years there was some regional variation with females and males in eastern Europe and central Asia having accelerated growth resulting in catching up to those in HICs by 19 years. Adolescents in Latin America and the Caribbean have an earlier plateau in height, around the age of 13 years. By 19 years the mean heights of females and males vary substantially by world region and country (appendix pp 34–35). One should note that although growth in utero and during the first 5 years after birth are similar in well nourished populations around the world,38, 39 this has not been shown for growth from 5–19 years.
Figure 3.
Mean length or height and body-mass index for females and males from birth to 19 years by world region
Mean length or height for 0–59-month-old females (A), 5–19-year-old females (B), 0–59-month-old males (C), and 5–19-year-old males (D), and mean body-mass index 0–59-month-old females (E), 5–19-year-old females (F), 0–59-month-old males (G), and 5–19-year-old males (H). Data sources were Demographic and Health Survey, Multiple Indicator Cluster Survey, and the Non-Communicable Disease Risk Factor Collaboration.36 *High-income countries are listed in the appendix (pp 9–10).
The BMIs of females and males increased from birth to about 12 months in all regions, but they varied substantially: the highest BMIs were reported in Latin America and the Caribbean and the lowest in south Asia (figure 3). From 12 to 59 months, mean BMI in Latin America and the Caribbean and eastern Europe and central Asia exceeded the WHO standard, whereas mean BMI was far below the WHO standard in south Asia (appendix pp 32–35). From 5 to 19 years, females' BMI increased more than the WHO reference in HICs, Latin America and the Caribbean, and the Middle East and north Africa, whereas females in south Asia continued to have substantially lower average BMI (figure 3). Except for those in south Asia, west and central Africa, and eastern and southern Africa, by 19 years, males in most world regions had a mean BMI that exceeded the WHO reference, reflecting increased adiposity. Mean BMI for 19-year-old females and males varied substantially by region and country (appendix pp 36–37).
Nutritional status of adolescent females is important not only for their own health, but for the health and survival of their offspring. Short stature is associated with small pelvic size and increased risk of intrapartum stillbirths, and underweight is associated with poor fetal growth and low birthweight40 resulting in intergenerational transmission of undernutrition and poor health outcomes. Conversely, increased adiposity in both females and males increases the risk of nutrition-related non-communicable diseases in adulthood.41
Disability in children and adolescents
Disabilities beginning in childhood are a global concern with important effects on human capital; of note, they are not included in the Human Capital Index.3 Many disabilities can be prevented or their adverse consequences mitigated by environmental adaptation, rehabilitation, corrective services, and supportive families and workplaces. From a nurturing care perspective, providing services to prevent or mitigate the consequences of disabilities advances equity. Underlying differences in the health conditions leading to disabilities and in societal responses to disabilities result in substantial variation in their prevalence across world regions and consequences for individuals and their societies.
In LMICs the highest rates of years lived with disability for children and adolescents are in Africa and south Asia, and are lower in HICs, although the rates for the older adolescents are more similar across regions.42 Using broad categories of causes, the largest share of disabilities globally are undernutrition for children and mental disorders and substance use for adolescents.42 Considering disabilities with long-term impairments (excluding nutritional conditions and infections) in children younger than 20 years, the five most prevalent in 2019 were migraine, injuries, hearing impairment, asthma, and dermatitis.43
Educational measures and human capital
Global enrolment in primary schools has improved significantly over the past two decades, with increases in global literacy. However, school enrolment is not a good indicator of learning, and children in LMICs experience challenges in attaining literacy and numeracy.44 An indicator of the knowledge, skills, and problem-solving abilities obtained through education is often included in human capital indices, measured through test performance or grade attainment.45 Test performance is an indicator of cumulative learning influenced by environmental context, including school quality. Tests, such as the Programme for International Student Assessment, measure student knowledge and skills.46 Grades of formal school attained is used as a proxy for learning for the population aged 15 years and older.47 Attainment of higher grades in adolescents is often interpreted as indicating potential for future national economic growth.45
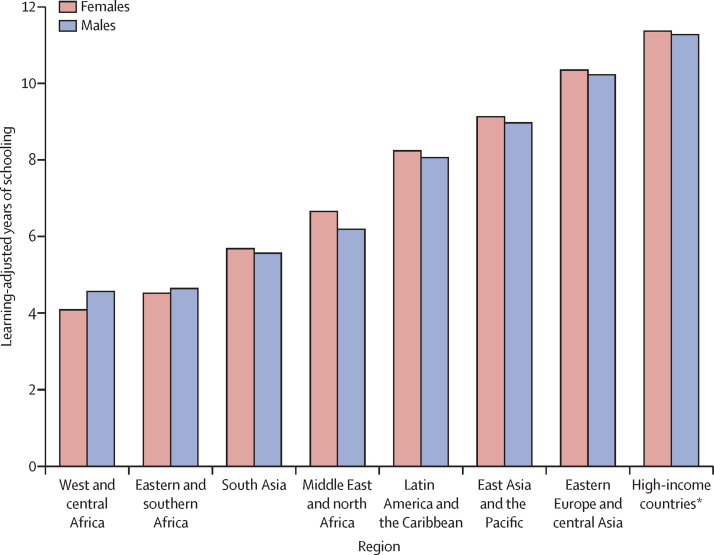
To incorporate both grade attainment and test performance into a single indicator, Filmer and colleagues48, 49 developed the learning-adjusted years of schooling (LAYS) indicator. The LAYS indicator adjusts the average number of grades attained for a given population to reflect the average learning acquired. Using scores on the Programme for International Student Assessment and the Trends in International Mathematics and Science Study, each country is compared with Singapore, a high-performing country. Each country's mean grade attainment by age cohort is then adjusted on the basis of their relative learning performance. For example, the average years of grade attainment for Chile (11·7 grades) is adjusted downwards to 8·1 LAYS.48
LAYS are used in the World Bank Human Capital Index to estimate population-level adjusted grades of schooling by sex for countries and world regions (figure 4). However, the information on the distribution of scores within countries, by factors such as geography, income, parental schooling attainment, and language, might be even more important to understanding educational quality and equity. Consistent with the principles of the Nurturing Care Framework, educational attainment can compensate for some factors, such as low socioeconomic status,50 and the potential for compensation is even higher when school quality is considered, as has been shown in Pakistan51 and elsewhere. Thus, LAYS might be useful in examining how grade attainment, and investments in school quality, can compensate for individual disparities, including early adversities.52
Figure 4.
Learning-adjusted years of schooling by world region
Adapted from Filmer and colleagues.49 *High-income countries are listed in the appendix (pp 9–10).
Life course analysis related to human capital
To illustrate the associations of some dimensions of early life to adult human capital, we analysed data from the COHORTS consortium, which includes six LMIC population-based birth cohorts with at least 20 years of follow-up and more than 1000 participants each. Two are from Brazil, and one each are from Guatemala, India, the Philippines, and South Africa.53 Available human capital outcomes in adulthood included height, completed school grades, intelligence, overweight or obesity, metabolic signs (eg, abdominal adiposity and raised blood pressure), and psychological symptoms (appendix pp 38–41).
The analyses addressed two questions. The first was how important is tracking from birth to adulthood when considering variables in the same domain? These results are highlighted table 2. One Z score increase in birth length was associated with an increase in adult height by 1·7 cm; for conditional length at 2 years (reflecting growth from birth to 2 years) the difference was 3·3 cm; and for height at 4 years (reflecting growth from 2 to 4 years), the corresponding difference was 2·3 cm, consistent with the importance in predictions of the first 1000 days from conception until the second birthday and a smaller predictive role of growth in later childhood (appendix pp 42–44). One Z score higher weight at birth was associated with an adult overweight prevalence ratio of 1·07; one Z score higher BMI at 2 years was associated with an adult overweight prevalence of 1·15; and a one Z score higher BMI at 4 years was associated with an adult overweight prevalence of 1·19 (appendix pp 42, 44). Previous COHORT studies described tracking in height and in adiposity.54, 55, 56 One Z score difference in the developmental quotient at 4 years was associated with 7·3 intelligence quotient (IQ) points in adulthood, consistent with literature from HICs (appendix pp 42, 45).57, 58
Table 2.
Summary of association results between early-life variables and adult outcomes
|
Height (cm) |
Intelligence quotient (harmonised units) |
Attained schooling (grades attained) |
Overweight and obesity (prevalence ratio) |
Metabolic syndrome (number of signs) |
Mental illness (number of symptoms) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regression coefficient | p value | Regression coefficient | p value | Regression coefficient | p value | Prevalence ratio | p value | Ratio of averages | p value | Regression coefficient | p value | ||
| Height | |||||||||||||
| Birth length | 1·68 (1·52 to 1·84)* | <0·0001 | 0·73 (0·35 to 1·10) | 0·0002 | 0·17 (0·07 to 0·27)† | 0·0010 | 1·05 (1·01 to 1·08) | 0·0050 | 1·00 (0·97 to 1·03) | 0·90 | −0·06 (−0·29 to 0·17)† | 0·60 | |
| Conditional length at 2 years | 3·34 (3·06 to 3·63)*† | <0·0001 | 1·52 (0·96 to 2·08)† | <0·0001 | 0·25 (0·10 to 0·40)† | 0·0010 | 1·01 (0·95 to 1·07)† | 0·75 | 1·05 (0·97 to 1·15)†‡ | 0·24 | −0·01 (−0·16 to 0·13) | 0·86 | |
| Conditional height at 4 years | 2·30 (2·03 to 2·58)*† | <0·0001 | 0·16 (−0·47 to 0·78) | 0·63 | 0·09 (−0·03 to 0·20)† | 0·15 | 1·05 (1·01 to 1·08) | 0·0060 | 1·03 (0·99 to 1·07)† | 0·14 | −0·03 (−0·18 to 0·13) | 0·75 | |
| Conditional height at adulthood | 4·19 (3·80 to 4·59)*† | <0·0001 | 0·05 (−0·91 to 1·00)† | 0·94 | −0·05 (−0·24 to 0·14)† | 0·63 | 0·90 (0·86 to 0·95)† | <0·0001 | 0·97 (0·93 to 1·02) | 0·31 | −0·10 (−0·23 to 0·04) | 0·18 | |
| Weight | |||||||||||||
| Birthweight | 1·07 (0·95 to 1·19) | <0·0001 | 0·74 (0·35 to 1·14) | 0·0002 | 0·13 (0·08 to 0·19) | <0·0001 | 1·07 (1·05 to 1·09)*† | <0·0001 | 1·00 (0·98 to 1·02) | 0·96 | −0·04 (−0·14 to 0·06) | 0·46 | |
| Conditional relative weight at 2 years | −0·14 (−0·44 to 0·15)*† | 0·34 | −0·26 (−1·21 to 0·69)† | 0·59 | 0·05 (−0·03 to 0·13) | 0·23 | 1·15 (1·12 to 1·18)*† | <0·0001 | 1·06 (1·02 to 1·11)† | 0·0040 | −0·05 (−0·59 to 0·48)† | 0·84 | |
| Conditional relative weight at 4 years | −0·24 (−0·54 to 0·06)*† | 0·12 | −0·19 (−0·69 to 0·32) | 0·47 | −0·09 (to 0·18 to −0·01) | 0·038 | 1·19 (1·12 to 1·25)*† | <0·0001 | 1·08 (1·02 to 1·14)† | 0·0080 | −0·02 (−0·13 to 0·10) | 0·76 | |
| Conditional relative weight at adulthood | −0·10 (−0·25 to 0·04) | 0·17 | 0·04 (−0·50 to 0·58)† | 0·88 | −0·13 (−0·26 to −0·01)† | 0·035 | 1·59 (1·49 to 1·69)*† | <0·0001 | 1·40 (1·28 to 1·53)† | <0·0001 | 0·03 (−0·29 to 0·35)† | 0·85 | |
| Cognitive development (4·0–8·5 years) | 0·77 (0·54 to 0·99) | <0·0001 | 7·30 (5·85 to 8·76)*† | <0·0001 | 0·82 (0·47 to 1·16)† | <0·0001 | 1·02 (0·96 to 1·08) | 0·58 | 0·97 (0·91 to 1·03) | 0·33 | −0·17 (−0·32 to −0·03) | 0·021 | |
Data are regression coefficients (95% CI).
Tracking result (results for which the early life variable and the adult outcome are in the same domain [eg, birth length and adult height])
Significant heterogeneity (ie, Cochran's Q test p<0·05) between cohorts.
Significant interaction (ie, interaction test p<0·05) between the sexes.
The second question was how well do early growth and development predict a broader range of adult human capital outcomes? The results are adjusted for family early-life socioeconomic position, parental education, and other confounding variables (table 2; appendix pp 38–41). Birth length and weight and conditional length at age 2 years were strongly positively associated with adult intelligence and schooling, but this was not the case for conditional height at 4 years, nor for adult height. These results suggest that linear growth up to the age of 2 years—but not later in childhood or during adolescence—is predictive of schooling and IQ in these cohorts, confirming previous results.59, 60, 61, 62 Cognitive development in childhood was positively associated with schooling. Also consistent with earlier COHORT findings, birthweight was positively associated with adult intelligence and schooling, but this was not the case for relative weights at any age. On the contrary, there were weak inverse associations of conditional relative weights at 2 and 4 years with attained schooling. The strong predictive power for early-life anthropometric measures in the COHORTS studies is also consistent with evidence suggesting that most of the structural and functional development of the brain takes place up to the age of 2 years, and that brain maturation that occurs after this age is much slower.63 The only long-term randomised trial on the topic found an effect of nutritional supplementation before, but not after, 36 months of age on adult intelligence in both sexes64 and on income for adult men.65 Additionally, trials of nutritional interventions suggest a greater benefit on adult cardiometabolic outcomes if they are done in early versus late childhood.41 Although the COHORTS results reported here and separately62 are observational, the sum of the available evidence suggests a causal role for good early nutrition on adult outcomes, including human capital.
In addition to being strongly and positively associated with all weight-derived indicators throughout the life course, the prevalence of adult overweight or obesity was positively associated with birth length and conditional length at 4 years. The number of metabolic syndrome signs was positively associated with con-ditional relative weight at 2 years, 4 years, and in adulthood, as earlier analyses had shown.66 Moreover, consistent with the literature,67 frequency of mental illness symptoms in adults was inversely associated with cognitive developmental scores in childhood.
Differences between the sexes were examined, but they were observed only for the association of conditional length at 2 years with metabolic syndrome, with a stronger association for males than for females. However, this might be due to multiple testing because only one of 56 interaction tests was statistically significant. Several associations showed heterogeneity across cohorts, but this was mostly in magnitudes rather than the directions of the estimates.
These results are consistent with substantial tracking in linear growth and in intellectual development from early childhood to adulthood. The positive associations of linear growth with intelligence, learning, and schooling are strongest up to the age of 2 years.62 Analyses of other LMICs cohorts found that growth later in childhood or adolescence also has some predictive power for cognitive function (appendix p 46).68, 69, 70, 71 Our results are also suggestive that early-life adiposity is predictive of adult overweight and of signs of metabolic illnesses. Studies of cohorts in high-income countries, such as New Zealand, show the importance of early-life adiposity and risk of cardiometabolic disease and mortality in adulthood and the association of adversities, such as maltreatment, in early childhood with adult mental health and chronic diseases (appendix pp 47–48).
Conclusions
Human capital encompasses the skills, knowledge, experience, and health of individuals and collectively of populations. The World Bank has highlighted the importance of human capital for global societal and economic development3 and created the Human Capital Index to monitor national progress.1 The Human Capital Index includes survival for children to 5 years and for adults from 15 to 60 years, thriving operationaliased as healthy growth in the first 5 years of life, and the quantity and quality of education. Others have developed an index of human capital focused on adult mortality, educational attainment, and adult functional health and correlate this with national economic growth.72 Our analyses, similar to the Human Capital Index, focus on the life course determinants of human capital before adulthood and suggest consideration of loss of life and undernutrition both before birth and for all children and adolescents, along with indicators of overweight and disabilities. We examine how key contributors to human capital differ by world regions and document the key periods of the life course for the optimal development of human capabilities and formation of human capital.
The probability of death from birth to 5 years of age is a commonly used indicator of human capital and the progress of countries. We propose instead the probability of death from the third trimester of pregnancy to the 20th birthday, an age range in which there were 8·6 million deaths in 2019. Regional disparities were large with mortality in west and central Africa 14-times higher than in HICs. These differences were largely due to complications of pregnancy and delivery, and high rates of acute lower-respiratory infections, diarrhoea, malaria, and other infections in the higher mortality regions.23 Undernutrition is an underlying factor in 45% of deaths in children under 5 years of age, increasing the risk of death from infectious diseases; it also especially effects adolescent mothers through cephalopelvic disproportion and anaemia in childbirth.73
Growth and development before adulthood are crucial for human capital, and conditions before conception and in utero are important for a healthy start in life. Low birthweight—a consequence of fetal growth restriction, preterm delivery, or both—is a global problem, but the prevalence of low birthweight in South Asia was nearly double that in sub-Saharan Africa.32 In the first 5 years after birth, gains in height in LMICs were lower than the WHO growth standard, with the lowest gain in heights reported in south Asia. Between 5 and 12 years, the gains in height were largely parallel across regions, but there was more variation in trajectories between 13 and 19 years. Elevated mean BMI in comparison with the WHO reference began in many regions in the preschool period and continued into adolescence. The highest mean BMI values were in HICs, the Middle East and north Africa, and Latin America and the Caribbean. Conversely, low BMI values through adolescence were found in south Asia. These analyses are based on historical cross-sectional data and might not reflect current growth trajectories if living conditions have changed rapidly.
The analyses of the COHORTS data show that there was strong tracking of size from birth to adolescence. Of the measures of size examined, birth length and growth from birth to 2 years were the strongest predictors of adult height. The association between BMI at 4 years and adult overweight and metabolic disease also suggest the importance of the early childhood in predicting adult health should be considered more strongly in human capital metrics. The importance of nurturing care from preconception through early childhood for human capital formation is shown by the finding that linear growth in the first 2 years of life was a very strong predictor of schooling, learning, and adult IQ.
Nurturing care provides a conceptual framework for our analysis, adopting a life course perspective from preconception through adolescence, highlighting developmental periods that are vulnerable to adversity and sensitive to interventions, and focusing on opportunities that promote equity, resilience, and human capital. Education, beginning during the preprimary school period and extending through secondary school and beyond, plays a crucial role in the accrual of human capital. The absence of quality education is particularly concerning because it most commonly occurs in regions with the highest rates of other early-life adversities (eg, south Asia and sub-Saharan Africa). Consistent with the Nurturing Care Framework, responsive caregiving and learning experiences can mitigate adversities.
Human capital depends on children surviving and thriving throughout their life course, with the first 1000 days following conception especially important, a crucial period of formative development. Investments to promote human capital should begin before conception and support healthy growth and development through adolescence by environments that promote health and nutrition, protect from threats, and provide opportunities for learning and responsive care and relationships.
The SDGs for children are aspirational but resonate with both nations and families. Achieving them, already challenging, will be set back by the COVID-19 pandemic and the resulting economic crisis.74, 75 Achieving the SDGs will need more investment, but would result in a greater return in human capital.
For the Optimising Child and Adolescent Health and Development Series see www.lancet.com/series/optimising-child-adolescent-health
Declaration of interests
REB serves on the Board of Directors of Vitamin Angels, a non-profit charitable organisation supporting maternal and child nutrition services in low-income and middle-income countries. ME reports a grant from AstraZeneca for the Young Health Programme, and personal fees from Prudential, outside the submitted work. REB, FV, LH, LL, ADS, DYo, and DYe report grants from the Bill & Melinda Gates Foundation. LH and DYo report grants from USAID, outside the submitted work.
Acknowledgments
Acknowledgments
Funding for research contributing to this paper was provided by the Bill & Melinda Gates Foundation in grants to the Johns Hopkins Bloomberg School of Public Health for the mortality analyses (OPP1172551), and to Emory School of Public Health for the COHORTS analyses (OPP1164115). Work at the Federal University of Pelotas for COHORTS and Demographic and Health Survey and Multi-indicator Cluster Survey analyses were supported by the Bill & Melinda Gates Foundation (OPP1199234) and the Wellcome Trust (101815/Z/13/Z). The COHORTS consortium was established through a grant from the Wellcome Trust (082554/Z/07/Z). The analysis of 5–19-year-olds by the School of Public Health, Imperial College London, London, UK, was supported by the Wellcome Trust (101506/Z/13/Z), AstraZeneca Young Health Programme, and the European Union (774548); a grant from the Bill & Melinda Gates Foundation to UNICEF USA supported the analyses contributing to this Series paper. The COHORTS study team (Fernando C Barros, Isabelita Bas, Santosh K Bhargava, Delia B Carba, Natália P Lima, Fernanda Kroker-Lobos, Sara Naicker, Lukhanyo H Nyati, Lakshmy Ramakrishnan, Harshpal Singh Sachdev, Bruna G C Silva, Bhaskar Singh, Sikha Sinha, Jithin Sam Varghese, and Fernando Wehrmeister) provided data. The administrative and editorial support by Brittany Furgal is appreciated. The sponsors had no role in analysis and interpretation of the evidence, writing the paper, or decision to submit for publication.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
REB conceptualised and coordinated the analyses, wrote the first draft of the paper, responded to reviewer comments, and incorporated all revisions until publication. MMB wrote the Nurturing care and human capital section. MMB and AG wrote the section on education. DYo, LH, and BM provided information on stillbirths and mortality. LL, FV, JP, DYe, SC, and REB analysed the UN-Inter-agency Group for Child Mortality Estimation mortality data and contributed the cause-of-death information. FV contributed the map with regional mortality estimates. EB provided the analysis of child mortality in fragile and conflict countries in Africa. HB and JEL contributed on preterm, low birthweight, and stillbirths. LPV and CGV contributed the analyses of height and body-mass index in children younger than 5 years. AR-M and ME contributed the analyses of height and body-mass index in 5–19-year-olds. FPH, CGV, and REB contributed the COHORTS analyses. ADS, RM, and CO advised on statistical analyses. LSA, CHDF, BH, AMBM, MR-Z, and LMR provided the COHORTS data. JB contributed the Young Lives Panel. TV and MMB contributed the Dunedin panel. GP and ZAB provided overall guidance and comments on draft manuscripts. All authors read the final draft and approved submission for publication. REB had full access to all data and final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Gatti RV, Kraay AC, Avitabile C, Collin ME, Dsouza R, Dehnen NAP. World Bank; Washington, DC: 2018. The Human Capital Project (English) [Google Scholar]
- 2.Clark H, Coll-Seck AM, Banerjee A, et al. A future for the world's children? A WHO-UNICEF-Lancet Commission. Lancet. 2020;395:605–658. doi: 10.1016/S0140-6736(19)32540-1. [DOI] [PubMed] [Google Scholar]
- 3.Kim JY. The human capital gap: getting governments to invest in people. Foreign Aff. 2018;97:92. [Google Scholar]
- 4.UN General Assembly Transforming our world: the 2030 Agenda for Sustainable Development. 2015. https://sustainabledevelopment.un.org/post2015/transformingourworld
- 5.UN Millennium Development Goals Report. 2015. https://www.un.org/millenniumgoals/2015_MDG_Report/pdf/MDG%202015%20rev%20(July%201).pdf
- 6.Moucheraud C, Owen H, Singh NS, et al. Countdown to 2015 country case studies: what have we learned about processes and progress towards MDGs 4 and 5? BMC Public Health. 2016;16(suppl 2):794. doi: 10.1186/s12889-016-3401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black MM, Walker SP, Fernald LCH, et al. Early childhood development coming of age: science through the life course. Lancet. 2017;389:77–90. doi: 10.1016/S0140-6736(16)31389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kioupi V, Voulvoulis N. Education for sustainable development: a systemic framework for connecting the SDGs to educational outcomes. Sustainability. 2019;11 [Google Scholar]
- 9.Victora CG, Hartwig FP, Vidaletti LP, et al. Effects of early-life poverty on health and human capital in children and adolescents: analyses of national surveys and birth cohort studies in LMICs. Lancet. 2022 doi: 10.1016/S0140-6736(21)02716-1. published online April 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaivada T, Lassi ZS, Irfan O, et al. What can work and how? An overview of evidence-based interventions and delivery strategies to support health and human development from before conception to 20 years. Lancet. 2022 doi: 10.1016/S0140-6736(21)02725-2. published online April 27. [DOI] [PubMed] [Google Scholar]
- 11.Kruk ME, Lewis TP, Arsenault C, et al. Improving health and social systems for all children in LMICs: structural innovations to deliver high-quality services. Lancet. 2022 doi: 10.1016/S0140-6736(21)02532-0. published online April 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhutta ZA, Boerma T, Black MM, et al. Optimising child and adolescent health and development in the post-pandemic world. Lancet. 2022 doi: 10.1016/S0140-6736(21)02789-6. published online April 27. [DOI] [PubMed] [Google Scholar]
- 13.Boyce WT, Levitt P, Martinez FD, McEwen BS, Shonkoff JP. Genes, environments, and time: the biology of adversity and resilience. Pediatrics. 2021;147 doi: 10.1542/peds.2020-1651. [DOI] [PubMed] [Google Scholar]
- 14.Evans GW, Li D, Whipple SS. Cumulative risk and child development. Psychol Bull. 2013;139:1342–1396. doi: 10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
- 15.WHO. UNICEF. World Bank Group . World Health Organization; Geneva: 2018. Nurturing care for early childhood development: a framework for helping children survive and thrive to transform health and human potential. [Google Scholar]
- 16.Black MM, Behrman JR, Daelmans B, et al. The principles of Nurturing Care promote human capital and mitigate adversities from preconception through adolescence. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2020-004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trude ACB, Richter LM, Behrman JR, et al. Effects of responsive caregiving and learning opportunities during pre-school ages on the association of early adversities and adolescent human capital: an analysis of birth cohorts in two middle-income countries. Lancet Child Adolesc Health. 2021;5:37–46. doi: 10.1016/S2352-4642(20)30309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross DA, Hinton R, Melles-Brewer M, et al. Adolescent well-being: a definition and conceptual framework. J Adolesc Health. 2020;67:472–476. doi: 10.1016/j.jadohealth.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharrow D, Hug L, You D, et al. Global, regional, and national trends in under-5 mortality between 1990 and 2019 with scenario-based projections until 2030: a systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet Glob Health. 2022;10:e195–e206. doi: 10.1016/S2214-109X(21)00515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.UN Inter-agency Group for Child Mortality Estimation . UNICEF; New York, NY: 2020. A neglected tragedy: the global burden of stillbirths: report of the UN Inter-Agency Group for Child Mortality Estimation 2020. [Google Scholar]
- 21.UN Inter-agency Group for Child Mortality Estimation . UNICEF; New York, NY: 2020. Levels and trends in child mortality: report 2020: estimates developed by the UN Inter-agency Group for Child Mortality Estimation. [Google Scholar]
- 22.Hug L, You D, Blencowe H, et al. Global, regional, and national levels and trends in stillbirths from 2000 to 2019: a systematic assessment. Lancet. 2021;398:772–785. doi: 10.1016/S0140-6736(21)01112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Villavicencio F, Yeung D, et al. National, regional, and global causes of mortality in 5–19-year-olds from 2000 to 2019: a systematic analysis. Lancet Glob Health. 2022;10:E337–E347. doi: 10.1016/S2214-109X(21)00566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masquelier B, Hug L, Sharrow D, et al. Global, regional, and national mortality trends in youth aged 15–24 years between 1990 and 2019: a systematic analysis. Lancet Glob Health. 2021;9:e409–e417. doi: 10.1016/S2214-109X(21)00023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perin J, Mulick A, Yeung D, et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child & Adols Health. 2022;6:106–115. doi: 10.1016/S2352-4642(21)00311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burke M, Heft-Neal S, Bendavid E. Sources of variation in under-5 mortality across sub-Saharan Africa: a spatial analysis. Lancet Glob Health. 2016;4:e936–e945. doi: 10.1016/S2214-109X(16)30212-1. [DOI] [PubMed] [Google Scholar]
- 27.World Bank Classification of fragile and conflict-affected situations. FY20 FCS List. 2020. http://pubdocs.worldbank.org/en/179011582771134576/FCS-FY20.pdf (Aug 2, 2021).
- 28.Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 29.Lee AC, Kozuki N, Cousens S, et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21st standard: analysis of CHERG datasets. BMJ. 2017;358 doi: 10.1136/bmj.j3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz J, Lee ACC, Kozuki N, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet. 2013;382:417–425. doi: 10.1016/S0140-6736(13)60993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christian P, Lee SE, Donahue Angel M, et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int J Epidemiol. 2013;42:1340–1355. doi: 10.1093/ije/dyt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blencowe H, Krasevec J, de Onis M, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. 2019;7:e849–e860. doi: 10.1016/S2214-109X(18)30565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGuire S. Comprehensive implementation plan on maternal, infant, and young child nutrition. Geneva, Switzerland, 2014. Adv Nutr. 2015;6:134–135. doi: 10.3945/an.114.007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.UNICEF. WHO. World Bank Group Joint child malnutrition estimates, levels and trends in child malnutrition: key findings of the 2021 edition. 2021. https://data.unicef.org/resources/jme-report-2021/
- 35.Fan J, Gijbels I. Taylor Francis; London: 1996. Local polynomial modelling and its applications. [Google Scholar]
- 36.NCD Risk Factor Collaboration Height and body-mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: a pooled analysis of 2181 population-based studies with 65 million participants. Lancet. 2020;396:1511–1524. doi: 10.1016/S0140-6736(20)31859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO . World Health Organization; Geneva: 2006. WHO child growth standards: length/height for age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. [Google Scholar]
- 38.Villar J, Papageorghiou AT, Pang R, et al. The likeness of fetal growth and newborn size across non-isolated populations in the INTERGROWTH-21st Project: the Fetal Growth Longitudinal Study and Newborn Cross-Sectional Study. Lancet Diabetes Endocrinol. 2014;2:781–792. doi: 10.1016/S2213-8587(14)70121-4. [DOI] [PubMed] [Google Scholar]
- 39.de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO multicentre growth reference study: planning, study design, and methodology. Food Nutr Bull. 2004;25(suppl):S15–S26. doi: 10.1177/15648265040251S103. [DOI] [PubMed] [Google Scholar]
- 40.Victora CG, Christian P, Vidaletti LP, Gatica-Domínguez G, Menon P, Black RE. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet. 2021;397:1388–1399. doi: 10.1016/S0140-6736(21)00394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He S, Stein AD. Early-life nutrition interventions and associated long-term cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2021;12:461–489. doi: 10.1093/advances/nmaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guthold R, White Johansson E, Mathers CD, Ross DA. Global and regional levels and trends of child and adolescent morbidity from 2000 to 2016: an analysis of years lost due to disability (YLDs) BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-004996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cieza A, Kamenov K, Sanchez MG, et al. Burden of disability in children and adolescents must be integrated into the global health agenda. BMJ. 2021;372:n9. doi: 10.1136/bmj.n9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ball J, Paris SG, Govinda R. In: Learning and Education in Developing Countries: Research and Policy for the Post-2015 UN Development Goals. Wagner DA, editor. Palgrave Macmillan; New York, NY: 2014. Literacy and numeracy skills among children in developing countries; pp. 26–41. [Google Scholar]
- 45.Fraumeni BM. Choosing a human capital measure: educational attainment gaps and rankings. 2015. https://www.nber.org/papers/w21283 (Aug 2, 2021).
- 46.Nohara D, Goldstein AA. US Department of education, National Center for Education Statistics; Washington DC: 2001. A comparison of the national assessment of educational progress (NAEP), the third international mathematics and science study repeat (TIMSS-R), and the programme for international student assessment (PISA) [Google Scholar]
- 47.Barro RJ, Lee JW. A new data set of educational attainment in the world, 1950–2010. J Dev Econ. 2013;104:184–198. [Google Scholar]
- 48.Filmer D, Rogers H, Angrist N, Sabarwal S. Learning-adjusted years of schooling (LAYS): defining a new macro measure of education (English) 2018. https://openknowledge.worldbank.org/handle/10986/30464
- 49.Filmer D, Rogers H, Angrist N, Sabarwal S. Learning-adjusted years of schooling (LAYS): defining a new macro measure of education. Econ Educ Rev. 2020;77 [Google Scholar]
- 50.Montez JK, Hayward MD. Cumulative childhood adversity, educational attainment, and active life expectancy among U.S. adults. Demography. 2014;51:413–435. doi: 10.1007/s13524-013-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das J, Pandey P, Zajonc T. Learning levels and gaps in Pakistan: a comparison with Uttar Pradesh and Madhya Pradesh. Econ Polit Wkly. 2012;47:228–240. [Google Scholar]
- 52.Hughes K, Bellis MA, Hardcastle KA, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017;2:e356–e366. doi: 10.1016/S2468-2667(17)30118-4. [DOI] [PubMed] [Google Scholar]
- 53.Richter LM, Victora CG, Hallal PC, et al. Cohort profile: the consortium of health-orientated research in transitioning societies. Int J Epidemiol. 2012;41:621–626. doi: 10.1093/ije/dyq251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adair LS, Fall CH, Osmond C, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382:525–534. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9:474–488. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 56.Stein AD, Wang M, Martorell R, et al. Growth patterns in early childhood and final attained stature: data from five birth cohorts from low- and middle-income countries. Am J Hum Biol. 2010;22:353–359. doi: 10.1002/ajhb.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flensborg-Madsen T, Mortensen EL. Associations of early developmental milestones with adult intelligence. Child Dev. 2018;89:638–648. doi: 10.1111/cdev.12760. [DOI] [PubMed] [Google Scholar]
- 58.Schneider W, Niklas F, Schmiedeler S. Intellectual development from early childhood to early adulthood: the impact of early IQ differences on stability and change over time. Learn Individ Differ. 2014;32:156–162. [Google Scholar]
- 59.Hoddinott J, Behrman JR, Maluccio JA, et al. Adult consequences of growth failure in early childhood. Am J Clin Nutr. 2013;98:1170–1178. doi: 10.3945/ajcn.113.064584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horta BL, Victora CG, de Mola CL, et al. Associations of linear growth and relative weight gain in early life with human capital at 30 years of age. J Pediatr. 2017;182:85–91.e3. doi: 10.1016/j.jpeds.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baptista Menezes AM, Oliveira PD, Wehrmeister FC, et al. Associations between growth from birth to 18 years, intelligence, and schooling in a Brazilian cohort. Am J Clin Nutr. 2020;112:187–194. doi: 10.1093/ajcn/nqaa047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poveda NE, Hartwig F, Victora C, et al. Patterns of growth in childhood in relation to adult schooling attainment and IQ in 6 birth cohorts in low and middle-income countries: evidence from COHORTS. J Nutr. 2021;151:2342–2352. doi: 10.1093/jn/nxab096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gilmore JH, Knickmeyer RC, Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 2018;19:123–137. doi: 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martorell R, Melgar P, Maluccio JA, Stein AD, Rivera JA. The nutrition intervention improved adult human capital and economic productivity. J Nutr. 2010;140:411–414. doi: 10.3945/jn.109.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoddinott J, Maluccio JA, Behrman JR, Flores R, Martorell R. Effect of a nutrition intervention during early childhood on economic productivity in Guatemalan adults. Lancet. 2008;371:411–416. doi: 10.1016/S0140-6736(08)60205-6. [DOI] [PubMed] [Google Scholar]
- 66.Umer A, Kelley GA, Cottrell LE, Giacobbi P, Jr, Innes KE, Lilly CL. Childhood obesity and adult cardiovascular disease risk factors: a systematic review with meta-analysis. BMC Public Health. 2017;17:683. doi: 10.1186/s12889-017-4691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rankin J, Matthews L, Cobley S, et al. Psychological consequences of childhood obesity: psychiatric comorbidity and prevention. Adolesc Health Med Ther. 2016;7:125–146. doi: 10.2147/AHMT.S101631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen PH, Tran LM, Khuong LQ, et al. Child linear growth during and after the first 1000 days is positively associated with intellectual functioning and mental health in school-age children in Vietnam. J Nutr. 2021;151:2816–2824. doi: 10.1093/jn/nxab182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Georgiadis A, Benny L, Crookston BT, et al. Growth trajectories from conception through middle childhood and cognitive achievement at age 8 years: evidence from four low- and middle-income countries. SSM Popul Health. 2016;2:43–54. doi: 10.1016/j.ssmph.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crookston BT, Schott W, Cueto S, et al. Postinfancy growth, schooling, and cognitive achievement: young lives. Am J Clin Nutr. 2013;98:1555–1563. doi: 10.3945/ajcn.113.067561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aurino E, Schott W, Behrman JR, Penny M. Nutritional status from 1 to 15 years and adolescent learning for boys and girls in Ethiopia, India, Peru, and Vietnam. Popul Res Policy Rev. 2019;38:899–931. [Google Scholar]
- 72.Lim SS, Updike RL, Kaldjian AS, et al. Measuring human capital: a systematic analysis of 195 countries and territories, 1990–2016. Lancet. 2018;392:1217–1234. doi: 10.1016/S0140-6736(18)31941-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 74.Roberton T, Carter ED, Chou VB, et al. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8:e901–e908. doi: 10.1016/S2214-109X(20)30229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Headey D, Heidkamp R, Osendarp S, et al. Impacts of COVID-19 on childhood malnutrition and nutrition-related mortality. Lancet. 2020;396:519–521. doi: 10.1016/S0140-6736(20)31647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.