Abstract
Dialysate leakage is one of the causes of peritoneal dialysis (PD)-related peritonitis. The rate of catheter removal in PD-related peritonitis caused by dialysate leakage (PDPDL) is high, and the correct treatment is unclear. We experienced a case of PDPDL that was treated with intravenous and intraperitoneal antibiotic therapy. A 44-year-old Japanese man had high glucose discharge from the exit site after 14 days of initiating PD, and he had a fever and cloudy effluent with a high white cell count. We diagnosed him with PDPDL and began to administer vancomycin and ceftazidime intraperitoneally. However, the peritonitis could not be ameliorated. A culture examination showed Staphylococcus aureus from the effluent of peritoneal cavity and exit site cultures. We began intraperitoneal cefazolin administration according to a drug susceptibility test, but the effluent cell count remained high. As we added intravenous cefazolin administration, his symptoms and cloudy effluent improved, and the effluent cell count normalized. He has not developed any recurrence of dialysate leakage or peritonitis. Our findings suggest that PD-related peritonitis accompanied by other infectious sites, such as PDPDL, should be treated with additional intravenous antibiotic therapy to taking effect on the infectious sites except for peritoneum and to keep plasma concentration of antibiotics sufficient especially in cases with preserved residual kidney function.
Keywords: Cefazolin, Cloudy effluent, Exit site discharge, Plasma antibiotic concentration, Residual kidney function
Introduction
Peritonitis is a significant complication of peritoneal dialysis (PD). PD-related peritonitis is a cause of PD failure and conversion to hemodialysis [1]. One of the causes of PD-related peritonitis is dialysate leakage. Dialysate leakage is likely to develop in the period of PD initiation especially with short duration between PD catheter insertion and intraperitoneal dialysate storage. Exit site discharge of high glucose levels can occur, and the exit site communicates with the peritoneal cavity by dialysate leakage. These effects increase the risk of PD-related peritonitis caused by dialysate leakage (PDPDL) [2].
International Society for Peritoneal Dialysis (ISPD) recommendations have little advice on managing PDPDL [3]. With PDPDL, only intraperitoneal antibiotic administration may be insufficient because of other infectious sites, except for the peritoneal cavity. Therefore, we attempted to treat a case of PDPDL with intraperitoneal and intravenous antibiotic therapy and obtained a successful result.
Case report
A 44-year-old Japanese man without no past history of abdominal surgery was admitted our hospital to initiate PD. He had type 2 diabetes mellitus and was treated by oral anti-diabetic drugs, but kidney dysfunction had been progressing.
On admission, his height was 169 cm, body weight was 81.9 kg, body temperature was 37.3 °C, and blood pressure was 138/88 mmHg. A physical examination showed normal breathing sounds, heart sounds, abdomen, and nervous system. He had mild edema at his lower limbs. The urinary occult blood of qualitative test was 1 + , and the count of red blood cells in urine was 1–4/HPF. The count of white blood cells in urine was 1–4/HPF. The urinary protein of qualitative test was 3 + , and the urinary protein-to-creatinine ratio 6.6 g/g Cr. A full blood examination showed the following: white blood cells, 6,830/µL; hemoglobin, 10.4 g/dL; hematocrit, 30.7%; and platelets, 24.1 × 104/µL. Serum biochemical analyses showed the following: total protein, 5.4 g/dL; serum albumin, 2.9 g/dL; blood urea nitrogen, 69 mg/dL; serum creatinine, 7.50 mg/dL; sodium, 140 mmol/L; potassium, 4.1 mmol/L; chloride, 106 mmol/L; calcium 8.0 mg/dL; phosphate 5.3 mg/dL; and C-reactive protein, 0.19 mg/dL (Table. 1). The venous gas analysis showed the following: pH 7.355, pO2 50.3 mmHg, pCO2 33.4 mmHg, HCO3− 18.2 mmol/L, base excess – 6.5 mmol/L.
Table 1.
Laboratory data at the time of the admission
| Parameter | Level |
|---|---|
| Urinalysis | |
| Specific gravity | 1.014 |
| pH | 6.5 |
| Occult blood | (1 +) |
| Protein | (3 +) |
| Red blood cell, /HPF | 3–5 |
| White blood cell, /HPF | 3–4 |
| Protein-to-creatinine ratio, g/g Cr | 6.6 |
| Complete blood count | |
| White blood cell, μL | 6830 |
| Neutrophils, % | 70.3 |
| Lymphocyte, % | 18.5 |
| Eosinophils, % | 2.6 |
| Basophils, % | 0.4 |
| Monocyte, % | 7.0 |
| Hemoglobin, g/dL | 10.4 |
| Hematocrit, % | 30.7 |
| Platelets, × 104/μL | 24.1 |
| Serum biochemistry | |
| Total protein, g/dL | 5.4 |
| Albumin, g/dL | 2.9 |
| Urea nitrogen, mg/dL | 69 |
| Creatinine, mg/dL | 7.5 |
| Sodium, mmol/L | 140 |
| Potassium, mmol/L | 4.1 |
| Chloride, mmol/L | 106 |
| Calcium, mg/dL | 8.0 |
| Phosphate, mg/dL | 5.3 |
| Aspartate transaminase, U/L | 13 |
| Alanine aminotransferase, U/L | 28 |
| γ-Glutamyl transpeptidase, U/L | 30 |
| Triglyceride, mg/dL | 177 |
| Total cholesterol, mg/dL | 168 |
| C-reactive protein, mg/dL | 0.19 |
Although the patient had a slight fever, he had no symptoms, and his laboratory test showed none of inflammation. Therefore, we performed PD catheter implantation. A transverse incision in rectus abdominis was made through the skin and the subcutaneous tissue. A subcutaneous adipose layer was thick and preperitoneal adipose layer was found. After reaching the peritoneal cavity, catheter was inserted without difficulties. We placed purse-string sutures of peritoneum twice to preventing leakage. A subcutaneous catheter tunnel was made through swan-neck abdominal pathway. Exit site was made with a piercing trocar. This sequential procedure of surgery did not have any problem and catheter was flushed once a day for 3 days before PD was initiated. Storage of the peritoneal dialysate that contains 1.5% glucose concentration took 10 days, gradually increasing up to 1500 mL × 4/day, and the patient’s Kt/V was 2.008 (residual kidney: 1.003; dialysate: 1.005)/week.
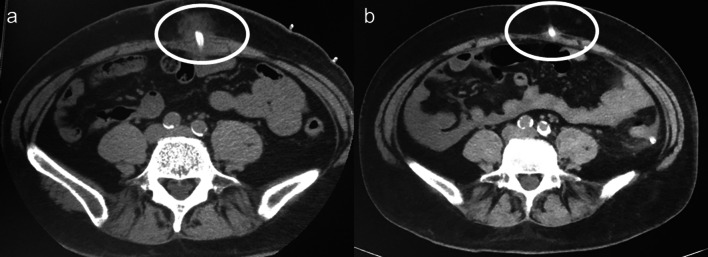
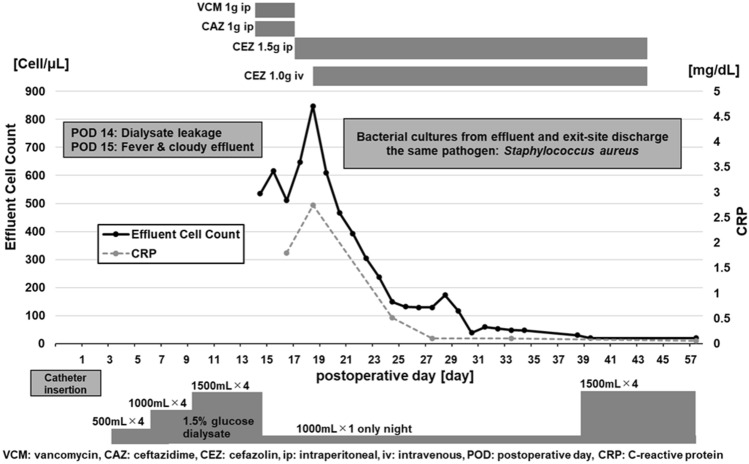
On 14 days after initiating PD, exit site discharge appeared. Fluid of the discharge showed high glucose levels and abdominal computed tomography (CT) showed dialysate leakage in the abdominal wall (Fig. 1a). We ceased intraperitoneal dialysate storage in the daytime without transferring to temporary hemodialysis because he had preserved residual kidney function with sufficient urinary volume. On the following day, he had a fever and cloudy effluent, with 535/µL of effluent cell count. We diagnosed him with PD-related peritonitis and began to administer intraperitoneal vancomycin 1 g/day and ceftazidime 1 g/day. The storage of the peritoneal dialysate was only once a day during night time with supine posture, and these antibiotics were administrated into peritoneal dialysate of 1000 mL. The dwelling time of antibiotics was more than 6 h. However, the cloudy effluent was not ameliorated, and the effluent cell count remained high 4 days after beginning treatment. Both cultures from the effluent of peritoneal cavity and the exit–site discharge showed Staphylococcus aureus. The result of blood culture was negative. We changed intraperitoneal vancomycin and ceftazidime to intraperitoneal cefazolin 1.5 g/day according to a drug susceptibility test. However, the effluent cell count reached 848/µL 6 days after the onset of peritonitis. Therefore, we added intravenous cefazolin 1.0 g/day. Subsequently, his symptoms and cloudy effluent improved. Additionally, the effluent cell count decreased and achieved a normal count 17 days after the onset of peritonitis. After 4 weeks of cefazolin administration, we ceased antibiotics and resumed dialysate storage in the peritoneal cavity (Fig. 2). The patient had no recurrence of peritonitis until kidney transplantation, and abdominal CT showed no trace of dialysate leakage 2 years after initiating PD (Fig. 1b).
Fig. 1.
Computed tomography showing peritoneal dialysate leakage. a Peritoneal dialysate leakage can be seen around the catheter in the abdominal wall (circle). b No recurrence of peritoneal dialysate leakage after 2 years (circle)
Fig. 2.
Clinical course of the present case
Discussion
We report a patient with PDPDL who was treated with both intraperitoneal and intravenous antibiotic therapy. Intraperitoneal antibiotic therapy alone did not improve the peritonitis, but both intraperitoneal and intravenous antibiotics therapy was effective.
Peritoneal dialysate leakage, defined as any dialysate loss from the peritoneal cavity other than through the lumen of the catheter, is arbitrarily classified as early (< 30 days) or late (> 30 days), following catheter implantation and the start of PD [4]. Early leakage like the present case tends to be associated with catheter implantation technique, the timing of PD initiation, high-dialysate volumes used, some medication with adverse effect of treatment delay of acute wound, and a weak abdominal wall such as obesity, a history of multiple surgeries or pregnancies [4–6]. In catheter implantation technique, the abdominal midline incision has been reported to be cause of dialysate leakage [7, 8]. We performed surgery with paramedian site approach. Moreover, we placed double purse-string sutures of peritoneum to preventing leakage and, therefore, it is quite unlikely that implantation technique was the cause of leakage. Delaying start of dialysis for 2 weeks following catheter placement prevents developing a leak [4, 9, 10]. Moreover, initial subcutaneous embedding of PD catheter decreases the early leakage [11, 12]. Any medication with adverse effect of treatment delay of acute wound such as corticosteroid or inhibitor of mammalian target of rapamycin was not used in this case. Diabetes might had an effect on the wound healing and cause the dialysate leakage, but no previous reports have shown the relation between diabetes and dialysate leakage. Obesity in the present case corresponded to overweight in World Health Organization classification [13] might be considered to be one of the reasons for developing dialysate leakage. In the present case, patient had multiple risks including diabetes mellitus and obesity. In addition, he had little symptom of severe uremia except for mild volume overload and trivial metabolic acidosis due to chronic renal failure. Therefore, delaying start of PD by subcutaneous embedding of catheter should have been considered.
If dialysate leakage is developed, dialysate storage should be ceased or decreased with supine posture. We thought at this time that the dialysate storage only during night with supine posture could contribute to reduce the harmful effect of dwell of dialysate, but the intraperitoneal storage of the present case should have been ceased completely to prevent exacerbation of dialysate leakage and infection. On the following day of development of dialysate leakage, peritonitis was developed and it was necessary to storage the dialysate for the route of intraperitoneal antibiotics administration. As an alternative, only intravenous antibiotic therapy with complete discontinuance of PD should have been considered. However, only intravenous antibiotic therapy might fail the treatment for peritonitis because it is preferable to treat with peritonitis by intraperitoneal antibiotic therapy in ISPD recommendations.
The infectious sources of PD-related peritonitis include intraluminal contamination such as touch contamination, periluminal contamination such as extension from exit site or tunnel infection, and secondary peritonitis such as transvisceral migration from gastrointestinal tract [14]. It is difficult to apply PDPDL to these kinds of sources. In PDPDL case, microbes were probably transmitted to peritoneal cavity through the fluid of not intraluminal but periluminal catheter. However, the absence of purulent drainage from exit site or erythema of the skin over the subcutaneous pathway does not suggest exit site or tunnel infection. The characteristics that differ from a general PD-related peritonitis suggest that the case of PDPDL requires the distinctive therapy.
The treatment for PDPDL is unclear, and the ISPD recommendations about peritonitis [3] and about creating and maintaining optimal PD access in the adult patient [15] shows the way not for treating infection with dialysate leakage but for preventing it. We started to treat by intraperitoneal vancomycin and ceftazidime in accordance with ISPD recommendation about treatment for general PD-related peritonitis. Even though glycopeptide such as vancomycin plus ceftazidime was considered to be superior to other regimens in a proportional meta-analysis [16], peritonitis of the present case was not ameliorated. A total of 42% patients with peritoneal leakage suffer from catheter infection or PDPDL, and the catheter removal rate of PDPDL can reach 52% [2]. Therefore, the method of treating PDPDL should be essentially established. Unfortunately, the treatment for PDPDL is unclear and even case reports of PDPDL are very few. We think that our report serves as one example of the treatments for PDPDL.
The present PDPDL case with successful treatment by intravenous and intraperitoneal antibiotics had two implications in the treatment for PDPDL. The first implication is the route of antibiotics. The ISPD recommendations about peritonitis shows that intraperitoneal administration of antibiotics is the preferred route unless the patient has features of systemic sepsis [3]. In the present case, peritoneal administration alone was insufficient because he had dialysate leakage, which might have infectious dialysate around the catheter. Therefore, additional intravenous antibiotic treatment was performed and effective. The findings suggest that PD-related peritonitis accompanied by other infectious sites, such as dialysate leakage, should be treated with additional intravenous antibiotics.
The second implication is the concentrations of antibiotics. The ISPD recommendations suggest that adjustment of the intraperitoneal antibiotic dose for residual kidney function is unnecessary [3]. A previous report showed that the correlation between urinary volume and vancomycin concentrations in dialysate was poor [17]. Another report showed that plasma vancomycin and gentamicin concentrations were similar in patients who were cured of PD-related peritonitis compared with those who required catheter removal [18]. In case of PDPDL, intraperitoneal antibiotics has effect on infection in peritoneum, but it is not certain that the dosage of the intraperitoneal antibiotics for treating general PD-related peritonitis can keep plasma concentration enough for treating infectious fluid around catheter. Indeed, antibiotics are absorbed from the intraperitoneal cavity to plasma, but plasma concentrations may be influenced by residual kidney function, permeability of the peritoneum, and the type of the antibiotics. In particular, the present case had preserved residual kidney function. Therefore, additional intravenous cefazolin administration was reasonable to maintain plasma antibiotic concentrations certainly.
A case report cannot establish the manner of treatment for disease. Not all cases of PDPDL should be treated by the same manner of the present case. We thought that the cause of ineffectiveness of only intraperitoneal antibiotics therapy was inadequate serum concentration of antibiotics because the patient had obesity and residual kidney function. Therefore, we planned to remove the catheter if the combination therapy of both intraperitoneal and intravenous antibiotics failed or the side effect of antibiotics was shown.
In summary, we treated a case of PDPDL using intraperitoneal and intravenous antibiotic therapy. Our findings suggest that PD-related peritonitis accompanied by other infectious sites, such as PDPDL, should be treated with additional intravenous antibiotic therapy to taking effect on the infectious sites except for peritoneum and to keep plasma concentration of antibiotics sufficient especially in cases with preserved residual kidney function.
Acknowledgements
We thank Ellen Knapp, from Edanz Group (www.edanzediting.com/ac) for editing the English text of a draft of this manuscript.
Declarations
Conflict of interest
All authors have declared that no conflict of interest exists.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Written informed consent was obtained from the patient included in this case study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ghali JR, Bannister KM, Brown FG, Rosman JB, Wiggins KJ, Johnson DW, et al. Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Perit Dial Int. 2011;31:651–662. doi: 10.3747/pdi.2010.00131. [DOI] [PubMed] [Google Scholar]
- 2.Holley JL, Bernardini J, Piraino B. Characteristics and outcome of peritoneal dialysate leaks and associated infections. Adv Perit Dial. 1993;9:240–243. [PubMed] [Google Scholar]
- 3.Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, Figueiredo AE, et al. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;36:481–508. doi: 10.3747/pdi.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzamaloukas AH, Gibel LJ, Eisenberg B, Goldman RS, Kanig SP, Zager PG, et al. Early and late peritoneal dialysate leaks in patients on CAPD. Adv Perit Dial. 1990;6:64–71. [PubMed] [Google Scholar]
- 5.Newman LN, Tessman M, Hanslik T, Schulak J, Mayes J, Friedlander M. A retrospective view of factors that affect catheter healing: four years of experience. Adv Perit Dial. 1993;9:217–222. [PubMed] [Google Scholar]
- 6.Perl J, Jassal SV, Bargman JM. Persistent peritoneal dialysis catheter exit-site leak in a patient receiving maintenance immunosuppression with sirolimus. Clin Transplant. 2008;22:672–673. doi: 10.1111/j.1399-0012.2008.00823.x. [DOI] [PubMed] [Google Scholar]
- 7.Helfrich BG, Pechan WB, Alijani MR, Barnard WF, Rakowski TAWJ. Reduction of catheter complications with lateral placement. Perit Dial Bull. 1983;3(Suppl 4):S2–4. doi: 10.1177/089686088300304S01. [DOI] [Google Scholar]
- 8.Spence PA, Mathews RE, Khanna ROD. Improved results with a paramedian technique for the insertion of peritoneal dialysis catheters. Surg Gynecol Obs. 1985;161:585–587. [PubMed] [Google Scholar]
- 9.Leblanc M, Ouimet D, Pichette V. Dialysate leaks in peritoneal dialysis. Semin Dial. 2001;14:50–54. doi: 10.1046/j.1525-139x.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- 10.Winchester JFKF. Fluid leaks: prevention and treatment. Perit Dial Int. 1994;14(3):S43–48. doi: 10.1177/089686089401403S09. [DOI] [PubMed] [Google Scholar]
- 11.Moncrief JW, Popovich RP, Broadrick LJ, He ZZ, Simmons EE, Tate RA. The Moncrief-Popovich catheter. A new peritoneal access technique for patients on peritoneal dialysis. ASAIO J. 1993;39:62–65. doi: 10.1097/00002480-199339010-00014. [DOI] [PubMed] [Google Scholar]
- 12.Prischl FC, Wallner M, Kalchmair H, Povacz F, Kramar R. Initial subcutaneous embedding of the peritoneal dialysis catheter - A critical appraisal of this new implantation technique. Nephrol Dial Transplant. 1997;12:1661–1667. doi: 10.1093/ndt/12.8.1661. [DOI] [PubMed] [Google Scholar]
- 13.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London, England) 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 14.Ito Y, Tawada M, Yuasa H, Ryuzaki M. New Japanese society of dialysis therapy guidelines for peritoneal dialysis. Contrib Nephrol. 2019;198:52–61. doi: 10.1159/000496523. [DOI] [PubMed] [Google Scholar]
- 15.Crabtree JH, Shrestha BM, Chow KM, Figueiredo AE, Povlsen JV, Wilkie M, et al. Creating and maintaining optimal peritoneal dialysis access in the adult patient: 2019 update. Perit Dial Int. 2019;39:414–436. doi: 10.3747/pdi.2018.00232. [DOI] [PubMed] [Google Scholar]
- 16.Barretti P, Doles JVP, Pinotti DG, El Dib R. Efficacy of antibiotic therapy for peritoneal dialysis-associated peritonitis: A proportional meta-analysis. BMC Infect Dis. 2014;14:445. doi: 10.1186/1471-2334-14-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fish R, Nipah R, Jones C, Finney H, Fan SLS. Intraperitoneal vancomycin concentrations during peritoneal dialysis-associated peritonitis: correlation with serum levels. Perit Dial Int. 2012;32:332–338. doi: 10.3747/pdi.2010.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blunden M, Zeitlin D, Ashman N, Fan SL. Single UK centre experience on the treatment of PD peritonitis — antibiotic levels and outcomes. Nephrol Dial Transplant. 2007;22:1714–1719. doi: 10.1093/ndt/gfm079. [DOI] [PubMed] [Google Scholar]