Abstract
A 37-year-old African–British man was referred to our hospital for detailed examination because of persistent fever, swelling and pain in both ankle joints, and blurred vision for two months. Inguinal lymph node biopsy showed a large number of epithelioid granulomas without necrosis. Granulomatous anterior uveitis, nephropathy, high serum angiotensin-converting enzyme activity, and high serum-soluble interleukin-2 receptor were observed, and the diagnosis of systemic sarcoidosis was made. His serum creatinine was 1.4 mg/dL and hematuria, leukocyturia, and urine protein were also seen. The renal biopsy finding was mesangial proliferative glomerulonephritis, with no findings of granuloma formation or tubular interstitial nephritis. Immunofluorescence staining showed deposition of IgG, C3, and C1q in the mesangial region. IgG3 was dominant in subclass staining. There was no monoclonality on kappa and lambda staining. Electron microscopy showed predominant deposition in the mesangial region with some subepithelial and endothelial deposition. His hematuria and leukocyturia disappeared with steroid therapy, suggesting sarcoidosis-related nephropathy. A case of systemic sarcoidosis with mesangial proliferative glomerulonephritis showing predominant deposition of IgG in the mesangial region is presented. No cases of such histological findings have been reported so far, and it is necessary to analyze further cases to clarify the pathogenic significance of the renal biopsy findings observed in this case.
Keywords: Systemic sarcoidosis, Mesangial proliferative glomerulonephritis, IgG
Introduction
Sarcoidosis is an unexplained inflammatory disease that forms epithelioid cell granulomas without necrosis. Renal lesions in sarcoidosis cases include lesions associated with abnormal calcium metabolism, glomerular lesions due to the background of systemic inflammatory diseases, and granulomatous interstitial nephritis. As to the glomerular lesions, a variety of different lesions have been described, including membranous nephropathy, IgA nephropathy, minimal-change disease, a proliferative or crescentic glomerulonephritis, and focal segmental glomerulosclerosis [1–3]. A case of systemic sarcoidosis with mesangial proliferative glomerulonephritis showing predominant deposition of IgG in the mesangial region is presented.
Case report
A 37-year-old African–British man had swelling and bilateral ankle pain, blurred vision, intermittent fever, and weight loss of 10 kg in 6 months. He visited a local doctor and was found to have renal dysfunction and a high CRP level, and he was referred to our hospital for detailed examination. At the time of admission, the patient was 164 cm tall and weighed 75.0 kg. Vital signs were as follows: temperature, 37.3 °C; pulse rate, 68 beats/min; blood pressure, 112/68 mmHg; respiratory rate, 16 breaths/min; and oxygen saturation, 98% (room air). Physical examination showed severe swelling and tenderness of bilateral ankles and swelling of the inguinal lymph nodes. The patient had a history of asthma, and eczema. He had no family history of renal disease.
Laboratory data at the time of admission are presented in Table 1. In addition to decreased renal function, laboratory examination showed elevated C-reactive protein (CRP), angiotensin-converting enzyme (ACE), and soluble interleukin-2 receptor (sIL2R) levels. Negative results were obtained for antinuclear antibodies, antineutrophil cytoplasmic antibodies, and rheumatoid factor. Urinalysis showed proteinuria of 0.7 g per gram creatinine, hematuria of 20–29 red blood cells per high-power field (HPF), and white blood cells of 5–9/HPF. No dysmorphic red blood cells or casts were observed. Urinary excretion of β2-microglobulin was in the normal range. Axillary and inguinal lymphadenopathy and a large number of patchy ground-glass shadows were observed on computed tomography (CT), mainly in the lower lobes of both lungs. No mediastinal hilar lymphadenopathy was observed. On close examination by an ophthalmologist demonstrated anterior ocular uveitis with lard-like posterior corneal deposits and Busacca nodules. Inguinal lymph node biopsy showed a large number of epithelioid granulomas without necrosis. Given all of these findings, he was diagnosed with systemic sarcoidosis.
Table 1.
Laboratory data at the time of admission
| WBCs | 6200/μL | CRP | 8.7 mg/dL |
|---|---|---|---|
| Neutrophils | 72% | IgG | 1780 mg/dL |
| Eosinophils | 4% | IgA | 262 mg/dL |
| Basophils | 1% | IgM | 34 mg/dL |
| Monocytes | 11% | C3 | 162 mg/dL |
| Lymphocytes | 14% | C4 | 51 mg/dL |
| RBCs | 506 × 104/μL | CH50 | 56 U/mL |
| Hb | 12.2 g/dL | ANA | < 1:40 |
| Hct | 36.7% | P-ANCA | < 0.5 IU/mL |
| Plt | 32.5 × 104/μL | C-ANCA | 0.7 IU/mL |
| TP | 7.7 g/dL | ACE | 30.7 IU/L |
| Alb | 3.5 g/dL | sIL2R | 2320 U/mL |
| AST | 68 IU/L | HBS-Ag | Negative |
| ALT | 73 IU/L | HCV-Ab | Negative |
| LDH | 237 IU/L | HIV-AgAb | Negative |
| BUN | 15.7 mg/dL | ||
| Cre | 1.35 mg/dL | Urinary P/C ratio | 0.7 g/gCr |
| Ca | 10.1 mg/dL | Urinary RBC | 20–29/HPF |
| T-Chol | 191 mg/dL | Urinary WBC | 5–9/HPF |
| LDL-Chol | 125 mg/dL | Urinary NAG | 15.1 U/L |
| Glu | 86 mg/dL | Urinary β2MG | 227 μg/L |
| HbA1c | 4.1% |
ANA antinuclear antibody, P-ANCA perinuclear-antineutrophil cytoplasmic antibody, C-ANCA cytoplasmic-antineutrophil cytoplasmic antibody, ACE angiotensin-converting enzyme, sIL2R soluble interleukin-2 receptor, HBS hepatitis B surface, Ag antigen, HCV hepatitis C virus, Ab antibody, HIV human immunodeficiency virus, P/C protein/creatinine, RBC red blood cell, WBC white blood cell, NAG N-acetyl-glucosaminidase, β2MG beta 2 microglobulin
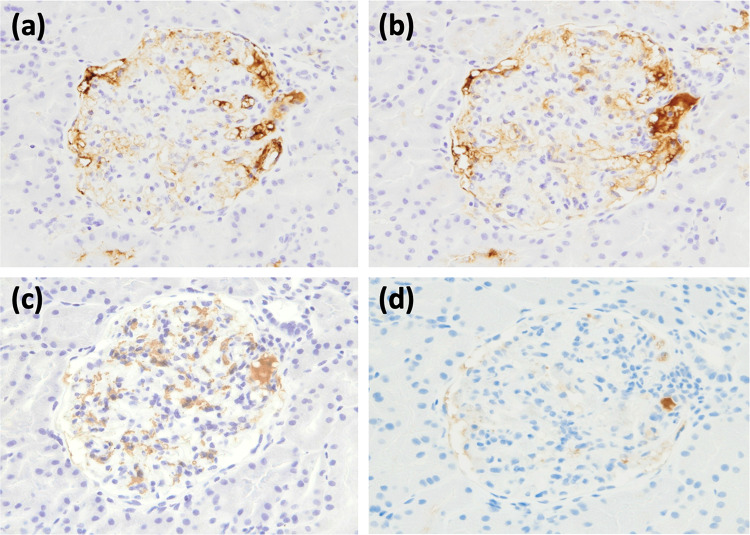
A renal biopsy was performed to investigate the pathology of this renal disease. Light microscopy examination of ten glomeruli showed one with global sclerosing changes, and mesangial proliferative change was noted in seven glomeruli, with no findings of granuloma formation or tubular interstitial nephritis (Fig. 1, a–c). Immunofluorescence staining showed deposition of IgG, C3, and C1q in the mesangial region (Fig. 1, d–h). There was no monoclonality on kappa and lambda staining (Fig. 1 i, j). Electron microscopy showed predominant deposition in the mesangial region with some subepithelial and endothelial deposition (Fig. 1, k–m). IgG3 was dominant in subclass staining (Fig. 2). From the above observations, the pathologic diagnosis was mesangial proliferative glomerulonephritis with predominant deposition of IgG in the mesangial region.
Fig. 1.
Renal biopsy specimens. a, b Periodic acid–Schiff staining. c Periodic acid–methenamine silver staining. d Immunofluorescent IgG staining. e Immunofluorescent IgA staining. f Immunofluorescent IgM staining. g Immunofluorescent C1q staining. h Immunofluorescent C3 staining. i Immunofluorescent kappa staining. j Immunofluorescent lambda staining. k, l, m Electron microscopy
Fig. 2.
Immunohistochemistry of paraffin tissue for IgG1 to 4. Immunofluorescence staining shows IgG3-dominant deposition in the mesangial region. a IgG1, b IgG2, c IgG3, d IgG4
Treatment was started with prednisolone 60 mg per day (0.8 mg/kg/day), and the patient’s general condition improved, CRP became negative, and ocular findings, lung lesion and bilateral ankle arthritis also improved. As to the nephropathy, hematuria and leukocyturia disappeared, and urinary protein decreased from 0.7 to 0.4 g. The creatinine level remained stable at about 1.3 to 1.5 mg/dL.
Discussion
It has been reported that clinically important renal lesions are found in about 35–50% of systemic sarcoidosis cases [4–7]. Renal lesions in sarcoidosis cases include lesions associated with abnormal calcium metabolism, glomerular lesions due to the background of systemic inflammatory diseases, and granulomatous interstitial nephritis [7]. As to the glomerular lesions, a variety of different lesions have been described, including membranous nephropathy, IgA nephropathy, minimal-change disease, a proliferative or crescentic glomerulonephritis, and focal segmental glomerulosclerosis [1–3]. A cohort study by Loffler et al. reported that 26% of patients had sarcoidosis with IgA nephropathy, and in all cases, treatment with prednisolone improved renal function and decreased urinary protein [2]. Auinger et al. also reported a case of sarcoidosis with ANCA-positive crescentic glomerulonephritis, suggesting some association between glomerulitis and sarcoidosis, although the details are unknown [8]. Table 2 shows a summary of case reports and observational studies of sarcoidosis with glomerular lesions published since 2000. According to this, membranous nephropathy was the most common in 20 cases, followed by IgA nephropathy in 16 cases. In addition, multiple cases of focal segmental glomerulosclerosis, necrotizing crescentic glomerulonephritis, and minimal-change nephrotic syndrome have also been reported. Based on the data in Table2, we illustrated the literature review of glomerulonephritis with sarcoidosis (after 2000) (Fig. 3). From this figure, it can be understood that most of the mesangial proliferative glomerulonephritis associated with sarcoidosis was IgA nephropathy, which was 16 out of 21 cases (76%). The other five cases were lupus nephritis with a full-house pattern, one case with C3, C4d, and C5b-9 deposits in the mesangial region, one case with IgM-immune complex in the mesangial region, and our case. From this, it can be said that the mesangial proliferative glomerulonephritis showing predominant deposition of IgG in the mesangial region seen in this case is rare.
Table 2.
Literature review of glomerulonephritis with sarcoidosis (after 2000)
| Year | Author | Type of glomerulonephritis | Reference |
|---|---|---|---|
| 2002 | Altiparmak et al | FSGS | [10] |
| 2002 | Evangelista et al | Necrotizing crescentic GN | [11] |
| 2003 | Hamada et al | IgAN | [12] |
| 2004 | Soylu et al | Membranous nephropathy | [13] |
| 2005 | Hagiwara et al | Mesangial proliferative GN (C3, C4d, and C5b-9 deposits) | [14] |
| 2005 | Kahn et al | IgAN | [15] |
| 2005 | Kornev et al | IgAN | [16] |
| 2006 | Kanamori et al | IgM-immune complex GN | [17] |
| 2007 | Polaina et al | FSGS | [18] |
| 2011 | Knehtl et al | Membranous nephropathy (PLA2R-related) | [19] |
| 2013 | Martin-Navarro et al | FSGS | [20] |
| 2013 | Larsen et al | Membranous nephropathy (4 cases) | [21] |
| 2013 | Kabara et al | Necrotizing crescentic GN | [22] |
| 2013 | Stehle et al | Membranous nephropathy (11 cases), IgAN (6 cases), FSGS (4 cases), MCNS (3 cases), proliferative lupus nephritis (2 cases) | [1] |
| 2013 | Svobodova et al | Membranous nephropathy (PLA2R-related) | [23] |
| 2014 | Zilberman et al | Membranous nephropathy | [24] |
| 2015 | Löffler et al | IgAN (7 cases) | [2] |
| 2015 | Stehle et al | Membranous nephropathy (PLA2R-related) | [25] |
| 2018 | Al-Hwiesh et al | Necrotizing crescentic GN | [26] |
| 2019 | Akbari et al | IgAN | [27] |
| 2019 | Tsuchiya et al | Necrotizing crescentic GN (MPO-ANCA-positive) | [28] |
| 2020 | Sugai et al | Necrotizing crescentic GN | [29] |
| 2021 | Yoshida et al | Membranous nephropathy | [30] |
| Present case | Watanabe et al | Mesangial proliferative GN with IgG deposition | – |
FSGS focal segmental glomerulosclerosis, GN glomerulonephritis, IgAN immunoglobulin A nephropathy, MCNS minimal-change nephrotic syndrome
Fig. 3.

Literature review of glomerulonephritis with sarcoidosis (after 2000). MN membranous nephropathy, IgAN IgA nephropathy, FSGS focal segmental glomerulosclerosis, NCGN necrotizing crescentic glomerulonephritis, Mes PGN mesangial proliferative glomerulonephritis, MCNS minimal-change nephrotic syndrome
In this report, a case of systemic sarcoidosis with mesangial proliferative glomerulonephritis showing predominant deposition of IgG in the mesangial region was presented. The glomerulus showed an increase in substrate and cell proliferation in the mesangial region, and the basement membrane showed no changes, such as spike formation or double contour. In addition, no findings suggestive of interstitial inflammation were found in the interstitial region. The characteristic finding of mesangial proliferative nephritis in this case was that IgG and complement were deposited on immunostaining, and IgG3 was predominant on subclass staining. An IgG3-dominant staining pattern is found in autoimmune diseases such as SLE and rheumatoid arthritis [9]. The IgG3-dominant deposition pattern in the present case may have been associated with sarcoidosis in some way, but the details are unknown. Since some kind of deposition disease such as light chain deposition disease (LCDD) was also in the differential diagnosis, κ and λ staining was also performed, but there was no monoclonality. No cases of such histological findings have been reported so far, and it is necessary to analyze further cases to clarify the pathogenic significance of the renal biopsy findings observed in this case.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not describe any studies with human participants or animals performed by any of the authors.
Consent for publication
Informed consent was obtained from the patient for publication of the case details.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stehle T, Joly D, Vanhille P, et al. Clinicopathological study of glomerular diseases associated with sarcoidosis: a multicenter study. Orphanet J Rare Dis. 2013;8:65. doi: 10.1186/1750-1172-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loffler C, Loffler U, Tuleweit A, et al. Renal sarcoidosis: epidemiological and follow-up data in a cohort of 27 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2015;31:306–315. [PubMed] [Google Scholar]
- 3.Maroz N, Field H. Necrotizing crescentic glomerulonephritis related to sarcoidosis: a case report. J Med Case Rep. 2015;9:282. doi: 10.1186/s13256-015-0764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lofgren S, Snellman B, Lindgren A. Renal complications in sarcoidosis; functional and biopsy studies. Acta Med Scand. 1957;159:295–305. doi: 10.1111/j.0954-6820.1957.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 5.Lebacq E, Desmet V, Verhaegen H. Renal involvement in sarcoidosis. Postgrad Med J. 1970;46:526–529. doi: 10.1136/pgmj.46.538.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacSearraigh ET, Doyle CT, Twomey M, O'Sullivan DJ. Sarcoidosis with renal involvement. Postgrad Med J. 1978;54:528–532. doi: 10.1136/pgmj.54.634.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergner R, Hoffmann M, Waldherr R, Uppenkamp M. Frequency of kidney disease in chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:126–132. [PubMed] [Google Scholar]
- 8.Auinger M, Irsigler K, Breiteneder S, Ulrich W. Normocalcaemic hepatorenal sarcoidosis with crescentic glomerulonephritis. Nephrol Dial Transplant. 1997;12:1474. doi: 10.1093/ndt/12.7.1474. [DOI] [PubMed] [Google Scholar]
- 9.Haas M. IgG subclass deposits in glomeruli of lupus and nonlupus membranous nephropathies. Am J Kidney Dis. 1994;23:358–364. doi: 10.1016/S0272-6386(12)80997-8. [DOI] [PubMed] [Google Scholar]
- 10.Altiparmak MR, Oyger D, Bilir M, Kilicarslan I, Serdengecti K. A rare cause of focal segmental glomerulosclerosis: sarcoidosis. Nephron. 2002;90:211–212. doi: 10.1159/000049044. [DOI] [PubMed] [Google Scholar]
- 11.Evangelista DF, Hernandez-Jaras J, Gordo CC, Perez HG. Pulmonary Sarcoidosis associated to crescentic glomerulonephritis: a case report. An Med Interna. 2002;19:632–634. [PubMed] [Google Scholar]
- 12.Hamada K, Nagai S, Ono T, et al. Sarcoidosis complicated with IgA nephropathy. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:69–73. [PubMed] [Google Scholar]
- 13.Soylu A, Turkmen M, Kasap B, et al. Sarcoidosis with an uncommon presentation: apropos of a case. Turk J Pediatr. 2004;46:366–369. [PubMed] [Google Scholar]
- 14.Hagiwara S, Ohi H, Eishi Y, et al. A case of renal sarcoidosis with complement activation via the lectin pathway. Am J Kid Dis. 2005;45:580–587. doi: 10.1053/j.ajkd.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Kahn A, Hodges N, Lord M. A case of sarcoidosis in a patient with IgA nephropathy. MedGenMed. 2005;7:7. [PMC free article] [PubMed] [Google Scholar]
- 16.Kornev BM, Kozlovskaia LV, Fomin VV, et al. IGA-nephropathy with nephrotic syndrome as an extrapulmonary manifestation of sarcoidosis. Klin Med (Mosk) 2005;83:83–86. [PubMed] [Google Scholar]
- 17.Kanamori H, Ota M, Takeoka H, et al. IgM-immune complex glomerulonephritis associated with sarcoidosis. Clin Exp Nephrol. 2006;10:68–73. doi: 10.1007/s10157-005-0390-7. [DOI] [PubMed] [Google Scholar]
- 18.Polaina M, Perez del Barrio MP, Ramirez C, Borrergo FJ. Nephrotic syndrome secondary to focal and segmental glomerulosclerosis in a patient with alveolar sarcoidosis. Nefrologia. 2007;27:83–84. [PubMed] [Google Scholar]
- 19.Knehtl M, Debiec H, Kamgang P, et al. A case of phospholipase A2 receptor-positive membranous nephropathy preceding sarcoid-associated granulomatous tubulointerstitial nephritis. Am J Kid Dis. 2011;57:140–143. doi: 10.1053/j.ajkd.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Navarro JA, Gutierrez-Sanchez MJ, Petkov-Stoyanov V, Justo-Avila P, Ionela-Stanescu R. Focal segmental glomerulonephritis in patients with pulmonary sarcoidosis. Nefrologia. 2013;33:431–433. doi: 10.3265/Nefrologia.pre2012.Sep.11753. [DOI] [PubMed] [Google Scholar]
- 21.Larsen CP, Messias NC, Silva FG, Messias E, Walker PD. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol. 2013;26:709–715. doi: 10.1038/modpathol.2012.207. [DOI] [PubMed] [Google Scholar]
- 22.Kabara M, Nakagawa N, Matsuki M, Chinda J, Fujino T, Hasebe N. Mizoribine for crescentic glomerulonephritis with sarcoidosis: effectiveness not only for urinalysis abnormalities but also for hilar lymph node enlargement. Mod Rheumatol. 2013;23:146–150. doi: 10.3109/s10165-012-0614-0. [DOI] [PubMed] [Google Scholar]
- 23.Svobodova B, Honsova E, Ronco P, Tesar V, Debiec H. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant. 2013;28:1839–1844. doi: 10.1093/ndt/gfs439. [DOI] [PubMed] [Google Scholar]
- 24.Zilberman T, Zahavi T, Osadchy A, Nacasch N, Korzets A. Membranous nephropathy associated with sarcoidosis: a primary or secondary glomerulopathy? Isr Med Assoc J. 2014;16:390–392. [PubMed] [Google Scholar]
- 25.Stehle T, Audard V, Ronco P, Debiec H. Phospholipase A2 receptor and sarcoidosis-associated membranous nephropathy. Nephrol Dial Transplant. 2015;30:1047–1050. doi: 10.1093/ndt/gfv080. [DOI] [PubMed] [Google Scholar]
- 26.Al-Hweish AK, Abdul-Rahman IS, Alhweish A, et al. Sarcoidosis with multiple organ involvement associated with necrotizing crescentic glomerulonephritis. Saudi J Kidney Dis Transpl. 2018;29:979–984. doi: 10.4103/1319-2442.239649. [DOI] [PubMed] [Google Scholar]
- 27.Akbari R, Shahani M, Ranae M. Acute renal failure due to IgA nephropathy in sarcoidosis. Iran J Kidney Dis. 2019;13:340–342. [PubMed] [Google Scholar]
- 28.Tsuchiya K, Karayama M, Sato T, et al. Simultaneous occurrence of sarcoidosis and anti-neutrophil cytoplasmic antibody-associated vasculitis in a patient with lung cancer. Intern Med. 2019;58:3299–3304. doi: 10.2169/internalmedicine.3004-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugai M, Murata O, Oikawa H, et al. A case of bone marrow involvement in sarcoidosis with crescentic glomerular lesions. Respir Med Case Rep. 2020;31:101202. doi: 10.1016/j.rmcr.2020.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida H, Takahashi N, Horiguchi T, et al. Development of myeloperoxidase anti-neutrophil cytoplasmic antibody-positive necrotizing crescentic glomerulonephritis in an elderly patient with immunological kidney disease. Intern Med. [Online ahead of print] [DOI] [PMC free article] [PubMed]