Abstract
A 67-year-old woman with transverse myelitis and seizure disorder secondary to suspected central nervous system (CNS) systemic lupus erythematosus (SLE) and seropositive rheumatoid arthritis had two episodes of severe nephrotic syndrome 15 years apart. She underwent a renal biopsy in both episodes, showing tip lesion variant focal segmental glomerulosclerosis (FSGS). The patient responded both times to prednisone treatment, achieving a complete remission within 2 months in the first episode and remission 4 months in the second episode. A year after her second episode, the patient had a third episode of severe nephrotic syndrome. She achieved an equally rapid complete remission in 3 months without steroid treatment, as she was concomitantly treated with the Janus Kinase (JAK) inhibitor tofacitinib for a flare of rheumatoid arthritis. This case report suggests that JAK inhibitors may have therapeutic use in FSGS, which is supported by experimental data in the medical literature.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13730-021-00658-y.
Keywords: FSGS, JAK inhibitor, Tofacitinib, Tip lesion variant, Rhupus, Complete remission
Introduction
Focal segmental glomerulosclerosis (FSGS) is a heterogeneous disorder, where focal scarring of the filtering units in kidneys is the initial site of injury and disease progression [1]. Existing treatment is often ineffective from preventing progression and FSGS is an important cause of end stage kidney disease (ESRD) [2]. There is evidence of increased activation of the Janus Kinase signal transducer and activator of transcription (JAK/STAT) pathway in patients with FSGS [3], suggesting that JAK inhibitors could be useful to target this mechanism of injury [4]. JAK inhibitors are in clinical use for several autoimmune diseases [5]; however, only limited data exists of the use of JAK inhibitors for kidney disease in humans. Here we describe the case of a woman with recurrent tip variety FSGS that responded to coincident treatment of rheumatoid arthritis (RA) with the JAK inhibitor tofacitinib.
Case report
First episode of nephrotic syndrome
A woman with a history of hypertension and suspected central nervous system (CNS) systemic lupus erythematosus (SLE) presented at age 47 to the renal clinic with foamy urine, pitting edema and weight gain of about 25 lbs. Her blood pressure had been well controlled on amlodipine 10 mg daily and she had mostly recovered from her CNS disease except a neurogenic bladder. She had been treated with prednisone for almost 5 years and her symptoms developed while on a dose of 10 mg daily. She also received risedronate to preserve bone density. The patient did not use non-steroidal anti-inflammatory drugs (NSAIDS).
Her BP was 138/90, and her urine sediment revealed a few hyaline and granular casts, epithelial cells and few RBC of normal morphology. No acanthocytes were seen. A spot urine protein/creatinine ratio was 15, a serum albumin was 1.5 g/dl and her serum creatinine was 0.9 mg/dl. Her CBC was normal. Her ANA was negative, and complement levels were normal. The patient’s serum creatinine transiently increased to 1.8 mg/dl after she was started on an ACEI and a loop diuretic, which resolved following cessation of the ACEI and reduction in the diuretic dose.
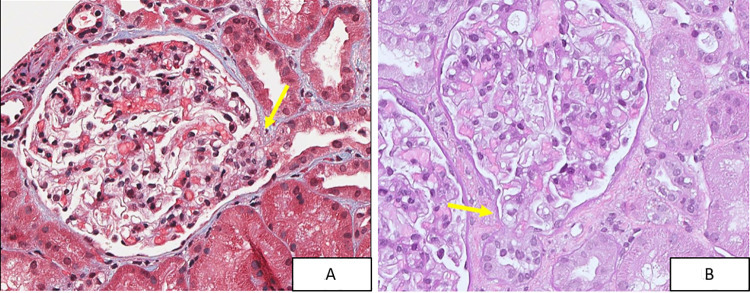
A kidney biopsy revealed tip lesion variant FSGS with at least three lesions in 18 glomeruli, consisting of adhesions between the glomerular tuft and Bowman’s capsule at the proximal tubular pole. Foam cells and hyaline droplets were present within the segmental lesions. Immunofluorescence (IF) was negative, and electron microscopy (EM) showed diffuse foot process effacement. Importantly, there were no perihilar or collapsing lesions and no histologic, immunofluorescence or ultrastructural features of lupus nephritis (Fig. 1).
Fig. 1.
Segmental sclerotic tip lesions (arrows) from the 2014 renal biopsy, stained by trichrome (A) and PAS (B). (40 ×)
Following the kidney biopsy, the patient was treated with prednisone 80 mg daily to which she responded in 4 weeks and achieved a complete remission in 8 weeks with a spot urine protein/creatinine ration of 0.3 and a serum albumin back into the normal range. At follow-up visits, the patient sometimes had microscopic hematuria as she self-catheterized to provide samples but usually her urine tested dipstick negative for blood. Repeated exams of the urine sediment showed no acanthocytes, and her urine tested negative for microalbuminuria. Her BP remained well controlled on 5 mg of amlodipine. The patient stopped following up with her nephrologist after 6 years.
Neurological disease suspected CNS lupus
Five years before she presented to the renal clinic (timeline graph), the patient had developed paraplegia, dysesthesias and neurogenic bladder and was diagnosed with transverse myelitis secondary to probable CNS SLE. The patient had a positive ANA (1:320), speckled pattern, and positive anti-Ro and anti-La antibodies but no sicca symptoms or other manifestation of Sjogren’s syndrome. The patient had no fever and no hematologic, mucocutaneous or joint manifestations of SLE. Complement was normal and anti-dsDNA, anti-Smith, anti-cardiolipin and other serologies for a vasculitis workup were negative. The patient responded to prednisone and pulse cyclophosphamide and recovered from her paraplegia. Unfortunately she has had to self-catheterize her bladder ever since. The patient had to stop cyclophosphamide after a year because of mental status changes and hyponatremia and did not tolerate azathioprine because of hepatitis. The patient suffered recurrent CNS symptoms with attempts to taper prednisone. She was started on Methotrexate (MTX), which was stopped after 3 years when she had a sinus infection, because it did not permit her to taper prednisone. Since her initial presentation, subsequent ANA had been negative and a differential diagnosis of demyelinating disease was entertained because of suggestive cerebral and spinal cord lesions on repeated MRI exams. Her neurological symptoms were stable on prednisone 10 mg daily when she developed her first episode of nephrotic syndrome and had her first kidney biopsy.
Following complete remission of tip lesion FSGS, the patient was unable to taper prednisone below 10 mg/day because of recurrent neurological symptoms, such as foot drop and dysesthesias. The patient developed episodes of garbled speech and visual migraines that were diagnosed as a seizure disorder, and she has been treated with topiramate since. Mycophenolate mofetil (MMF) was introduced as a steroid sparing agent, and prednisone was tapered to 6 mg daily. Seven and a half years later MMF was discontinued because of cytomegalovirus (CMV) hepatitis. The patient did well on 6 mg prednisone per day for more than 6 years with stable peripheral neuropathy and minor weakness in her lower extremities.
Rheumatological disease
The patient has been followed by the rheumatological service since her initial presentation with transverse myelitis and suspected vasculitis. Since then, the patient experienced a traumatic rotator cuff injury for which she declined surgery and has developed chronic shoulder pain. She was treated with bisphosphonates for osteopenia and once had anserine bursitis. Years later, she had an exacerbation of lumbar radiculopathy from degenerative changes of her spine that resolved with a short course of prednisone. The patient had been with stable neurological disease on 6 mg daily of prednisone for years when she developed joint pain and swelling in her hands, prominently on metacarpal–phalangeal joints but also distal phalange–phalangeal joints. Hand X-rays showed synovitis, erosive changes and concomitant osteoarthritis. The patient had an elevated CRP and positive rheumatoid factor (RF). The patient was treated with prednisone and methotrexate for rheumatoid arthritis (RA). Having tested negative for ANA repeatedly for years since her initial presentation, the patient then had positive ANA again with titers varying between 1:80 and 1:320 and speckled pattern. Complement was normal and anti-dsDNA and anti-Smith antibodies were negative. Anti-Ro and anti-La were positive as before. Cyclic citrullinated peptid antibody (CCP-Ab) was ambiguous with one positive and subsequent negative tests.
Second episode of nephrotic syndrome
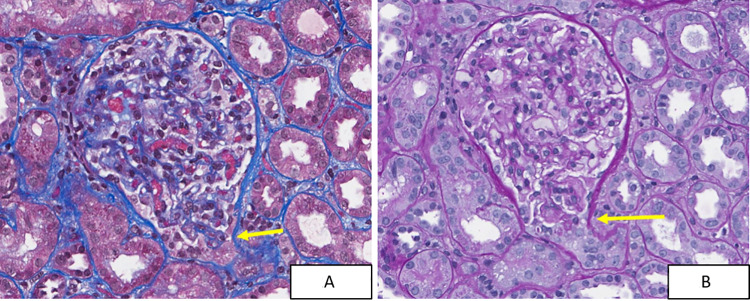
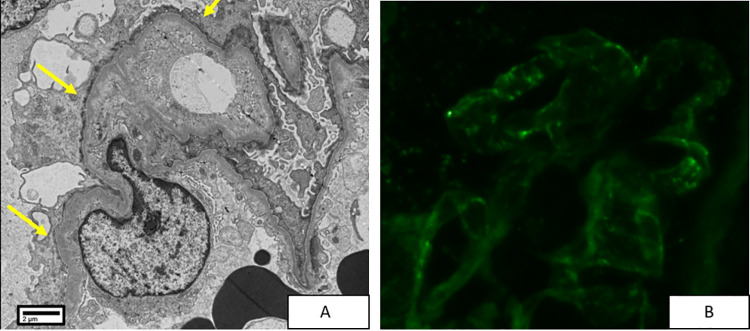
Almost 15 years since her initial visit and 5 months since her diagnosis of RA, the patient returned to the renal clinic with the same symptoms of foamy urine, pitting edema and weight gain. Her arthritis and neurological disease were treated with 10 mg daily of prednisone and 15 mg MTX. The patient was found to have a recurrent nephrotic syndrome with a spot urine protein/creatinine ratio of 16, and a serum albumin of 1.4 mg/dl. Her serum creatinine was normal (0.53 mg/dl). The urine dipstick showed 3 + protein and 1 + blood, and the sediment showed epithelial cells, as well as a few WBC’s and RBC’s of normal morphology. Acanthocytes were not seen. Complement levels were normal, ANA was positive (1:320) with speckled pattern, Anti-Ro and -La antibodies were positive and anti-dsDNA and anti-Smith antibodies were negative. A renal biopsy was performed and, as in her first biopsy, revealed a diagnosis of tip lesion FSGS with two tip variant lesions in 21 glomeruli (Fig. 2). IF showed trace to 1 + granular variable staining in peripheral capillary loops for anti-IgG, anti-IgA and anti-C3. IF was negative for C1q and IgM. Tip lesions were not present in tissue submitted for IF. EM showed widespread foot process effacement without any evidence of electron dense deposits (Fig. 3). The minimal glomerular immunoglobulin and complement staining seen by IF was deemed of uncertain significance and not considered diagnostic of lupus nephritis. The patient was very compliant with her medications and salt restricted diet. As a result, her edema was mild despite the severity of proteinuria and hypoalbuminemia, and no diuretics were required. The patient was treated again with prednisone (80 mg daily) and achieved a remission of her nephrotic syndrome in 4 months with normalization of her serum albumin and a gradual decline of her proteinuria. She tested dipstick negative for protein at 5 months. Eight months after her biopsy, the patient tested urine dipstick negative for protein.
Fig. 2.
Segmental sclerotic tip lesion (arrows) from the 2019 renal biopsy, stained by trichrome (A) and PAS (B). (40 ×)
Fig. 3.
Transmission electron photomicrograph showing diffuse podocyte foot process effacement (arrows in A) and absence of electron dense immune complex deposits. Immunofluorescence for anti-IgG (B) showed trace granular peripheral staining of uncertain significance
Initiation of tofacitinib
The patient was unable to taper prednisone below 20 mg daily because of severe arthritis limiting her ability to use her right hand. Methotrexate eventually was stopped due to inefficacy and side effects. The patient consulted a different rheumatologist because of increased pain and swelling. At her initial visit CRP, RF and CCP-Ab were all positive. ANA and complement were not tested. The patient had a serum albumin of 1.5 mg/dl, but her urine was not checked for protein. This was 3 months after her last dipstick negative urine test. The patient was started on tofacitinib 5 mg twice a day.
Third episode of nephrotic syndrome
Fourteen month since her last kidney biopsy, the patient returned to the renal clinic because of recurrence of foamy urine and dependent edema. By the time she was seen in the renal clinic, she had been treated with tofacinitib for 2 months already. Her prednisone dose was unchanged at 20 mg per day. Clinically, the edema and her arthritis symptoms had improved. A spot urine protein/creatinine ratio of 4 was in the nephrotic range. Her serum creatinine was normal 0.59 mg/dl. Complement was negative and ANA was positive (1:80) in a speckled pattern. Her serum albumin was 3.1 mg/dl, lower than normal, but improved from the previous serum albumin level of 1.5 mg/dl 3 months earlier. As the improved serum albumin and decreased edema suggested remission of the nephrotic syndrome, her prednisone dose was not increased and a renal biopsy was deferred. At the next follow-up visit 1 month later, the patient continued treatment with tofacitinib, and her prednisone dose had been tapered to 15 mg daily. The patient was in complete remission of her nephrotic syndrome with normal serum albumin and only microalbuminuria (100 mcg/mg) detectable in a spot urine, 3 months since tofacitinib was started. Half a year later, prednisone has been tapered to 5 mg daily, and the patient has stable rheumatological and neurological disease and remains in complete remission of her renal disease.
Discussion
FSGS is a pathological diagnosis that characterizes a heterogeneous group of disorders that in most patients progresses to ESRD within 5–8 years. The patient in discussion has tip variant FSGS, which has a more benign course [1]. Despite her initial presentation with massive proteinuria and extremely low serum albumin concentration, which are markers of poor prognosis [6], she responded to treatment. In fact, the patient never had a decrease in renal function and still has normal renal function after three recurrences and 17 years later. Among treatments of glomerular disease, there are few responses as dramatic and rapid as the decrease in proteinuria that may occur in minimal change disease (MCD) and tip lesion variant FSGS with prednisone. In this case we report an equally dramatic and rapid response of tip variant FSGS to the JAK inhibitor tofacitinib.
One could argue that in some cases of FSGS proteinuria can gradually decrease with the continued use of moderate amounts of prednisone and that the third episode of nephrotic syndrome remitted because of 20 mg of prednisone and not the tofacitinib. Against this possibility is that the nephrotic syndrome developed on the same 20 mg dose of prednisone. The patient was so compliant with her medications and salt restricted diet that, despite the severity of proteinuria and extremely low serum albumin levels, she only had modest edema and did not require diuretics during her second and third episodes. Moreover, her proteinuria decreased extremely rapidly with complete remission after only 3 months of tofacitinib and, remarkably, only a 1-month interval between a mid-course protein/creatinine ratio of 4 and microalbuminuria. It is important to note that the patient did not use NSAIDS, which can produce a nephrotic syndrome with tip lesions, which can resolve rapidly once the NSAIDS are stopped [7]. Finally, one has to consider that FSGS can remit spontaneously [8]. This also would be a much slower process and unlikely to happen three times in the same patient.
The patient’s neurological disease was attributed to SLE, lacking a better explanation. This is despite the absence of constitutional, hematologic, mucocutaneous, serosal and musculoskeletal manifestations. Moreover, the patient never had low complement, antiphospholipid or SLE specific antibodies. The patient developed her first episode of nephrotic syndrome during the period of several years that she tested ANA negative and had no biopsy features of SLE. Thus, her tip lesion variant FSGS was most likely primary rather than secondary to SLE. The second biopsy showed a trace amount of granular IF staining for IgG, C3, IgA, kappa and lambda, but there was no C1q or IgM deposition. No electron dense immune complex deposits were identified by electron microscopy. The trace or 1 + staining seen by immunofluorescence is of unknown significance, and there was no definite biopsy evidence for lupus nephritis. However, because this patient’s neurologic disease could represent SLE, we cannot completely exclude the possibility that the FSGS in this case represents an unusual atypical lupus podocytopathy, even though she has none of the typical clinical and serological signs of SLE typically observed with lupus podocytopathy [9]. The treatment would be the same as in non-lupus associated minimal change disease/FSGS.
As this patient later went on to develop seropositive RA and has possible SLE neurological disease, the possibility of Rhupus syndrome has to be considered. Rhupus describes the presence of two autoimmune diseases, SLE and RA in the same patient, suggesting a potential overlap with common pathophysiological mechanisms [10]. A recent comparative study of clinical and serologic characteristics between patients with rhupus and those with SLE and RA, which included a review of all available case series, indicated that cutaneous and hematological manifestations and serositis are the most frequent extra articular manifestations of rhupus [11]. Compared to SLE, rhupus patients have a lower incidence of renal and neurological manifestations. All rhupus patients with renal manifestations have proteinuria, but, different from SLE, nephrotic range proteinuria has not been described [11]. A case report from Italy describes the effect of Jak inhibitors baricitinib and tofacitinib in two patients with rhupus who have renal disease. One patient with class V SLE nephritis had a decrease in proteinuria from 750 to 230 mg/day in 6 months. A second patient with class III SLE nephritis had a decrease in proteinuria from 420 to 270 mg/day in 6 months; both patient had normal serum creatinine [12]. These two patients had a different patter of renal pathology than our patient and a much slower and modest response. It is clear that our patient is not a typical rhupus patient, but autoimmune disease is dynamic. In the absence of official defining characteristics, we cannot exclude this possibility. Renal disease in patients with RA alone is usually non-autoimmune and related to side effects of treatment [13]. Hence, our observation of the occurrence of concomitant flares of RA and tip lesion variant FSGS, which allows for observation of the effect of tofacitinib on both conditions, is rare and serendipitous.
Tofacitinib is a small molecule oral Janus Kinase (JAK) inhibitor that was developed initially as a JAK 3 inhibitor for maintenance immunosuppression in transplantation but proved more of a pan JAK inhibitor with increased risk of malignancy and infection in clinical trials [14]. The FDA approved tofacitinib for treatment of RA in 2012 and since then for psoriatic arthritis and ulcerative colitis [5].
There is only limited data of the effect of JAK inhibitors on kidney disease in humans. A pilot study of the JAK inhibitor baricitinib in patients with diabetic nephropathy found after 24 weeks a 41% reduction of average pretreatment albuminuria of 820 mg/g compared to placebo [15]. We mentioned the case report of JAK inhibitors in two patients with rhupus [12]. In a second case report, tofacitinib treatment for RA in a patient with biopsy proven concomitant IgA nephropathy and amyloidosis lowered proteinuria from 2.6 to 0.04 g/day, and serum creatinine from 1.18 to 0.97 mg/dl [16]. On the other hand, there is a case report of a patient with RA developing IgA vasculitis with tofacitinib treatment [17].
There is experimental evidence for the activation of STAT signaling in a variety of kidney diseases, including FSGS. Interestingly, the main pathway implicated is JAK2/STAT3 [18]. Tofacitinib is more active against JAK 1 and 3 but inhibits JAK2 as well. Thus there is a potential molecular mechanism that may explain the apparent beneficial activity of tofacitinib in tip variant FSGS. The serendipitous clinical observation reported here raises the question whether this agent or related agents might be therapeutically useful against steroid-resistant FSGS variants that may have worse prognosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to acknowledge and thank the patient who has taught us many valuable lessons about life with chronic disease and has allowed us to share her experience with Tofacitinib in this case report.
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from the patient whose case is reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Martin Sedlacek, Email: martin.sedlacek@mssm.edu.
Jason R. Pettus, Email: Jason.R.Pettus@hitchcock.org
References
- 1.Fogo AB. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat Rev Nephrol. 2015;11(2):76–87. doi: 10.1038/nrneph.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bose B, Cattran D, Toronto Glomerulonephritis Registry Glomerular diseases: FSGS. Clin J Am Soc Nephrol. 2014;9(3):626–632. doi: 10.2215/CJN.05810513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tao PMJ, Mariani L, Eddy S, Maecker H, Kambham N, Mehta K, Hartman J, Wang W, Kretzler M, Lafayette RA. JAK-STAT signaling is activated in the kidney and peripheral blood cells of patients with focal segmental glomerulosclerosis. Kidney Int. 2018;94(4):795–808. doi: 10.1016/j.kint.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trachtman H. Emerging drugs for treatment of focal segmental glomerulosclerosis. Expert Opin Emerg Drugs. 2020;25(3):367–375. doi: 10.1080/14728214.2020.1803276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O'Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16(12):843–862. doi: 10.1038/nrd.2017.201. [DOI] [PubMed] [Google Scholar]
- 6.Korbet SM. Treatment of primary FSGS in adults. J Am Soc Nephrol. 2012;23(11):1769–1776. doi: 10.1681/ASN.2012040389. [DOI] [PubMed] [Google Scholar]
- 7.Sekhon I, Munjal S, Croker B, Johnson RJ, Ejaz AA. Glomerular tip lesion associated with nonsteroidal anti-inflammatory drug-induced nephrotic syndrome. Am J Kidney Dis. 2005;46(4):e55–e58. doi: 10.1053/j.ajkd.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Oo SZMWH, Freese ME, Holanda DG, Thomas CP. Spontaneous remission of genetic, apparent primary, FSGS presenting with nephrotic syndrome challenges traditional notions of primary FSGS. J Nephrol. 2020 doi: 10.1007/s40620-020-00837-7. [DOI] [PubMed] [Google Scholar]
- 9.Oliva-Damaso N, Payan J, Oliva-Damaso E, Pereda T, Bomback AS. Lupus podocytopathy: an overview. Adv Chronic Kidney Dis. 2019;26(5):369–375. doi: 10.1053/j.ackd.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonini L, Le Mauff B, Marcelli C, Aouba A, de Boysson H. Rhupus: a systematic literature review. Autoimmun Rev. 2020;19(9):102612. doi: 10.1016/j.autrev.2020.102612. [DOI] [PubMed] [Google Scholar]
- 11.Frade-Sosa B, Narváez J, Salman-Monte TC, Castellanos-Moreira R, Ortiz-Santamaria V, Torrente-Segarra V, Castellvi I, Magallares B, Reina D, Minguez S, Sallés M, de Manrique LMG, Ordoñez S, Riera E, Schur PH, Gómez-Puerta JA. A comparative study on clinical and serological characteristics between patients with rhupus and those with systemic lupus erythematosus and rheumatoid arthritis. Lupus. 2020;29(10):1216–1226. doi: 10.1177/0961203320938456. [DOI] [PubMed] [Google Scholar]
- 12.Garufi C, Mancuso S, Spinelli FR, Truglia S, Ceccarelli F, Alessandri C, Conti F. Janus kinases inhibitors for treating patients with rhupus. Joint Bone Spine. 2020;87(6):673–674. doi: 10.1016/j.jbspin.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Kapoor T, Bathon J. Renal manifestations of rheumatoid arthritis. Rheum Dis Clin N Am. 2018;44(4):571–584. doi: 10.1016/j.rdc.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Brosius FC, 3rd, He JC. JAK inhibition and progressive kidney disease. Curr Opin Nephrol Hypertens. 2015;24(1):88–95. doi: 10.1097/MNH.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuttle KR, Brosius FC, 3rd, Adler SG, Kretzler M, Mehta RL, Tumlin JA, Tanaka Y, Haneda M, Liu J, Silk ME, Cardillo TE, Duffin KL, Haas JV, Macias WL, Nunes FP, Janes JM. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial. Nephrol Dial Transpl. 2018;33(11):1950–1959. doi: 10.1093/ndt/gfx377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe T, Hattori T, Ogawa Y, Jodo S. Successful treatment with tofacitinib for renal disorder due to amyloid A amyloidosis and immunoglobulin A nephropathy in a patient with rheumatoid arthritis. Clin Exp Rheumatol. 2018;36(4):683–684. [PubMed] [Google Scholar]
- 17.Itoh I, Kasuno K, Yamamoto C, Takahashi N, Shimizu H, Ojima T, Hayashi S, Kimura H, Iwano M. IgA vasculitis developed as an adverse effect of tofacitinib taken for rheumatoid arthritis. Intern Med. 2020;59(6):817–821. doi: 10.2169/internalmedicine.3668-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pace J, Paladugu P, Das B, He JC, Mallipattu SK. Targeting STAT3 signaling in kidney disease. Am J Physiol Renal Physiol. 2019;316(6):F1151–F1161. doi: 10.1152/ajprenal.00034.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.