Abstract
A 17-year-old lady was diagnosed with proteinuria and microscopic hematuria within the prior 2 years to this presentation. Further tests revealed nephrotic syndrome with hematuria and hypocomplementemia. Renal histopathology showed membranoproliferative glomerulonephritis. Immunostaining and electron microscopy, suggested C3 glomerulonephritis (C3GN). Nephritis-associated plasmin receptor (NAPlr) and plasmin activity (PA), markers of infection-related glomerulonephritis, were identified in the aforementioned pathology specimens. There are several reports suggesting a causal relationship between group A streptococcal infection and C3 glomerulopathy (C3G). There are many intractable cases, whereas some have responded to immunosuppressive therapy, as did our case. However, there is currently no established gold standard treatment for this disease. We herein report a case of C3G with glomerular positive NAPlr and PA despite the absence of a streptococcal infection. Accumulation of cases such as this may help advance treatment by clarifying the etiology and pathogenic mechanism of C3G and future prognosis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13730-021-00662-2.
Keywords: C3 glomerulonephritis, C3 glomerulopathy, Nephritis-associated plasmin receptor, Plasmin activity, Group A streptococcal infection
Introduction
C3 glomerulopathy (C3G) is a recently identified disease entity caused by dysregulation of the alternative complement pathway; dense deposit disease (DDD) and C3 glomerulonephritis (C3GN) constitute its components [1, 2]. C3G is a form of proliferative glomerulonephritis, with a membranoproliferative glomerulonephritis (MPGN) pattern on light microscopy. Immunohistologically, it has C3 staining alone, implicating hyperactivity of the alternative complement pathway. C3 convertase dysregulation can develop as a result of genetic mutations in the alternative complement pathway, or secondary to autoantibodies known as C3 nephritic factors.
(C3Nefs) [3]. Environmental triggers of C3G are poorly documented. Some reports suggest that C3G can be triggered by streptococcal infection [4–6]. There are several reports suggesting a causal relationship between glomerular deposition of nephritis-associated plasmin receptor (NAPlr), a nephritogenic protein isolated from group A streptococcus, and C3G [4, 5]. We report a case of C3G with a positive NAPlr despite no apparent history of streptococcal infection.
Case report
A previously healthy 17-year-old lady presented to our hospital because proteinuria and microscopic hematuria were identified at a school medical examination. She had been diagnosed with proteinuria 2 years earlier, and hematuria 1 year before this presentation, respectively. She had no family history of kidney diseases.
On a physical examination, it revealed the following findings: body mass index was 23.7, blood pressure was 102/69 mmHg, heart rate was 90 beats per min, respiratory rate was 15 breathes per min and body temperature was 36.8 °C, respectively. Auscultation found normal S1 and S2, no murmur was heard. Her breaths sounds were clear. Her abdomen was soft and non-distended with normal bowel sounds. Except for lower extremity edema, her physical examination was normal. There was no evidence of tonsillitis.
Laboratory findings are shown in Table 1. The urinalysis showed 4 + proteinuria and 2 + hematuria. Quantitative protein excretion was 4.4 g/day. The urinary sediment showed 5 to 10 red blood cells/high power field.
Table 1.
Laboratory data on admission
| Urinalysis | Blood chemistry | Immunology and serology | |||
|---|---|---|---|---|---|
| Sp. gravity | 1.034 | TP | 4.6 g/dL | IgG | 740 mg/dL |
| Protein | 4 + | Alb | 2.9 g/dL | IgA | 158 mg/dL |
| Occult blood | 2 + | AST | 13 IU/L | IgM | 140 mg/dL |
| Sugar sediment | – | ALT | 10 IU/L | C3 | 6 mg/dL |
| RBC | 5–10/HPF | LDH | 154 IU/L | C4 | 15 mg/dL |
| WBC | 1–5/HPF | BUN | 11.6 mg/dL | CH50 | < 12 |
| Granular cast | 1–4/HPF | Cr | 0.72 mg/dL | ANA | 20 × |
| Protein excretion | 4.4 g/day | Na | 141 mEq/L | Anti-DNA antibody | < 2.0 IU/mL |
| Hematology | K | 3.7 mEq/L | Cryoglobulin | Negative | |
| WBC | 6700/μL | Cl | 108 mEq/L | ASK | 320 × |
| RBC | 368 × 104/μL | T-Cho | 304 mg/dL | ASO | 105 IU/mL |
| Hb | 7.5 g/dL | Glucose | 82 mg/dL | HBs antibody | Negative |
| Hct | 31.6% | CRP | 0 mg/dL | HBs antigen | 0.00 U/mL |
| Plt | 44 × 104/μL | Renal function | HCV antibody | Negative | |
| 24 h CCr | 89 mL/min | HIV antibody | Negative | ||
Sp. gravity specific gravity, RBC red blood cells, WBC white blood cells, Hb hemoglobin, Hct hematocrit, Plt platelets, TP total protein, ALB albumin, BUN blood urea nitrogen, Cr creatinine, T-Cho total cholesterol, CRP C-reactive protein, CCr creatinine clearance, ANA antinuclear antibody, ASK antistreptokinase antibody titer, ASO antistreptolysin O titer, HBs hepatitis B surface, HCV hepatitis C virus, HIV human immunodeficiency virus
Laboratory blood tests were as follows: blood urea nitrogen 11.6 mg/dL, creatinine 0.72 mg/dL, total protein 4.6 g/dL, albumin 2.9 g/dL, sodium 141 mEq/L, potassium 3.7 mEq/L, leukocytes 6,700/mm3, hemoglobin 11.0 g/dL, platelets 170,000/mm3, IgG 740 mg/dL (normal range 870–1700 mg/dL), IgA 158 mg/dL (normal range 110–410 mg/dL), IgM 140 mg/dL (normal range 33–190 mg/dL), C3 6 mg/dL (normal range 69–128 mg/dL), C4 15 mg/dL (normal range 14–36 mg/dL) and CH50 < 12 U/mL (normal range 30–45 U/mL). Antinuclear antibody, anti-SS-A/Ro antibody, and anti-SS-B/La antibody were all negative. The antistreptolypsin O (ASO) titer was 105 IU/mL (normal < 240 IU/mL) and antistreptokinase antibody (ASK) titer was 320 times (normal < 1280 times), respectively.
Renal and cardiac ultrasonography revealed no abnormalities. Chest and abdominal computed tomography showed no obvious abnormal findings.
A renal biopsy was performed to assess for nephrotic syndrome. A sample of renal cortex contained 66 glomeruli, none of which showed global sclerosis.
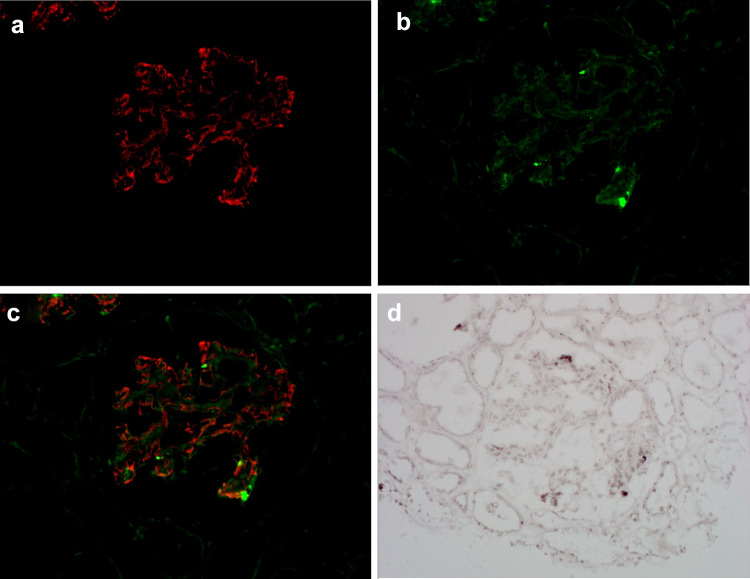
All glomeruli showed severe proliferation of glomerular mesangial cells, increased mesangial matrix, and adhesion and lobulation of the glomerular capillary tufts (Fig. 1a–c). Immunofluorescent analysis showed granular deposition of C3 in the glomerular capillary walls and mesangial areas (Fig. 2a). There was no immunoglobulin deposition. Electron microscopy revealed subendothelial and subepithelial electron-dense deposits in the capillary basement membranes and mesangial areas (Fig. 1d). These findings were consistent with a diagnosis of MPGN type III. Immunofluorescent analysis of nephritis-associated plasmin receptor (NAPlr) and in situ zymography for plasmin activity (PA) were performed. Localization of NAPlr and C3 was essentially different (Fig. 2a–c), while NAPlr and PA were identified in glomeruli of sequential sections with a similar distribution pattern (Fig. 2b, d). Colocalization of NAPlr and PA was further confirmed by double staining on the single section (Supplemental Fig. 1). Prior to commencing treatment, C3 nephritic factor (C3Nef) was evaluated according to a previous report [7], but the result was negative. The diagnosis of C3GN was made, and the patient was treated with prednisolone 2 mg/kg on alternate days. After commencing treatment, urinary protein decreased significantly. Losartan 100 mg once daily, atorvastatin 10 mg once daily, and dipyridamole 100 mg twice daily were used in combination. Prednisolone was gradually tapered, and after 2 years, the urinary protein remained at about 0.5 g/g of creatinine. One year passed when prednisolone lowered by 5 mg every other day, urinary protein increased without any particular infection. Mizoribine 150 mg once daily was added and proteinuria decreased again. There was no deterioration in renal function, while the low C3 state continued for more than 5 years after onset.
Fig. 1.
Renal light microscopic findings. a Glomeruli showing severe proliferation of glomerular mesangial cells, increased mesangial matrix, and adhesion and lobulation of the glomerular capillary tufts. Periodic acid-Schiff and myeloperoxidase staining. (original magnification × 200). b The glomerular capillary walls are diffusely thickened. Periodic acid silver-methenamine staining. (original magnification × 200). c Double-contour appearance of capillary walls. Periodic acid silver-methenamine staining. (original magnification × 400). d Electron microscopy revealed subendothelial and subepithelial electron-dense deposits in the capillary basement membranes and mesangial areas
Fig. 2.
Immunofluorescent findings. a Granular deposition of C3 in the glomerular capillary walls and mesangial areas. C3 staining. b Granular nephritis-associated plasmin receptor (NAPlr) staining showing segmental positive staining of NAPlr. c Merged image of C3 and NAPlr showing localization of NAPlr and C3, which was essentially different. d Plasmin activity (PA) by in situ zymography was found in a similar area as NAPlr staining
The clinical course is summarized in Fig. 3.
Fig. 3.
The clinical course
Discussion
A major pathological mechanism underlying C3G is excessive activation of the alternative complement pathway. This results in the deposition of multiple complement components in the glomeruli [2]. Normally, the activity of the alternative complement pathway is directly related to the activity of the C3 convertase with its baseline turn over and amplification loop tightly regulated by complement regulators [8]. In contrast, in some patients with C3G, the activity of C3 convertase is increased. This unrestrained complement activation may be driven by genetic abnormalities in complement genes, autoantibodies against complement components, or nephritic factors that stabilize C3 and/or C5 convertases [2]. Regarding genetic abnormalities, previous reports showed that small population of C3G accompanied with pathogenic mutations, differed from aHUS [9]. In our case, it was unclear whether there was genetic mutation or not because genetic analysis was not performed. C3 nephritic factor (C3NeF) is found in 80 percent of patients with DDD and somewhat less often in C3GN in 1 report [10]. However, C3NeF is not specific or unique to these patients, as it has also been found in the serum of healthy individuals [11] and patients with the other diseases such as lupus nephritis and acquired lipodystrophy [12]. How these factors contribute to the development of C3G is unknown as they are insufficient themselves to cause disease. Some environmental triggers may be involved in the onset, albeit poorly documented. There are reported cases possibly triggered by streptococcal pharyngitis. The reports suggesting a relationship between group A streptococcal infection and C3G are summarized in the Table 2. Two of the five cases had both positive C3Nef and positive risk alleles of complement factor H (CFH) gene. (Table 2, Case 1 and Case2) [6]. Complement gene screening was performed in another case, and heterozygous complement factor H-related protein 5 (CFHR5) deficiency was detected. However, the presence of low serum C3 level in this case cannot be explained by the heterozygous sequence variant in CFHR5 alone because serum C3 level were normal in the patient’s mother and sister who also carried the sequence variant (Table 2, Case 3) [13].
Table 2.
Reports suggesting a relationship between group A streptococcal infection and C3G laboratory data on admission
| Age sex | Diagnosis | NAPlr | PA | Preceding APSGN | C3Nef | Serum CFH level | Treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 7 F | DDD | Not inspected | Yes | + | Normal | Steroid, ARB | Normal renal function 5 years after onset | |
| Case 2 | 6 F | DDD | Not inspected | Yes | + | Normal | Steroid, RB | Normal renal function 2.5 years after onset | |
| Case 3 | 7 F | C3GN | Not inspected | Yes | − | Normal | Steroid, RB |
Normal renal function 50 months after onset Proteinuria improved with steroid therapy |
|
| Case 4 | 12 M | DDD | + | + | Yes | Unmeasured | Steroid | Complete remission; only mild hypocomplementemia 7 years after onset | |
| Case 5 | 14 F | DDD | + | + | Yes | − | Normal | Steroid, ARB, MZR | Urine protein 0.5 g/g·Cr and normal renal function 26 months after onset |
| Our case | 17 F | C3GN | + | + | No | − | Unmeasured | Steroid, ARB, MZR | Normal renal function 5 years after onset |
NAPlr nephritis-associated plasmin receptor, PA plasmin activity, DDD dense deposit disease, C3GN C3 glomerulonephritis, C3Nef C3 nephritic factors, CFH Complement Factor H, ARB angiotensin II receptor blocker, MZR mizoribine
NAPlr is a nephritogenic protein isolated from Group A streptococcus that is homologous to streptococcal glyceraldehyde-3-phosphate dehydrogenase (GAPDH). NAPlr binds plasmin and maintains PA by protecting it from its physiological inhibitors, and plasmin is considered to cause glomerular damage [14]. Also, NAPlr has been shown to activate the alternative complement pathway with C3 conversion in vitro [15]. According to the previous report, 22 of the 22 PSAGN renal biopsy specimens obtained in the early stage [1 to 14 day(s) after the onset] and 9 of the 18 PSAGN renal biopsy specimens obtained in the late stage (15 to 32 days after onset) were positive for glomerular NAPlr, respectively. Glomerular NAPlr was not present in ten normal kidney specimens. Also, it was found in 4 of the 100 non-PSAGN nephritis specimens: 2 cases of MPGN, one case of Henoch-Schonlein purpura nephritis, and one case of lupus nephritis. Two of the four positive patients were retrospectively found to have evidence of a recent streptococcal infection [16]. Another study proved that no glomerular NAPlr staining was observed in seven PSAGN specimens obtained in 31 to 90 days after the onset [15].
NAPlr deposition and PA are usually detected with a similar distribution pattern in the glomeruli of acute phase poststreptococcal glomerulonephritis (PSAGN). However, similar findings are found in infection-related glomerulonephritis of other bacterial origin probably due to the homology of the GAPDH molecule among various micro-organisms, and its universal plasmin-binding capacity [14, 17]. NAPlr and PA usually disappear within 30 days following onset of PSAGN [15]. However, previous reports detected that NAPlr and PA still tested positive at 2 months and 6 months post-disease onset of nephritis in C3G (Table 2, Case 4 and Case 5), respectively [4, 5]. Another report described three patients with C3G who had definite antecedent streptococcal infection with the phenotype of PSAGN, although NAPlr was not investigated (Table 2, Case 1, Case 2 and Case 3) [6, 13]. These cases suggest a causal relationship between group A streptococcal infection and C3G.
We herein demonstrate a patient who was eventually diagnosed with C3 glomerulopathy with a positive NAPlr, despite no apparent history of streptococcal infection, but without C3Nef. There was no apparent history of pharyngitis through entire life and no increase in ASO and ASK titers were observed. It is considered that potential group A streptococcal infection or infection of bacteria other than streptococcus can trigger the onset of C3G. Although such an infection was not clearly observed in this case, common isolation of various bacteria such as H. influenzae, S. pyogenes, and S. aureus has been reported in the core of hypertrophic tonsillar tissue with no apparent clinical tonsilitis, the complete negation of asymptomatic infection is almost impossible [18]. Persistent positivity of NAPlr and PA may be involved in activation of the alternative complement pathway and glomerular damage.
The long-term renal prognosis of C3G is generally unfavorable. It was reported that 47% of 17 patients with DDD and 23% of 53 patients with C3GN progressed to ESRD during a median follow-up period of 28 months, respectively [19]. In our case, there was no deterioration in renal function 5 years after onset. Interestingly, all five cases suggesting a relationship between group A streptococcal infection had a good prognosis similar to our case. Corticosteroid treatment seemed to be effective in our case, as it was for the other four cases. It is also reported that combined therapy with prednisolone and mizoribine was effective to reduce urinary protein [5]. In our case, mizoribine was added instead of increasing the dose of corticosteroid when urinary protein increased, and it seemed to be effective. C3G with group A streptococcal infection may be sensitive to immunosuppressive treatment. It is, however, impossible to clearly separate C3 nephropathy from atypical post-infectious glomeruronephritis. Several cases of APSGN that takes longer to resolve or persists with renal dysfunction have been reported, and spontaneous remission has been observed in such cases [20–22]. Some of the cases of C3G with group A streptococcal infection may also spontaneously remit without corticosteroids.
Clearly, more studies are necessary to determine the relationship of C3G and group A streptococcal infection. Future case observations may lead to more decisive treatment and prognosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Supplemental Fig.1 Immunofluorescent findings. (a) Plasmin activity (PA) by in situ zymography was positive in the segmental areas. (black arrows) (b) Nephritis-associated plasmin receptor (NAPlr) was also positive in segmental area. (yellow arrows) (c) Merged image of PA and NAPlr indicates colocalization of NAPlr and PA. (yellow arrows) (TIF 3245 KB)
Acknowledgements
The authors thank Prof. C.L. Harris (University of Newcastle, U.K.) for kindly evaluating the C3 nephritic factor for this patient.
Declarations
Conflict of interest
All the authors have declared no competing interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ito N, Ohashi R, Nagata M. C3 glomerulopathy and current dilemmas. Clin Exp Nephrol. 2017;21(4):541–551. doi: 10.1007/s10157-016-1358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caravaca-Fontan F, Lucientes L, Cavero T, Praga M. Update on C3 glomerulopathy: a complement-mediated disease. Nephron. 2020;144(6):272–280. doi: 10.1159/000507254. [DOI] [PubMed] [Google Scholar]
- 3.Rabasco-Ruiz C, Huerta-Arroyo A, Caro-Espada J, Gutierrez-Martinez E, Praga-Terente M. C3 glomerulopathies. A new perspective on glomerular diseases. Nefrologia. 2013;33(2):164–170. doi: 10.3265/Nefrologia.pre2012.Nov.11802. [DOI] [PubMed] [Google Scholar]
- 4.Sawanobori E, Umino A, Kanai H, Matsushita K, Iwasa S, Kitamura H, et al. A prolonged course of Group A streptococcus-associated nephritis: a mild case of dense deposit disease (DDD)? Clin Nephrol. 2009;71(6):703–707. doi: 10.5414/cnp71703. [DOI] [PubMed] [Google Scholar]
- 5.Suga K, Kondo S, Matsuura S, Kinoshita Y, Kitano E, Hatanaka M, et al. A case of dense deposit disease associated with a group A streptococcal infection without the involvement of C3NeF or complement factor H deficiency. Pediatr Nephrol. 2010;25(8):1547–1550. doi: 10.1007/s00467-010-1479-0. [DOI] [PubMed] [Google Scholar]
- 6.Prasto J, Kaplan BS, Russo P, Chan E, Smith RJ, Meyers KE. Streptococcal infection as possible trigger for dense deposit disease (C3 glomerulopathy) Eur J Pediatr. 2014;173(6):767–772. doi: 10.1007/s00431-013-2245-7. [DOI] [PubMed] [Google Scholar]
- 7.Paixao-Cavalcante D, Lopez-Trascasa M, Skattum L, Giclas PC, Goodship TH, de Cordoba SR, et al. Sensitive and specific assays for C3 nephritic factors clarify mechanisms underlying complement dysregulation. Kidney Int. 2012;82(10):1084–1092. doi: 10.1038/ki.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizuno M, Suzuki Y, Ito Y. Complement regulation and kidney diseases: recent knowledge of the double-edged roles of complement activation in nephrology. Clin Exp Nephrol. 2018;22(1):3–14. doi: 10.1007/s10157-017-1405-x. [DOI] [PubMed] [Google Scholar]
- 9.Osborne AJ, Breno M, Borsa NG, Bu F, Fremeaux-Bacchi V, Gale DP, et al. Statistical validation of rare complement variants provides insights into the molecular basis of atypical hemolytic uremic syndrome and C3 glomerulopathy. J Immunol. 2018;200(7):2464–2478. doi: 10.4049/jimmunol.1701695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Servais A, Noel LH, Roumenina LT, Le Quintrec M, Ngo S, Dragon-Durey MA, et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82(4):454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- 11.Gewurz AT, Imherr SM, Strauss S, Gewurz H, Mold C. C3 nephritic factor and hypocomplementaemia in a clinically healthy individual. Clin Exp Immunol. 1983;54(1):253–258. [PMC free article] [PubMed] [Google Scholar]
- 12.Corvillo F, Okroj M, Nozal P, Melgosa M, Sanchez-Corral P, Lopez-Trascasa M. Nephritic factors: an overview of classification, diagnostic tools and clinical associations. Front Immunol. 2019;10:886. doi: 10.3389/fimmu.2019.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vernon KA, Goicoechea de Jorge E, Hall AE, Fremeaux-Bacchi V, Aitman TJ, Cook HT, et al. Acute presentation and persistent glomerulonephritis following streptococcal infection in a patient with heterozygous complement factor H-related protein 5 deficiency. Am J Kidney Dis: Off J Natl Kidney Found. 2012;60(1):121–125. doi: 10.1053/j.ajkd.2012.02.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida T, Oda T. Glomerular deposition of nephritis-associated plasmin receptor (NAPlr) and related plasmin activity: key diagnostic biomarkers of bacterial infection-related glomerulonephritis. Int J Mol Sci. 2020 doi: 10.3390/ijms21072595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshizawa N, Yamakami K, Fujino M, Oda T, Tamura K, Matsumoto K, et al. Nephritis-associated plasmin receptor and acute poststreptococcal glomerulonephritis: characterization of the antigen and associated immune response. J Am Soc Nephrol. 2004;15(7):1785–1793. doi: 10.1097/01.asn.0000130624.94920.6b. [DOI] [PubMed] [Google Scholar]
- 16.Yamakami K, Yoshizawa N, Wakabayashi K, Takeuchi A, Tadakuma T, Boyle MD. The potential role for nephritis-associated plasmin receptor in acute poststreptococcal glomerulonephritis. Methods. 2000;21(2):185–197. doi: 10.1006/meth.2000.0990. [DOI] [PubMed] [Google Scholar]
- 17.Oda T, Yamakami K, Omasu F, Suzuki S, Miura S, Sugisaki T, et al. Glomerular plasmin-like activity in relation to nephritis-associated plasmin receptor in acute poststreptococcal glomerulonephritis. J Am Soc Nephrol. 2005;16(1):247–254. doi: 10.1681/ASN.2004040341. [DOI] [PubMed] [Google Scholar]
- 18.Jeong JH, Lee DW, Ryu RA, Lee YS, Lee SH, Kang JO, et al. Bacteriologic comparison of tonsil core in recurrent tonsillitis and tonsillar hypertrophy. Laryngoscope. 2007;117(12):2146–2151. doi: 10.1097/MLG.0b013e31814543c8. [DOI] [PubMed] [Google Scholar]
- 19.Medjeral-Thomas NR, O'Shaughnessy MM, O’Regan JA, Traynor C, Flanagan M, Wong L, et al. C3 glomerulopathy: clinicopathologic features and predictors of outcome. Clin J Am Soc Nephrol. 2014;9(1):46–53. doi: 10.2215/CJN.04700513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sethi S, Fervenza FC, Zhang Y, Zand L, Meyer NC, Borsa N, et al. Atypical postinfectious glomerulonephritis is associated with abnormalities in the alternative pathway of complement. Kidney Int. 2013;83(2):293–299. doi: 10.1038/ki.2012.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchida T, Oda T, Watanabe A, Izumi T, Higashi K, Kushiyama T, et al. Clinical and histologic resolution of poststreptococcal glomerulonephritis with large subendothelial deposits and kidney failure. Am J Kidney Dis. 2011;58(1):113–117. doi: 10.1053/j.ajkd.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Iseri K, Iyoda M, Yamamoto Y, Kobayashi N, Oda T, Yamaguchi Y, et al. Streptococcal infection-related nephritis (SIRN) manifesting membranoproliferative glomerulonephritis type I. Intern Med. 2016;55(6):647–650. doi: 10.2169/internalmedicine.55.5409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Supplemental Fig.1 Immunofluorescent findings. (a) Plasmin activity (PA) by in situ zymography was positive in the segmental areas. (black arrows) (b) Nephritis-associated plasmin receptor (NAPlr) was also positive in segmental area. (yellow arrows) (c) Merged image of PA and NAPlr indicates colocalization of NAPlr and PA. (yellow arrows) (TIF 3245 KB)