Abstract
Hypouricemia in kidney transplant (KT) recipients is rare since they usually have subnormal kidney function which raises serum uric acid level. Recently, interests in pathogenesis of hypouricemia have been increasing due to the understanding of the role of uric acid transporter in renal hypouricemia (RHUC). We herein report the case of RHUC consequently developed in a KT recipient from a living donor with RHUC diagnosed by the detailed urinary and genetic test. A 73-year-old Japanese man underwent KT, and the donor was his wife who had hypouricemia [serum uric acid (S-UA) 0.6 mg/dL]. Nine months after KT, the recipient’s S-UA was low (1.5 mg/dL) with serum creatinine (S-Cr) of 1.56 mg/dL, and fractional excretion of UA (FEUA) was high (59.7%; normal < 10%), indicating RHUC. Regarding the donor’s information, S-Cr, S-UA, and FEUA were 0.95 mg/dL, 1.0 mg/dL, and 54.5%, respectively. To investigate further on the pathogenesis of RHUC in both the recipient and the donor, we performed genetic tests. The donor had a homozygous mutation of W258X in the SLC22A12 gene and the recipient had a wild type of W258X. Finally, we reviewed the previous literature on RHUC among KT recipients and discussed the strategy of follow-up for these patients.
Keywords: Hypouricemia, Renal hypouricemia, Kidney transplantation, Living donor
Introduction
In daily clinical practice, hyperuricemia gained more focus than hypouricemia, since the former is more common and is related not only to gout and nephrolithiasis but also to cardiovascular disease [1]. On the other hand, hypouricemia is overlooked owing to its rarity and the usual presentation of no symptoms. Recently, hypouricemia has gained more interests due to recent progress in understanding of the role of uric acid transporters, and its recognition of renal hypouricemia (RHUC) as a disease, which often complicates kidney stones and exercise-induced acute kidney injury (EIAKI) [2] The diagnosis of RHUC is based on hypouricemia (serum uric acid (UA) < 2.0 mg/dL) and an increase in both fractional excretion of uric acid (FEUA) and clearance of UA (CUA), without other secondary causes of hypouricemia. RHUC type 1 is caused by a mutation in the SLC22AA12 gene which encodes a renal urate-anion exchanger, named URAT1 (urate transporter 1), along the apical membrane of the proximal tubule. While RHUC type 2 is caused by a defect in the SLC2A9 gene that encodes a high-capacity urate transporter, named GLUT9 (glucose transporter like protein 9), along the basolateral membrane of the proximal tubule. The prevalence of RHUC is less than 0.5% in general population [3, 4], and its prevalence among women is higher than that among men in Japan [4]. Hypouricemia, especially RHUC, is often accompanied with kidney stones and EIAKI [5], with an incidence of 8.5% and of 6.5%, respectively [6].
Kidney transplantation (KT) from donors with RHUC could possibly put recipients on the risk of hypouricemia since they excrete urine form affected donor kidney with aforementioned mutations. RHUC in KT recipients may cause kidney stones or EIAKI after KT. A few case reports have documented RHUC in either recipients or living donors following KT. However, comprehensive clinical data are scarce regarding KT recipients and donors in the literature.
We herein report a case of RHUC that developed in a recipient from a living donor with hypouricemia. We also underwent a literature review of RHUC in KT recipients and discussed an ideal follow-up strategy for these patients.
Case
A 73-year-old Japanese man with a 2 year history of maintenance hemodialysis underwent living-donor and ABO-compatible KT 1 year ago in our transplant center. The numbers of human leukocyte antigen (HLA) mismatches was 4. The etiology of his renal failure was suspected to be IgA nephritis without kidney biopsy. No past history of hypouricemia, kidney stones, and EIAKI were documented. His family history also was not remarkable for hypouricemia. His laboratory results before KT showed a serum UA (S-UA) level of 8.1 mg/dL even with febuxostat (Table 1).
Table 1.
Laboratory data before and after kidney transplantation of the recipient
| Pre-kidney transplantation | 9 months after kidney transplantation | |
|---|---|---|
| Complete blood cell count and serum chemistries | ||
| White blood cell (×109/L) | 5.3 | 5.4 |
| Hemoglobin (g/dL) | 10.0 | 13.7 |
| Hematocrit (%) | 29.2 | 43.1 |
| Platelets (×109/L) | 29.2 | 10.4 |
| Total protein (g/L) | 6.4 | 6.8 |
| Albumin (g/L) | 3.7 | 4.2 |
| Blood urea nitrogen (mg/dL) | 33.0 | 25.6 |
| Creatinine (mg/dL) | On dialysis | 1.56 |
| Uric acid (mg/dL) | 8.1 | 1.5 |
| Sodium (mEq/L) | 138 | 140 |
| Potassium (mEq/L) | 5.1 | 4.1 |
| Chloride (mEq/L) | 112 | 107 |
| Calcium (mg/dL) | 8.9 | 9.2 |
| Phosphate (mg/dL) | 3.4 | 3.0 |
| Urinalysis | ||
| pH | 5.5 | 6.0 |
| Urinary protein | (2+) | (−) |
| Occult blood | ( ±) | (−) |
| RBC sediment (per HPF) | 1–4 | < 1 |
| WBC sediment (per HPF) | 1–4 | < 1 |
| CCr (mL/min/1.73 m2) | On dialysis | 49.3 |
| Urine UA (mg/day) | N/A | 629.3 |
| UA clearance (mL/min) | N/A | 29 |
| FEUA (%) | N/A | 59.7 |
HPF high power field, RBC red blood cell, WBC white blood cell, CCr creatinine clearance, UA uric acid, FEUA fractional excretion of uric acid, N/A not available
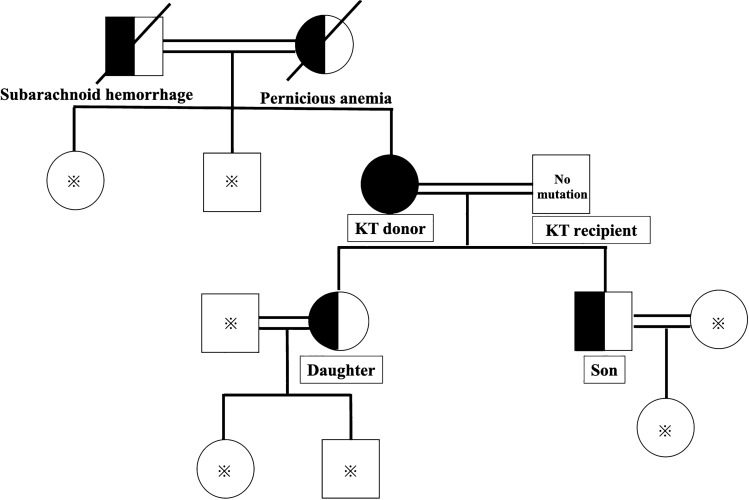
The living donor was his 71-year-old wife. Before KT, her serum creatinine (S-Cr) was 0.64 mg/dL, S-UA was 0.6 mg/dL, and FEUA was 24.9% which was consistent with RHUC (Table 2) but without genetic testing. The donor and her family had no history of hypouricemia or related diseases such as kidney stones and EIAKI (Fig. 1). As there was no consensus in terms of UA threshold of each low and high level for living kidney donation, we did not perform a genetic test for the definitive diagnosis of hypouricemia prior to donation, and accepted her as an eligible living donor.
Table 2.
Laboratory data before and after kidney transplantation of the donor
| Pre-kidney transplantation | Nine month after-kidney transplantation | |
|---|---|---|
| Complete blood cell count and serum chemistries | ||
| White blood cell (×109/L) | 7.8 | 7.2 |
| Hemoglobin (g/dL) | 13.3 | 13.3 |
| Hematocrit (%) | 39.6 | 40.6 |
| Platelets (×109/L) | 22.4 | 21.8 |
| Total protein (g/L) | 7.5 | 7.6 |
| Albumin (g/L) | 4.6 | 4.4 |
| Blood urea nitrogen (mg/dL) | 13.1 | 16.0 |
| Creatinine (mg/dL) | 0.64 | 0.95 |
| Uric acid (mg/dL) | 0.6 | 1.0 |
| Sodium (mEq/L) | 141 | 140 |
| Potassium (mEq/L) | 3.9 | 4.4 |
| Chloride (mEq/L) | 105 | 104 |
| Calcium (mg/dL) | 9.6 | 9.3 |
| Phosphate (mg/dL) | 4.2 | 4.0 |
| Urinalysis | ||
| pH | 6.5 | 6.0 |
| Urinary protein | (−) | (−) |
| Occult blood | (−) | (−) |
| RBC sediment (per HPF) | < 1 | < 1 |
| WBC sediment (per HPF) | < 1 | < 1 |
| CCr (mL/min/1.73 m2) | 111.8 | 77.1 |
| Urine UA (mg/day) | 539.0 | 488.6 |
| UA clearance (mL/min) | 27.9 | 42.0 |
| FEUA (%) | 24.9 | 54.9 |
HPF high power field, RBC red blood cell, WBC white blood cell, CCr creatinine clearance, UA uric acid, FEUA fractional excretion of uric acid, N/A not available
Fig. 1.
Family tree of both the recipient and donor. * represents no information regarding mutation of RHUC. Circles represent female gender and squares represent male gender. Whole Black circle represents homozygous mutation of W258X in the SLC22A12 gene. Half black circle and square represent heterozygous mutation of W258X in the SLC22A12 gene. Crosses represent dead family members
The clinical course of KT regarding both donor and recipient was stable. Conventional maintenance immunosuppressive therapy comprising steroids, extended-release tacrolimus, and mycophenolate mofetil was administered. Two weeks after KT, the recipient’s S-Cr was 1.72 mg/dL and his S-UA was 1.5 mg/dL without any medication for hyperuricemia. Nine months after KT, the recipient’s S-Cr was stable (1.56 mg/dL), while level of S-UA remained low (1.5 mg/dL), with high FEUA (59.7%; normal < 10%), and high CUA (29 mL/min) (Table 1). In terms of donor’s UA status after donation, S-UA was 1.0 mg/dL when s-Cr was 0.95 mg/dL, with high FEUA (54.5%) and high CUA (42 mL/min) (Table 2). Table 3 also shows the changes in the FEUA, S-UA, and S-Cr levels, and glomerular filtration rate (GFR) in both the recipient and the donor.
Table 3.
Laboratory data regarding kidney function and uric acid-related parameters of the recipient and donor at 2 weeks, 3 months, 9 months, and 1 year post-kidney transplantation
| 2 weeks | 3 months | 9 months | 1 year | |
|---|---|---|---|---|
| Recipient | ||||
| CCr (mL/min/1.73 m3) | N/A | N/A | 49.3 | N/A |
| UA clearance (mL/min) | N/A | N/A | 29 | N/A |
| FEUA (%) | N/A | N/A | 59.4 | 60.4 |
| Serum UA (mg/dL) | 1.5 | 1.6 | 1.5 | 1.8 |
| Serum Cr (mg/dL) | 1.72 | 1.68 | 1.56 | 1.79 |
| eGFR(mL/min/1.73 m2) | 31.4 | 32.2 | 34.8 | 30.0 |
| Donor | ||||
| CCr (mL/min/1.73 m3) | N/A | N/A | 77.1 | N/A |
| UA clearance (mL/min) | N/A | N/A | 42 | N/A |
| FEUA (%) | N/A | N/A | 54.9 | 50.3 |
| Serum UA (mg/dL) | 1.2 | 1.0 | 1.0 | 1.1 |
| Serum Cr (mg/dL) | 1.02 | 1.0 | 0.95 | 0.93 |
| eGFR (mL/min/1.73 m2) | 41.4 | 42.4 | 44.6 | 45.7 |
CCr creatinine clearance, UA uric acid, FEUA fractional excretion of uric acid, eGFR estimated glomerular filtration rate, N/A not available
Hypouricemia developed in the recipient was apparently due to implanted donor kidney, which strongly indicates the diagnosis of RHUC, we decided to perform a genetic test after obtaining informed consent. The donor had a homozygous mutation of W258X in the SLC22A12 gene and the recipient had a wild type W258X. Based on these genetic results, hypouricemia in the donor resulted in RHUC type 1 and the recipient was consequently affected owing to the donated kidney.
The donor’s and recipient’s S-UA and S-Cr levels did not significantly change during the first year following KT. The protocol (non-indication) allograft biopsies of the recipient were done at the bench (0 h,) 2 months, and 1 year after KT. There was no evidence of rejection, recurrence of IgA nephritis, any casts, any crystals/calcified lesions or nephrolithiasis. Furthermore, both the recipient and donor did not have any stones in the urinary tract by the CT scanning. Based on the definitive diagnosis, we have strived to prevent RHUC-related complications in both the donor and recipient during post-KT follow-ups. In particular, we have advised avoiding extreme anaerobic exercise with non-steroidal anti-inflammatory drugs (NSAIDs) to prevent EIAKI and drinking water (1.5–2 L/day), and we ensured to check their urinary pH (ideally pH > 6.5) during every checkup to prevent the formation of urinary stones.
Discussion and the literature review
We reported a case of KT from a donor with a homogeneous mutation of W258X in the SLC22A12 gene diagnosed as RHUC type 1. Although we noticed extremely low levels of UA before donation which suspected RHUC without the genetic test, there has been no consensus in terms of UA threshold of each low and high level for living kidney donation [7, 8]. We indicated any harms of RHUC to both the donor and recipient after donor nephrectomy and deeply discussed that with transplant team. The donor and recipient consented to undergo KT; and thus, we decided the eligibility for donation. After KT, the recipient had relatively low levels of UA even with mild kidney dysfunction. We suspected the cause and transmission of hypouricemia to be the donated kidney and suspected a diagnosis of RHUC. We performed a genetic test for both the donor and recipient to obtain the accurate diagnosis and assess the risk of urinary stones formation and EIAKI development, since both the donor and recipient had a single kidney. Furthermore, the genetic type of RHUC is important for risk assessments. RHUC type 1, a mutation in the SLC22A12 gene, is more frequent than RHUC type 2 [6, 9, 10] W258X is the most prevalent mutation in RHUC type 1 and it has been reported in several reports (74.1% [11] and 79.7% [11]). Particularly, homozygous W258X mutation is the most frequent mutation in Japan (54.8%) and in Korea (42%) [11]. RHUC patients having at least one W258X mutation tended to experience urinary stones, AKI, or hematuria [11]. However, these reports were on the general population and not from kidney transplant patients. Interestingly, the donor’s FEUA after donation was increased compared to that prior to donation, which represents reduction in her GFR, in accordance with the principle of fractional excretion (Table 2). FEUA is the ratio of urinary UA concentration and urine volume to the product of serum UA concentration and GFR. Thus, the decreased GFR reflects the increased FEUA.
Moreover, we indirectly proved the transmission of RHUC via the donated kidney with a mutation of W258X in the SLC22A12 gene, since the recipient’s FEUA and GFR, which reflects fractional excretion, was almost the same with the donor’s FEUA, despite of no mutation of W258X in the SLC22A12 gene in recipient (Table 1).
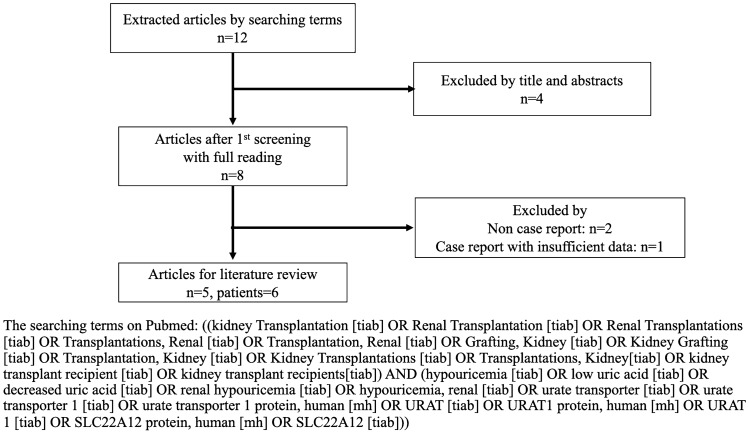
Since there are scarce clinical data on RHUC in KT, we performed a literature review based on past case reports on MEDLINE (PubMed). Literature review was separately done by two authors (T. M. and M. Y.) according to the searching terms describing on Fig. 2. We eventually found 5 reports and 6 cases, this data is summarized in Tables 4 and 5. Cases of living KT donors occupied the majority (4 out of 6 cases). All genetic abnormalities were in the SLC22A12 gene and only one of these patients experienced urinary stone. The differences between our case as compared to the previous reports are as follows: we have (1) detailed urinary data (FEUA and UA clearance) of both the donor and recipient and (2) detailed genetic information (mutation of W258X in SLC22A12 gene). Only our case demonstrated high FEUA and UA clearance in both the recipient and donor consisting of RHUC. According to the review of the literature, the incidence of urinary stone and EIAKI in either the KT recipient or donor with RHUC were not concluded due to a small number of patients in previous studies.
Fig. 2.
Flowchart diagram of the literature searching among kidney transplant recipients with renal hypouricemia
Table 4.
Literature review of renal hypouricemia among kidney transplant recipients and our case (references [14–18])
| References | Country | Type of donor | Recipient age and gender | S-UA (mg/dL) Post-KT |
FEUA (%) Post-KT |
UA clearance (mL/min) Post-KT |
Urinary stone Post-KT |
Impaired kidney function (including EIAKI) |
|---|---|---|---|---|---|---|---|---|
| Yamamoto et al. [14] | Japan | Living |
24 Male |
1.0 | 57.9 | 39.9 | No | No |
| Juraschek et al. [15] | USA | Living |
71 Female |
2.7 | N/A | N/A | No | No |
| Okabayashi et al. [16] | Japan | Living |
41 Male |
1.9 | 29 | 26.8 | Yes | No |
| Teng et al. [17] | China | Deceased |
41 Male |
0.92–1.1 | 44 | 35.9 | No | No |
|
37 Female |
0.76–0.92 | 75 | 73.3 | No | No | |||
| Tsuji et al. [18] | Japan | Living |
40 Male |
3.9 | 11.7 | N/A | No | No |
| Our case | Japan | Living | 73 Male | 1.5 | 59.7 | 29 | No | No |
S-UA serum uric acid, FEUA fractional excretion of uric acid, KT kidney transplantation, EIAKI exercise-induced acute kidney injury, N/A not available
Table 5.
Literature review of renal hypouricemia among kidney transplant recipients and our case with donors’ characteristics (references [14–18])
| References | Donor’s genetic abnormality | Age and gender | S-UA (mg/dL) Pre-KT |
S-UA (mg/dL) Post-KT |
FEUA (%) Pre-KT |
FEUA (%) Post-KT |
UA clearance (mL/min) Pre-KT |
UA clearance (mL/min) Post-KT |
Urinary stone Post-KT |
Donor impaired kidney function (including EIAKI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Yamamoto et al. [14] | SLC22A12 (W258X homozygote) | Father Male | N/A | 1.0 | N/A | N/A | N/A | N/A | No | No |
| Juraschek et al. [15] | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | No | No |
| Okabayashi et al. [16] | N/A | Father Male | N/A | 2.4 | N/A | 10.3 | N/A | N/A | No | No |
| Teng et al. [17] | SLC22A12 (R89H, L181V compound heterozygote) | 30 Male | 0.8 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Tsuji et al. [18] | SLC22A12 (R90H heterozygote) | 42 Female | 1.7 | N/A | 15.8 | 20.0 | N/A | 41.0 | No | No |
| Our case | SLC22A12 (W258X homozygote) | 71 Female | 0.6 | 1.0 | 24.9 | 54.5 | 87.3 | 42.0 | No | No |
S-UA serum uric acid, FEUA fractional excretion of uric acid, KT kidney transplantation, EIAKI exercise-induced acute kidney injury, N/A not available
Finally, although we do not have data on whether the recipient and donor of KT with RHUC have high incidence of kidney stones or EIAKI, we should focus on preventing these complications based on the evidence obtained from the general population, since the recipient and donor both have a single kidney and a mutation of W258X, which is highly likely to cause those complications. In this regard, it is best to avoid extremely hard and anaerobic exercise, to be adequately hydrated [12], and to have urine alkalizers [13]. Our donor and recipient are above the age of 70 years and only had the habit of aerobic walking thrice a week; thus, they were less likely to perform hard or anaerobic exercise. We therefore especially focused on preventing urinary stones by keeping a check on urinary pH and maintaining it between 6.5 and 7.0 in accordance with the recommendation to maintain the uric acid solubility [13]. The recipient and donor did not reach target range of urinary pH even though we recommend enough consumption of vegetables and fruits (Tables 1 and 2). We would recommend the use of potassium citrate or sodium bicarbonate in the near future.
Acknowledgements
This case report has not been published previously in whole or part and supported by any grant.
Data availability
All clinical data are available on the electronic medical record at St. Marianna University Hospital. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
Authors have no conflicts of interest to declare.
Informed consent
We obtained informed consent from both the recipient and donor and they accepted the presentation of their clinical course, including genetic testing. The consent details are stated in the electron medical record at St. Mariann University Hospital.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Takamasa Miyauchi, Email: whitebear496@gmail.com.
Maho Terashita, Email: mahoterashita@gmail.com.
Masatomo Ogata, Email: mutd7001@gmail.com.
Marie Murata, Email: truthvillage@gmail.com.
Kiyomi Osako, Email: kiyomi.osako@gmail.com.
Naohiko Imai, Email: naohiko.imai@gmail.com.
Yuko Sakurai, Email: yuko-saku0907@marianna-u.ac.jp.
Hideo Sasaki, Email: sr20det@marianna-u.ac.jp.
Yuki Ohashi, Email: lily084bys@gmail.com.
Kimiyoshi Ichida, Email: ichida@toyaku.ac.jp.
Yugo Shibagaki, Email: yugoshibagaki@gmail.com.
Masahiko Yazawa, Email: masahikoyazawa@gmail.com.
References
- 1.Rahimi-Sakak F, Maroofi M, Rahmani J, Bellissimo N, Hekmatdoost A. Serum uric acid and risk of cardiovascular mortality: a systematic review and dose-response meta-analysis of cohort studies of over a million participants. BMC Cardiovasc Disord. 2019;19:218. doi: 10.1186/s12872-019-1215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakayama A, Matsuo H, Ohtahara A, et al. Clinical practice guideline for renal hypouricemia (1st edition) Hum Cell. 2019;32:83–87. doi: 10.1007/s13577-019-00239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuwabara M, Niwa K, Ohtahara A, et al. Prevalence and complications of hypouricemia in a general population: a large-scale cross-sectional study in Japan. PLoS One. 2017 doi: 10.1371/journal.pone.0176055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakasugi M, Kazama JJ, Narita I, et al. Association between hypouricemia and reduced kidney function: a cross-sectional population-based study in Japan. Am J Nephrol. 2015;41:138–146. doi: 10.1159/000381106. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu Y, Wakabayashi K, Totsuka A, et al. Exercise-induced acute kidney injury in a police officer with hereditary renal hypouricemia. Case Rep Nephrol Dial. 2019;9:92–101. doi: 10.1159/000501877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichida K, Hosoyamada M, Kamatani N, et al. Age and origin of the G774A mutation in SLC22A12 causing renal hypouricemia in Japanese. Clin Genet. 2008;74:243–251. doi: 10.1111/j.1399-0004.2008.01021.x. [DOI] [PubMed] [Google Scholar]
- 7.Lentine KL, Kasiske BL, Levey AS, et al. KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Transplantation. 2017;101:S1–s109. doi: 10.1097/TP.0000000000001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ethics Committee of the Transplantation Society The consensus statement of the Amsterdam forum on the care of the live kidney donor. Transplantation. 2004;78:491–492. doi: 10.1097/01.tp.0000136654.85459.1e. [DOI] [PubMed] [Google Scholar]
- 9.Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 10.Ichida K, Hosoyamada M, Hisatome I, et al. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol. 2004;15:164–173. doi: 10.1097/01.ASN.0000105320.04395.D0. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Z, Ma L, Zhou J, et al. Renal hypouricemia caused by novel compound heterozygous mutations in the SLC22A12 gene: a case report with literature review. BMC Med Genet. 2018;19:142. doi: 10.1186/s12881-018-0595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5 year randomized prospective study. J Urol. 1996;155:839–843. doi: 10.1016/S0022-5347(01)66321-3. [DOI] [PubMed] [Google Scholar]
- 13.Rodman JS. Prophylaxis of uric acid stones with alternate day doses of alkaline potassium salts. J Urol. 1991;145:97–99. doi: 10.1016/S0022-5347(17)38258-7. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto I, Yamamoto H, Ichida K, et al. Successful living-related kidney transplantation in hereditary renal hypouricaemia. Nephrol Dial Transplant. 2006;21:2041. doi: 10.1093/ndt/gfk103. [DOI] [PubMed] [Google Scholar]
- 15.Juraschek SP, Kurano T, Rosen A, Gelber AC. Acute gout after renal transplantation with notable hypouricemia. Am J Med. 2013;126:e5–6. doi: 10.1016/j.amjmed.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Okabayashi Y, Yamamoto I, Komatsuzaki Y, et al. Rare case of nephrocalcinosis in the distal tubules caused by hereditary renal hypouricaemia 3 months after kidney transplantation. Nephrology (Carlton) 2016;21(Suppl 1):67–71. doi: 10.1111/nep.12774. [DOI] [PubMed] [Google Scholar]
- 17.Teng L, Zhang Y, Ye L, et al. Donor-derived hypouricemia in irrelevant recipients caused by kidney transplantation. Ann Transl Med. 2020;8:330. doi: 10.21037/atm.2020.02.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuji K, Kitamura M, Muta K, et al. Transplantation of a kidney with a heterozygous mutation in the SLC22A12 (URAT1) gene causing renal hypouricemia: a case report. BMC Nephrol. 2020;21:282. doi: 10.1186/s12882-020-01940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All clinical data are available on the electronic medical record at St. Marianna University Hospital. The data that support the findings of this study are available from the corresponding author upon reasonable request.