Summary
Background
The World Health Organization recommends protease inhibitor (PI)-based antiretroviral therapy (ART) as second-line and third-line regimens in pregnant women living with HIV (WLHIV). US, European, and UK guidelines include PI-based ART as first-line regimens, but advise against the use of lopinavir/ritonavir (LPV/r)-based ART, citing an increased risk of preterm birth (PTB). We aimed to assess the risk of adverse perinatal outcomes in WLHIV receiving PI-ART and the comparative risks associated with different PI-ART regimens.
Methods
We conducted a systematic literature review by searching PubMed, CINAHL, Global Health, and EMBASE for studies published between Jan 1, 1980, and April 20, 2020. Two investigators independently selected studies and extracted data from studies reporting on the association of pregnant WLHIV receiving PI-ART with 11 perinatal outcomes: PTB, very PTB (VPTB), spontaneous PTB (sPTB), low birth weight (LBW), very LBW (VLBW), term LBW, preterm LBW, small for gestational age (SGA), very SGA (VSGA), stillbirth, and neonatal death. Pairwise random-effects meta-analyses examined the risk of each adverse perinatal outcome in WLHIV receiving PI-ART compared to non-PI-based ART (non-PI-ART), and comparisons of different PI-ART regimens. Quality assessments of studies were performed, subgroup and sensitivity analyses were conducted based on country income status and study quality, heterogeneity assessed, and the effect of adjustment for confounding factors assessed. The protocol is registered with PROSPERO, CRD42021248987.
Findings
Of 94,594 studies identified, 34 cohort studies including 57,546 women met the inclusion criteria. Random-effects meta-analyses showed that PI-ART was associated with a significantly increased risk of SGA (Relative Risk [RR] 1.24, 95% CI 1.08–1.43; I2=66.7%) and VSGA (RR 1.40, 1.09–1.81; I2=0.0%), but not PTB (RR 1.09, 0.95–1.24; I2=68.3%), VPTB (RR 1.30, 0.78–2.18; I2=43.0%), sPTB (RR 1.91, 0.61–5.99; I2=95.7%), LBW (RR 1.04, 0.85–1.27; I2=63.9%), VLBW (RR 0.72, 0.37–1.43; I2=37.9%), term LBW (RR 0.94, 0.30–3.02; I2=0.0%), stillbirth (RR 1.04, 0.60–1.79; I2=0.0%), and neonatal death (RR 1.82, 0.97–3.40; I2=0.0%), compared to non-PI-ART. We found no significant differences in perinatal outcomes between ART regimens containing LPV/r, atazanavir/ritonavir (ATV/r), and darunavir/ritonavir (DRV/r), which are the most commonly used PIs.
Interpretation
PI-ART is associated with an increased risk of SGA and VSGA, but not PTB or other perinatal outcomes. No significant differences in perinatal outcomes were found between LPV/r, ATV/r, and DRV/r. These findings should inform clinical guidelines, and further efforts should be made to improve perinatal outcomes among pregnant WLHIV.
Funding
None.
Keywords: HIV, Antiretroviral therapy, Protease inhibitor, Perinatal outcome, Preterm birth, Small for gestational age, Systematic review, Meta-analysis
Research in context.
Evidence before this study
We searched PubMed, CINAHL, Global Health, and EMBASE for studies reporting on the association of pregnant women living with HIV (WLHIV) receiving protease inhibitor(PI)-based antiretroviral therapy (ART) with adverse perinatal outcomes, published between Jan 1, 1980, and April 20, 2020, using search terms for “pregnancy outcome”, “specific perinatal outcomes”, “HIV”, and “antiretroviral therapy”. Some previous systematic reviews and meta-analyses have reported an increased risk of preterm birth (PTB) associated with PIs, but lacked information on other perinatal outcomes, included non-ART regimens (i.e. mono- and dual-therapy) in the analysis, or did not include recent data. No previous systematic review and/or meta-analysis compared perinatal outcomes associated with ART regimens containing different PI drugs.
Added value of this study
We conducted the largest systematic review and meta-analysis to date to our knowledge, including 57,546 WLHIV from 34 studies. We found that PI-ART was associated with a significantly increased risk of small for gestational age (SGA, <10th centile) and very small for gestational age (VSGA, <3rd centile), but not PTB or any of the other perinatal outcomes assessed, compared to non-PI-ART. We found no significant differences for any of the perinatal outcomes assessed between ART regimens containing the three most commonly used PIs: lopinavir/ritonavir, atazanavir/ritonavir, and darunavir/ritonavir.
Implications of all the available evidence
ART in pregnancy has clear benefits for maternal health and prevention of HIV transmission to the child, but we found that PI-ART is associated with an increased risk of SGA and VSGA, but not PTB or other perinatal outcomes. PI drugs remain an important option for pregnant WLHIV if other regimens are contraindicated or unavailable, and we found no differences in perinatal outcomes between the most commonly used PI drugs. More evidence is needed regarding the comparative safety and efficacy of different ART regimens in pregnancy and further efforts should be made to improve perinatal outcomes among pregnant WLHIV worldwide.
Alt-text: Unlabelled box
Introduction
Globally, 37.7 million people were living with HIV in 2020, including 19.3 million women of childbearing age.1 Each year, an estimated 1.3 million women living with HIV (WLHIV) are pregnant, the vast majority of whom reside in sub-Saharan Africa.1 Pregnancies in untreated WLHIV are associated with an increased risk of preterm birth (PTB), low birthweight (LBW), small for gestational age (SGA), and stillbirth, compared to HIV-negative women.2
Adverse perinatal outcomes are major contributors to neonatal and child mortality and morbidity, with the highest rates found in sub-Saharan Africa.3 PTB is the leading cause of neonatal and child mortality and morbidity globally.4 SGA contributes to 21.9% of neonatal deaths in low-income and middle-income countries (LMICs).5 PTB and SGA are both causes of LBW, a perinatal outcome measure frequently used in LMICs, as gestational age at birth is often unknown, and associated with increased neonatal mortality.6 The United Nations’ Sustainable Development Goal 3 (SDG3) target 3.2 aims to reduce neonatal and under-5 mortality to 12 and 25 per 1000 live births, respectively, in all countries by 2030. These targets are set to be missed by the vast majority of countries in sub-Saharan Africa, highlighting an urgent need to address the adverse perinatal outcomes that lead to neonatal and child mortality.7
Antiretroviral therapy (ART, i.e. triple drug therapy) is crucial for WLHIV to improve maternal health and to reduce perinatal HIV transmission. In 2013 the World Health Organization (WHO) recommended that all pregnant WLHIV should receive ART.8 This led to an increase in the global proportion of pregnant women with HIV who received ART during pregnancy from 44% in 2010 to 82% in 2018, resulting in a 41% reduction in perinatal HIV transmission in the same period.9 Since 2015, WHO have recommended that all people living with HIV should initiate lifelong ART as soon as possible after diagnosis, including pregnant WLHIV.10 This resulted in a dramatic increase in the proportion of pregnant WLHIV who received ART at the time of conception, from 7% in 2010 to 51% in 2018, in the 23 focus countries which harbour 86% of global pregnant WLHIV.11
The WHO currently recommends integrase inhibitor dolutegravir (DTG)-based ART as first-line regimen for adults, including pregnant women.12 Non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz (EFV)-based ART is an alternative first-line regimen. ART containing protease inhibitors (PIs), preferably ritonavir-boosted atazanavir (ATV/r), ritonavir-boosted lopinavir (LPV/r) or ritonavir-boosted darunavir (DRV/r) are designated as second-line or third-line regimens.12 US guidelines recommend integrase inhibitor DTG-based or raltegravir (RAL)-based ART or protease inhibitor ATV/r or DRV/r-based ART in pregnancy.13 European guidelines recommend integrase inhibitor DTG-based or RAL-based ART or protease inhibitor DRV/r-based ART in pregnancy.14 UK guidelines recommend EFV-based or ATV/r-based ART regimens, with rilpivirine-, DRV/r-, RAL- and DTG-based regimens as alternatives.15 US, European, and UK guidelines advise against the use of LPV/r in pregnancy, citing concerns about an associated increased risk of PTB.13, 14, 15 As the number of pregnant WLHIV receiving ART increases, understanding of the impact of different ART regimens on perinatal outcomes is crucial.
A recent network meta-analysis of seven randomised controlled trials (RCTs) compared seven distinct mono-, dual- and triple drug regimens initiated during pregnancy.16 Among the ART (i.e. triple drug) regimens assessed, LPV/r-based ART was associated with an increased risk of spontaneous PTB compared to zidovudine/lamivudine/abacavir (ZDV/3TC/ABC; a nucleoside reverse transcriptase inhibitor [NRTI] ART regimen which is no longer recommended),17 but no other significant differences in perinatal outcomes between the ART regimens assessed were found. Moreover, LPV/r is the only PI analysed in RCTs conducted in pregnant WLHIV to date, precluding comparison to other PIs.16
Observational studies have reported conflicting findings, with some studies reporting an increased risk of some adverse outcomes associated with PI-based ART (PI-ART),18, 19, 20, 21, 22, 23, 24, 25, 26 whereas others did not find this.27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 Some previous systematic reviews and meta-analyses have reported an increased risk of PTB associated with PIs, but lacked information on other perinatal outcomes, included non-ART (i.e. mono- and dual-therapy) regimens in the analysis, or did not include recent data.44, 45, 46 Other reviews did not include a meta-analysis47,48 or lacked a comparator group altogether.49,50 In addition, to our knowledge, no systematic review and/or meta-analysis has compared perinatal outcomes associated with ART regimens containing different PI drugs.
International HIV treatment guidelines highlight the limited data available regarding the safety and pregnancy outcomes associated with antiretroviral drugs in pregnancy.12,13,15 To help fill this evidence gap we conducted a systematic review and meta-analysis of cohort studies to examine the risk of a broad range of adverse perinatal outcomes associated with PI-ART compared to non-PI-based ART (non-PI-ART). In addition, we assessed the risk of perinatal outcomes associated with different PI-based ART regimens.
Methods
Search strategy
The systematic review and meta-analyses were conducted according to a protocol developed based on the Cochrane guidelines51 and registered online (PROSPERO, number CRD42021248987). The systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.52 We searched PubMed, CINAHL (Ebscohost), Global Health (Ovid), and EMBASE (Ovid) for studies published between Jan 1, 1980, and April 20, 2020, using a comprehensive search strategy adapted for each database, developed by a specialist librarian (SK). Both free text and controlled vocabulary search terms for “pregnancy outcome”, “specific perinatal outcomes”, “HIV”, and “antiretroviral therapy” were used. No methodological, country, or language filters were applied. Full-text articles and abstracts, including conference abstracts, were considered. The references of included studies were assessed for additional relevant studies. For full search terms see Appendix pp3–15. Retrieved articles were imported into EndNote reference manager (EndNote X9; Clarivate Analytics, Philadelphia, Pennsylvania, USA) and deduplicated.
Eligibility criteria
Studies that contained information on the association of pregnant WLHIV receiving PI-ART with adverse perinatal outcomes were deemed eligible. Inclusion criteria were study design (prospective and retrospective cohort studies), population (pregnant WLHIV), exposure (PI-ART) and comparator (either non-PI-ART or PI-ART containing a different PI drug than the exposure). ART was defined as any triple antiretroviral drug therapy. PI-ART regimens were defined as two backbone drugs plus any type of boosted or unboosted PI as a third drug. Studies were not included if less than 95% of WLHIV in an exposure or comparator group conformed to the exposure/comparator definition (e.g., <95% of WLHIV received PI-ART) or if additional treatment was received by one exposure/comparator group only (e.g. anti-tuberculosis treatment). Any timing of ART initiation (preconception and/or antenatal) was eligible. Perinatal outcomes were defined as follows: PTB (birth <37+0 weeks gestation); very PTB (VPTB, birth <32+0 weeks gestation); spontaneous PTB (sPTB, spontaneous birth <37+0 weeks gestation); LBW (<2500 g); very LBW (VLBW, <1500 g); SGA (birthweight for gestational age <10th centile) or very SGA (VSGA, birthweight for gestational age <3rd centile) according to the reference chart used at the study site, stillbirth (delivery of an infant without any signs of life with birthweight ≥1000 g or gestational age ≥24+0 weeks or body length ≥35 cm); and neonatal death (NND, death of an infant in the first 28 days of life).2 Term and preterm LBW were defined according to definitions of PTB and LBW, although no data for preterm LBW was found.
Study selection
The titles and abstracts of studies retrieved by the literature searches were screened and full text manuscripts of relevant citations were obtained and assessed against the eligibility criteria by at least two independent investigators (CP, HS, MK and ZB). Studies were not included if outcomes were not defined or if defined differently from our definitions. If a cohort was reported more than once, the study containing the most recent and complete data was included. If different studies reported different perinatal outcomes for the same cohort, each study was included. References of included studies were assessed for additional relevant studies. Any ambiguities or disagreements regarding inclusion of studies were resolved through discussion with the senior investigator (JH). Details of excluded papers are available upon request.
Data extraction
Data on study and population characteristics, ART exposures and perinatal outcomes were independently extracted from eligible studies by at least two investigators (CP, HS, MK and ZB), and reviewed by the senior investigator (JH). Outcome data according to ART exposure were extracted, as well as information on methods used to adjust for confounders, including regression analysis, risk factor analysis, and matching. Reported unadjusted and adjusted relative risks (RR), odds ratios (OR), and 95% confidence intervals (CIs) of perinatal outcomes according to type of ART exposure were also extracted. Any ambiguities or disagreements were resolved through discussion with the senior investigator (JH).
Quality assessment
The quality of individual studies was independently assessed by at least two investigators (CP, HS, MK and ZB), using an adapted Newcastle-Ottawa Scale. Nine criteria were assessed in three groups: Selection of study participants (maximum 4 points), Comparability of comparator groups (maximum 2 points), and Assessment of outcomes of interest, including methods to assess gestational age at birth (maximum 3 points). Studies were classified as ‘good’, ‘average’, or ‘poor’ quality according to predefined criteria (Appendix pp16–22).
Statistical analysis
Perinatal outcomes were compared between WLHIV receiving PI-ART and WLHIV receiving non-PI-ART. Dichotomous outcome data according to ART exposure from individual studies were used to generate RRs and 95% CIs. Pairwise meta-analyses were carried out if two or more studies reported data for the same perinatal outcome, using a random-effects model to calculate a weighted summary effect estimate (RR) and 95% CI. Meta-analyses were represented in forest plots and the I2 statistic was used to quantify heterogeneity due to clinical and methodological variability between studies. The degree of heterogeneity was classified as none (<25%), low (25–49%), moderate (50–74%), or high (≥75%). Further analyses were carried out of subgroups of PI-ART, including head-to-head comparisons of ART regimens containing specific PIs, and boosted compared to non-boosted PI-ART. Subgroup and sensitivity analyses were also performed to assess the effects of country income status and study quality on associations of PI-ART/non-PI-ART, boosted/non-boosted PI-ART, and LPV/r-ART/ATV/r-ART with perinatal outcomes. Sensitivity analyses were also carried out to assess the effect of adjustment for confounders. The Peters test was utilised to assess publication bias in meta-analyses containing ten or more studies. All statistical analyses were done with Stata version 17 (College Station, Texas, USA).
Role of the funding source
This study received no funding. All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication.
Results
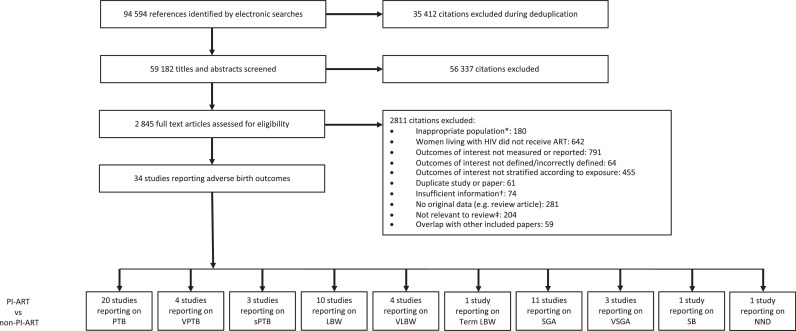
The literature search yielded 94,594 citations, of which 34 studies reported relevant data (Figure 1). The perinatal outcomes reported for WLHIV receiving PI-ART compared to WLHIV receiving non-PI-ART were PTB (20 studies), VPTB (4 studies), sPTB (3 studies), LBW (10 studies), VLBW (4 studies), term LBW (1 studies), SGA (11 studies), VSGA (3 studies), SB (1 studies), and NND (6 studies) (Figure 1).
Figure 1.
Study selection.
* For example, women living with HIV were not pregnant. † For example, paper did not provide relevant outcome data. ‡ For example, Assisted Reproductive Technology (ART). Abbreviations: ART = antiretroviral therapy, HIV = human immunodeficiency virus, LBW = low birthweight, NND = neonatal death, PI = protease inhibitor, PTB = preterm birth, SB = stillbirth, SGA = small for gestational age, sPTB = spontaneous preterm birth, VLBW = very low birthweight, VPTB = very preterm birth, VSGA = very small for gestational age. See Methods for definitions of perinatal outcomes.
Characteristics of included studies are summarised in Table 1.18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43,53, 54, 55, 56, 57, 58, 59, 60 11 prospective (32%) and 23 retrospective (68%) cohort studies analysed data from 57,546 WLHIV in 22 countries (Table 1). 24 studies (71%) took place in high income countries (HICs), and 10 studies (29%) took place in LMICs (Table 1). Quality assessments classified 20 studies (59%) as average quality and 14 studies (41%) as poor quality (Table 1, Appendix pp19–22). 26 studies (74%) reported the methods used to determine gestational age, with only two studies using first trimester ultrasound,29,41 the most accurate method to establish or confirm gestational age,61 for all women (Table 1). 28 studies (82%) used methods to assess potential confounding factors, with 19 studies conducting regression analysis, 19 studies performing risk factor analysis, and one study matched participants (Table 1, Appendix pp23–26). Of the 35 comparisons which were adjusted for covariates in individual studies, only one resulted in a change in the significance of the effect estimate (Appendix pp86–88).
Table 1.
Study characteristics.
| Study | Country | Country income status | Cohort study design | Recruitment period | Number of women on ART in study | Population characteristics* | Method to correct for confounders | Method to estimate gestational age | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|
| Aaron et al.27 | USA | High | Prospective | 1/2000 to 1/2011 | 183 | First born twin included, 38.3% smoking, 18.0% IDU, urban setting | Regression analysis | LNMP confirmed by second trimester ultrasound | Average |
| Albert et al.28 | Canada | High | Retrospective | 1/1/1997 to 31/1/2018 | 421 | Twins excluded, women recruited from a provincial surveillance database, 46.1% smoking, 23.3% alcohol use, 26.0% IDU | Risk factor analysis | Ultrasound in first and/or second trimester | Average |
| Bailey18 | UK, Ireland, Ukraine, Russia, Belgium, Romania, Spain and Switzerland | High | Retrospective | 2008 to 2014 | 7193 | Twins excluded, women recruited across eight countries in Western and Eastern Europe, 6.7% history of IDU in entire cohort | Regression analysis | Ultrasound (unspecified) | Average |
| Boer et al.29 | Netherlands | High | Retrospective | 12/1997 to 7/2003 | 143 | First born twin included, women recruited from an academic medical centre, 12.9% smoking, 1.7% history of IDU | Regression analysis, matching | LNMP confirmed by first trimester ultrasound | Poor |
| Carceller et al.30 | Canada | High | Retrospective | 1997 to 2005 | 206 | Recruited from a tertiary hospital in Montreal, urban setting, hospital deliveries | None | No description | Poor |
| Chen et al.31 | Botswana | Middle | Retrospective | 1/5/2009 to 30/4/2011 | 3290 | First born twin included, hospital deliveries, 5.3% alcohol use, 1.7% smoking | Regression analysis, risk factor analysis | LNMP, symphysis-fundal height, or ultrasound (unspecified) | Average |
| Delicio et al.32 | Brazil | Middle | Retrospective | 2000 to 2015 | 714 | Women recruited from obstetrics clinic serving pregnant women without health insurance from low socioeconomic status, 5.8% alcohol use, 14.3% smoking, 14.3% IDU | Risk factor analysis | No description | Average |
| Ejigu et al.33 | Ethiopia | Low | Retrospective | 2/2010 to 10/2016 | 1464 | Twins excluded, women recruited from public hospitals and public healthcare centres | Regression analysis, risk factor analysis | Ultrasound, LNMP or fundal height | Average |
| Ezechi et al.19 | Nigeria | Middle | Retrospective | 7/2004 to 6/2010 | 847 | Twins included | Regression analysis, risk factor analysis | LNMP | Average |
| Favarato et al.20 | UK and Ireland | High | Prospective | 2007 to 2015 | 6073 | Twins excluded, 1.7% IDU | Regression analysis | No description | Poor |
| Favarato et al.34 | UK and Ireland | High | Prospective | 2007 to 2015 | 6952 | Twins excluded, 1.58% IDU | Risk factor analysis | No description | Poor |
| Floridia et al.53 | Italy | High | Retrospective | 1/12/2001 to 10/6/2013 | 428 | Twins included, 11.2% smoking, 5.4% IDU | Risk factor analysis | LNMP and ultrasound | Average |
| Floridia et al.54 | Italy | High | Retrospective | 5/2004 to 6/2017 | 500 | 5.6% IDU | Risk factor analysis | Ultrasound, LNMP or both | Average |
| Floridia et al.21 | Italy | High | Retrospective | 2008 to 2018 | 794 | Twins excluded, 18.8% smoking, 4.0% recent substance abuse | Risk factor analysis | Ultrasound, LNMP or both | Average |
| Kakkar et al.55 | Canada | High | Prospective | 1988 to 2011 | 364 | Twins excluded, women recruited from a tertiary referral centre and the largest maternal-health centre in the province | Regression analysis, risk factor analysis |
Other method: LNMP and ultrasound (unspecified) |
Average |
| Kowalska et al.35 | Poland | Middle | Prospective | 1/1995 to 2/2003 | 46 | Twins included, women recruited from an outpatient HIV clinic, 47.1% IDU | Risk factor analysis | LNMP | Poor |
| Lopez et al.36 | Spain | High | Prospective | 1/2006 to 12/2011 | 156 | Twins excluded, women recruited in a tertiary hospital, 31.4% smoking, 15.4% history of IDU | Risk factor analysis | First trimester ultrasound and earliest available ultrasound in late gestation | Average |
| Machado et al.37 | Brazil | Middle | Prospective | 1996 to 2006 | 313 | Twins excluded, women recruited from a HIV referral centre, 21.3% smoking, 5.4% alcohol use, 9% IDU | Regression analysis, risk factor analysis | LNMP or ultrasound | Poor |
| Montgomery-Taylor et al.38 | UK | High | Retrospective | 1/2008 to 12/2012 | 61 | 13.0% alcohol use, 3.0% smoking, recruited in a tertiary hospital, urban setting, all hospital deliveries | None | No description | Poor |
| Perry et al.56 | England | High | Retrospective | 1/9/2007 to 30/8/2012 | 493 | Twins included, women recruited from 10 London HIV centres, urban setting, 2.0% smoking, 0.4% alcohol, 0.2% recreational drug use | None | LNMP | Poor |
| Rough et al.57 | USA | High | Prospective | Two cohorts: one from April 1st 2007 - March 1st 2016, one from 2002 to 2013 | 1621 | Twins included, 18.4% alcohol use, 19.6% smoking, 12.2% IDU | Regression analysis | Ultrasound, physical examination or LNMP | Average |
| Schulte et al.39 | USA | High | Retrospective | 1989 to 2004 | 2563 | 27.6% history of IDU | Regression analysis | LNMP, ultrasound (unspecified), neonatal assessment (unspecified) | Poor |
| Shapiro et al.22 | Botswana | Middle | Prospective | 7/2006 to 5/2008 | 730 | Recruited from government run antenatal clinics in urban and rural communities | None | LNMP, ultrasound (in 1st, 2nd and 3rd trimester) | Poor |
| Short et al.23 | UK | High | Retrospective | 1996 to 2010 | 331 | Twins included, women recruited from a HIV antenatal clinic, urban setting, deliveries in a tertiary hospital,13.0% smoking | None | No description | Poor |
| Sibiude et al.58 | France | High | Retrospective | 1990 to 2009 | 6738 | Twins excluded, recruited from obstetric centres, 94.1% history or active IDU | Regression analysis, risk factor analysis | LNMP confirmed by ultrasound | Average |
| Sibiude et al.40 | France | High | Retrospective | 2005 to 2015 | 1597 | Women enrolled from French Perinatal Cohort | Regression analysis, risk factor analysis | LNMP confirmed by ultrasound | Average |
| Smith et al.59 | USA | High | Retrospective | 1997 to 2009 | 158 | Twins excluded, data from Children's Hospital Immunodeficiency Program (CHIP), 12% IDU | None | No description | Poor |
| Snijdewind et al.41 | Netherlands | High | Retrospective | 1/1997 to 2/2015 | 1392 | Twins excluded, women recruited from 26 nationwide sites, 10.8% smoking, 11.7% alcohol use, 0.6% IDU | Risk factor analysis | Early ultrasound or LNMP | Average |
| Szyld et al.24 | Argentina, Bahamas, Brazil and Mexico |
Middle | Prospective | 1/9/2002 to 1/3/2005 | 587 | Twins excluded, 9.4% alcohol use, 21.4% smoking, 2.3% IDU | Regression analysis, risk factor analysis | LNMP with/without ultrasound, neonatal assessment (unspecified) | Average |
| Townsend et al.60 | UK and Ireland | High | Prospective | 1990 to 2005 | 3384 | Twins excluded, 5.0% IDU | Regression analysis | No description | Poor |
| Van der Merwe et al.25 | South Africa | Middle | Retrospective | 10/2004 to 3/2007 | 946 | Twins excluded, women recruited from HIV referral centres including a tertiary hospital, 3.7% smoking, 3.9% alcohol use | Regression analysis, risk factor analysis | LNMP, ultrasound (unspecified), symphysis-fundal height, neonatal assessment (unspecified) | Poor |
| Watts et al.43 | USA and Puerto Rico | High | Retrospective | 2007 to 31/10/2010 | 1672 | Twins excluded, 17% smoking, 8.0% alcohol use, 8.0% IDU | Regression analysis | Clinical method (unspecified) and ultrasound (unspecified) | Average |
| Williams et al.42 | USA | High | Retrospective | 1/7/2000 to 1/11/2007 | 188 | Twins excluded, hospital deliveries, 38.3% smoking, 25.0% IDU | Regression analysis, risk factor analysis | LNMP, clinical assessment (unspecified) and ultrasound | Average |
| Zash et al.26 | Botswana | Middle | Retrospective | 15/8/2014 to 15/8/2016 | 4995 | Twins excluded, obstetric records extracted at 8 national government hospitals, 6.3% alcohol consumption or smoking | Regression analysis | LNMP confirmed by ultrasound where possible | Average |
* Details on the inclusion of twins, recruitment centre, urban/rural setting, deliveries at home/hospital, smoking, alcohol use, and IDU were sought and reported here if provided by each study.
Abbreviations: ART = antiretroviral therapy, HIV = human immunodeficiency virus, IDU = illicit drug use, LNMP = last normal menstrual period, NSHPC, the National Study of HIV in Pregnancy and Childhood; USA, United States of America; UK, United Kingdom.
The ART regimens received by WLHIV, ART regimen comparisons reported, and perinatal outcomes analysed are displayed for each study in Table 2. 27 studies (79%) reported perinatal outcomes in WLHIV receiving PI-ART compared to non-PI-ART, 10 studies (29%) compared ART regimens containing different PIs, and five studies (15%) compared boosted PI-ART with non-boosted PI-ART. 12 studies (35%) did not specify which specific PI was used in the PI-ART regimen. In most studies (24, 71%) ART was initiated either preconception or antenatal (mixed), in two studies (6%) ART was initiated preconception, in four studies (12%) antenatally, and unspecified in four studies (12%; Table 2).
Table 2.
Antiretroviral therapy characteristics, treatment comparisons, and perinatal outcomes.
| Study | ART regimens | Protease inhibitors | Timing of ART initiation | PI-ART vs non-PI- ART | Comparison of specific protease inhibitors | Boosted PI-ART vs non-boosted PI-ART | Perinatal outcomes |
|---|---|---|---|---|---|---|---|
| Aaron et al.27 | 63.9% PI-ART, 36.1% non-PI-ART (NNRTI/NRTI-ART) |
38.5% NFV, 26.6% RTV, 26.6% ATV, 4.6% APV, 1.8% FPV, 1.8% DRV |
Unspecified | Yes | No | No | SGA, VSGA |
| Albert et al.28 | 77.1% PI-ART, 23.9% non-PI-ART (INSTI/NNRTI-ART) Amongst PI-ART: 65.2% boosted PI-ART, 34.8% non-boosted PI-ART |
IDV, NFV, DRV, RTV, ATV/r, LPV/r, SQV/r, DRV/r, unspecified proportions | Mixed | Yes | No | Yes | sPTB |
| Bailey18 | 90.3%% PI-ART, 9.7% non-PI-ART (NNRTI-ART) |
85.6% LPV/r, 14.4% other | Antenatal | Yes | No | No | PTB, SGA |
| Boer et al.29 | 64.3% PI-ART, 35.7% non-PI-ART (NNRTI-ART) |
NFV, unspecified proportion | Mixed | Yes | No | No | PTB, LBW, VLBW |
| Carceller et al.30 | 85.4% PI-ART, 14.6% non-PI-ART (NNRTI/NRTI-ART) |
85.8% NFV, 11.9% IDV, 4.5% LPV/r, 2.8% RTV 8.5% SQV |
Unspecified | Yes | No | No | PTB, Term LBW |
| Chen et al.31 | 7.9% PI-ART, 92.4% non-PI-ART (NNRTI-ART) |
LPV/r, Unspecified proportion |
Mixed | Yes | No | No | PTB, SGA |
| Delicio et al.32 | 61.8% boosted PI-ART, 18.2% non-boosted PI-ART, 19.2% non-PI-ART (NNRTI-ART) |
73.1% LPV/r, 22.5% NFV, 4.3% ATV/r |
Mixed | Yes | Yes | Yes | PTB, LBW, VLBW |
| Ejigu et al.33 | 2.2% PI-ART, 97.8% non-PI-ART (NNRTI-ART) |
Unspecified | Mixed | Yes | No | No | PTB, LBW, SGA |
| Ezechi et al.19 | 6.7% PI-ART, 93.3% non-PI-ART (NNRTI-ART) |
Unspecified | Mixed | Yes | No | No | sPTB |
| Favarato et al.20 | 68% PI-ART, 32% non-PI-ART (NNRTI-ART) |
55% LPV/r, 45% other PI | Mixed | Yes | No | No | PTB, SGA |
| Favarato et al.34 | 67.5% PI-ART, 32.5% non-PI-ART (NNRTI-ART) |
26.9% ATV/r, 63.7% LPV/r, 9.4% DRV/r |
Mixed | Yes | Yes | No | SB |
| Floridia et al.53 | 100% PI-ART | 75.2% LPV/r, 24.8% ATV/r | Mixed | No | Yes | No | PTB, LBW, VLBW, SGA |
| Floridia54 | 100% PI-ART | 81.8% ATV/r 18.2% DRV/r | Mixed | No | Yes | No | PTB, VPTB, LBW, VLBW, SGA |
| Floridia et al.21 | 78.5% PI-ART, 21.5% non-PI-ART (INSTI/NNRTI-ART) |
46.7% ATV/r, 43.8% LPV/r, 7.5% DRV/r, 2.0% other |
Mixed | Yes | No | No | PTB, VPTB, LBW, VLBW, SGA |
| Kakkar et al.55 | 39.6% boosted PI-ART, 60.4% non-boosted PI-ART |
49.8% NFV, 31.9% LPV/r, 5.5% ATV/r, 1.1% FPV/r, 1.1% TPV/r, 5.2% SQV, 5.5% IDV |
Unspecified | No | Yes | Yes | PTB |
| Kowalska et al.35 | 39.1% PI-ART, 60.1% non-PI-ART (unspecified) |
Unspecified | Mixed | Yes | No | No | PTB, LBW |
| Lopez et al.36 | 67.9% PI-ART, 32.1% non-PI-ART (NNRTI-ART) |
Unspecified | Mixed | Yes | No | No | SGA |
| Machado et al.37 | 68.1% PI-ART, 31.9% non-PI-ART (NNRTI-ART) |
Unspecified | Mixed | Yes | No | No | PTB, LBW |
| Montgomery-Taylor et al.38 | 60.1% PI-ART, 39.9% non-PI-ART (NNRTI-ART) |
64.9% LPV/r, 13.5% ATV/r, 5.4% DRV/r, 2.7% FPV/r, 2.7% SQV/r, 2.7% IDV, 2.7% RAL, 5.4% other |
Mixed | Yes | No | No | SGA |
| Perry et al.56 | 100% PI-ART | 62.1% LPV/r 37.9% ATVr | Mixed | No | Yes | No | PTB, VPTB, LBW, VLBW |
| Rough et al.57 | 100% PI-ART | 66.7% LPV/r, 33.3% ATV/r | Antenatal | No | Yes | No | PTB, LBW, VLBW |
| Schulte et al.39 | 30.6% PI-ART, 69.4% non-PI-ART (unspecified) |
Unspecified | Unspecified | Yes | No | No | PTB, LBW |
| Shapiro et al.22 | 37.7% PI-ART, 62.3% non-PI-ART (NNRTI/NRTI-ART) |
100% LPV/r | Antenatal | Yes | No | No | PTB, VPTB, LBW, VLBW |
| Short et al.23 | 38.4% PI-ART, 61.6% non-PI-ART (NNRTI/NRTI-ART) |
Unspecified | Mixed | Yes | No | No | PTB |
| Sibiude et al.58 | 85.1% boosted PI-ART 14.9% non-boosted PI-ART |
Non-boosted PI: 92.0% NFV, 5.9% ATV Boosted PI: 81.8% LPV/r, 9.9% SQV/r, 5.0% IDV/r, 1.3%, ATV/r, 2.0% FPV/r |
Antenatal | No | Yes | Yes | PTB, VPTB, sPTB |
| Sibiude et al.40 | 96.0% PI-ART, 4.0% non-PI-ART (INSTI-ART) |
46.3% LPV/r 34.8% ATV/r 18.7% DRV/r |
Preconception | Yes | Yes | No | VSGA |
| Smith et al.59 | 100% PI-ART | 50.9% LPV/r, 13.8% ATV/r, 35.3% NFV |
Mixed | No | Yes | Yes | PTB, VSGA |
| Snijdewind et al.41 | 66.7% PI-ART, 33.3% non-PI-ART (NNRTI-ART) |
Unspecified | Mixed | Yes | No | No | PTB, VPTB, LBW, VLBW, SGA |
| Szyld et al.24 | 56.2% PI-ART, 43.8% non-PI-ART (NNRTI-ART) |
Unspecified | Mixed | Yes | No | No | PTB, LBW |
| Townsend et al.60 | 39.7% PI-ART, 60.3% non-PI-ART (NNRTI/NRTI-ART) |
Unspecified | Mixed | Yes | No | No | PTB, VPTB, SB, NND |
| Van der Merwe et al.25 | 44.5% PI-ART, 55.4% non-PI-ART (NNRTI-ART) |
100% LPV/r | Mixed | Yes | No | No | PTB, LBW, VLBW, SGA |
| Watts et al.43 | 78.6% PI-ART, 21.4% non-PI-ART (NNRTI/NRTI-ART) |
Unspecified | Mixed | Yes | No | No | PTB, sPTB, SGA |
| Williams et al.42 | 68.6% PI-ART, 31.4% non-PI-ART (unspecified) |
Unspecified | Mixed | Yes | No | No | PTB |
| Zash et al.26 | 8.0% PI-ART, 92.0% non-PI-ART (NNRTI-ART) |
100% LPV/r | Preconception | Yes | No | No | PTB, VPTB, SGA, VSGA, SB, NND |
Abbreviations: ART = antiretroviral therapy (≥ 3 antiretroviral drugs), INSTI = integrase strand transfer inhibitor, NNRTI = non-nucleoside reverse transcriptase inhibitor, NRTI = nucleoside reverse transcriptase inhibitor, PI = protease inhibitor.
Protease inhibitors: APV = amprenavir, ATV = atazanavir, DRV = darunavir, FPV = fosamprenavir, IDV = indinavir, LPV = lopinavir, NFV = nelfinavir, SQV = saquinavir, TPV = tipranavir, RAL = raltegravir, RTV = ritonavir, /r = ritonavir boosted.
Perinatal outcomes: LBW = low birthweight, NND = neonatal death, PTB = preterm birth, SB = stillbirth, SGA = small for gestational age, sPTB = spontaneous preterm birth, VLBW = very low birthweight, VPTB = very preterm birth, VSGA = very small for gestational age.
Random-effects meta-analyses were conducted to compare perinatal outcomes in WLHIV receiving PI-ART regimens and non-PI-ART regimens, head-to-head comparisons of ART regimens containing specific PIs, and boosted compared to non-boosted PI-ART. The summary effect estimates and 95% confidence intervals are presented in Figure 2, Figure 3 and Table 3, and the forest plots in Appendix pp27–74. Subgroup analyses were carried out according to country income status (Appendix p80–82), and study quality (Appendix p83–85).
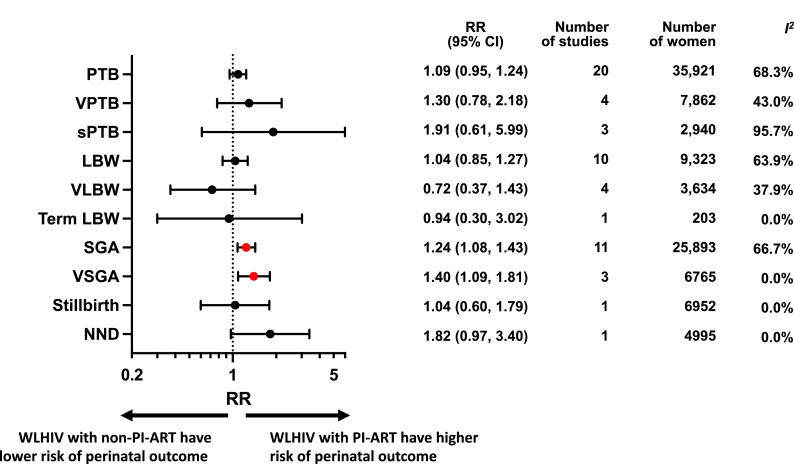
Figure 2.
Perinatal outcomes of women living with HIV receiving PI-ART compared to non-PI-ART.
Random-effects meta-analysis results for perinatal outcomes associated with women living with HIV receiving PI-ART compared to non-PI-ART. Relative risk and 95% confidence interval, number of studies and women included in the analysis of each perinatal outcome, and I2 value for heterogeneity are displayed. Statistically significant results are presented with red dots and non-significant effects with black dots. Forest plots of the meta-analyses of PI-ART compared to non-PI-ART for each perinatal outcome can be found in Appendix pp 27–32.
Abbreviations: ART = antiretroviral therapy, CI = confidence interval, LBW = low birthweight, NND = neonatal death, PI = protease inhibitor, PTB = preterm birth, RR = relative risk, SGA = small for gestational age, sPTB = spontaneous preterm birth, VLBW = very low birthweight, VPTB = very preterm birth, VSGA = very small for gestational age, WLHIV= women living with HIV.
Figure 3.
Perinatal outcomes of women living with HIV receiving different PI-ART regimens.
Random-effects meta-analysis results for perinatal outcomes associated with women living with HIV receiving ART regimens containing LPV/r vs ATV/r (a), ATV/r vs DRV/r (b), LPV/r vs DRV/r (c), LPV/r vs NFV (d), ATV/r vs NFV (e), boosted-PI vs non-boosted-PI (f). Relative risk and 95% confidence interval, number of studies and women included in the analysis of each perinatal outcome, and I2 value for heterogeneity are displayed for each PI comparison. Statistically significant results are presented with red dots and non-significant effects with black dots. Forest plots of pairwise meta-analyses of ART regimens containing different protease inhibitors for each perinatal outcome can be found in Appendix pp 33–74.
Abbreviations: ART = antiretroviral therapy, CI = confidence interval, PI = protease inhibitor, RR = relative risk, WLHIV= women living with HIV.
Protease inhibitors: ATV/r = atazanavir/ritonavir, DRV/r = darunavir/ritonavir, LPV/r = lopinavir/ritonavir, NFV = nelfinavir.
Perinatal outcomes: LBW = low birthweight, PTB = preterm birth, SGA = small for gestational age, sPTB = spontaneous preterm birth, VLBW = very low birthweight, VPTB = very preterm birth, VSGA = very small for gestational age.
Table 3.
Risk of preterm birth of women living with HIV receiving different PI-ART regimens.
| ATV/r-ART | NFV-ART | DRV/r-ART | FPV/r-ART | IDV/r-ART | IDV-ART | SQV/r-ART | SQV-ART | TPV/r-ART | |
|---|---|---|---|---|---|---|---|---|---|
| RR (95%CI) | RR (95%CI) | RR (95%CI) | RR (95%CI) | RR (95%CI) | RR (95%CI) | RR (95%CI) | RR (95%CI) | RR (95%CI) | |
| LPV/r-ART | 0.98 (0.75, 1.27) |
1.33 (1.03, 1.72) |
0.83 (0.36, 1.95) |
1.09 (0.54, 2.22) |
1.68 (0.43, 6.65) |
1.08 (0.65, 1.81) |
2.99 (0.43, 20.92) |
1.90 (0.13, 27.28) |
|
| ATV/r-ART | 1.63 (0.91, 2.92) |
0.92 (0.55, 1.55) |
0.90 (0.12, 6.98) |
0.54 (0.07, 4.04) |
2.70 (0.62, 11.72) |
0.54 (0.08, 3.77) |
4.80 (0.64, 35.91) |
3.10 (0.21, 46.34) |
|
| NFV-ART | 0.52 (0.20, 1.32) |
0.66 (0.28, 1.53) |
1.06 (0.27, 4.19) |
0.65 (0.33, 1.30) |
1.89 (0.27, 13.19) |
1.20 (0.08, 17.25) |
|||
| DRV/r-ART | |||||||||
| FPV/r-ART | 1.44 (0.47, 4.42)) |
0.76 (0.04, 13.43) |
1.43 (0.52, 3.91) |
1.13 (0.05, 23.72) |
|||||
| IDV/r-ART | 0.99 (0.43, 2.31) |
||||||||
| IDV-ART | 1.78 (0.18, 17.80) |
1.32 (0.07, 23.26) |
|||||||
| SQV/r-ART | |||||||||
| SQV-ART | 0.88 (0.04, 18.47) |
||||||||
Random-effects meta-analysis results for risk of preterm birth associated with women living with HIV receiving ART regimens containing different protease inhibitors (PIs). Relative risk (RR) and 95% confidence interval (95% CI) are displayed. A RR > 1 indicates increased risk of a preterm birth associated with the ART regimen in the row compared to the regimen in the column. For example, LPV/r-ART is associated with an increased risk of preterm birth compared to NFV-ART (RR 1.33, 95% CI 1.03–1.72). Forest plots of pairwise meta-analyses of ART regimens containing different protease inhibitors can be found in Appendix pp 36–74.
Abbreviations: ART = antiretroviral therapy, ATV/r = atazanavir/ritonavir, DRV/r = darunavir/ritonavir, FPV/r = fosamprenavir/ritonavir, HIV = human immunodeficiency virus, IDV = indinavir, IDV/r = indinavir/ritonavir, LPV/r = lopinavir/ritonavir, NFV = nelfinavir, PI = protease inhibitor, SQV/r = saquinavir/ritonavir, SQV = saquinavir, TPV/r = tipranavir/ritonavir.
PI-ART compared to non-PI-ART
In the analysis of 35,921 WLHIV from 20 studies, PI-ART was not significantly associated with PTB compared to non-PI-ART (RR 1.09, 95% CI 0.95–1.24) (Figure 2, Appendix p27). There was moderate heterogeneity between studies (I2=68.3%), but no evidence of publication bias (Peters test, p = 0.761; Appendix p27). This association remained non-significant in subgroup analyses conducted in HICs (1.06, 0.94–1.19) and LMICs (1.10, 0.83–1.46; Appendix p80), and average quality (1.09, 0.98–1.22) and poor quality (1.08, 0.83–1.41) studies (Appendix p83).
PI-ART was not significantly associated with VPTB compared to non-PI-ART (1.30, 0.78–2.18) in 4 studies with 7862 WLHIV (Figure 2, Appendix p28), with low heterogeneity between studies (I2=43%; Appendix p28). However, two studies conducted in LMICs found that PI-ART was associated with an increased risk of VPTB (1.49, 1.04–2.14; Appendix p80), without heterogeneity (I2=0.0%; Appendix p82).
PI-ART was not significantly associated with sPTB (1.91, 0.61–5.99) in 3 studies with 2940 WLHIV (Figure 2, Appendix p28), with high heterogeneity between studies (I2=95.7%; Appendix p28). One study conducted in a LMIC found an increased risk of sPTB with PI-ART (5.02, 3.62–6.98; Appendix p80).
PI-ART was not significantly associated with LBW (1.04, 0.85–1.27) in 10 studies with 9323 WLHIV (Figure 2, Appendix p29). There was moderate heterogeneity between studies (I2=63.9%), but no evidence of publication bias (Peters test, p = 0.465; Appendix p29).
PI-ART was not significantly associated with VLBW (0.72, 0.37–1.43) in 4 studies with 3634 WLHIV (Figure 2, Appendix p29). In two average quality studies conducted in HICs PI-ART was associated with a decreased risk of VLBW (0.59, 95% CI 0.36-0.98) (Appendix p80, p83), without heterogeneity (I2=0.0%; Appendix p82, p85).
PI-ART was not significantly associated term LBW (0.94, 0.30–3.02) in one study with 203 WLHIV (Figure 2, Appendix p30).
In the analysis of 25,893 WLHIV from 11 studies, PI-ART was associated with a significantly increased risk of SGA compared to non-PI-ART (1.24, 1.08–1.43; Figure 2, Appendix p30). There was moderate heterogeneity (I2=66.7%), but no evidence of publication bias (Peters test, p = 0.435; Appendix p30). This association remained significant in subgroup analyses conducted in HICs (1.20, 1.04–1.37; Appendix p80) and average quality studies (1.13, 1.01–1.27; Appendix p83), but not in LMICs (1.30, 0.94–1.78; Appendix p80) and poor quality studies (1.51, 0.91–2.49; Appendix p83).
In the analysis of 6765 WLHIV from three studies, PI-ART was also associated with a significantly increased risk of VSGA compared to non-PI-ART (1.40, 1.09–1.81; Figure 2, Appendix p31), without heterogeneity (I2=0.0%; Appendix p31). This association remained significant in the subgroup analysis of average quality studies (1.39, 1.08–1.80; Appendix p83) and studies conducted in LMICs (1.37, 1.05–1.80; Appendix p80), but not in poor quality studies (1.93, 0.27–13.66; Appendix p83) or studies conducted in HICs (1.65, 0.74–3.68; Appendix p80).
One study examined stillbirth and another neonatal death, both finding no significant associations with PI-ART (Figure 2, Appendix pp31–32).
Comparisons between different PI-ART regimens
10 studies compared PI-ART regimens containing nine different PIs. PIs assessed included the three PIs recommended in current treatment guidelines, namely ATV/r, DRV/r, and LPV/r, as well as other PIs fosemprenavir/ritonavir (FPV/r), indinavir (IND), indinavir/ritonavir (IDV/r), nelfinavir (NFV), saquinavir/ritonavir (SQV/r) and tipranavir/ritonavir (TPV/r; Figure 3, Table 3). There was data for the outcomes PTB, VPTB, LBW, VLBW, SGA VSGA and stillbirth, but no other perinatal outcomes.
Compared to ATV/r-ART, LPV/r-ART was not significantly associated with PTB (0.98, 0.75–1.27), VPTB (1.04, 0.42–2.59), LBW (1.15, 0.95–1.38), VLBW (0.76, 0.24–2.42), SGA (0.95, 0.53–1.72), VSGA (1.35, 0.79–2.31) or stillbirth (1.87, 0.82–4.23; Figure 3A, Appendix pp36–39). There were also no significant associations of LPV/r-ART, compared to ATV/r-ART, with any of the perinatal outcomes in the subgroup analyses according to country income status or study quality (Appendix p80, p83).
Compared to DRV/r-ART, ATV/r-ART was not significantly associated with PTB (0.92, 0.55–1.55), VPTB (0.72, 0.15–3.38), LBW (1.21, 0.69–2.11), VLBW (0.62, 0.17–2.24), SGA (1.06, 0.52–2.17), VSGA (0.70, 0.35–1.43) and stillbirth (0.60, 0.31–1.13; Figure 3B, Appendix pp51–54).
Compared to DRV/r-ART, LPV/r-ART was not significantly associated with VSGA (0.99, 0.52–1.86) or stillbirth (1.53, 0.47–4.98; Figure 3C, Appendix p42), but there were no data for other outcomes.
Compared to NFV-ART, LPV/r-ART was associated with a significantly increased risk of PTB (1.33, 1.03–1.72; Figure 3D, Appendix p40), without heterogeneity (I2=0.0%; Appendix p40). However, LPV/r-ART was not associated with an increased risk of LBW (1.36, 0.91–2.02) or VSGA (1.09, 0.35–3.47; Figure 3D, Appendix pp40–41).
Compared to NFV-ART, ATV/r-ART was not significantly associated with PTB (1.63, 0.91–2.92), LBW (1.06, 0.44–2.52) or VSGA (1.09, 0.22–5.37; Figure 3E, Appendix pp49–50).
ART regimens containing other specific PIs were only reported in relation to PTB, but no other perinatal outcomes. No significant associations with PTB were found when comparing FPV/r-ART, IDV-ART, IDV/r-ART, SQV-ART, SQV/r-ART or TPV/r-ART with any other specific PI assessed, including LPV/r-ART, ATV/r-ART, DRV/r-ART, and NFV-ART, although data were not reported for all possible pairwise PI comparisons (Table 3, Appendix pp43–48 and pp55–74).
Boosted PI-ART compared to non-boosted PI-ART
In the analysis of 3333 WLHIV from five studies, boosted PI-ART was associated with a significantly increased risk of PTB compared to non-boosted PI-ART (1.36, 1.12–1.65; Figure 3F, Appendix p33), without heterogeneity (I2=0.0%; Appendix p33).
In the analysis of 2347 WLHIV from two studies, boosted PI-ART was also associated with a significantly increased risk of VPTB compared to non-boosted PI-ART (1.85, 1.02–3.37) (Figure 3F, Appendix p33), with a high degree of heterogeneity (I2=76.0%; Appendix p33).
In the analysis of two studies with 1578 WLHIV, boosted PI-ART was not significantly associated with sPTB (1.31, 0.87–1.97), compared to non-boosted PI-ART (Figure 3F, Appendix p34). Compared to non-boosted PI-ART, boosted PI-ART was also not significantly associated with LBW (1.34, 0.90–1.99) in one study with 590 WLHIV or VSGA (1.09, 0.35–3.47) in one study with 111 WLHIV (Figure 3F, Appendix pp34–35).
No data comparing boosted PI-ART with non-boosted PI-ART were found for other outcomes. There were no data directly comparing unboosted (e.g. IDV) with boosted (e.g. IDV/r) formulations of the same primary PI.
Discussion
This meta-analysis shows that PI-ART is associated with a significantly increased risk of SGA and VSGA compared to non-PI-ART. In contrast to previous reports, PI-ART was not associated with PTB, VPTB or sPTB, or other perinatal outcomes, including LBW, VLBW, term LBW, stillbirth and NND. In the comparisons between ART regimens containing different PIs, no differences were observed between ART regimens containing ATV/r, LPV/r or DRV/r, which are the most commonly used PIs.
The finding that PI-ART was not associated with an increased risk of PTB compared to non-PI-ART contrasts with older meta-analyses of cohort studies which examined PTB in relation to PI-containing regimens.44,45 This difference is attributable foremost to the large number of new studies included in our PTB analysis (21 studies, compared to 844 and 1045 studies). In addition, we included only ART (i.e. triple drug) regimens in our analyses, thereby excluding studies using monotherapy and dual therapy, which were included in previous analyses.44,45 This highlights the importance of regularly updating meta-analyses as new data becomes available and inclusion of relevant regimens in analyses.
This is the first meta-analysis to show that PI-ART is significantly associated with both SGA and VSGA, compared to non-PI-ART, as these outcomes were not assessed in previous meta-analyses of cohort studies.44,45 The association with SGA is particularly solid, as it is based on 11 studies with 25,893 WLHIV. In contrast, in the network meta-analysis of RCTs no RCTs comparing ART regimens reported data on SGA and no RCTs reported on VSGA.16 The current data therefore represents the best available evidence regarding the association of PI-ART with both SGA and VSGA. WLHIV receiving PI-ART may benefit from increased surveillance of fetal growth, which should be assessed against international standards.62
UK, US and European guidelines advise against the use of LPV/r, and instead recommend ATV/r and DRV/r, citing an increased risk of PTB with LPV/r.13, 14, 15 However, we found that the risks of PTB, VPTB, LBW, VLBW, SGA, VSGA and stillbirth associated with ART containing ATV/r were comparable with those associated with ART containing LPV/r or DRV/r. The comparison of ATV/r with LPV/r for PTB was based on 7 studies including nearly 4000 WLHIV, giving an effect estimate close to 1 (RR 0.98, 0.75–1.27), a result which was confirmed in the 5 higher quality studies. The comparison of LPV/r with DRV/r did not show a difference for VSGA and stillbirth in a single study and no data was available for other outcomes - the evidence for this comparison is therefore limited. To ensure an adequate assessment of the evidence for all PIs, we also analysed PIs (IDV, IDV/r, NFV, SQV, SQV/r, TPV, FPV, FPV/r) which are older and no longer recommended for use in pregnancy for a variety of reasons, including lower efficiency, toxicities, pharmacokinetics, and limited data and use in pregnancy.13 For many comparisons involving these agents there was very limited evidence, often only relating to PTB, and only revealed an increased risk of PTB for LPV/r compared with NFV, which is no longer recommended because of inferior virological efficacy.13 The association between boosted PI-ART with an increased risk of PTB compared to non-boosted PI-ART was largely based on the data comparing LPV/r with NFV, in addition to data based on non-boosted IDV and SQV (Appendix pp36–74). The limited use and evidence for these older PIs limits the interpretation and application of these findings.
Overall, these data indicate that ART regimens containing LPV/r or DRV/r are associated with similar perinatal outcomes as ART regimens containing ATV/r. It should be borne in mind that LPV/r is the only PI that has been assessed in RCTs in pregnancy, and that LPV/r-containing regimens were ranked as having the highest risk of the perinatal outcomes assessed.16 However, among the ART regimens assessed in RCTs, ZDV/3TC/LPV/r was associated with a significantly increased risk of spontaneous PTB compared to ZDV/3TC/ABC, but no other significant differences in perinatal outcomes between ART regimens assessed were found. LPV/r-based ART regimens were significantly associated with PTB, LBW and VLBW, compared to ZDV monotherapy.16 However, ZDV monotherapy, dual therapy, and NRTI-ART, such as ZDV/3TC/ABC, are no longer recommended for use in pregnancy in international treatment guidelines.12, 13, 14, 15 Therefore, RCTs provide no evidence regarding the comparative perinatal outcomes associated with different PI-ART regimens and the available data from cohort studies presented here indicate that ATV/r-ART, LPV/r-ART, and DRV/r-ART are comparable with regard to perinatal outcomes, which should inform international treatment guidelines.
This study has several strengths. Our systematic review and meta-analysis is the largest to date to our knowledge, assessing a comprehensive range of 10 perinatal outcomes in WLHIV receiving ART, including 57,546 WLHIV from 34 studies. The analyses of PTB, LBW and SGA for the comparison between WLHIV receiving PI-ART and non-PI-ART were each supported by ≥10 studies, including 20 studies with 35,921 WLHIV for the analysis of PTB, providing strong evidence for the results found. As well as including many more studies and WLHIV compared to previous meta-analyses, our study focussed on ART regimens and specific PI drugs, assessed a wide range of perinatal outcomes, and overcame several methodological limitations of previous studies by conducting quality assessments, subgroup and sensitivity analyses, and assessment of correction for confounders.44,45 Our study was conducted according to Cochrane guidelines,51 with exposures and outcomes predefined to minimise selection and misclassification bias and promote consistency across studies. A random-effects meta-analysis model was used and several subgroup and sensitivity analyses were conducted. In particular, the higher quality studies confirmed our findings in the main analyses. Where applicable, the Peters test confirmed an absence of publication bias, and the systematic review was reported according to the PRISMA guidelines.52
We acknowledge several limitations of the included studies. All included studies are observational and are therefore associated with risks of bias, including indication bias linked to the fact the PI-ART are second-line regimens in many LMICs and hence WLHIV on PI-ART are more likely to have failed other regimens, and chronological bias among older PI-ART regimens. We could not assess the effect of certain important confounders (e.g., maternal viral load and CD4 cell count), which are associated with adverse outcomes, because of limited reporting of these confounders in included studies. We extensively assessed the methods used to assess potential confounding in each study and found that adjustment for covariates by regression analysis rarely resulted in a change in the significance of the effect estimate in individual studies. However, we cannot exclude the potential of residual confounding. The perinatal outcomes of VPTB, sPTB, VLBW, term LBW, VSGA, SB and NND were reported in a limited number of studies (<5). There were also fewer studies reporting perinatal outcomes for ART regimens containing specific protease inhibitors. In particular, there were few data for older PI drugs and limited data on boosted compared to non-boosted PIs. Consequently, several of the meta-analyses included few studies, and the results from these analyses are less reliable. There was no data comparing the same primary PI with and without RTV boosting, and no data was found for PIs boosted with cobicistat. We did not assess the drugs in the ART backbone. Moreover, eight studies did not give information on how gestational age was established, while only two studies used a universal first trimester ultrasound, the most accurate method to establish or confirm gestational age.61 Certain perinatal outcomes, such as PTB and SGA, may therefore have been vulnerable to mis-classification bias due to inaccurate assessment of gestational age. SGA and VSGA were defined according to the reference chart used at each study site, rather than an international reference standard.63 Further, differences in populations and settings between studies may have contributed to the heterogeneity observed in our analyses. Timing of ART initiation may have contributed to heterogeneous findings as a meta-analysis reported that preconception ART initiation was associated with an increased risk of PTB, VPTB and LBW compared to antenatal ART initiation.64 This finding may be due to selection bias, as WLHIV who start ART during pregnancy will have less opportunity to experience adverse birth outcomes compared to women who start ART preconception.65 In most studies included in our meta-analysis there was a mixture of preconception and antenatal initiation of ART and we were unable to conduct subgroup analyses according to timing of ART initiation.
The biological mechanisms responsible for the observed association of PI-ART with SGA among WLHIV are unclear. HIV infection is associated with CD4 depletion and chronic immune activation.66 Several innate immune cells, including innate lymphoid cells and mucosal associated invariant T cells, are depleted during early HIV infection and fail to recover with ART, and may be associated with increased risk of adverse perinatal outcomes.67,68 ART may promote a pro-inflammatory shift in T-cell function, counteracting the Th1 to Th2 shift required in pregnancy.69,70 The immunological changes in pregnancy are in part driven by placental progesterone. Potential effects of PIs on progesterone levels, as well as direct effects on placenta and decidua have recently been reviewed.71 It has been reported that WLHIV receiving PI-ART have lower plasma progesterone levels, which may be due to effects of PIs on placental cytochrome P450 enzymes and/or increase in placental expression of 20-alpha-hydroxysteroid dehydrogenase, which inactivates progesterone.71,72 In both mouse-models and WLHIV receiving PI, reduced progesterone levels are associated with increased risk of SGA.73 A potential role for low progesterone as a mediator of adverse outcomes in WLHIV inspired a recent RCT of progesterone supplementation in pregnant WLHIV on ART (mostly NNRTI-ART, only 3% PI-ART).74 Interestingly, this RCT showed that administration of 17-alpha-hydroxyprogesterone had no effect on the primary outcomes of PTB or stillbirth, but was instead associated with a reduction in the risk of VSGA.74 In summary, the available mechanistic data are limited and complex, and highlight the need to firmly establish the epidemiological associations between HIV/ART and specific perinatal outcomes before embarking on mechanistic and intervention studies.
To our knowledge, this is the first systematic review and meta-analysis comparing ART regimens containing different specific PIs. It is clear that more and larger studies are needed to compare ART regimens containing different PI drugs and we recommend that future cohort studies stratify results by specific PI-regimens, including information regarding timing of ART initiation, to enable comparative analysis amongst PI-ART regimens. Accurate assessment of perinatal outcomes is paramount and should involve first trimester ultrasound to establish or confirm gestational age, accurate assessment of birthweight, and use of international standards to assess SGA and VSGA, to aid international comparisons.63,75 Furthermore, data on potential confounders should be collected and corrected for in adjusted analyses.
ART in pregnancy has clear benefits for maternal health, prevention of HIV transmission to the child, and prevention of horizontal HIV transmission. Choice of ART regimen for pregnant women should take into consideration viral load suppression, adverse drug effects and adherence, ART drug resistance, drug interactions, pharmacokinetics, comorbidities, drug cost and availability, dosing regimens, and safety and perinatal outcome data. Current WHO guidance recommends DTG-containing regimens as preferred first-line ART, including for women of childbearing potential and pregnant women. A retrospective cohort study from Botswana showed that perinatal outcomes were comparable between WLHIV receiving DTG-based and EFV-based ART.76,77 Recent randomised controlled trials of ART regimens initiated during pregnancy showed that DTG-based ART had superior virological efficacy compared to EFV-based ART, and that a regimen containing DTG, emtricitabine and tenofovir alafenamide fumarate had the lowest rate of adverse pregnancy outcomes.78,79 We found that PI-ART is associated with an increased risk of SGA and VSGA, but not PTB or other perinatal outcomes. However, no significant differences in perinatal outcomes were found between LPV/r, ATV/r, and DRV/r. PI drugs remain an important option for pregnant WLHIV if other regimens are contraindicated, for example due to drug resistance, or unavailable. WLHIV should be assisted in making an informed decision about the use of ART in pregnancy, taking account of all available evidence. More evidence is needed regarding the comparative safety and efficacy of different ART regimens in pregnancy and further efforts should be made to improve perinatal outcomes among pregnant WLHIV worldwide in order to make progress towards achieving Sustainable Development Goal target 3.2, particularly in sub-Saharan Africa.7
Contributors
IC and KB selected relevant studies, conducted the meta-analyses, subgroup and sensitivity analyses, interpreted the data and wrote the first draft of the manuscript. IC and KB contributed equally to this study. CP, HS, MK, and ZB screened the literature search results for relevant manuscripts and assessed their eligibility, verified and extracted data and conducted methodological quality assessments. SK designed and conducted the literature search. JH conceived, designed and coordinated the study, developed the systematic review protocol, assisted with the literature search, assessment of eligibility of manuscripts, data extraction, methodological quality assessment, designed the meta-analysis plan, interpreted the data and wrote the manuscript. All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication.
Data sharing statement
Study data are available on reasonable request to the corresponding author. The protocol for this review is available on the PROSPERO website (CRD42021248987).
Declaration of interests
We declare no competing interests.
Funding
None.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101368.
Appendix. Supplementary materials
References
- 1.UNAIDS. Global AIDS update 2021. 2021.
- 2.Wedi C.O., Kirtley S., Hopewell S., Corrigan R., Kennedy S.H., Hemelaar J. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. Lancet HIV. 2016;3(1):e33–e48. doi: 10.1016/S2352-3018(15)00207-6. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2015 Child Mortality Collaborators Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1725–1774. doi: 10.1016/S0140-6736(16)31575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L., Oza S., Hogan D., et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388(10063):3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee A.C., Kozuki N., Cousens S., et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21st standard: analysis of CHERG datasets. BMJ. 2017;358:j3677. doi: 10.1136/bmj.j3677. (Clinical research ed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee A.C., Katz J., Blencowe H., et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1(1):e26–e36. doi: 10.1016/S2214-109X(13)70006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GBD 2019 Under-5 Mortality Collaborators Global, regional, and national progress towards sustainable development goal 3.2 for neonatal and child health: all-cause and cause-specific mortality findings from the Global Burden of Disease Study 2019. Lancet. 2021;398(10303):870–905. doi: 10.1016/S0140-6736(21)01207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO . Recommendations for a Public Health Approach. World Health Organisation; Geneva: 2013. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. [PubMed] [Google Scholar]
- 9.UNAIDS. UNAIDS data. 2020.
- 10.WHO . Recommendations for a Public Health Approach. 2nd ed. World Health Organisation; Geneva: 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. [PubMed] [Google Scholar]
- 11.UNAIDS. Start Free, Stay Free, AIDS Free. 2019.
- 12.WHO. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach, 2021. [PubMed]
- 13.Panel on treatment of pregnant women with HIV infection and prevention of perinatal transmission - A Working Group of the Office of AIDS Research Advisory Council (OARAC). Recommendations for the use of antiretroviral drugs in pregnant women with HIV infection and interventions to reduce perinatal HIV transmission in the United States, 2021.
- 14.European AIDS Clinical Society. Treatment of pregnant women living with HIV or women considering pregnancy. EACS Guidelines 2021; v11.
- 15.BHIVA. British HIV Association guidelines for the management of HIV in pregnancy and postpartum 2018 (2020 third interim update). 2020. [DOI] [PubMed]
- 16.Tshivuila-Matala C.O.O., Honeyman S., Nesbitt C., Kirtley S., Kennedy S.H., Hemelaar J. Adverse perinatal outcomes associated with antiretroviral therapy regimens: systematic review and network meta-analysis. AIDS. 2020;34(11):1643–1656. doi: 10.1097/QAD.0000000000002593. [DOI] [PubMed] [Google Scholar]
- 17.Powis K.M., Kitch D., Ogwu A., et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. J Infect Dis. 2011;204(4):506–514. doi: 10.1093/infdis/jir307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey H. European pregnancy and paediatric HIV cohort collaboration (EPPICC) study group. Nucleoside reverse transcriptase inhibitor backbones and pregnancy outcomes. AIDS. 2019;33(2):295–304. doi: 10.1097/QAD.0000000000002039. [DOI] [PubMed] [Google Scholar]
- 19.Ezechi O.C., David A.N., Gab-Okafor C.V., et al. Incidence of and socio-biologic risk factors for spontaneous preterm birth in HIV positive Nigerian women. BMC Pregnancy Childbirth. 2012;12:93. doi: 10.1186/1471-2393-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favarato G., Townsend C.L., Bailey H., et al. Protease inhibitors and preterm delivery: another piece in the puzzle. AIDS. 2018;32(2):243–252. doi: 10.1097/QAD.0000000000001694. [DOI] [PubMed] [Google Scholar]
- 21.Floridia M., Dalzero S., Giacomet V., et al. Pregnancy and neonatal outcomes in women with HIV-1 exposed to integrase inhibitors, protease inhibitors and non-nucleoside reverse transcriptase inhibitors: an observational study. Infection. 2020;48(2):249–258. doi: 10.1007/s15010-019-01384-5. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro R.L., Hughes M.D., Ogwu A., et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362(24):2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Short C.E., Douglas M., Smith J.H., Taylor G.P. Preterm delivery risk in women initiating antiretroviral therapy to prevent HIV mother-to-child transmission. HIV Med. 2014;15(4):233–238. doi: 10.1111/hiv.12083. [DOI] [PubMed] [Google Scholar]
- 24.Szyld E.G., Warley E.M., Freimanis L., et al. Maternal antiretroviral drugs during pregnancy and infant low birth weight and preterm birth. AIDS. 2006;20(18):2345–2353. doi: 10.1097/01.aids.0000253362.01696.9d. [DOI] [PubMed] [Google Scholar]
- 25.van der Merwe K., Hoffman R., Black V., Chersich M., Coovadia A., Rees H. Birth outcomes in South African women receiving highly active antiretroviral therapy: a retrospective observational study. J Int AIDS Soc. 2011;14:42. doi: 10.1186/1758-2652-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zash R., Jacobson D.L., Diseko M., et al. Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr. 2017;171(10) doi: 10.1001/jamapediatrics.2017.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aaron E., Bonacquisti A., Mathew L., Alleyne G., Bamford L.P., Culhane J.F. Small-for-gestational-age births in pregnant women with HIV, due to severity of HIV disease, not antiretroviral therapy. Infect Dis Obstet Gynecol. 2012;2012 doi: 10.1155/2012/135030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albert A.Y.K., Elwood C., Wagner E.C., et al. Investigation of factors associated with spontaneous preterm birth in pregnant women living with HIV. AIDS. 2020;34(5):719–727. doi: 10.1097/QAD.0000000000002464. [DOI] [PubMed] [Google Scholar]
- 29.Boer K., Nellen J.F., Patel D., et al. The AmRo study: pregnancy outcome in HIV-1-infected women under effective highly active antiretroviral therapy and a policy of vaginal delivery. BJOG. 2007;114(2):148–155. doi: 10.1111/j.1471-0528.2006.01183.x. [DOI] [PubMed] [Google Scholar]
- 30.Carceller A., Ferreira E., Alloul S., Lapointe N. Lack of effect on prematurity, birth weight, and infant growth from exposure to protease inhibitors in utero and after birth. Pharmacotherapy. 2009;29(11):1289–1296. doi: 10.1592/phco.29.11.1289. [DOI] [PubMed] [Google Scholar]
- 31.Chen J.Y., Ribaudo H.J., Souda S., et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012;206(11):1695–1705. doi: 10.1093/infdis/jis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delicio A.M., Lajos G.J., Amaral E., Cavichiolli F., Polydoro M., Milanez H. Adverse effects in children exposed to maternal HIV and antiretroviral therapy during pregnancy in Brazil: a cohort study. Reprod Health. 2018;15(1):76. doi: 10.1186/s12978-018-0513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ejigu Y., Magnus J.H., Sundby J., MC Magnus. Pregnancy outcome among HIV-infected women on different antiretroviral therapies in Ethiopia: a cohort study. BMJ Open. 2019;9(8) doi: 10.1136/bmjopen-2018-027344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Favarato G., Townsend C.L., Peters H., et al. Stillbirth in women living with HIV delivering in the United Kingdom and Ireland: 2007–2015. J Acquir Immune Defic Syndr. 2019;82(1):9–16. doi: 10.1097/QAI.0000000000002087. [DOI] [PubMed] [Google Scholar]
- 35.Kowalska A., Niemiec T., El Midaoui A., Burkacka E. Effect of antiretroviral therapy on pregnancy outcome in HIV-1 positive women. Med Wieku Rozwoj. 2003;7(4 Pt 1):459–468. [PubMed] [Google Scholar]
- 36.López M., Palacio M., Goncé A., et al. Risk of intrauterine growth restriction among HIV-infected pregnant women: a cohort study. Eur J Clin Microbiol Infect Dis. 2015;34(2):223–230. doi: 10.1007/s10096-014-2224-6. [DOI] [PubMed] [Google Scholar]
- 37.Machado E.S., Hofer C.B., Costa T.T., et al. Pregnancy outcome in women infected with HIV-1 receiving combination antiretroviral therapy before versus after conception. Sex Transm Infect. 2009;85(2):82–87. doi: 10.1136/sti.2008.032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montgomery-Taylor S., Hemelaar J. Management and outcomes of pregnancies among women with HIV in Oxford, UK, in 2008–2012. Int J Gynaecol Obstet. 2015;130(1):59–63. doi: 10.1016/j.ijgo.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 39.Schulte J., Dominguez K., Sukalac T., Bohannon B., Fowler M.G., Pediatric Spectrum of HIV Disease Consortium Declines in low birth weight and preterm birth among infants who were born to HIV-infected women during an era of increased use of maternal antiretroviral drugs: pediatric spectrum of HIV disease, 1989–2004. Pediatrics. 2007;119(4):e900–e906. doi: 10.1542/peds.2006-1123. [DOI] [PubMed] [Google Scholar]
- 40.Sibiude J., Dialla O., Tubiana R., Blanche S., et al. Comparison of four classical PI- and raltegravir-based regimens during pregnancy. Top Antivir Med. 2018;26(1s):359s. [Google Scholar]
- 41.Snijdewind I.J.M., Smit C., Godfried M.H., et al. Preconception use of cART by HIV-positive pregnant women increases the risk of infants being born small for gestational age. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0191389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams S.F., Holland B., Bozdogan U., Alvarez J.R., Apuzzio J.J., Bardeguez A.D. Do protease inhibitors increase preterm births in human immunodeficiency virus-infected patients? Adv Infect Dis. 2013;03(03):172–176. [Google Scholar]
- 43.Watts D.H., Williams P.L., Kacanek D., et al. Combination antiretroviral use and preterm birth. J Infect Dis. 2013;207(4):612–621. doi: 10.1093/infdis/jis728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kourtis A.P., Schmid C.H., Jamieson D.J., Lau J. Use of antiretroviral therapy in pregnant HIV-infected women and the risk of premature delivery: a meta-analysis. AIDS. 2007;21(5):607–615. doi: 10.1097/QAD.0b013e32802ef2f6. [DOI] [PubMed] [Google Scholar]
- 45.Mesfin Y.M., Kibret K.T., Taye A. Is protease inhibitors based antiretroviral therapy during pregnancy associated with an increased risk of preterm birth? Systematic review and a meta-analysis. Reprod Health. 2016;13:30. doi: 10.1186/s12978-016-0149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veroniki A.A., Antony J., Straus S.E., et al. Comparative safety and effectiveness of perinatal antiretroviral therapies for HIV-infected women and their children: systematic review and network meta-analysis including different study designs. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saleska J.L., Turner A.N., Maierhofer C., Clark J., Kwiek J.J. Use of antiretroviral therapy during pregnancy and adverse birth outcomes among women living with HIV-1 in low- and middle-income countries: a systematic review. J Acquir Immune Defic Syndr. 2018;79(1):1–9. doi: 10.1097/QAI.0000000000001770. [DOI] [PubMed] [Google Scholar]
- 48.Alemu F.M., Yalew A.W., Fantahun M., Ashu E.E. Antiretroviral therapy and pregnancy outcomes in developing countries: a systematic review. Int J MCH AIDS. 2015;3(1):31–43. [PMC free article] [PubMed] [Google Scholar]
- 49.Pasley M.V., Martinez M., Hermes A., d'Amico R., Nilius A. Safety and efficacy of lopinavir/ritonavir during pregnancy: a systematic review. AIDS Rev. 2013;15(1):38–48. [PubMed] [Google Scholar]
- 50.Huang X., Xu Y., Yang Q., et al. Efficacy and biological safety of lopinavir/ritonavir based anti-retroviral therapy in HIV-1-infected patients: a meta-analysis of randomized controlled trials. Sci Rep. 2015;5:8528. doi: 10.1038/srep08528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higgins J.P.T., Thomas J., Editors . Cochrane Handbook For Systematic Reviews of Interventions. Wiley-Blackwell Publishing; Chichester: 2008. Cochrane Handbook For Systematic Reviews of Interventions. [Google Scholar]
- 52.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Floridia M., Ravizza M., Masuelli G., et al. Atazanavir and lopinavir profile in pregnant women with HIV: tolerability, activity and pregnancy outcomes in an observational national study. J Antimicrob Chemother. 2014;69(5):1377–1384. doi: 10.1093/jac/dkt497. [DOI] [PubMed] [Google Scholar]
- 54.Floridia M., Masuelli G., Ravizza M., et al. Atazanavir and darunavir in pregnant women with HIV: evaluation of laboratory and clinical outcomes from an observational national study. J Antimicrob Chemother. 2018;73(4):1025–1030. doi: 10.1093/jac/dkx478. [DOI] [PubMed] [Google Scholar]
- 55.Kakkar F., Boucoiran I., Lamarre V., et al. Risk factors for pre-term birth in a Canadian cohort of HIV-positive women: role of ritonavir boosting? J Int AIDS Soc. 2015;18:19933. doi: 10.7448/IAS.18.1.19933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perry M.E., Taylor G.P., Sabin C.A., et al. Lopinavir and atazanavir in pregnancy: comparable infant outcomes, virological efficacies and preterm delivery rates. HIV Med. 2016;17(1):28–35. doi: 10.1111/hiv.12277. [DOI] [PubMed] [Google Scholar]
- 57.Rough K., Seage G.R., Williams P.L., et al. Birth outcomes for pregnant women with HIV using Tenofovir-Emtricitabine. N Engl J Med. 2018;378(17):1593–1603. doi: 10.1056/NEJMoa1701666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sibiude J., Warszawski J., Tubiana R., et al. Premature delivery in HIV-infected women starting protease inhibitor therapy during pregnancy: role of the ritonavir boost? Clin Infect Dis. 2012;54(9):1348–1360. doi: 10.1093/cid/cis198. [DOI] [PubMed] [Google Scholar]
- 59.Smith C., Weinberg A., Forster J.E., et al. Maternal Lopinavir/ritonavir is associated with fewer adverse events in infants than Nelfinavir or Atazanavir. Infect Dis Obstet Gynecol. 2016;2016 doi: 10.1155/2016/9848041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Townsend C.L., Cortina-Borja M., Peckham C.S., Tookey P.A. Antiretroviral therapy and premature delivery in diagnosed HIV-infected women in the United Kingdom and Ireland. AIDS. 2007;21(8):1019–1026. doi: 10.1097/QAD.0b013e328133884b. [DOI] [PubMed] [Google Scholar]
- 61.Committee on Obstetric Practice, American Institute of Ultrasound in Medicine, Society for Maternal–Fetal Medicine Committee opinion No 700: methods for estimating the due date. Obstet Gynecol. 2017;129(5):e150–e1e4. doi: 10.1097/AOG.0000000000002046. [DOI] [PubMed] [Google Scholar]
- 62.Papageorghiou A.T., Ohuma E.O., Altman D.G., et al. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):869–879. doi: 10.1016/S0140-6736(14)61490-2. [DOI] [PubMed] [Google Scholar]
- 63.Villar J., Cheikh Ismail L., Victora C.G., et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 64.Uthman O.A., Nachega J.B., Anderson J., et al. Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta-analysis. Lancet HIV. 2017;4(1):e21–e30. doi: 10.1016/S2352-3018(16)30195-3. [DOI] [PubMed] [Google Scholar]
- 65.Stringer J.S., Stoner M.C., Kasaro M.P., Vwalika B., Cole S.R. Preconception ART and preterm birth: real effect or selection bias? Lancet HIV. 2017;4(4):e150. doi: 10.1016/S2352-3018(17)30046-2. [DOI] [PubMed] [Google Scholar]
- 66.Paiardini M., Müller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev. 2013;254(1):78–101. doi: 10.1111/imr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akoto C., Chan C., Tshivuila-Matala C., et al. Innate Lymphoid cells are reduced in pregnant HIV positive women and are associated with preterm birth. Front Immunol. 2020;10(1):13265. doi: 10.1038/s41598-020-69966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ravi K., Chan C.Y.S., Akoto C., et al. Changes in the Vα7.2+ CD161++ MAIT cell compartment in early pregnancy are associated with preterm birth in HIV-positive women. Am J Reprod Immunol. 2020;83(6):e13240. doi: 10.1111/aji.13240. [DOI] [PubMed] [Google Scholar]
- 69.Fiore S., Ferrazzi E., Newell M.L., Trabattoni D., Clerici M. Protease inhibitor-associated increased risk of preterm delivery is an immunological complication of therapy. J Infect Dis. 2007;195(6):914–916. doi: 10.1086/511983. [DOI] [PubMed] [Google Scholar]
- 70.Akoto C., Norris S.A., Hemelaar J. Maternal HIV infection is associated with distinct systemic cytokine profiles throughout pregnancy in South African women. Sci Rep. 2021;11(1):10079. doi: 10.1038/s41598-021-89551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dunk C.E., Serghides L. Protease inhibitor-based antiretroviral therapy in pregnancy: effects on hormones, placenta, and decidua. Lancet HIV. 2021;2(2):e120–e129. doi: 10.1016/S2352-3018(21)00249-6. [DOI] [PubMed] [Google Scholar]
- 72.Papp E., Balogun K., Banko N., et al. Low prolactin and high 20-alpha-hydroxysteroid dehydrogenase levels contribute to lower progesterone levels in HIV-infected pregnant women exposed to protease inhibitor-based combination antiretroviral therapy. J Infect Dis. 2016;213(10):1532–1540. doi: 10.1093/infdis/jiw004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Papp E., Mohammadi H., Loutfy M.R., et al. HIV protease inhibitor use during pregnancy is associated with decreased progesterone levels, suggesting a potential mechanism contributing to fetal growth restriction. J Infect Dis. 2015;211(1):10–18. doi: 10.1093/infdis/jiu393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Price J.T., Vwalika B., Freeman B.L., et al. Weekly 17 alpha-hydroxyprogesterone caproate to prevent preterm birth among women living with HIV: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2021;8(10):e605–ee13. doi: 10.1016/S2352-3018(21)00150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santosa W.B., Staines-Urias E., Tshivuila-Matala C.O.O., Norris S.A., Hemelaar J. Perinatal outcomes associated with maternal HIV and antiretroviral therapy in pregnancies with accurate gestational age in South Africa. AIDS. 2019;33(10):1623–1633. doi: 10.1097/QAD.0000000000002222. [DOI] [PubMed] [Google Scholar]
- 76.Zash R., Holmes L., Diseko M., et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med. 2019;381(9):827–840. doi: 10.1056/NEJMoa1905230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zash R., Jacobson D.L., Diseko M., et al. Comparative safety of dolutegravir-based or efavirenz-based antiretroviral treatment started during pregnancy in Botswana: an observational study. Lancet Glob Health. 2018;6(7):e804–ee10. doi: 10.1016/S2214-109X(18)30218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lockman S., Brummel S.S., Ziemba L., et al. Efficacy and safety of dolutegravir with emtricitabine and tenofovir alafenamide fumarate or tenofovir disoproxil fumarate, and efavirenz, emtricitabine, and tenofovir disoproxil fumarate HIV antiretroviral therapy regimens started in pregnancy (IMPAACT 2010/VESTED): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet. 2021;397(10281):1276–1292. doi: 10.1016/S0140-6736(21)00314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kintu K., Malaba T.R., Nakibuka J., et al. Dolutegravir versus efavirenz in women starting HIV therapy in late pregnancy (DolPHIN-2): an open-label, randomised controlled trial. Lancet HIV. 2020;7(5):e332–e3e9. doi: 10.1016/S2352-3018(20)30050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.