Abstract
To assess the potential for emergence of resistance during the use of linezolid, we tested 10 clinical isolates of vancomycin-resistant enterococci (VRE) (four Enterococcus faecalis, five Enterococcus faecium, and one Enterococcus gallinarum) as well as a vancomycin-susceptible control (ATCC 29212) strain of E. faecalis. The enterococci were exposed to doubling dilutions of linezolid for 12 passes. After the final passage, the linezolid plate growing VRE contained a higher drug concentration with E. faecalis than with E. faecium. DNA sequencing of the 23S rRNA genes revealed that linezolid resistance in three E. faecalis isolates was associated with a guanine to uracil transversion at bp 2576, while the one E. faecium isolate for which the MIC was 16 μg/ml contained a guanine to adenine transition at bp 2505.
Vancomycin-resistant enterococci (VRE) have emerged as important nosocomial pathogens in medical centers throughout the United States. Over the last decade, the incidence of VRE has been increasing, and currently nearly one-quarter of enterococci isolated from patients in intensive care units are vancomycin resistant (2, 9). In addition, these organisms are capable of prolonged survival on the hands of healthcare workers as well as on environmental surfaces commonly encountered in the healthcare setting (11).
Linezolid is a member of a new class of antibacterial agents called the oxazolidinones, which are chemically unrelated to currently available agents. This agent selectively binds to the 50S ribosomal subunit, thereby resulting in inhibition of bacterial protein synthesis (6). These compounds are unique in that they do not inhibit elongation (3, 4, 14) but instead inhibit the formation of the initiation complex constructed with 70S ribosomes, mRNA, initiation factors IF2 and IF3, and formylmethionyl-tRNA (15). Linezolid is highly active against gram-positive organisms, including VRE (10). The purposes of this study were to select for VRE resistant to linezolid in vitro and to investigate the stability of this resistance, if it could be developed.
We chose 10 clinical isolates of VRE, 5 of which were Enterococcus faecium (EF208, EF1347, EF1401, EF1509, and EF1644), 4 of which were Enterococcus faecalis (F118, F177, F217, and F317), and 1 of which was Enterococcus gallinarum (Z393). All were isolated from patients at Northwestern Memorial Hospital, Chicago, Ill. Vancomycin resistance (screened for by using a MIC of ≥6 μg/ml as recommended by the NCCLS) was determined by agar dilution (Difco Laboratories, Detroit, Mich.) testing according to the National Committee for Clinical Laboratory Standards (8). E. faecalis ATCC 29212 was tested as a vancomycin-susceptible control. All isolates were initially tested for their baseline activity against linezolid (Pharmacia Corp., Kalamazoo, Mich.) according to a standard tube dilution method (8). Susceptibility testing was performed using doubling dilutions ranging from 0.25 to 8 μg/ml. The MIC was defined as the lowest concentration of the antibiotic that exhibited no visible turbidity. The susceptibility breakpoint for linezolid is ≤4 μg/ml.
The basic approach used to select for resistance utilized serial passage on successively higher concentrations, similar to the methods previously applied to the selection of E. faecium highly resistant to quinupristin-dalfopristin (7). To examine the stability of resistance, the isolates were subcultured to drug-free agar twice weekly for one month and then tested by the agar dilution method (8) to determine the final linezolid MIC.
Cultures were grown overnight in 50 ml of brain heart infusion broth at 37°C. Chromosomal DNA was isolated as described by Baele and colleagues (1), except that the cell lysate was extracted twice with phenol-chloroform-isoamyl alcohol (24:25:1) before the DNA was precipitated with ethanol. PCR was then carried out using the primers 5′-GACGGAAAGACCCCATGG-3′ and 5′-ACACTTAGATGCTTT-3′ corresponding to bp 2049 to 2767. Amplification was carried out using Taq DNA polymerase (Gibco) according to the manufacturer's directions.
PCR products were sequenced directly by using an ABI377 fluorescence sequencer (Perkin-Elmer Applied Biosystems, Foster City, Calif.) and the ABI BigDye Terminator Cycle Sequencing Ready Reaction kit with AmpliTaq FS DNA polymerase (Perkin-Elmer Applied Biosystems).
A summary of the results of this investigation is provided in Table 1. The initial linezolid MIC, tested by broth dilution, for all VRE and the ATCC 29212 strain was 1 μg/ml. Selection of linezolid-resistant mutants occurred for all four of the vancomycin-resistant E. faecalis strains and the vancomycin-susceptible control strain. The final MIC for one of the vancomycin-resistant E. faecium strains was 16 μg/ml, while the final MICs for the remaining E. faecium isolates and the E. gallinarum isolate were ≤8 μg/ml, despite multiple attempts to select for resistant mutants. Two of these isolates (EF 1401 and EF 1509) were from patients who were previously treated with linezolid for VRE bacteremia. In neither of these strains were we able to select for resistance despite prior clinical exposure to this antimicrobial agent.
TABLE 1.
Results of in vitro selection of linezolid-resistant enterococci
| Strain | MIC (μg/ml)a for strain at pass:
|
Antibiotic-free MIC (μg/ml)b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| E. faecalis | |||||||||||||
| F118 | 1 | 2 | 8 | 16 | 16 | 16 | 16 | 32 | 16 | 16 | 32 | 32 | 32 |
| F177 | 1 | 1 | 2 | 2 | 2 | 4 | 4 | 16 | 32 | 32 | 64 | 64 | 64 |
| F217 | 1 | 2 | 8 | 16 | 32 | 32 | 32 | 64 | 64 | 128 | 128 | 128 | 128 |
| F317 | 1 | 1 | 2 | 4 | 16 | 16 | 16 | 32 | 32 | 32 | 32 | 32 | 16 |
| ATCC 29212 | 1 | 1 | 2 | 8 | 16 | 32 | 32 | 64 | 64 | 128 | 128 | 128 | 128 |
| E. gallinarum | |||||||||||||
| Z393 | 1 | 1 | 2 | 2 | 4 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| E. faecium | |||||||||||||
| EF208 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 4 | 4 | 4 | 8 | 8 | 8 |
| EF1347 | 1 | 1 | 2 | 2 | 4 | 4 | 4 | 4 | 4 | 8 | 8 | 8 | 8 |
| EF1401 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| EF1509 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| EF1644 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 4 | 8 | 16 | 16 | 16 |
MIC by broth dilution.
MIC by agar dilution following one month of growth on antibiotic-free media.
Interestingly, the vancomycin-resistant E. faecalis strains developed resistance to linezolid more rapidly and to a greater extent than did E. faecium strains. For E. faecalis, the range for the final-pass MICs was 32 to 128 μg/ml; however, for E. faecium, the range was 2 to 16 μg/ml. Selection of linezolid-resistant mutants occurred for all strains of E. faecalis tested, but at various rates; the MICs developed for three of the four strains by passage 5 were ≥16 μg/ml.
For only one isolate (F317) was there a change in the final linezolid MIC following growth on drug-free media for one month, and this represented a twofold change from the final-pass MIC after one month of growth on drug-free media.
Resistance to linezolid is uncommon based on in vitro results as well as those from clinical trials. A relatively low spontaneous mutation frequency has been observed with linezolid and is consistent with the results obtained with other oxazolidinones (4, 12). Previous investigations with staphylococci failed to identify spontaneous mutants at twice the MIC and higher, resulting in a calculated spontaneous mutational frequency of less than 10−9 to 10−11 (5, 16). In addition, serial passage of both enterococci and staphylococci on gradient plates has failed to produce a high frequency of resistant mutants (16).
Our results suggest that it is more difficult to select for enterococci resistant to linezolid than to quinupristin-dalfopristin (7). For E. faecalis, we were able to select for resistance in all four of the isolates, which began to develop after three passes. In contrast, for vancomycin-resistant E. faecium, resistance did not begin to develop until at least 10 passes. When similar selection studies were performed with quinupristin-dalfopristin, high-level resistance developed very rapidly with all isolates tested (7). The data also suggest that when significant resistance (above the MIC breakpoint, 4 μg/ml) develops, it is stable, as it was with experiments performed with quinupristin-dalfopristin.
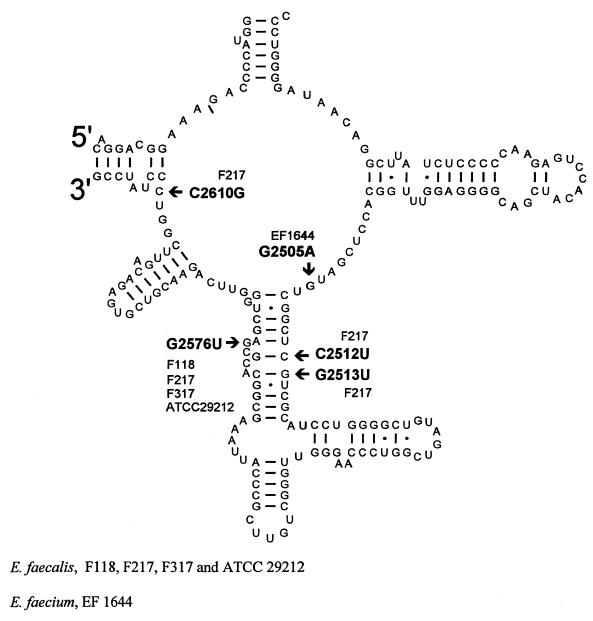
PCR amplification of the 23S rRNA gene region corresponding to the peptidyl transferase site revealed that the linezolid-resistant E. faecalis isolates F118, F217, F317, and ATCC 29212 all had a guanine to uracil conversion at bp 2576 (Fig. 1). This G2576U mutation had been previously described for two E. faecium isolates obtained from two separate patients who developed resistance to linezolid while on therapy (G. E. Zurenko, W. M. Todd, B. Hafkin, B. Meyers, C. Kauffman, A. Bock, J. Slightom, and D. Shinabarger, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-848, 1999). Both of these patients received prolonged courses of linezolid for VRE bacteremia with infected intravascular devices that could not be removed. In addition, G2576U has occurred in both E. faecalis and S. aureus selected for resistance to the oxazolidinone eperezolid in the laboratory (13; S. M. Swaney, D. L. Shinabarger, R. D. Schaadt, J. H. Bock, J. L. Slightom, and G. E. Zurenko, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-104, 1998). E. faecalis F217 was particularly unusual in that it contained three mutations (C2512U, G2513U, and C2610G) in addition to G2576U. The contribution of these three unusual mutations to the level of linezolid resistance is unknown since the MIC for F217 and ATCC 29212 was the same.
FIG. 1.
Location of linezolid-resistant mutations in the enterococcal 23S rRNA peptidyl transferase region.
In order to determine whether linezolid-resistant E. faecium isolates contained 23S rRNA mutations, EF1644 was sequenced because it exhibited the highest level of resistance (16 μg/ml) compared to the other four E. faecium isolates from this study. Interestingly, EF1644 was found to contain a new mutation at G2505A not previously found in oxazolidinone-resistant bacteria. DNA sequencing of E. gallinarum Z393 (MIC for the strain, 8 μg/ml) failed to detect any mutations in the peptidyl transferase region. We are continuing to sequence the genes which encode 23S and 16S rRNAs, as well as the genes which encode ribosomal proteins, in Z393 in order to determine whether a mutation is indeed present.
Overall, our investigation demonstrates the potential for development of resistance to linezolid among vancomycin-resistant E. faecalis strains; however, there was difficulty in selecting for resistance in vancomycin-resistant E. faecium isolates. In addition, these results suggest that the mechanism of resistance for E. faecalis and E. faecium may be different. Our data suggest that linezolid has a finite but low propensity for the selection of resistance in vancomycin-resistant E. faecium strains.
Acknowledgments
This work was supported in part by the Medical School Research Grant Program, Northwestern University Medical School, and Pharmacia.
We thank C. Bannigan and J. Slightom for DNA sequencing.
REFERENCES
- 1.Baele M, Baele P, Vaneechoutte M, Storms V, Butaye P, Devriese L A, Verschraegen G, Gillis M, Haesebrouck F. Application of tRNA intergenic spacer PCR for identification of enterococcus species. J Clin Microbiol. 2000;38:4201–4207. doi: 10.1128/jcm.38.11.4201-4207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Nosocomial Infection Surveillance (NNIS) system report, data summary from January 1990–May 1999, issued June 1999. Am J Infect Control. 1999;27:520–532. doi: 10.1016/s0196-6553(99)70031-3. [DOI] [PubMed] [Google Scholar]
- 3.Daly J S, Eliopoulos G M, Reiszner E, Moellering R C., Jr Activity and mechanism of action of DuP 105 and DuP 721, new oxazolidinone compounds. J Antimicrob Chemother. 1988;21:721–730. doi: 10.1093/jac/21.6.721. [DOI] [PubMed] [Google Scholar]
- 4.Daly J S, Eliopoulos G M, Willey S, Moellering R C., Jr Mechanism of action and in vitro and in vivo activities of S-6123, a new oxazolidinone compound. Antimicrob Agents Chemother. 1988;32:1341–1346. doi: 10.1128/aac.32.9.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaatz G W, Seo S M. In vitro activities of oxazolidinone compounds U-100592 and U-100766 against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1996;40:799–801. doi: 10.1128/aac.40.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin, A. H., R. W. Murray, T. J. Vidmar, and K. R. Marotti. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob. Agents Chemother. 41:2127–2131. [DOI] [PMC free article] [PubMed]
- 7.Millichap J, Ristow T A, Noskin G A, Peterson L R. Selection of Enterococcus faecium strains with stable and unstable resistance to the streptogramin RP 59500 using step-wise in vitro exposure. Diagn Microbiol Infect Dis. 1996;25:15–20. doi: 10.1016/0732-8893(96)00067-3. [DOI] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard. M7–A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 9.Noskin G A. Vancomycin-resistant enterococci: clinical, microbiologic, and epidemiologic features. J Lab Clin Med. 1997;130:14–20. doi: 10.1016/s0022-2143(97)90054-8. [DOI] [PubMed] [Google Scholar]
- 10.Noskin G A, Siddiqui F, Stosor V, Hacek D, Peterson L R. In vitro activity of linezolid against important gram-positive bacterial pathogens including vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1999;43:2059–2062. doi: 10.1128/aac.43.8.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noskin G A, Stosor V, Cooper I, Peterson L R. Recovery of vancomycin resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol. 1995;16:577–581. doi: 10.1086/647011. [DOI] [PubMed] [Google Scholar]
- 12.Schaadt R D, Batts D H, Daley-Yates P T, Pawsey S D, Stalker D J, Zurenko G E. Serum inhibitory titers and serum bactericidal titers for human subjects receiving multiple doses of the antibacterial oxazolidinones eperezolid and linezolid. Diagn Microbiol Infect Dis. 1997;28:201–204. doi: 10.1016/s0732-8893(97)00071-0. [DOI] [PubMed] [Google Scholar]
- 13.Shinabarger D. Mechanism of action of the oxazolidinone antibacterial agents. Exp Opin Investig Drugs. 1999;8:1195–1202. doi: 10.1517/13543784.8.8.1195. [DOI] [PubMed] [Google Scholar]
- 14.Shinabarger D L, Marotti K R, Murray R W, Lin A H, Melchior E P, Swaney S M, Dunyak D S, Demyan W F, Buysse J M. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob Agents Chemother. 1997;41:2132–2136. doi: 10.1128/aac.41.10.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swaney S M, Aoki H, Ganoza M C, Shinabarger D L. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob Agents Chemother. 1998;42:3251–3255. doi: 10.1128/aac.42.12.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zurenko G E, Yagi B H, Schaadt R D, Allison J W, Kilburn J O, Glickman S E, Hutchinson D K, Barbachyn M R, Brickner S J. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob Agents Chemother. 1996;40:839–845. doi: 10.1128/aac.40.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]