Abstract
A novel and efficient copper-catalyzed synthesis of dihydro-6H-indolo[2,3-b]quinoline derivatives has been developed by using 3-alkyl-1-alkylindolin-2-imine hydrochlorides as the building blocks. Furthermore, easy reduction of dihydro-6H-indolo[2,3-b]quinolines with diisobutylaluminum hydride provided tetrahydro-6H-indolo[2,3-b]quinoline derivatives in excellent yields. The present method shows some advantages including use of cheap cuprous chloride as the catalyst and tolerance of wide functional groups.
A novel and efficient copper-catalyzed synthesis of dihydro-6H-indolo[2,3-b]quinoline derivatives has been developed by using 3-alkyl-1-alkylindolin-2-imine hydrochlorides as the building blocks.
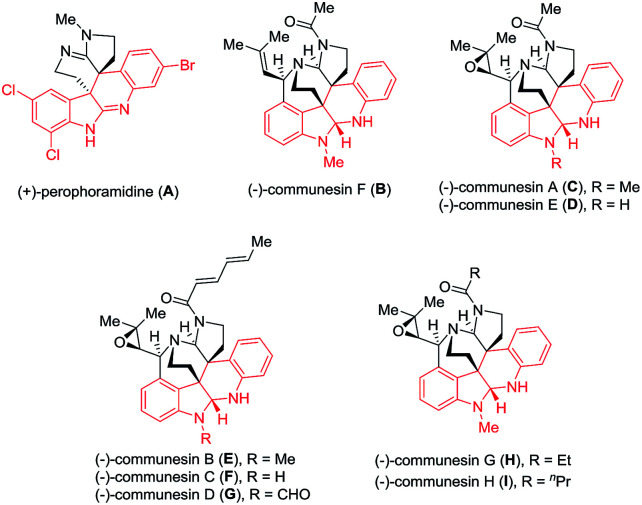
Indole alkaloids widely occur in nature and exhibit diverse and interesting biological and pharmacological activities.1 For example, perophoramidine (A) and communesins (B–I), isolated from Penicillium species,2 a marine fungal strain, and Philippine ascidian Perophora namei,3 show important cytotoxicity and insecticidal properties (Fig. 1). Both intriguing structural complexity and interesting biological activities of these alkaloids attract much attention for organic synthetic chemists.4
Fig. 1. Structures of representative perophoramidine and communesin alkaloids with diverse biological activities.
In previous synthesis of indole alkaloids, dearomatization of readily available indoles is often used in the construction of complex indole-containing structural motifs.5 In particular, dearomatizing C3-alkylation/arylation of 3-substituted indoles first provides C3-quaternary indolenines, and then the indolenines are used as the versatile building blocks for the synthesis of complex indole alkaloids and related compounds.6 However, this strategy often needs long multi-step and tedious processes. As an alternative, dearomatizing alkylation of tryptamine derivatives yields the C3-quaternary indolenines followed spontaneous cyclization to afford pyrroloindolines (Scheme 1a).7 To the best of our knowledge, 1-alkyl-3-alkylindolin-2-imine hydrochlorides as a kind of indole derivatives have not been used in synthesis of indole alkaloids thus far. Herein, we report application of 1-alkyl-3-alkylindolin-2-imine hydrochlorides as the useful building blocks in copper-catalyzed synthesis of polycyclic indoline scaffolds (Scheme 1b).
Scheme 1. Synthesis of polycyclic indoline scaffolds using tryptamine derivatives (a) or 1-alkyl-3-alkylindolin-2-imine hydrochlorides (b) as the useful building blocks.
At the outset, copper-catalyzed reaction of 3-benzyl-1-methylindolin-2-imine hydrochloride (1h) with 2-iodobenzyl bromide (2a) leading to 10b-benzyl-6-methyl-10b,11-dihydro-6H-indolo[2,3-b]quinoline (3h) was selected as the model to optimize conditions including catalysts, base, solvents and temperature. As shown in Table 1, six catalysts, CuI, CuBr, CuCl, Cu2O, Cu(OAc)2 and Cu(TFA)2, were tested using NaOBut as the base and HOBut as the solvent under nitrogen atmosphere at 100 °C for 20 h (entries 1–6), and CuCl gave the highest yield (90%) (entry 3). Subsequently, other four solvents, MeCN, HOPri, toluene and 1,4-dioxane, were attempted (entries 7–10), and they were inferior to HOBut (compare entries 3, 7–10). Next, effect of bases including LiOBut, K2CO3, Cs2CO3, K3PO4, NaOAc and diisopropylethylamine (DIPEA) was investigated (entries 11–16), and the results showed that K2CO3 was a suitable base (entry 12). Finally, we attempted variation of temperature and found that 100 °C was an optimal temperature (compare entries 12, 17 and 18). Therefore, the copper-catalyzed optimal conditions for synthesis of 10b-benzyl-6-methyl-10b,11-dihydro-6H-indolo[2,3-b]quinoline are as follows: 10 mol% CuCl as the catalyst, K2CO3 as the base, and HOBut as the solvent under nitrogen atmosphere at 100 °C for 20 h.
Optimization of conditions for copper-catalyzed reaction of 3-benzyl-1-methylindolin-2-imine hydrochloride (1h) with 2-iodobenzyl bromide (2a) leading to 10b-benzyl-6-methyl-10b,11-dihydro-6H-indolo[2,3-b]quinoline (3h)a.

| ||||
|---|---|---|---|---|
| Entry | Cat. | Base | Solvent | Yieldb (%) |
| 1 | CuI | t BuONa | t BuOH | 73 |
| 2 | CuBr | t BuONa | t BuOH | 78 |
| 3 | CuCl | t BuONa | t BuOH | 90 |
| 4 | Cu2O | t BuONa | t BuOH | 75 |
| 5 | Cu(OAc)2 | t BuONa | t BuOH | 83 |
| 6 | Cu(TFA)2 | t BuONa | t BuOH | 81 |
| 7 | CuCl | t BuONa | CH3CN | 46 |
| 8 | CuCl | t BuONa | iPrOH | 81 |
| 9 | CuCl | t BuONa | Toluene | 42 |
| 10 | CuCl | t BuONa | 1,4-Dioxane | 38 |
| 11 | CuCl | t BuOLi | t BuOH | 89 |
| 12 | CuCl | K 2 CO 3 | t BuOH | 91 |
| 13 | CuCl | Cs2CO3 | t BuOH | 88 |
| 14 | CuCl | K3PO4 | t BuOH | 86 |
| 15 | CuCl | NaOAc | t BuOH | 43 |
| 16 | CuCl | DIPEA | t BuOH | Trace |
| 17c | CuCl | K2CO3 | t BuOH | 80 |
| 18d | CuCl | K2CO3 | t BuOH | 91 |
Reaction conditions: under nitrogen atmosphere, 3-benzyl-1-methylindolin-2-imine hydrochloride (1h) (0.33 mmol, 1.1 equiv.), 2-iodobenzyl bromide (2a) (0.3 mmol, 1.0 equiv.), catalyst (30 μmol, 10 mol%), base (1.2 mmol, 4.0 equiv.), solvent (3.0 mL), temperature (100 °C), time (20 h) in a sealed Schlenk tube.
Isolated yield.
Temperature (80 °C).
Temperature (120 °C).
After obtaining the optimized conditions, we surveyed substrate scope for the copper-catalyzed reactions of 1-alkyl-3-alkylindolin-2-imine hydrochlorides (1) with substituted 2-iodobenzyl bromides (2) leading to dihydro-6H-indolo[2,3-b]quinolines (3). As shown in Table 2, we first surveyed reactivity of substrates (1) using 2-iodobenzyl bromide (2a) as the partner. When substituents R3 in 1 were aliphatic alkyls (see 3a–3e), substituted benzyls (see 3f–3l), substituted phenylethyls (see 3m–3o), phenylpropyl (see 3p) and phenyl (see 3q), and the reactions were performed well. Subsequently, variation of substituent groups R2 including ethyl (see 3r), propyl (see 3s), butyl (see 3t), allyl (see 3u), benzyl (see 3v) and phenylpropyl (see 3w) in 1 was investigated, and the substrates provided the corresponding target products (3r–3w) in 82–92%. Next, several substrates 1 containing different R1 substituents including electron-donating (see 3x–3ab), poor electron-withdrawing (see 3ac–3ae), strong electron-withdrawing (see 3af–3ah) groups were tested, and they afforded 3x–3ah in 80–90%. Finally, several substituted 2-iodobenzyl bromide (2) were applied with 3-benzyl-1-methylindolin-2-imine hydrochloride (1h) as the partner, and the target products (3ai–3an) were obtained in high yields. The copper-catalyzed reactions showed tolerance of various functional groups including C–F (see 3i, 3ac, 3aj and 3ak), C–Cl (see 3j, 3ad and 3al), C–Br (see 3k, 3o, 3ae and 3am) bonds, ether (see 3f, 3m, 3ab and 3ai), trifluoromethyl (see 3l and 3af), cyano (see 3ag) and nitro (see 3ah and 3an) groups.
Substrate scope for copper-catalyzed synthesis of dihydro-6H-indolo[2,3-b]quinolines (3)a.

|
|---|
| 3 (time, yieldb) |

|
Reaction conditions: under nitrogen atmosphere, 3-alkyl-1-alkylindolin-2-imine hydrochloride (1) (0.33 mmol, 1.1 equiv.), substituted 2-iodobenzyl bromide (2) (0.3 mmol, 1.0 equiv.), CuCl (30 μmol, 10 mol%), K2CO3 (1.2 mmol, 4.0 equiv.), tBuOH (3.0 mL), temperature (100 °C), time (20 h) in a sealed Schlenk tube.
Isolated yield.
To explore mechanism on the copper-catalyzed reactions of 1 with 2, two control experiments were carried out as follows: (a) reaction of 3-benzyl-1-methylindolin-2-imine hydrochloride (1h) with 2-iodobenzyl bromide (2a) produced 4 in 95% yield in the absence of copper catalyst at room temperature (Scheme 2a). (b) Copper-catalyzed intramolecular N-arylation of 4 gave the target product (3h) in 93% yield under the standard conditions (Scheme 2b). According to the results above, the copper-catalyzed reaction mechanism is proposed in Scheme 3.8 First, 1 transforms into anion I in the presence of base (K2CO3), and nucleophilic attack of I to 2 yields II. Coordination of CuCl with nitrogen in imine group of II provides III, and oxidative addition of III forms IV in the presence of base. Finally, reductive elimination of IV gives the target product (3) freeing copper catalyst.
Scheme 2. (a) Reaction of 3-benzyl-1-methylindolin-2-imine hydrochloride (1g) with 2-iodobenzyl bromide (2a) in the absence of copper catalyst leading to 4. (b) Copper-catalyzed intramolecular cyclization of 4 under the standard conditions.
Scheme 3. Reaction mechanism for the copper-catalyzed synthesis of dihydro-6H-indolo[2,3-b]quinolines (3).
Furthermore, easy reduction of dihydro-6H-indolo[2,3-b]quinolines (3) with diisobutylaluminum hydride (DIBAL-H) in toluene at 0 °C led to another kind of N-heterocycles, tetrahydro-6H-indolo[2,3-b]quinolines (5a–5f) with wide biological activities2,3 (Scheme 4). However, the traditional methods for synthesis of this kind of compounds need long multi-step processes by using common indoles as the starting materials. Therefore, the present method using 3-alkyl-1-alkylindolin-2-imine hydrochlorides as the building blocks is very simple and practical strategy for construction of dihydro-6H-indolo[2,3-b]quinoline and tetrahydro-6H-indolo[2,3-b]quinoline derivatives.
Scheme 4. Reduction of dihydro-6H-indolo[2,3-b]quinolines (3) with DIBAL-H leading to tetrahydro-6H-indolo[2,3-b]quinolines (5).
In summary, we have developed a novel and efficient copper-catalyzed synthesis of dihydro-6H-indolo[2,3-b]quinoline derivatives by using 3-alkyl-1-alkylindolin-2-imine hydrochlorides as the building blocks. Furthermore, easy reduction of dihydro-6H-indolo[2,3-b]quinolines with DIBAL-H provided tetrahydro-6H-indolo[2,3-b]quinolines. The present method shows some advantages including use of cheap CuCl as the catalyst, and tolerance of wide functional groups. We believe that 3-alkyl-1-alkylindolin-2-imine hydrochlorides as the building blocks will find wide application in synthesis of complex polycyclic indoline scaffolds.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We thank the National Natural Science Foundation of China (Grant No. 21772108) for financial support.
Electronic supplementary information (ESI) available: Synthetic procedures, characterization data and 1H, 13C, 19F NMR spectra of these synthesized compounds. See DOI: 10.1039/c9ra00995g
Notes and references
- (a) Anthoni U., Christophersen C. and Nielsen P. H., in Alkaloids: Chemical and Biological Perspectives, ed. S. W.Pelletier, Wiley, New York, 1999, vol. 13, p. 163 [Google Scholar]; (b) Pearce H. L., in The Alkaloids, ed. A.Brossi and M.Suffness, Academic, San Diego, CA, 1990, vol. 37, p. 145 [Google Scholar]
- (a) Dalsgaard P. W. Blunt J. W. Munro M. H. G. Frisvad J. C. Christophersen C. J. Nat. Prod. 2005;68:258. doi: 10.1021/np049646l. [DOI] [PubMed] [Google Scholar]; (b) Andersen B. Smedsgaard J. Frisvad J. C. J. Agric. Food Chem. 2004;52:2421. doi: 10.1021/jf035406k. [DOI] [PubMed] [Google Scholar]; (c) Hayashi H. Matsumoto H. Akiyama K. Biosci., Biotechnol., Biochem. 2004;68:753. doi: 10.1271/bbb.68.753. [DOI] [PubMed] [Google Scholar]; (d) Jadulco R. Edrada R. A. Ebel R. Berg A. Schaumann K. Wray V. Steube K. Proksch P. J. Nat. Prod. 2004;67:78. doi: 10.1021/np030271y. [DOI] [PubMed] [Google Scholar]; (e) Numata A. Takahashi C. Ito Y. Takada T. Kawai K. Usami Y. Matsumura E. Imachi M. Ito T. Hasegawa T. Tetrahedron Lett. 1993;34:2355. doi: 10.1016/S0040-4039(00)77612-X. [DOI] [Google Scholar]
- Verbitski S. M. Mayne C. L. Davis R. A. Concepcion G. P. Ireland C. M. J. Org. Chem. 2002;67:7124. doi: 10.1021/jo026012f. [DOI] [PubMed] [Google Scholar]
- For a recent review, see: ; (a1) Trost B. M. Osipov M. Chem. –Eur. J. 2015;21:16318. doi: 10.1002/chem.201501735. [DOI] [PMC free article] [PubMed] [Google Scholar]; ; For selected papers, see:; (b) Yang J. Wu H. Shen Y. Qin Y. J. Am. Chem. Soc. 2007;129:13794. doi: 10.1021/ja075705g. [DOI] [PubMed] [Google Scholar]; (c) Liu P. Seo J. H. Weinreb S. M. Angew. Chem., Int. Ed. 2010;49:2000. doi: 10.1002/anie.200906818. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Seo J. H. Artman III G. D. Weinreb S. M. J. Org. Chem. 2006;71:8891. doi: 10.1021/jo061660a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Belmar J. Funk R. L. J. Am. Chem. Soc. 2012;134:16941. doi: 10.1021/ja307277w. [DOI] [PubMed] [Google Scholar]; (f) Fuchs J. R. Funk R. L. J. Am. Chem. Soc. 2004;126:5068. doi: 10.1021/ja049569g. [DOI] [PubMed] [Google Scholar]; (g) Han S.-J. Vogt F. Krishnan S. May J. A. Gatti M. Virgil S. C. Stoltz B. M. Org. Lett. 2014;16:3316. doi: 10.1021/ol5013263. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Han S.-J. Vogt F. May J. A. Krishnan S. Gatti M. Virgil S. C. Stoltz B. M. J. Org. Chem. 2015;80:528. doi: 10.1021/jo502534g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Zuo Z. Xie W. Ma D. J. Am. Chem. Soc. 2010;132:13226. doi: 10.1021/ja106739g. [DOI] [PubMed] [Google Scholar]; (j) Zuo Z. Ma D. Angew. Chem., Int. Ed. 2011;50:12008. doi: 10.1002/anie.201106205. [DOI] [PubMed] [Google Scholar]; (k) Sabahi A. Novikov A. Rainier J. D. Angew. Chem., Int. Ed. 2006;45:4317. doi: 10.1002/anie.200601278. [DOI] [PubMed] [Google Scholar]
- For reviews of dearomatization reactions, see: ; (a) Roche S. P. Porco Jr J. A. Angew. Chem., Int. Ed. 2011;50:4068. doi: 10.1002/anie.201006017. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhuo C.-X. Zhang W. You S.-L. Angew. Chem., Int. Ed. 2012;51:12662. doi: 10.1002/anie.201204822. [DOI] [PubMed] [Google Scholar]
- For selected examples, see: ; (a) Robinson R. Suginome H. J. Chem. Soc. 1932:298. doi: 10.1039/JR9320000298. [DOI] [Google Scholar]; (b) Woodward R. B. Cava M. P. Ollis W. D. Hunger A. Daeniker H. U. Schenker K. J. Am. Chem. Soc. 1954;76:4749. doi: 10.1021/ja01647a088. [DOI] [Google Scholar]; (c) Stork G. Dolfini J. E. J. Am. Chem. Soc. 1963;85:2872. doi: 10.1021/ja00901a061. [DOI] [Google Scholar]; (d) He F. Bo Y. Altom J. D. Corey E. J. J. Am. Chem. Soc. 1999;121:6771. doi: 10.1021/ja9915201. [DOI] [Google Scholar]; (e) Kozmin S. A. Iwama T. Huang Y. Rawal V. H. J. Am. Chem. Soc. 2002;124:4628. doi: 10.1021/ja017863s. [DOI] [PubMed] [Google Scholar]
- For a recent review, see: ; (a1) Ruiz-Sanchis P. Savina S. A. Albericio F. Álvarez M. Chem.–Eur. J. 2011;17:1388. doi: 10.1002/chem.201001451. [DOI] [PubMed] [Google Scholar]; ; For selected papers, see:; (b) Kawahara M. Nishida A. Nakagawa M. Org. Lett. 2000;2:675. doi: 10.1021/ol005513p. [DOI] [PubMed] [Google Scholar]; (c) Tan G. H. Zhu X. Ganesan A. Org. Lett. 2003;5:1801. doi: 10.1021/ol034516+. [DOI] [PubMed] [Google Scholar]; (d) Austin J. F. Kim S.-G. Sinz C. J. Xiao W.-J. MacMillan D. W. C. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5482. doi: 10.1073/pnas.0308177101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected reviews on copper-catalyzed reactions, see: ; (a) Ley S. V. Thomas A. W. Angew. Chem., Int. Ed. 2003;42:5400. doi: 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]; (b) Beletskaya I. P. Cheprakov A. V. Coord. Chem. Rev. 2004;248:2337. doi: 10.1016/j.ccr.2004.09.014. [DOI] [Google Scholar]; (c) Evano G. Blanchard N. Toumi M. Chem. Rev. 2008;108:3054. doi: 10.1021/cr8002505. [DOI] [PubMed] [Google Scholar]; (d) Ma D. Cai Q. Acc. Chem. Res. 2008;41:1450. doi: 10.1021/ar8000298. [DOI] [PubMed] [Google Scholar]; (e) Monnier F. Taillefer M. Angew. Chem., Int. Ed. 2009;48:6954. doi: 10.1002/anie.200804497. [DOI] [PubMed] [Google Scholar]; (f) Surry D. S. Buchwald S. L. Chem. Sci. 2010;1:13. doi: 10.1039/C0SC00107D. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Rao H. Fu H. Synlett. 2011:745. [Google Scholar]; (h1) Liu T. Fu H. Synthesis. 2012;44:2805. doi: 10.1055/s-0032-1316763. [DOI] [Google Scholar]; and references cited therein
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.