Abstract
Responding quickly to HIV outbreaks is one of four pillars of the United States Ending the HIV Epidemic initiative (EHE). Inclusion of cluster detection and response (CDR) in the fourth pillar of EHE has led to public discussion concerning bioethical implications of CDR and molecular HIV surveillance (MHS) among public health authorities, researchers, and community members. This study reports on findings from a qualitative analysis of interviews with community members and providers regarding their knowledge and perspectives of MHS. We identified five key themes: 1) context matters, 2) making sense of MHS, 3) messaging, equity, and resource prioritization, 4) operationalizing confidentiality, and 5) stigma, vulnerability, and power. Inclusion of community perspectives in generating innovative approaches that address bioethical concerns related to the use of MHS data is integral to ensure that widely accessible information about the use of these data is available to a diversity of community members and providers.
Keywords: cluster detection and response, consent for use of biospecimens, molecular HIV surveillance, thematic analysis
HIV genetic sequence data allow health departments to rapidly analyze and identify molecular HIV clusters (e.g., HIV-TRACE; Kosakovsky Pond et al., 2018). The widespread availability of this technology, coupled with the Centers for Disease Control and Prevention’s (CDC) mandate to utilize molecular HIV surveillance (MHS) as a means for identifying emerging HIV transmission clusters, creates an opportunity for health departments to target HIV prevention efforts in a new way. HIV molecular epidemiology—the use of genetic data to understand epidemiology in the context of HIV—has been used previously to identify social and demographic factors implicated in emerging outbreaks among transmission networks, or clusters (Mehta et al., 2018, 2015; Poon et al., 2016; Stecher et al., 2018). Genetic distance analyses provide a means for comparing HIV genetic sequences from different people with HIV to analyze the evolutionary relationships among the sequences (Dawson et al., 2020). Cluster detection and response (CDR) is highlighted as one pillar of the national Ending the HIV Epidemic (EHE) initiative (Fauci et al., 2019). Linkages among individuals in a “molecular cluster” are determined by genetic similarity (sometimes assessed and reported via phylogenetic analyses), where a high degree of genetic similarity indicates that infections are closely related (Wertheim et al., 2019). Insofar as multiple recently diagnosed people living with HIV (PLWH) are part of identifiable, growing clusters, focusing scarce prevention resources on these PLWH may be an efficient way to contain the HIV epidemic. HIV molecular studies may provide a rapid and cost-effective way to prioritize HIV prevention efforts and can elucidate underlying patterns of viral transmission that may increase our understanding of epidemics, without such analyses necessarily resulting in direct public health action.
The inclusion of CDR as the fourth pillar of the national EHE initiative has led to intensified public discussion around CDR among public health authorities, researchers, and community members. The use of MHS data and molecular analyses has also prompted discussions regarding the bioethical implications of MHS (Evans & Benbow, 2018; Farrow, n.d.; Gilbert et al. 2016; Kempner, n.d.; Mehta et al., 2019; Molldrem & Smith, 2020; Ryan, 2018). Critics have expressed concern that the use of MHS data signals a “shift back to the early days of the AIDS epidemic where certain groups were singled out as infectious and characterized as ‘dangerous’ and the virus (not those affected) was a central focus for public health intervention” (McClelland et al., 2020, p. 4).
Requirements for HIV prevention funding received as part of the EHE initiative include community engagement around CDR. The health department in Seattle, Washington, Public Health–Seattle & King County (PHSKC), has initiated community engagement in several ways, including presenting information about MHS to the Ryan White Planning Council and other organizations and posting information about MHS on its website (“Drug resistance surveillance and molecular HIV surveillance [MHS],” n.d.). Additionally, using local media channels, PHSKC has alerted community members regarding molecular clusters that included multi-class drug-resistant HIV (Kahle et al., 2009). Continuing community engagement is a vital component of the work being undertaken by PHSKC to develop a local protocol for cluster detection and response.
The present study reports on findings from a formative qualitative analysis of key informant interviews with community members and medical and social service providers regarding their knowledge and perspectives of MHS. Our goal was to identify how PHSKC can effectively provide information about MHS and CDR to community members, explore potential barriers to implementing molecular cluster investigations, and identify recommendations from participants for messaging and dissemination.
METHODS
Design
We conducted key informant interviews with community members living with or at risk for HIV who access care in Seattle, Washington, PHSKC staff, and medical and social service providers. All study procedures were approved by the University of Washington Institutional Review Board prior to recruitment and data collection.
Participants
Recruitment of community members took place through a low barrier primary care clinic that offers medical care and social services to PLWH (Dombrowski et al., 2018) and HIV uninfected persons at high risk for HIV infection. Potential participants were purposively sampled in order to include a diverse representation of HIV serostatuses, ages, races/ethnicities, and the various populations of individuals whose perspectives we were interested in capturing (men who have sex with men [MSM], people who inject drugs [PWID], and women who are living homeless and/or engaged in sex work). Potential participants were contacted by a research nurse with whom many had existing clinical relationships, provided brief information about the goals and procedures of this study, and were asked to verify inclusion criteria. PHSKC staff and medical and social service providers (hereafter all referred to simply as “providers”) were identified by the research team and contacted directly. Providers included physicians, disease intervention specialists, needle exchange coordinators, HIV test counselors, HIV prevention service coordinators, and HIV research community engagement coordinators. In some cases, providers also represented communities of people living with or at risk for HIV, many of whom noted that their perspectives were representative of both their positions as community members and as providers. Community members were provided a $50 cash incentive.
Data Collection and Analysis
Key informant interviews were carried out with community members living with or at risk for HIV and included MSM, PWID, and women who are living homeless and/or engage in transactional sex work. Additional interviews were conducted with PHSKC staff (e.g., disease intervention specialists, needle exchange coordinators) and medical and social service providers. A semi-structured interview guide was developed by the study team and included questions across four topical areas (see Table 1 for content areas and examples of specific interview questions). Participants were recruited and interviewed between January, 2020 and March, 2020. Interviews were conducted by the first author, a nurse researcher with experience conducting in-depth interviews and familiarity with both HIV prevention services and the CDC’s molecular HIV surveillance guidance. All community member interviews were conducted in private rooms at the primary care clinic from which they were recruited; this included PLWH and participants who were HIV uninfected or status unknown at the time of interview. PHSKC staff and provider interviews were conducted in participants’ offices or through videoconference. Participants were provided a brief information sheet and invited to ask questions. All participants provided oral consent prior to the interviews. Interviews were audiotaped, transcribed verbatim by a professional transcriptionist, and lasted between 30 and 60 minutes.
Table 1.
Community Members and Providers’ Knowledge, Awareness of, and Experiences With Molecular HIV Surveillance—Content Areas and Interview Questions
| Content Area | Interview Questions |
|---|---|
| Awareness of and/or experience with MHS and how it might be used for HIV prevention | 1. Do you know what molecular HIV surveillance is? 2. [If yes to #1] How much do you know about it? Please share with me what you know. 3. Do you know what HIV cluster detection and response is? 4. [If yes to #3] How much do you know about it? Please share with me what you know. 5. Did you know that the health department would like to start using information collected for molecular HIV surveillance to help with HIV prevention work? |
| Concerns about MHS and the use of MHS for cluster detection and response |
1. How do you feel about the health department using molecular HIV surveillance information in this way? 2. What concerns (if any) regarding potential legal issues do you have? 3. [For PLWH only] Were you contacted for an interview when you were diagnosed with HIV? How was that for you? |
| Perceived benefits of the use of MHS for cluster detection and response | 1. How do you think this type of information might be helpful in supporting HIV prevention efforts? |
| Information that key informants identified as important for PHSKC to provide to the community in general—and to cluster members and their contacts specifically—about MHS-based interventions when they are implemented | 1. What should we tell people in HIV clusters and their partners about how they were identified or why they are being contacted by the health department? 2. What kinds of information should they be provided? 3. If the health department starts doing this, what do you think people in your community [or the community you serve] should know? 4. How could the health department get this information to people who need it? |
Note. MHS = molecular HIV surveillance; PHSKC = Public Health–Seattle & King County; PLWH = person living with HIV.
Thematic analysis was utilized to guide the analytic process. The two primary goals of analysis included 1) characterizing the stories about and reactions to MHS within and across participant narratives, and 2) describing how participants’ positions relative to MHS (e.g., as a person whose data might be used, as a person who might carry out an intervention, etc.) related to concerns and perceived benefits of the utilization of MHS information for HIV prevention and response.
We adopted an inductive approach to coding, in which the themes identified were firmly grounded in the data and did not necessarily reflect researchers’ particular theoretical orientations or opinions (Willig, 2008). This approach involved a process of coding the data without attempting to fit them into a pre-existing coding frame (Braun & Clarke, 2006). Our analysis included the steps outlined by Braun and Clarke: familiarization with the data, generating initial codes, searching for themes among the initial codes, reviewing themes that may fit together as subthemes, and defining and naming final themes (Braun & Clarke, 2006). Two of the authors (A.S. and R.K.) served as data analysts for this study. We used a process of triangulation in which two different analysts independently coded the same segments of data in order to ensure reliability and confirmation of findings through convergence of different perspectives. The process of analysis continued until data saturation was achieved (i.e., no new categories could be identified). The interviewer kept a reflective journal throughout the study process to record any biases and assumptions he may have had. This journal, the illustrations used to facilitate understanding of MHS during interviews, and the codebook are available for auditing.
RESULTS
See Table 2 for participant demographics. We interviewed a total of 19 participants, with substantially more male participants (n = 13) than female (n = 6). Of the 12 community members we interviewed, half were between the ages of 46 and 55 years. Half disclosed current or previous injection drug use (n = 6), half were living homeless (n = 6), and approximately 60% were PLWH (n = 7). Of the seven providers we interviewed, the majority worked for PHSKC in some capacity (n = 4). Given that we were interested in describing how participants’ positions relative to MHS related to concerns and perceived benefits of the utilization of MHS, we did not collect HIV status, age, and sexual orientation information from providers. Instead, we asked providers to share details regarding their professional role as it related to MHS (i.e., professional job title and specific ways their work may be impacted by MHS). Roughly 40% of all participants were White (n = 8), 21% were Black/African-American (n = 4), and 10% were Multiracial. We provide verbatim quotes in Table 3 to support the validity of themes identified by the analysts (Cho & Trent, 2006).
Table 2.
Characteristics of Community Members and Provider Participants–King County, Washington
| Community Members | Providers | Total | |||
|---|---|---|---|---|---|
| Group | PLWH | HIV uninfected/status unknown | All | ||
| N | 7 | 5 | 12 | 7 | 19 |
| Gender | |||||
| Male | 5 | 3 | 8 | 5 | 13 |
| Female | 2 | 2 | 4 | 2 | 6 |
| Transgender | 0 | 0 | 0 | 0 | 0 |
| Sexual orientation | |||||
| MSM | 3 | 2 | 5 | - | 5 |
| Heterosexual | 4 | 2 | 6 | - | 6 |
| Other sexuality (i.e., bisexual, queer) | 0 | 1 | 1 | - | 1 |
| Race/ethnicity | |||||
| White | 2 | 3 | 5 | 3 | 8 |
| Black/African-American | 3 | 0 | 3 | 1 | 4 |
| Latino | 0 | 0 | 0 | 1 | 1 |
| Multiracial | 0 | 2 | 2 | 0 | 2 |
| Asian | 1 | 0 | 1 | 0 | 1 |
| Native American | 1 | 0 | 1 | 0 | 1 |
| Not reported | 0 | 0 | 0 | 2 | 2 |
| Age (years) | |||||
| 25–35 | 0 | 1 | 1 | - | 1 |
| 36–45 | 1 | 2 | 3 | - | 3 |
| 46–55 | 6 | 0 | 6 | - | 6 |
| 56+ | 1 | 2 | 3 | - | 3 |
| History of injection drug use | 5 | 1 | 6 | - | 6 |
| Living homeless | 3 | 3 | 6 | - | 6 |
| History of exchange sex | 1 | 0 | 1 | - | 1 |
| Practice area | - | - | - | ||
| Private practice | - | - | - | 1 | 1 |
| PHSKC | - | - | - | 4 | 4 |
| CBO | - | - | - | 2 | 2 |
Note. HIV serostatus, age, and sexual orientation not collected from providers. CBO = community-based organization; MSM = men who have sex with men; PHSKC = Public Health–Seattle and King County; PLWH = person living with HIV.
Table 3.
Representative Quotes From Identified Themes
| Theme 1: Context Matters | “There are a variety of people that are served within this building. There is maternity support services. There’s primary care. There’s refugee screening. There’s travel clinic. There’s dental. Within that, you would think, ‘Okay, we’re public health. We are here to serve the public’. Our motto is, ‘Healthy people, healthy communities’ so everybody’s welcome. Well, that’s not the case because there are people in this building that think that [PWIDs] aren’t necessarily deserving of service. So public health isn’t just one entity. That’s just not a reality.” |
| “It would be nice if the health department would say to [providers], ‘Thank you, we’ve contacted everybody that went to that party and we’ve identified three more cases.’” | |
| “Cultural norms in various communities who are most impacted by HIV will impact how the community feels about MHS.” | |
| Theme 2: Making Sense of MHS | “It kind of reminds me of an ancestry tree, how it shows different branches and how it shows the root of one thing.” |
| “Basically, what you guys are doing is going back and seeing the ancestors of the virus so to speak.” | |
| “It’s the study and research of HIV patterns and the way it’s spread, and how to keep [HIV] from being spread by studying smaller groups and smaller genetic makeups or genetic tendencies of HIV in groups of two or three, or maybe more.” | |
| Theme 3: Messaging, Equity, and Resource Prioritization | “There’s often times when, having been in this business for 24 years, and being at sort of the big table, with the people with lots of PhDs and whatever, MPHs, being at that table, it was like ‘I don’t know what you’re talking about.’ [I had to] get over that fear of being like, ‘I don’t know what you’re talking about.’ The first time I heard about molecular surveillance, it was just sort of thrown out there, and everybody else at the table knew exactly what they were talking about. And I was like, ‘Okay, over my head.” |
| “I think that if an HIV cluster is identified and [the health department] finds a common behavior pattern or source …for example, if they identify a cluster and they trace it to a [sex] party, or if they identify a cluster over time and they trace it to a particular bath house or a particular app, I think that it would be nice [for the health department] to inform the community.” | |
| “I know when it comes to health a lot of poor people are more surveilled because of getting benefits but if you take a step back it’s unfair because it’s like if people do have money they get that right to privacy sometimes.” | |
| “You don’t want to put panic into the community. But then again, we’ve got to let people be aware.” | |
| “I think when we look at MHS, the difference is there is a sense from the community that this is being done without their knowledge and without their consent. I think that’s probably the biggest impediment.” | |
| Theme 4: Operationalizing Confidentiality | “I don’t think that the expectation would be a static amount of resources necessarily, but that it would be the appropriate amount of resources. This kind of work could help make [HIV prevention services] more robust when they need to be, and maybe just kind of a baseline level when [resources] donť need to be as robust when there’s still preventative services and care services for people who are positive, maybe not as much intensive outreach or incentivized testing as there is needed when there’s a cluster [outbreak].” |
| “I don’t mind health information being used for the advancement of health. But if any of that information is shared with other institutions, I think that’s problematic. I think if researchers are just focused on the virus, clusters of people – removing that person in that social role then it’s fine because they’re trying to go after something that’s honestly a virus in a person.” | |
| Theme 5: Stigma, Vulnerability, and Power | “[In some communities], the sexual networks are really large because there’s a lot of app use and anonymous sex. In terms of outing someone based on a cluster identification, I feel like there’s less of these close connections and more anonymity among people who are getting diagnosed here at [clinic name] that are coming new to care. Compared to the people that I see at [CBO name] where there’s a more closed group of individuals with whom there’s essentially smaller sexual networks that are not mediated by online or anonymous modes. Even though these women are having a number of clients, they’re probably not getting HIV from their clients. They’re probably getting HIV from other partners that are known partners in the community.” |
| “When [a previous] cluster was identified, there was a lot of discussion [within the community] about who do you think this is: ‘Well I heard it’s so and so.’ A lot of speculation and a lot of ‘oh so and so I heard just got HIV and she spins with so and so, so that must be the person that she got it from.’ That definitely had a negative impact on people coming into care because they were worried about who’s going to find out.” | |
| “[MHS guided interventions] could reinscribe certain types of assumptions about communities.” | |
| “Once you disclose to the police, it’s often a whole different parallel universe that is irrespective of what public health would have…should have…could have done.” |
Note. PWID = people who inject drugs; MHS = molecular HIV surveillance; PhD = Doctor of Philosophy; MPH = Master of Public Health; CBO = community-based organization.
Theme 1: Context Matters
Community members’ perspectives of the health department in the context of MHS and CDR were largely based on previous experiences with public health departments, both PHSKC and others. Those that had existing positive relationships with PHSKC clinics or staff, for example, felt more comfortable with the idea of MHS and CDR and tended to regard it as an important strategy for identifying emergent molecular clusters, reducing HIV infections, and increasing access to care. Alternatively, participants who felt distrustful of health departments tended to refer to health departments generally (i.e., distrust of health departments other than PHSKC) and had limited exposure to and engagement with PHSKC services specifically. A similar tension was present in how providers regarded MHS and CDR but reflected their specific service roles and previous experiences with PHSKC in the setting of MHS and CDR. For providers, their conceptualization of the services they provided (e.g., primary care, HIV testing and counseling, linkage to care, needle exchange, jail health services, mobile clinics serving sex workers) influenced how they envisioned MHS and CDR and, specifically, their role(s), if any, in MHS and CDR. Many providers viewed the clinical services they provided as connected to MHS and CDR; for example, reporting new HIV infections and facilitating linkage to PHSKC for partner services. When providers conceptualized the services they provided as distinctly separate from public health efforts, they expressed more concern about the implications of MHS and CDR, particularly for vulnerable populations, such as incarcerated individuals and people engaged in survival sex work.
Theme 2: Making Sense of MHS
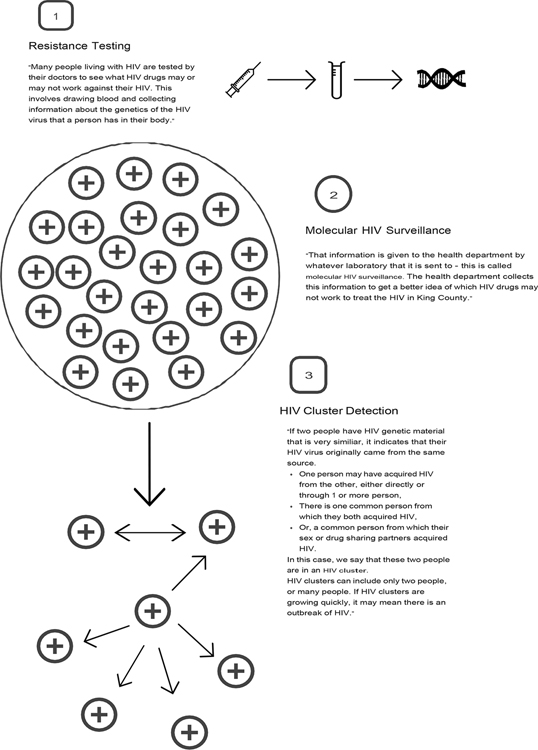
In general, community members lacked awareness of MHS and had a tendency to conflate it with related public health activities (e.g., existing partner services) but could understand the idea once presented with information about MHS. When participants had no previous knowledge of MHS or CDR, the interviewer provided brief information about MHS and utilized graphics drawn by hand in real time to visually render examples of molecular clusters, cluster outbreaks, and the various processes involved in MHS (see Figure 1). Participants frequently conceptualized MHS as an “ancestry tree” and of molecular clusters in multiple ways (e.g., clusters as a way of identifying HIV outbreaks, as transmission chains, personal sexual networks, a connection between two or more people, and as a close group that shares genetic material). Notably, community members commented that the word “surveillance” made them wary of MHS. Medical providers and clinicians defined MHS without the personal associations common among community members, speaking less of people in their definitions than they did about genotypes, molecules, etc. (i.e., readily placing it within a familiar scientific context). For example, concern about the word “surveillance” did not come up among most providers as it did among community members. However, disease intervention specialists and social service providers, such as needle exchange providers and HIV test counselors, shared similar concerns about language held by community members.
Figure 1.
Example of graphic & accompanying information provided to participants regarding Molecular HIV Surveillance and Cluster Detection and Response processes
Theme 3: Messaging, Equity, and Resource Prioritization
From the perspective of the community members we interviewed, the goals of MHS intervention should include 1) determining where public health resources might be needed most, and 2) improving PHSKC’s efforts to prevent the spread of HIV. Community members also felt that MHS interventions should include supporting community members’ awareness of their risk, which they viewed as potentially empowering and part of a larger operation to test, treat, and engage or re-engage in care. The majority of community members noted the complexity of interventions guided by MHS data, particularly for populations disproportionately impacted by HIV who may also be the focus of additional surveillance activities, such as care linkage activities or referral to a low-barrier clinic. Given that the nuances of MHS may be difficult for communities to understand, participants felt that clear messaging from PHSKC would be integral to reducing fear among community members. Similarly, providers felt that phylogenetic analyses are useful inasmuch as they determine general patterns of transmission in a community or geographic area and that the primary goals of MHS intervention should be to 1) improve/expand HIV prevention efforts, and 2) improve HIV resource allocation. Providers also suggested that MHS data and CDR could be helpful in reaching people who are multiply marginalized; for example, women who inject drugs and engage in survival sex work. Furthermore, MHS interventions could be used to support sustained resource allocation in areas that need them most over time and not only in response to cluster outbreaks.
Theme 4: Operationalizing Confidentiality
The desire for confidentiality among community members was frequently connected to fears of private information being shared publicly. Concerns about confidentiality and the use of MHS data centered on issues related to transmission and directionality, as well as disclosure and potential violence. The majority of participants felt that cluster investigations might lead to community members blaming one another for HIV transmission. Paradoxically, many noted that they would want to know who transmitted HIV to them, if possible. Many participants felt that the use of anonymized identifiers, such as case numbers, strengthened their trust in health department surveillance practices, and enhanced measures to protect community members’ confidentiality leads to more trusting relationships with individuals, clinics, and broader structures that comprise health departments. Many PLWH recounted their initial experiences disclosing HIV to sex and/or drug-sharing partners, noting how they were socially ostracized. They spoke of experiencing verbal, sexual, and/or physical abuse, particularly women who are living homeless and MSM. These participants emphasized the need to protect community members who may disproportionately experience violence as a result of disclosure, suggesting that protective services (e.g., referral to domestic violence services) be offered as a part of MHS interventions.
Providers noted that the size and relationship of social networks play a role in how confidentiality is operationalized and/or the extent to which confidentiality can realistically be ensured when using MHS data to guide cluster investigations. When clinics or sites of service provision (i.e., needle exchange sites) offer a diversity of services in addition to HIV prevention and treatment (e.g., vaccines, buprenorphine, etc.), there is less potential for inadvertent disclosure of one’s HIV status. Community members who are most marginalized might be less concerned about confidentiality because they have other unmet needs, such as housing and/or food insecurity. Providers also recounted experiences in which community members were weary of engaging with disease intervention specialists or medical service providers out of fear that any association with these providers might result in unwanted disclosure. Frequently, this fear prevented community members from linking to HIV care.
Theme 5: Stigma, Vulnerability, and Power
Community members worried that cluster investigations might potentially reinscribe assumptions about communities disproportionately impacted by HIV and exacerbate existing HIV-related stigma. For example, a number of participants talked about their experiences being perceived as somehow inherently dangerous or infectious because of their HIV status and expressed concerns specifically related to the potential of being identified as part of a growing cluster. Many participants felt that MHS data might be used as “evidence” of intent to harm in legal cases where HIV is criminalized. Similarly, providers felt that MHS data might be used to determine directionality of infection, which might discourage community members from accessing HIV services. Although providers noted that offering services in communities most marginalized might be a way of repairing past harm, they also worried that utilizing MHS data to guide interventions may, in fact, lead to less engagement between health departments and communities, rather than increased engagement and collaboration. Multiple providers noted the need to change laws related to HIV criminalization to keep MHS data truly confidential. They expressed concern that MHS data—used to determine directionality—might be used to layer other crimes, and that these practices are tied up with larger systems of oppression, particularly for communities disproportionately impacted by HIV where histories of police brutality and increased surveillance practices exist.
DISCUSSION
Our findings are consistent with previous studies that explored key stakeholders’ perspectives of HIV molecular epidemiology and highlight critical issues that should be considered when using MHS data for developing interventions targeted at CDR. At the center of concerns related to the ethical use of MHS data are issues broadly related to the transparency and accessibility of information about MHS, which participants in our study viewed as barriers to utilizing MHS data to guide prevention efforts. A need for ongoing transparency about the potential uses of MHS data remains, as well as meaningful community engagement to address community concerns and how best to share information about HIV molecular epidemiology. Regarding accessibility of information about the use of MHS data, community activists have questioned whether PLWH who receive drug resistance testing have been provided adequate information about how their genotype tests are aggregated for public health surveillance. McClelland et al., for example, have argued that “people living with HIV should be made aware that their personal health data is being mobilized and combined with other data sources to identify people for intervention” (McClelland et al., 2020, p. 4). A number of participants in our study echoed this concern and felt strongly that information about the use of biospecimens should be shared at the time of blood draw, with individuals being given the opportunity to opt out of such practices. Additionally, researchers have highlighted distinctions between the collection and use of data to protect the health of populations and the use of public health surveillance data for research purposes, both of which are subject to different rules and regulatory standards regarding data security, data access, and data sharing (Dawson et al., 2020; Lee et al., 2012; Sweeney et al., 2013).
In regards to community concerns around the confidentiality of MHS data, some public health advocates have noted that similar concerns were raised about potential negative effects on HIV testing prior to widespread name-based HIV surveillance. These effects have not played out in practice, however, and name-based HIV surveillance has become the norm in the United States (Hecht et al., 2000; Lansky et al., 2002; Nakashima et al., 1998; Tesoriero et al., 2008). MHS has provided public health benefits beyond cluster detection; MHS data have been utilized to monitor transmission in outbreaks after they have been identified, and retrospective characterization of transmission dynamics using MHS have been used to inform health policy (Alpren et al., 2020; Campbell et al., 2017; Golden et al., 2019; Pillay et al., 2015; Sizemore et al., 2020). For example, in 2018, PHSKC staff identified a cluster of cases of HIV infection among individuals living homeless, most of whom injected drugs and, after interventions were implemented to reduce further transmission, MHS was used to detect additional onward transmission (Golden et al., 2019).
Additionally, our findings suggest that because health department efforts to prevent the spread of HIV comprise many individuals delivering a diversity of services, information regarding HIV molecular epidemiology and the use of MHS data should be developed to meet the needs of a diversity of individuals, not only within the community but also within health departments. This is particularly true for public health staff, such as disease intervention specialists and/or peer educators, who will likely be tasked with carrying out an intervention guided by MHS data. Based on our experience providing information to both community members and providers, it is possible that graphic illustrations outlining the processes involved in HIV molecular epidemiology, and potential social issues that might arise in response to cluster investigations, will help to make the complexities of MHS and CDR more accessible to a broad range of individuals with varying relationships to health departments.
Regarding HIV criminalization, researchers, scholars, and community activists have highlighted the potential misuse of MHS data in criminal cases. Although Washington State passed HIV criminalization law changes in 2020 (Straube, 2020), it is possible that providers’ concerns about HIV criminalization may not reflect this change and that provider participants were not aware of this change at the time of interview. One of the primary concerns in this context is the possibility of estimating the probability of specific HIV transmission events between two individuals (Dawson et al., 2020). Assuaging community concerns about increased HIV criminalization has often centered on the idea that phylogenetic analyses cannot be used to determine directionality. Although recent studies have demonstrated increased technical ability to predict directionality of transmission, this only applies in aggregate between subpopulations and between individuals under very specific circumstances that are, at present, rare in the context of public health surveillance (Dawson et al., 2020; Ratmann et al., 2020; Zhang et al., 2020). Nevertheless, researchers have noted that the “the existence of HIV criminalization necessitates tight control over the use of [MHS] data to minimize risks of misuse, and heightens the need for transparency and good communication, to allay fears about misuse and protect community relationships with public health agencies, health care providers, and health researchers” (Dawson et al., 2020, p. 4). Community perceptions regarding the potential misuses of MHS data may exacerbate existing medical mistrust—particularly among communities disproportionately impacted by HIV—ultimately leading to disengagement with existing HIV prevention services. Based on these findings, we include several recommendations in Table 4 regarding the use of MHS and CDR in regards to communicating about MHS, protecting confidentiality, and addressing stigma and resource prioritization.
Table 4.
Recommendations for Messaging and Dissemination
| Recommendation | Theme |
|---|---|
| Establish closed loop communication with primary care providers regarding HIV case reports | Theme 1: Context Matters |
| Provide information about MHS to a variety of interventionists and public health service workers | Theme 1: Context Matters |
| Avoid the word "surveillance" in public messaging campaigns regarding MHS and CDR | Theme 2: Making Sense of MHS |
| Messaging should be developed specific to various populations’ needs | Theme 3: Messaging, Equity, and Resource Prioritization |
| Make information about health departments’ use of MHS data widely available to community members and accessible at a diversity of sites and locations (e.g., community meetings, via homeless health care advocates, needle exchange sites, shelters, clinics, and routine HIV testing sites) | Theme 3: Messaging, Equity, and Resource Prioritization |
| Prioritize community education regarding the use of MHS data prior to intervention | Theme 3: Messaging, Equity, and Resource Prioritization |
| Utilize peer educators to share information about MHS | Theme 4: Operationalizing Confidentiality |
| Disease intervention specialists should focus on providing information about the confidential nature of contact with potential cluster members | Theme 4: Operationalizing Confidentiality |
| Share information about the use of biospecimens in the context of MHS at the time of blood draw | Theme 4: Operationalizing Confidentiality |
| Address concerns about unwanted disclosure of one’s HIV status and safety | Theme 5: Stigma, Vulnerability, and Power |
Note. CDR = cluster detection and response; MHS = molecular HIV surveillance.
LIMITATIONS
The community member participants were all recruited from the same primary care clinic in Seattle, Washington, where the majority had existing positive relationships with clinic staff. This is likely to have impacted participants’ motives to share their perspectives of MHS and possible barriers to MHS-guided interventions and may not represent the perspectives of a broader sample of individuals not engaged in care. Additionally, none of the community members we interviewed had recently been diagnosed with HIV or were transgender. Transgender community activists living with HIV have figured prominently in discussions regarding the ethics and values of MHS and CDR. Representation of their perspectives in research and messaging is of critical importance when considering possible social and political implications of MHS/CDR, as well as future possible messaging. All providers that we interviewed had existing relationships with PHSKC and, thus, may have more knowledge of MHS than medical and social service providers without explicit connections to PHSKC. Given that an understanding of MHS was low among the community members we interviewed, this should be taken into consideration when interpreting our findings because it points to the need for increased transparency and information about MHS.
Moving forward, cluster investigations may help to highlight aspects of social vulnerability (e.g., homelessness, injection drug use, transactional and survival sex work) that drive cluster outbreaks in communities disproportionately impacted by HIV. As we have previously noted, a number of participants felt that MHS data might be utilized to determine where scarce HIV prevention resources are needed most and should be allocated. Our findings also suggest, however, that in some populations in which MHS-directed interventions are most likely to happen, community members are most marginalized and may not be afforded the “luxury of confidentiality.” Continued community engagement—for example, via community advisory boards or stakeholder meetings involving community representatives and advocates—is likely to support the generation of innovative approaches for addressing some of the bioethical concerns related to the use of MHS data.
KEY CONSIDERATIONS.
As local jurisdictions begin to operationalize CDC’s mandate to utilize molecular HIV surveillance (MHS) data to identify emerging HIV transmission clusters, health departments should engage community members and medical and HIV service providers in their efforts to develop public messaging regarding the use of MHS data to guide public health interventions. Focus groups, community advisory boards, and stakeholder meetings represent possible avenues for community engagement in the future.
Specific messaging should be developed to address the particular concerns of various communities (e.g., people living with HIV [PLWH], people who inject drugs [PWID], etc.), the goals of MHS-guided interventions should be made clear to community members (i.e., are MHS-guided interventions aimed at improving HIV prevention efforts, improving HIV resource allocation, or both?), and easily accessible information about confidentiality in the setting of MHS should be included in public messaging.
Given that MHS-guided interventions are likely to involve the delivery of a diversity of public health services, training and information regarding HIV molecular epidemiology and the use of MHS data should be developed to meet the needs of a diversity of individuals working within public health departments, particularly public health staff who are likely to be tasked with carrying out MHS-guided interventions.
Acknowledgments
We gratefully acknowledge and thank Lindsay Legg, RN, for her support recruiting and scheduling participants for this study. We also acknowledge the participants, who generously shared their experiences and perspectives. Support for this work was provided to J. T. Herbeck and R. P. Kerani by the University of Washington Center for AIDS Research (NIH P30 AI027757) and NIAID NIH R01AI127232.
Footnotes
Disclosures
The authors report no real or perceived vested interests related to this article that could be construed as a conflict of interest.
Contributor Information
Alic G. Shook, College of Nursing, Seattle University Seattle, Washington, USA.
Susan E. Buskin, Department of Epidemiology, University of Washington, Seattle, Washington, USA; Epidemiologist, Public Health – Seattle & King County, Seattle, Washington, USA.
Matthew Golden, Public Health – Seattle King County HIV/STD Program; Department of Medicine, University of Washington, Seattle, Washington, USA.
Julia C. Dombrowski, Public Health-Seattle & King County HIV/STD Program; Department of Medicine, University of Washington, Seattle, Washington, USA.
Joshua Herbeck, Department of Global Health, University of Washington, Seattle, Washington, USA.
Richard J. Lechtenberg, Public Health – Seattle & King County, Seattle, Washington, USA.
Roxanne Kerani, Department of Medicine, University of Washington, Seattle, Washington, USA.
REFERENCES
- Alpren C, Dawson EL, John B, Cranston K, Panneer N, Dawn Fukuda H, Roosevelt R, Klevens M, Bryant J, Peters P, Lyss SB., Switzer WM., Burrage A, Murray A, Agnew-Brune C, Stiles T, McClung P, Campbell EM, Breen C, Randall LM, … Buchacz K (2020). Opioid use fueling HIV transmission in an urban setting: An outbreak of HIV infection among people who inject drugs-Massachusetts, 2015–2018. American Journal of Public Health, 110(1), 37–44. 10.2105/AJPH.2019.305366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V, & Clarke V (2006). Using thematic analysis in psychology. Qualitative Research in Psychology, 3(2), 77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- Campbell EM, Jia H, Shankar A, Hanson D, Luo W, Masciotra S, Owen SM, Oster AM, Galang RR, Spiller MW, Blosser SJ, Switzer WM (2017). Detailed transmission network analysis of a large opiate-driven outbreak of HIV infection in the United States. Journal of Infectious Diseases, 216(9), 1053–1062. 10.1093/infdis/jix307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, & Trent A (2006). Validity in qualitative research revisited. Qualitative Research, 6(3), 319–340. 10.1177/1468794106065006 [DOI] [Google Scholar]
- Dawson L, Benbow N, Fletcher FE, Kassaye S, Killelea A, Latham SR, Lee LM, Leitner T, LIttle SJ, Mehta SR, Martinez O, Minalga B, Poon A, Rennie S, Sugarman J, Sweeney P, Torian LV, Wertheim JO (2020). Addressing ethical challenges in US-based HIV phylogenetic research. The Journal of Infectious Diseases. 10.1093/infdis/jiaa107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski JC, Ramchandani M, Dhanireddy S, Harrington RD, Moore A, & Golden MR (2018). The Max Clinic: Medical care designed to engage the hardest-to-reach persons living with HIV in Seattle and King County, Washington. AIDS Patient Care and STDs, 32(4), 149–156. 10.1089/apc.2017.0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health - Seattle & King County (2021, January 11). Drug resistance surveillance and molecular HIV surveillance (MHS). https://kingcounty.gov/depts/health/communicable-diseases/hiv-std/patients/epidemiology/drug-resistance-surveillance.aspx
- Evans D, & Benbow N (2018). Ethical considerations for a public health response using molecular HIV surveillance: A multi-stakeholder approach. Project Inform and Northwestern University. https://www.cdc.gov/hiv/pdf/programresources/guidance/cluster-outbreak/cdc-hiv-Ethical-Considerations-Report.pdf [Google Scholar]
- Farrow K (2018, June 13). Data collection is revolutionizing HIV surveillance and care, but at the cost of privacy? https://www.thebodypro.com/article/data-collection-is-revolutionizing-hiv-surveillanc.
- Fauci A, Redfield RR, Sigounas G, Weahkee MD, & Giroir B (2019). Ending the HIV epidemic: A plan for the United States. JAMA, 321(9), 844–845. 10.1056/nejmms1513641 [DOI] [PubMed] [Google Scholar]
- Gilbert M, Swenson L, Unger D, Scheim A, & Grace D (2016). Need for robust and inclusive public health ethics review of the monitoring of HIV phylogenetic clusters for HIV prevention. The Lancet HIV, 3(10), e461. 10.1016/S2352-3018(16)30156-4 [DOI] [PubMed] [Google Scholar]
- Golden MR, Lechtenberg R, Glick SN, Dombrowski J, Duchin J, Reuer JR, Dhanireddy S, Neme S, Buskin SE (2019). Outbreak of human immunodeficiency virus infection among heterosexual persons who are living homeless and inject drugs — Seattle, Washington, 2018. MMWR. Morbidity and Mortality Weekly Report, 68(15), 344–349. 10.15585/mmwr.mm6815a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht FM, Chesney MA, Lehman JS, Osmond D, Vranizan K, Colman S, Keane D, Reingold A, Bindman AB (2000). Does HIV reporting by name deter testing? AIDS, 14(12), 1801–1808. 10.1097/00002030-200008180-00016 [DOI] [PubMed] [Google Scholar]
- Kahle EM, Barash EA, Page LC, Lansky A, Jafa K, Sullivan PS, & Buskin SE (2009). Evaluation of the impact of news coverage of an HIV multiclass drug-resistant cluster in Seattle, Washington. American Journal of Public Health, 99(Suppl 1), 131–137. 10.2105/AJPH.2007.126656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempner M (n.d.). New study triggers concerns over use of molecular HIV surveillance. Retrieved January 11, 2021, from https://www.thebodypro.com/article/concerns-over-use-of-molecular-hiv-surveillance
- Kosakovsky Pond SL, Weaver S, Leigh Brown AJ, & Wertheim JO (2018). HIV-TRACE (TRAnsmission Cluster Engine): A tool for large scale molecular epidemiology of HIV-1 and other rapidly evolving pathogens. Molecular Biology and Evolution, 35(7), 1812–1819. 10.1093/molbev/msy016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansky A, Lehman JS, Gatwood J, Hecht FM, & Fleming PL (2002). Changes in HIV testing after implementation of name-based HIV case surveillance in New Mexico. American Journal of Public Health, 92(11), 1757. 10.2105/AJPH.92.11.1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LM, Heilig CM, & White A (2012). Ethical justification for conducting public health surveillance without patient consent. American Journal of Public Health, 102(1), 38–44. 10.2105/AJPH.2011.300297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland A, Guta A, & Gagnon M (2020). The rise of molecular HIV surveillance: Implications on consent and criminalization. Critical Public Health, 30(4), 487–493. 10.1080/09581596.2019.1582755 [DOI] [Google Scholar]
- Mehta SR, Chaillon A, Gaines TL, Gonzalez-Zuniga PE, Stockman JK, Almanza-Reyes H, Chavez JR, Vera A, Wagner KD, Patterson TL, Scott B, Smith DM, Strathdee SA (2018). Impact of public safety policies on human immunodeficiency virus transmission dynamics in Tijuana, Mexico. Clinical Infectious Diseases, 66(5), 758–764. 10.1093/cid/cix884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SR, Schairer C, & Little S (2019). Ethical issues in HIV phylogenetics and molecular epidemiology. Current Opinion in HIV and AIDS, 14(3), 221–226. 10.1097/COH.0000000000000538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SR, Wertheim JO, Brouwer KC, Wagner KD, Chaillon A, Strathdee S, Patterson TL, Rangel MG, Vargas M, Murrell B, Garfein R, Little SJ, Smith DM (2015). HIV transmission networks in the San Diego-Tijuana border region. EBioMedicine, 2(10), 1456–1463. 10.1016/j.ebiom.2015.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molldrem S, & Smith AKJ (2020). Reassessing the ethics of molecular HIV surveillance in the era of cluster detection and response: Toward HIV data justice. American Journal of Bioethics, 20(10), 10–23. 10.1080/15265161.2020.1806373 [DOI] [PubMed] [Google Scholar]
- Nakashima AK, Horsley R, Frey RL, Sweeney PA, Weber JT, & Fleming PL (1998). Effect of HIV reporting by name on use of HIV testing in publicly funded counseling and testing programs. Journal of the American Medical Association, 280(16), 1421–1426. 10.1001/jama.280.16.1421 [DOI] [PubMed] [Google Scholar]
- Pillay D, Herbeck J, Cohen MS, Oliveira T. de, Fraser C, Ratmann O, Brown AL, Kellam P (2015). PANGEA-HIV: Phylogenetics for generalised epidemics in Africa. The Lancet Infectious Diseases, 15(3), 259–261. 10.1016/S1473-3099(15)70036-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon AFY, Gustafson R, Daly P, Zerr L, Demlow SE, Wong J, Woods CK, Hogg RS, Krajden M, Moore D, Kendall P, Montaner JSG, Harrigan PR (2016). Near real-time monitoring of HIV transmission hotspots from routine HIV genotyping: An implementation case study. The Lancet HIV, 3(5), e231–e238. 10.1016/S2352-3018(16)00046-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratmann O, Kagaayi J, Hall M, Golubchick T, Kigozi G, Xi X, Wymant C, Nakigozi G, Abeler-Dorner L, Bonsall D, Gall A, Hoppe A, Kellam P, Bazaale J, Kalibbala S, Laeyendecker O, Lessler J, Nalugoda F, Chang LW, de Oliveira T … Tobian A (2020). Quantifying HIV transmission flow between high-prevalence hotspots and surrounding communities: A population-based study in Rakai, Uganda. The Lancet HIV, 7(3), e173–e183. 10.1016/S2352-3018(19)30378-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan B (2018). Will the genetic analysis-based HIV surveillance safeguard privacy? https://www.poz.com/article/will-genetic-analysisbased-hiv-surveillance-safeguard-privacy
- Sizemore L, Fill MM, Mathieson SA, Black J, Brantley M, Cooper K, Garrett J, Switzer WM, Peters P, Wester C (2020). Using an established outbreak response plan and molecular epidemiology methods in an HIV transmission cluster investigation, Tennessee, January–June 2017. Public Health Reports, 135(3), 329–333. 10.1177/0033354920915445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher M, Chaillon A, Eberle J, Behrens GMN, Eis-Hübinger AM, Lehmann C, Jablonka A, Bogner J, Fatkenheuer G, Spinner CD, Wasmuth J-C, Kaiser R, Mehta S, Vehreschild JJ, Hoenigl M (2018). Molecular epidemiology of the HIV epidemic in three German metropolitan regions - Cologne/Bonn, Munich and Hannover, 1999–2016. Scientific Reports, 8(1), 1–9. 10.1038/s41598-018-25004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T (2020). Washington State legislature votes to modernize HIV crime laws. Retrieved June 3, 2021, from https://www.poz.com/article/washington-state-legislature-votes-modernize-hiv-crime-laws
- Sweeney P, Gardner LI, Buchacz K, Garland PM, Mugavero MJ, Bosshart JT, Shouse RL, Bertolli J (2013). Shifting the paradigm: Using HIV surveillance data as a foundation for improving HIV care and preventing HIV infection. Milbank Quarterly, 91(3), 558–603. 10.1111/milq.12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesoriero JM, Battles HB, Heavner K, Leung SYJ, Nemeth C, Pulver W, & Birkhead GS (2008). The effect of name-based reporting and partner notification on HIV testing in New York State. American Journal of Public Health, 98(4), 728–735. 10.2105/AJPH.2006.092742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheim JO, Chato C, & Poon AFY (2019). Comparative analysis of HIV sequences in real time for public health. Current Opinion in HIV and AIDS, 14(3), 213–220. 10.1097/COH.0000000000000539 [DOI] [PubMed] [Google Scholar]
- Willig C (2008). Introducing qualitative research in psychology. Open University Press. 10.4135/9780857029034 [DOI] [Google Scholar]
- Zhang Y, Wymant C, Laeyendecker O, Grabowski MK, Hall M, Hudelson S, Piwowar-Manning E, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Mills LA, Santos BR, Grinsztejn B, Pilotto JH, Chariyalertsak S, Makhema J, Chen YQ, Cohen MS, Fraser C, Eshleman SH (2020). Evaluation of phylogenetic methods for inferring the direction of human immunodeficiency virus (HIV) transmission: HIV Prevention Trials Network (HPTN) 052. Clinical Infectious Diseases, 21205(Xx), 1–8. 10.1093/cid/ciz1247 [DOI] [PMC free article] [PubMed] [Google Scholar]