Abstract
The purpose of this study was to evaluate the pharmacokinetics (PK) profile of oral levofloxacin in human immunodeficiency virus-positive patients in steady-state treatment with nelfinavir (NFV) or with efavirenz (EFV) and to determine the effects of levofloxacin on the PK parameters of these two antiretroviral agents. For levofloxacin, plasma samples were obtained at steady state during a 24-h dosing interval. Plasma NFV and EFV concentrations were evaluated before and after 4 days of levofloxacin treatment. Levofloxacin PK do not seem affected by NFV and EFV. There was no significant difference between NFV and EFV plasma levels obtained with and without levofloxacin.
Levofloxacin, the S(−) isomer of racemic ofloxacin, is a recently developed fluoroquinolone often used for the management of bacterial complications in human immunodeficiency virus (HIV)-infected patients (7). The pharmacokinetics (PK) of levofloxacin following single and multiple oral and intravenous doses have been widely studied (3–5, 12; M. L. Holland, S. C. Chien, M. L. Corrado, et al., 5th Int. Symp. New Quinolones, abstr., 1994) and the drug PK profile for HIV-infected patients has been shown to be similar to that of healthy subjects (2, 6). Several studies have suggested the potential for significant drug-drug interactions involving fluoroquinolones (5, 8, 9). However, no data are available about potential pharmacological interactions between levofloxacin and protease inhibitors (PIs) or non-nucleoside transcriptase inhibitors (NNRTIs). The objectives of this study were to evaluate the PK of levofloxacin after multiple oral doses of 500 mg/day in combination with a PI (nelfinavir [NFV]) or with an NNRTI (efavirenz [EFV]) in HIV-infected patients and to determine the effects of levofloxacin on the steady-state systemic exposure of NFV and EFV.
As a part of an ongoing prospective multicenter observational cohort study related to the evaluation of prognostic features and outcome markers of community-acquired pneumonia in HIV patients (POP-HIV study), 24 subjects on steady-state antiretroviral therapy affected with radiologically diagnosed bacterial pneumonia and treated with levofloxacin were recruited for the PK study. All patients were receiving highly active antiretroviral therapy, including two NRTIs (zidovudine, 300 mg × 2/day, and lamivudine, 150 mg × 2/day) and NFV (750 mg × 3/day) or EFV (600 mg/day). The patient population, in steady-state antiretroviral treatment, was stratified in two groups of 12 subjects each related to the highly active antiretroviral therapy (group 1, NFV; group 2, EFV). Levofloxacin was administered orally at the dosage of 500 mg once a day for at least 7 days. The patients' medical histories were recorded prior to enrollment, and a physical examination and hematological, biochemical, and virological tests were performed for each patient. Active drug addicts, pregnant women, and subjects with quinolone treatment for >24 h in the 5 days prior to study entry were excluded. All enrolling patients met the criteria of normal liver function (transaminase values, less than 2.5 times the upper normal limit; blood albumin levels, >2.5 g/liter) and no renal failure (creatinine clearance, >80 ml/min). Approval for the study was obtained from the local ethics committee, and each patient provided written informed consent.
For the determination of plasma levofloxacin concentrations, blood samples were obtained on treatment day 4 during the dosing interval at the following times: 0 (before levofloxacin administration) and 0.5, 1, 1.5, 2, 3, 4, 8, 12, 14, 16, and 24 h after administration. Plasma NFV concentrations were evaluated on two different occasions: before the start of levofloxacin therapy and on the fourth day of antimicrobial therapy. Sampling times were 0 (Ctrough), 1, and 2 h after NFV administration, where Ctrough is the trough plasma concentration.
Plasma EFV concentrations were evaluated before and after 4 days of levofloxacin treatment at the following times: 0, 2, and 12 h after EFV administration. Plasma samples were separated, inactivated in a bath at 56°C for 45 min, and then frozen at −20°C until analysis. Plasma samples were analyzed for levofloxacin concentrations with a validated high-pressure liquid chromatography assay with fluorimetric detection (11). Standard curves were linear from 0.4 to 10 μg/ml. The inter- and intraassay precision values (coefficient of variation [CV%]) of the quality control samples were each less than 10%. Plasma NFV and EFV concentrations were determined by previously described, validated high-pressure liquid chromatography methods with UV detection (13, 15). Quality control samples at different concentrations of both NFV and EFV, analyzed with each analytical run, had a CV% for precision and accuracy lower than 15% for all the concentrations examined (range, 2.8 to 14.2% for NFV and 1.4 to 9.3% for EFV).
The peak concentration (Cmax), time to Cmax (Tmax), and Ctrough of levofloxacin in plasma were determined by direct visual inspection of data. Plasma levofloxacin concentration-time data were analyzed by a noncompartmental model using the Pharm-NCA computer program (Simed, Creteil, France). The area under the plasma concentration-time curve from 0 to 24 h (AUC0–24) was calculated using the trapezoidal method. Apparent total body clearance (CL/F, with F as the fraction of the absorbed dose, assumed to be equal to 1) was calculated as dose/AUC; the terminal disposition half-life was calculated as 0.693/kel, where kel is the terminal rate constant, calculated from the slope of the terminal log-linear phase of the plasma concentration-time profile. The average plasma drug concentration during the dosing interval at steady state was obtained by AUC/τ (where τ is the dosing interval).
For NFV, the 2-h abbreviated AUC, calculated by plasma NFV concentration of the morning predose sample and of 1- and 2-h postdose samples, provides an estimate of the full AUC0–8 (10) by the equation
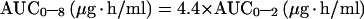 |
1 |
For EFV, by linear regression analysis, trough plasma concentrations are predictive of EFV total body systemic exposure, as previously demonstrated (14) by the good correlation between AUC(0–24) and Ctrough values:
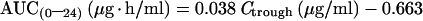 |
2 |
The two groups of patients were balanced for gender, age (median, 46.2 years in group 1 versus 42.4 years in group 2), weight (59 versus 61 kg), sex (five male and seven female versus six male and six female), CD4+ count (median, 123 versus 186 cells/mm3), and viral load (six patients with undetectable viremia in each group; median HIV RNA value in viremic subjects, 84,000 versus 78,000 copies/ml). Pharmacology histories were negative for the intake of drugs potentially interacting with PIs and NNRTIs in all patients.
The steady-state main PK parameters of levofloxacin (mean ± standard deviation) obtained after a regimen of 500 mg once daily in our two groups of HIV patients are reported in Table 1 and compared with data by Child et al. for healthy subjects with the same dosage (4). Pharmacokinetic results for NFV and EFV are reported in Table 2. No significant difference was observed for NFV and EFV measured parameters obtained before and during levofloxacin therapy.
TABLE 1.
Comparison of the main pharmacokinetic parameters of levofloxacin in two groups of HIV-positive patients and in healthy subjectsa
| Subject group | Pharmacokinetic parameter (mean ± SD)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (h) | AUC0–24 (μg · h/ml) | Css, avc (μg/ml) | Ctrough (μg/ml) | CL/F
|
t1/2 (h)d | ||
| liter/h/kg | liter/h | |||||||
| Levofloxacin + NFVb(n = 12) | 6.22 ± 1.98 | 1.4 ± 0.45 | 71.8 ± 32.45 | 2.99 ± 1.35 | 1.5 ± 1.13 | 0.152 ± 0.111 | 9.54 ± 8.0 | 8.6 ± 2.13 |
| Levofloxacin + EFVb(n = 12) | 5.75 ± 0.82 | 3.3 ± 1.1 | 65.51 ± 20.6 | 2.95 ± 1.48 | 0.86 ± 0.5 | 0.136 ± 0.056 | 8.7 ± 5.0 | 7.5 ± 2.6 |
| Healthy subjects | 6.55 ± 1.84 | 1.17 ± 0.52 | 53.5 ± 10.3 | 2.23 ± 0.43 | NRe | 9.69 ± 2.08 | 7.95 ± 1.35 | |
Data on healthy subjects are from Child et al. (4).
Values normalized to a dose of 8 mg/kg of body weight.
Css, av, average plasma drug concentration during the dosing interval at steady state.
t1/2, terminal disposition half-life.
NR, not reported.
TABLE 2.
Steady-state pharmacokinetic parameters of NFV and EFV with and without levofloxacin in two groups of HIV-positive patients
| HIV patient group and mean difference | Pharmacokinetic parameter (mean ± SD)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Ctrough (μg/ml) | C1h (μg/ml) | C2h (μg/ml) | C12h (μg/ml) | AUC0–2 (μg · h/ml)a | AUC0–8 (μg · h/ml)b | AUC0–12 (μg · h/ml)c | AUC0–24 (μg · h/ml)d | |
| NFV (n = 12) | 1.35 ± 0.71 | 1.34 ± 0.66 | 1.67 ± 1.26 | 2.85 ± 1.61 | 12.56 ± 7.08 | |||
| NFV + levofloxacin (n = 12) | 1.64 ± 0.73 | 1.72 ± 0.6 | 1.85 ± 0.6 | 3.47 ± 1.24 | 15.28 ± 5.5 | |||
| Mean difference (95% CI)e | −0.299 (−0.815; 0.218) | −0.379 (−0.806; 0.049) | −0.179 (−0.908; 0.551) | −0.614 (−1.619; 0.390) | −2.714 (−7.135; 1.707) | |||
| EFV (n = 12) | 1.77 ± 0.95 | 3.74 ± 1.6 | 2.57 ± 1.81 | 37.1 ± 19.03 | 66.86 ± 36.31 | |||
| EFV + levofloxacin (n = 12) | 2.23 ± 1.78 | 4.23 ± 3.23 | 2.59 ± 1.51 | 40.59 ± 28.53 | 84.35 ± 67.63 | |||
| Mean difference (95% CI) | −0.460 (−0.998; 0.077) | −0.494 (−1.534; 0.547) | −0.014 (−0.410; 0.382) | −3.494 (−9.344; 2.356) | −17.486 (−37.915; 2.942) | |||
Our results for 24 HIV-infected patients indicated that the systemic exposure to levofloxacin, when combined with NFV or with EFV, is not different from that previously observed in this population, when patients were not receiving these two antiretroviral agents (2, 6), or in healthy volunteers (3–6, 12). The longer levofloxacin Tmax observed in our patients taking EFV (3.3 h), if compared with the NFV group (1.4 h) or with historical controls (range, 0.9 to 1.7 h), may be referable to the delayed gastric-emptying phenomenon observed in a recent study of animals receiving EFV (1). However, this delay in absorption rate causes no significant differences in plasma levofloxacin levels, as the total body systemic exposure (AUC) to levofloxacin remains substantially unchanged. Moreover, no significant alterations were observed during the levofloxacin treatment for either plasma NFV or EFV concentrations. No unexpected clinical or laboratory adverse events occurred during the study, and the absence of potentially critical interactions between levofloxacin and PI and an NNRTI stresses the excellent safety data obtained. Since levofloxacin is likely to be used for the treatment of a variety of bacterial infections in HIV-infected subjects, our results suggest that a clinically important pharmacological interaction between levofloxacin and NFV or EFV is not likely to occur in this population treated with these agents simultaneously.
REFERENCES
- 1.Balani S K, Kauffman L R, deLuna F A, Lin J H. Nonlinear pharmacokinetics of efavirenz (DMP-266), a potent HIV-1 reverse transcriptase inhibitor, in rats and monkeys. Drug Metab Dispos. 1999;27:41–45. [PubMed] [Google Scholar]
- 2.Chien S C, Chow A T, Natarajan J, Williams R R, Wong F A, Rogge M C, Nayak R K. Absence of age and gender effects on the pharmacokinetics of a single 500-milligram oral dose of levofloxacin in healthy subjects. Antimicrob Agents Chemother. 1997;41:1562–1565. doi: 10.1128/aac.41.7.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chien S C, Chow A T, Rogge M C, Williams R R, Hendrix C W. Pharmacokinetics and safety of oral levofloxacin in human immunodeficiency virus-infected individuals receiving concomitant zidovudin. Antimicrob Agents Chemother. 1997;41:1765–1769. doi: 10.1128/aac.41.8.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Child J, Mortiboy D, Andrews J M, Chow A T, Wise R. Open-label crossover study to determine pharmacokinetics and penetration of two dose regimens of levofloxacin into inflammatory fluid. Antimicrob Agents Chemother. 1995;39:2749–2751. doi: 10.1128/aac.39.12.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fish D N, Chow A. The clinical pharmacokinetics of levofloxacin. Clin Pharmacokinet. 1997;32:101–119. doi: 10.2165/00003088-199732020-00002. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin S D, Gallis H A, Chow A T, Wong F A, Flor S C, Bartlett J A. Pharmacokinetics and safety of levofloxacin in patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1994;38:799–804. doi: 10.1128/aac.38.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirschtick R E, Glassroth J, Jordans M C. Bacterial pneumonia in person infected with the HIV. N Engl J Med. 1995;333:845–851. doi: 10.1056/NEJM199509283331305. [DOI] [PubMed] [Google Scholar]
- 8.Marchbanks C R. Drug-drug interactions with fluoroquinolones. Pharmacotherapy. 1993;13(Suppl.):23S–28S. [PubMed] [Google Scholar]
- 9.Radant J M, Marchbanks C R, Dudley M N. Interactions of fluoroquinolones with other drugs: mechanisms, variability, clinical significance and management. Clin Infect Dis. 1992;14:272–284. doi: 10.1093/clinids/14.1.272. [DOI] [PubMed] [Google Scholar]
- 10.Regazzi M B, Villani P, Maserati R, Seminari E, Pan A, LoCaputo F, Gambarana E, Fiocchio C. Clinical pharmacokinetics of nelfinavir combined with efavirenz and stavudine during the rescue treatment of heavily pretreated HIV-infected patients. J Antimicrob Chemother. 2000;45:343–347. doi: 10.1093/jac/45.3.343. [DOI] [PubMed] [Google Scholar]
- 11.Tobin C M, Sunderland J, White L O, MacGowan A P. A reverse-phase isocratic high-performance liquid chromatography assay for levofloxacin. J Antimicrob Chemother. 1999;43:434–435. doi: 10.1093/jac/43.3.434. [DOI] [PubMed] [Google Scholar]
- 12.Trampuz A, Wenk M, Rajacic Z, Zimmerli W. Pharmacokinetics and pharmacodynamics of levofloxacin against Streptococcus pneumoniae and Staphylococcus aureus in human skin blister fluid. Antimicrob Agents Chemother. 2000;44:1352–1355. doi: 10.1128/aac.44.5.1352-1355.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villani P, Pregnolato M, Banfo S, Rettani M, Burroni D, Seminari E, Maserati R, Regazzi M B. High-performance liquid chromatographic method for analysing the antiretroviral agent efavirenz in human plasma. Ther Drug Monit. 1999;21:346–350. doi: 10.1097/00007691-199906000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Villani P, Regazzi M B, Castelli F, et al. Pharmacokinetics of efavirenz (EFV) alone and in combination therapy with nelfinavir (NFV) in HIV-1 infected patients. Br J Clin Pharmacol. 1999;48:712–715. doi: 10.1046/j.1365-2125.1999.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu E Y, Wilkinson J M, Naret D G, Daniels V L, Williams L J, Khalil D A, Shetty B V. High-performance liquid chromatographic method for the determination of nelfinavir, a novel HIV-1 protease inhibitor, in human plasma. J Chromatogr B Biomed Sci Appl. 1997;695:373–380. doi: 10.1016/s0378-4347(97)00193-x. [DOI] [PubMed] [Google Scholar]


