Highlights
-
•
Elevated lipoprotein(a) [Lp(a)] is a common inherited condition associated with atherosclerotic cardiovascular disease.
-
•
Elevated Lp(a) is not routinely tested in clinical practice and most cases remain undiagnosed in the community.
-
•
We identified 124 relatives with elevated Lp(a) (≥50 mg/dL) from 83 affected adult probands who also had dyslipidemia.
-
•
We also demonstrate that follow-up management is effective in lowering low-density lipoprotein-cholesterol levels by 34% as a consequence of initiation of lipid-lowering therapy.
-
•
Cascade testing families for elevated Lp(a) from affected probands with dyslipidemia is an effective and acceptable approach for identifying new cases of elevated Lp(a) who will require management of modifiable risk factors, particularly hypercholesterolemia.
Keywords: Lipoprotein(a), Cascade testing, Cardiovascular disease
Abstract
Objective
Elevated lipoprotein(a) [Lp(a)] is a common inherited condition associated with cardiovascular disease. This study investigated whether cascade testing for Lp(a) was effective in detecting new cases of elevated Lp(a) in families.
Methods
Relatives from adult probands with Lp(a) concentration ≥100 mg/dL were tested for elevated Lp(a) (≥50 mg/dL) via a cascade testing program in a tertiary hospital setting. The prevalence and yield of detecting new cases of elevated Lp(a) among the relatives were assessed.
Results
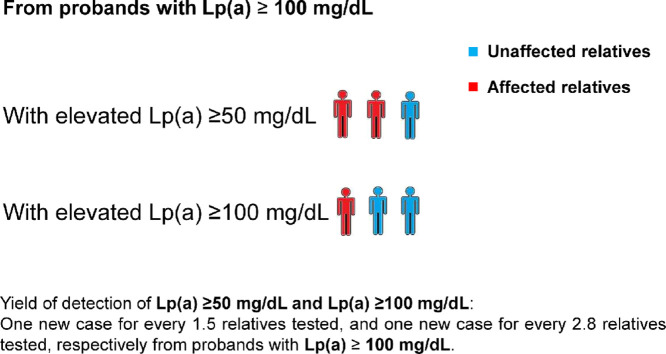
Of the 83 probands, 43.4% had familial combined hyperlipidemia (FCHL) and 34.9% common hypercholesterolemia (CH). Among 182 relatives tested (151 adults and 31 children), elevated Lp(a) was found in 68.1%, with 32.9% having Lp(a) between 50 and 99 mg/dL and 35.2% having Lp(a) ≥100 mg/dL. One new case of elevated Lp(a) ≥50 mg/dL was identified for every 1.5 relatives tested and 1 new case of elevated Lp(a) ≥100 mg/dL for every 2.8 relatives tested. The proportion of relatives detected with elevated Lp(a) was significantly higher when tested from probands with Lp(a) >150 mg/dL compared with those with Lp(a) between 100 and 150 mg/dL (81.1% vs. 55.5%; P = 0.001). The concordance rates (kappa coefficient) for the detection of elevated Lp(a) with FCHL and CH were 34.8% (0.026) and 53.2% (0.099), respectively.
Conclusion
Cascade testing for elevated Lp(a) from affected probands with phenotypic dyslipidemia is highly effective in identifying new cases of high Lp(a) in families. The yield of detecting elevated Lp(a) is greater when probands have higher levels of Lp(a) and exceeds the detection of relatives with FCHL and CH.
Central illusion
1. Introduction
Epidemiological and genetic studies confirm evidence that elevated plasma lipoprotein(a) [Lp(a)] concentration results in atherosclerotic cardiovascular disease (ASCVD) and calcific aortic valve disease [1], [2], [3]. Lp(a) has inflammatory, oxidative, and anti-fibrinolytic properties [4,5]. Current guidelines propose that plasma Lp(a) levels >50 mg/dL is a risk-enhancing factor for ASCVD [6], [7], [8], [9]. However, elevated Lp(a) is not routinely tested in clinical practice.
Lp(a) consists of a low-density lipoprotein (LDL)-particle covalently linked to a highly polymorphic apolipoprotein(a) [apo(a)] moiety [10]. Lp(a) concentrations are 70–90% heritable and determined by the LPA gene locus [4,5,10]. The plasma concentrations of Lp(a) tend to remain relatively constant throughout life [6]. Expert guidelines recommend testing for elevated Lp(a) in individuals at intermediate or high risk of ASCVD, and in patients with a personal or family history of premature coronary artery disease (CAD), including those with familial hypercholesterolemia (FH) [6], [7], [8], [9].
Genetic cascade testing is a cost-effective strategy for detecting FH in close relatives of affected index cases [11]. Cascade testing for elevated Lp(a) is also a feasible and effective approach for identifying new cases of elevated Lp(a) during genetic cascade testing for FH [12]. No studies have, however, been reported in families without FH.
In the present investigation, we examined the hypothesis, based on previous studies in FH [12,13], that cascade testing is effective in detecting new cases of elevated Lp(a) among close relatives of probands with elevated Lp(a) concentration and dyslipidemias other than FH. The primary objective was to describe the yield of detecting elevated Lp(a) from cascade testing relatives of probands with common dyslipidemias, other than FH, presenting to a lipid clinic. Secondary objectives were to describe the spectrum of cardiovascular factors and the initiation of lipid-lowering therapy (LLT) in affected relatives, as well as the experience of screenees of the cascade testing process.
2. Methods
We report on a selected group of patients who participated in an Lp(a) cascade testing program carried out in the Lipid Disorders Clinic between 2016 and 2021. Affected probands with Lp(a) ≥100 mg/dL were identified from patients with phenotypic dyslipidemias [e.g., familial combined hyperlipidemia (FCHL) and common hypercholesterolemia (CH) with no detectable FH gene variants], referred by general practitioners (GP), cardiologists and other specialists to the clinic. The selection of a cut-off Lp(a) concentration ≥100 mg/dL was based on a pilot study showing that the detection rate for relatives with elevated Lp(a) at this level was significantly higher than those with Lp(a) concentration between 50 and 99 mg/dL (Fig. 1 in online supplementary material; 88.9% vs 40.0%, P = 0.003). Accordingly, 83 probands with plasma concentration ≥100 mg/dL and their relatives agreed to participate in the cascade testing program, with at least one family member being tested for Lp(a). A total of 182 relatives, including children and adolescents (n = 31), were tested for elevated Lp(a) (defined as ≥50 mg/dL) and their clinical details, including cardiovascular risk factors were recorded. Details of the cascade testing protocol, assessment of patient-reported experience, clinical definition, biochemical and statistical analyses are given in supplementary material. The study was approved by the Royal Perth Hospital (Quality Activity 17,496) and University of Western Australia Human Research Ethics office RA/4/20/5553.
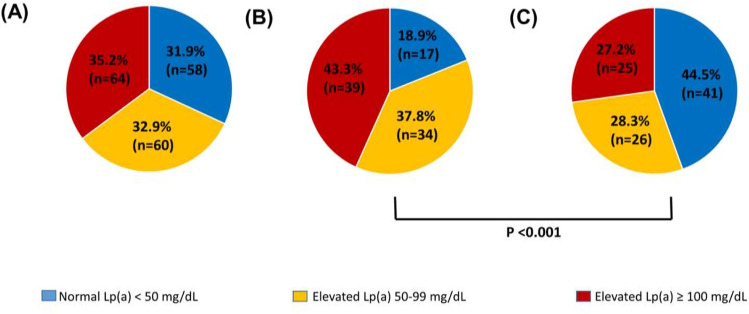
Fig. 1.
Outcomes of cascade testing for elevated lipoprotein(a) [Lp(a)] ≥50 mg/dL from probands with Lp (a) ≥100 mg/dL (n = 83) (A); Lp(a) >150 mg/dL (n = 42) (B) and Lp(a) 100–150 mg/dL (n = 41) (C).
3. Results
3.1. Subject characteristics
The demographic, clinical and biochemical characteristics of the 83 probands and 151 adult relatives are described in Table 1. Compared with adult relatives, probands were older (P < 0.001), more likely to be male (P < 0.05) and had a higher prevalence of CAD (P < 0.001), smoking (P < 0.01) and hypertension (P < 0.001). The proportion of receiving cholesterol-lowering therapy (statin and/or ezetimibe) was significantly higher in probands than relatives. Plasma concentrations of pre-treatment cholesterol, triglycerides, non-HDL-cholesterol, LDL-cholesterol and apolipoprotein B, as well as Lp(a), were significantly higher in probands than in adult relatives (P < 0.001 for all). There were no statistically significant differences in body mass index (BMI), plasma glucose concentration and estimated glomerular filtration rate (eGFR) between probands and adult relatives. Among subjects without a personal history of CAD (Table 1), the proportion with low risk of ASCVD was greater in relatives than probands (P < 0.01), the proportion with moderate risk of ASCVD being conversely greater in relatives (P < 0.01).
Table 1.
Demographic, clinical and biochemical characteristics of the probands and adult relatives tested for elevated lipoprotein(a).
| Characteristics | Probands | Adult relatives |
|---|---|---|
| Number | 83 | 151 |
| Age (years) Male, n (%) Body mass index (kg/m2) |
52.5 ± 12.5*** 45 (54.2)* 27.5 ± 4.7 |
41.1 ± 15.8 62 (40.8) 26.5 ± 4.6 |
| Family history of CAD, n (%) Family history of premature CAD, n (%) Personal history of CAD, n (%) |
76 (91.6) 43 (67.2) 38 (45.8)*** |
142 (93.4) 101 (66.9) 8 (5.3) |
| Smokers (current/ex), n (%) Hypertension, n (%) Type 2 Diabetes, n (%) Obesity (>30 kg/m2), n (%) |
40 (48.2)** 39 (47.0)*** 6 (7.2) 22 (26.5) |
40 (29.2) 18 (12.9) 4 (2.8) 23 (18.1) |
| On cholesterol-lowering medication, n (%) Statin, n (%) Ezetimibe, n (%) |
60 (72.3)*** 57 (68.7)*** 25 (30.1)*** |
22 (15.3) 22 (15.3) 3 (2.1) |
| Pre-treatment Total cholesterol (mmol/L) Triglycerides (mmol/L)1 HDL-cholesterol (mmol/L) Non-HDL-cholesterol (mmol/L) LDL-cholesterol (mmol/L) Apolipoprotein B (g/L) |
7.6 ± 1.6*** 1.9 (1.7 – 2.1) *** 1.4 ± 0.4 6.2 ± 1.4*** 5.4 ± 1.6*** 1.5 ± 0.4*** |
5.8 ± 1.3 1.3 (1.2 – 1.4) 1.4 ± 0.4 4.3 ± 1.3 3.7 ± 1.2 1.1 ± 0.3 |
| Lipoprotein(a) (mg/dL)1 | 156.3 (146.2 – 167.1) *** | 56.4 (47.6 – 66.8) |
| Plasma glucose (mmol/L) eGFR (mL/min/1.73 m2) |
5.3 ± 1.0 82.1 ± 12.0 |
5.1 ± 1.0 83.9 ± 10.6 |
| Estimated 5-year ASCVD risk2 Low, n (%) Moderate, n (%) High, n (%) |
29 (69.0)** 13 (31.0)** 0 (0.0) |
111 (87.4) 15 (11.8) 1 (0.8) |
ASCVD: atherosclerotic cardiovascular disease, CAD: coronary artery disease, HDL: high-density lipoprotein.
LDL: low-density lipoprotein, eGFR: estimated glomerular filtration rate.
Values represented as mean ± SD or geometric mean (95% confidence intervals) or number (%).
1Skewed variable with log transformation.
2Five-year cardiovascular risk assessment in probands and adult relatives: Low <3%, Moderate 3 – 14%, and High >15%.
*P < 0.05; **P < 0.01; and ***P < 0.001 when compared with adult relatives.
Compared with adults, children and adolescents tested for elevated Lp(a) (n = 31) were more likely to be male (62%), non-obese (BMI 22.0 ± 4.2 kg/m2) and normolipidemic (total cholesterol 4.7 ± 0.8 mmol/L, triglycerides 0.9 [0.7–1.1 mmol/L] and LDL-cholesterol 3.2 ± 0.6 mmol/L); none had a personal history of CAD, hypertension and type 2 diabetes, or were on cholesterol-lowering medication.
3.2. Outcome of cascade testing for elevated Lp(a)
Fig. 1 shows the proportion of relatives with elevated Lp(a) (≥ 50 mg/dL) according to the Lp(a) concentrations of probands. Overall, elevated Lp(a) was found in 68.1% of relatives, 32.9% having Lp(a) between 50 and 99 mg/dL and 35.2% ≥100 mg/dL (Fig. 1A). The proportion of relatives detected with elevated Lp(a) was significantly greater (81.1% vs 55.5%; P = 0.001) when tested from probands with Lp(a) >150 mg/dL (Fig. 1B) compared with probands with Lp(a) between 100 and 150 mg/dL (Fig. 1C). The proportion of first- and second-degree relatives of probands cascade tested for high Lp(a) were 94.0% (n = 171) and 6.0% (n = 11), respectively. Of the 11 second-degree relatives, 6 were first-degree relatives from an index case with elevated Lp(a) and the others we tested for practical and preferential reasons as second-degree relatives of the probands.
One new case of elevated Lp(a) ≥50 mg/dL was identified for every 1.5 relatives tested and 1 new case of elevated Lp(a) ≥100 mg/dL for every 2.8 relatives tested (see Central Illustration). One new case of elevated Lp(a) ≥50 mg/dL was also identified for every 1.8 relatives with probands having Lp(a) between 100 and 150 mg/dL and 1 new case for every 1.2 tested with probands Lp(a) concentration >150 mg/dL. The Lp(a) concentration of probands was a significant positive predictor of the probability of detecting a relative with elevated Lp(a) (odds ratio 1.45; 95% CI 1.19-1.81 for each 25 mg/dL increment of plasma Lp(a); P < 0.001).
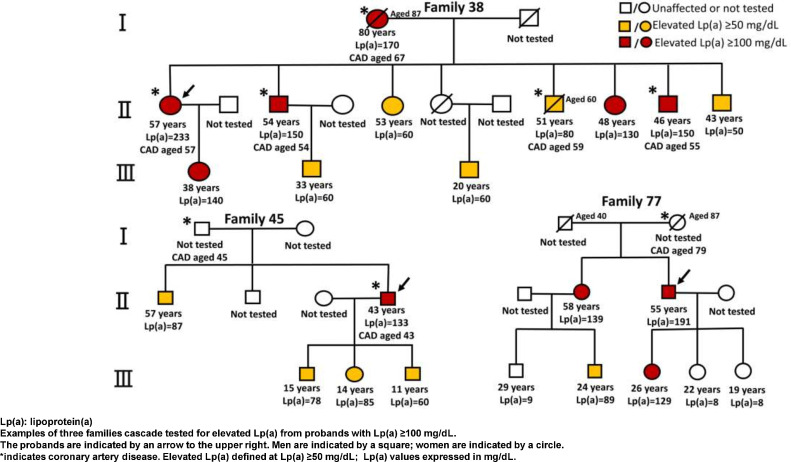
Fig. 2 shows the pedigrees of three selected families in whom the yield of detection of elevated Lp(a) was particularly high. In family 38 (in which the proband had a very high Lp(a) at 233 mg/dL), all ten relatives tested were found to have Lp(a) >50 mg/dL, with 5 having Lp(a) >100 mg/dL. In family 45, all four relatives tested were identified with Lp(a) ≥50 mg/dL. Of six relatives tested in family 77, three were identified with Lp(a) ≥50 mg/dL, with two ≥100 mg/dL.
Fig. 2.
Examples of pedigrees with elevated lipoprotein(a); relatives were tested over 2-to 3 generations from probands with high lipoprotein(a) [see arrow).
3.3. Cost estimation
The cost of Lp(a) assays for the 182 relatives was estimated to be A$3276. This cost was less than the estimated cost of staff time associated with contacting relatives, booking appointments, nurse review of relatives, consultant review, and counselling regarding cascade testing of relatives. The total staff costs associated with these activities were estimated to be A$68,381; the cost per case detected was A$551 (Table 1 in online supplementary material).
3.4. Association of elevated Lp(a) with lipid phenotypes in adult relatives
Of the 83 probands, 43.4% had FCHL and 34.9% CH, the remaining having isolated elevated Lp(a) and unclassified mixed hyperlipidemia. There was no significant difference (P = 0.449) in the proportion of relatives identified with elevated Lp(a) from probands with FCHL (67.0%) and CH (77.4%) (Fig. 2 in online supplementary material). The concordance rate between the detection of Lp(a) ≥50 mg/dL and the detection of FCHL among relatives of probands with FCHL was 34.8%, with a corresponding kappa statistic of 0.026. A low concordance rate was also observed with CH (53.2%, kappa coefficient 0.099). These data confirm the independent heritability of high Lp(a) and FCHL or CH (Table 1 in online supplementary material).
3.5. Characteristics of adult relatives according to Lp(a) concentration
Table 2 shows the characteristics of adult relatives according to their plasma Lp(a) concentrations. Relatives with Lp(a) between 50 and 99 mg/dL were significantly younger than those with Lp(a) ≥100 mg/dL (P < 0.05). Compared with relatives with Lp(a) <50 mg/dL, those with Lp(a) ≥100 mg/dL were more likely to have a family and personal history of CAD and be receiving treatment with cholesterol-lowering drugs. Plasma concentrations of pre-treatment cholesterol (P < 0.001), triglycerides (P < 0.05), non-HDL-cholesterol (P < 0.001), LDL-cholesterol (P < 0.001), apolipoprotein B and Lp(a) (P < 0.001) were also significantly higher in those with Lp(a) concentration ≥100 mg/dL compared with other groups. Relatives with Lp(a) concentration between 50 and 99 mg/dL had significantly higher LDL-cholesterol, apolipoprotein B and Lp(a) than those with Lp(a) <50 mg/dL (P < 0.05 for all). There were no significant differences in other variables among the groups (P > 0.05 for all).
Table 2.
Comparison of demographic, clinical and biochemical characteristics of adult relatives tested for elevated lipoprotein(a).
| Adult Relatives with Lp(a) concentration | P-value | |||
|---|---|---|---|---|
| <50 mg/dL | 50 – 99 mg/dL | ≥100 mg/dL | ||
| Number | 45 | 43 | 63 | |
| Age (years) Male, n (%) Body mass index (kg/m2) |
40.0 ± 16.8 16 (35.6) 27.1 ± 4.8 |
37.0 ± 13.8* 21 (48.8) 25.6 ± 3.9 |
45.1 ± 15.7 25 (39.7) 26.8 ± 4.9 |
0.023 0.492 0.335 |
| Family history of CAD, n (%) Personal history of CAD, n (%) |
38 (84.4) 1 (2.2) |
41 (95.3) 0 (0.0) |
63 (98.4)*** 7 (11.1) |
0.015 0.034 |
| Smokers (current/ex), n (%) Hypertension, n (%) Type 2 Diabetes, n (%) Obesity (>30 kg/m2), n (%) |
9 (23.7) 3 (7.5) 2 (5.0) 8 (24.2) |
9 (22.0) 5 (12.2) 2 (4.8) 4 (9.8) |
21 (36.8) 10 (17.2) 0 (0.0) 11 (21.2) |
0.252 0.358 0.177 0.197 |
| On cholesterol-lowering medication, n (%) Statin, n (%) Ezetimibe, n (%) |
2 (5.1) 2 (5.1) 0 (0.0) |
3 (7.1) 3 (7.1) 1 (2.4) |
17 (27.4)†† 17 (27.4)†† 2 (3.2) |
0.002 0.002 0.787 |
| Pre-treatment Total cholesterol (mmol/L) Triglycerides (mmol/L)1 HDL-cholesterol (mmol/L) Non-HDL-cholesterol (mmol/L) LDL-cholesterol (mmol/L) Apolipoprotein B (g/L) |
5.0 ± 1.3 1.2 (0.9 – 1.4) 1.4 ± 0.3 3.6 ± 1.3 3.0 ± 1.1 0.9 ± 0.3 |
5.5 ± 1.0 1.2 (1.1 – 1.4) 1.3 ± 0.3 4.1 ± 1.0 3.5 ± 1.0** 1.1 ± 0.3** |
6.4 ± 1.2††† 1.5 (1.3 – 1.7)† 1.5 ± 0.5 4.9 ± 1.2††† 4.2 ± 1.2†† 1.3 ± 0.3††† |
<0.001 0.044 0.162 <0.001 <0.001 <0.001 |
| Lipoprotein(a) (mg/dL)1 | 12.9 (10.9 – 15.3) | 71.9 (67.5 – 76.5)*** | 136.2 (129.0 – 143.9)†† | <0.001 |
| Plasma glucose (mmol/L) eGFR (mL/min/1.73 m2) |
5.0 ± 0.7 84.9 ± 9.7 |
5.2 ± 1.7 82.1 ± 11.7 |
5.0 ± 0.5 84.4 ± 10.4 |
0.469 0.572 |
| Estimated 5-year ASCVD risk2 Low, n (%) Moderate, n (%) High, n (%) |
32 (88.9) 4 (11.1) 0 (0.0) |
34 (85.0) 5 (12.5) 1 (2.5) |
44 (88.0) 6 (12.0) 0 (0.0) |
0.886 0.988 0.448 |
ASCVD: atherosclerotic cardiovascular disease CAD: coronary artery disease, HDL: high-density lipoprotein, LDL: low-density lipoprotein,.
eGFR: estimated glomerular filtration rate.
Values represented as mean ± SD or geometric mean (95% confidence intervals) or number (%).
Bold P values are significant between groups by ANOVA or Chi-square, where appropriate.
1Skewed variable with log transformation.
2Five-year cardiovascular risk assessment in probands and adult relatives: Low <3%, Moderate 3 – 14%, and High >15%.
*P <0.05 when compared with adult relatives with Lp(a) ≥100 mg/dL.
**P <0.05; and *** P <0.01 when compared with adult relatives with Lp(a) <50 mg/dL.
†P <0.05.
††P <0.01; and †††P <0.001 when compared with the other two groups.
3.6. Initiation of lipid-lowering therapy
Eighty-six of the relatives identified with Lp(a) ≥50 mg/dL were not on LLT (Table 2). Following participating in the Lp(a) detection program, 30.2% (n = 26) were initiated on LLT (Table 2 in online supplementary material). Further details of the characteristics of the relatives treated are given in supplementary material.
3.7. Participant reported experience of cascade testing
Of the 34 participants who completed the cascade testing questionnaire, 91.2% agreed they understood why they were approached for testing, 88.2% felt they were free to choose whether to participate in testing, 73.5% were satisfied with counselling received and 85.3% with the risk notification of result (Table 5 in online supplementary material).
4. Discussion
Consistent with our primary hypothesis derived from previous studies in FH [12,13], we demonstrated that cascade testing families for elevated Lp(a) from affected probands with dyslipidemia was an effective and acceptable approach for identifying new cases of elevated Lp(a). The yield of detecting elevated Lp(a) was also directly dependent on the Lp(a) concentration of probands. Our findings also suggest a potential role of cascade testing for elevated Lp(a) in improving the management of affected family members at risk of ASCVD.
4.1. Previous studies: familial hypercholesterolemia
By contrast to previous reports, our study is the first to investigate the outcome of cascade testing for elevated Lp(a) from probands without a diagnosis of FH. Two previous studies have demonstrated the effectiveness of testing for elevated Lp(a) when genetic cascade testing for FH [12,13]. We have extended previous reports by confirming the value of cascade testing for elevated Lp(a) from probands who principally had common dyslipidemia that can mimic FH (i.e., FCHL and CH, both polygenic dyslipidemias).
4.2. Detection and characteristics of relatives
The effectiveness of cascade testing in detecting elevated Lp(a) was underscored by our demonstration of an average of one new case of elevated Lp(a) per two relatives tested. This is consistent with the autosomal co-dominant heritability of Lp(a) that predicted in approximately 50% detection of elevated Lp(a) among relatives [10,14]. Consistent with previous reports [12,13], we found a higher proportion of relatives with high Lp(a) when testing from first-degree compared with second-degree relatives, although the population of relatives tested was relatively small. We also detected a higher proportion of relatives with elevated Lp(a) from probands with Lp(a) concertation >150 mg/dL than from probands with lower concentrations (i.e., between 100 and 150 mg/dL). These observations are compatible with the dilutional effect of the heritability of Lp(a) with an increase in separation of relatives from affected probands and with greater penetrance within families of genetic defects causative of high Lp(a) [e.g., copy number variation leading to smaller apo(a) isoform size] with probands with more severe elevation in Lp(a) [4,5,10].
The cost per case detected was A$551. If applied nationwide, the marginal cost per index case would be A$824 (i.e., a total cost of $68,381 with 83 probands). Given a population prevalence of elevated Lp(a) of 20%, of whom 2% have been hitherto identified in Australia (current population 25.7 million), this gives a one-off cost of cascade testing all known cases (n = 102,760) of A$84.687 million in Australia. Assuming early diagnosis and effective management of elevated Lp(a) reduces ASCVD risk, this is likely to represent a cost-effective use of resources. However, this estimate does not include the costs prevented from downstream events, and hence may over-estimate the net cost of cascade testing. A more detailed cost-utility analysis is required.
Probands with high Lp(a) were identified from patients with common dyslipidemias, typically FCHL and CH. We found comparable detection rates of elevated Lp(a) among relatives of probands with FCHL or CH, both being polygenic disorders [15], [16], [17]. Accordingly, there was a poor agreement between the detection of FCHL (or CH) and elevated Lp(a) among the relatives. A similar discordance between FH and elevated Lp(a) was previously reported [12,13]. These data accord with the notion that the heritability of Lp(a) is distinct and independent of other dyslipidemias, including FH, FCHL and CH [12,13,[15], [16], [17]].
We found that compared with probands, adult relatives tested for elevated Lp(a) had an overall lower prevalence of symptomatic CAD, smoking, hypertension, and were less likely to be receiving cholesterol-lowering medication. This was anticipated because they were younger than probands. Among relatives without symptomatic CAD, the estimated 5-year risk of ASCVD was also lower in relatives with probands. It is noteworthy that, as with other methods assessing ASCVD risk, PREDICT does not include Lp(a) as a predictor variable [18]. Hence, this approach may not fully reflect the absolute risk of ASCVD in relatives detected with elevated Lp(a). Elevated Lp(a) has been recognized as a risk-enhancing factor for ASCVD risk [6], [7], [8], [9]. Whether adding Lp(a) to the PREDICT algorithm can improve or reclassify estimated risk of ASCVD in relatives remains to be investigated, this being most relevant to those at moderate risk of ASCVD [19].
4.3. Initiation of lipid-lowering therapy in relatives
We showed that adult relatives with elevated Lp(a), particularly in those with Lp(a) ≥100 mg/dL, had a high frequency of smoking, hypertension, and dyslipidemia. This underscores the importance of lifestyle modification and LLT for mitigating modifiable cardiovascular risk in those who screen positive for Lp(a) [20]. We showed that among 30% of affected relatives initiated on LLT, there were clinically meaningful reductions in plasma concentrations of LDL-cholesterol and apoB-containing lipoproteins [21]. Beyond a higher Lp(a) concentration, those who started LLT were more likely to have FCHL, CH and a higher estimated absolute risk of ASCVD. Whether such changes in lipids and lipoproteins with treatment are associated with reductions in clinical outcomes remains to be determined. This information is important to inform the health economic assessment of cascade testing for Lp(a).
4.4. Strength and limitations: scope for further work
The strengths of our investigation include the use of a well-defined cohort of patients and a systematic approach to testing for elevated Lp(a), which was underpinned by previous experience with cascade testing for FH [12,13]. Based on a pilot study, we used a higher threshold of Lp(a) concentration ≥100 mg/dL in our study to define probands so as to enhance the detection rate of elevated Lp(a) among relatives. While ASCVD risk may begin at a threshold >30 mg/dL [2], our definition of elevated Lp(a) (i.e., ≥50 mg/dL) in relatives was based on current guidelines [6,8], corresponding to the 80th centile for Caucasian populations [6]. We carefully and phenotypically defined the dyslipidemias in our probands after excluding those with FH, noting FCHL and CH might have been more accurately defined using polygenic lipid scores [16,17]. The lack of a non-dyslipidemic control group is a potential limitation. However, we were limited to studying hyperlipidemia, non-FH patients who were referred to our clinical service by their family doctors and other specialists.
The sample size of our probands was relatively small, implying that the precision of our estimates of the detection of relatives with elevated Lp(a) might have been compromised. However, we purposely selected probands for having higher levels of Lp(a) in order to increase the yield and effectiveness of detecting relatives with Lp(a) ≥ 50 mg/dL. A potential limitation is that we based Lp(a) cascade testing using measurement of Lp(a) mass concentration instead of genetic testing. However, the genetics of Lp(a) is complex and does not improve prediction of CAD beyond mass or molar concentrations [22]. Lp(a) is also on average a quantitative genetic trait that reflects the impact of multiple gene variants or copy number variations within the LPA locus [10]. We concluded that participants were generally satisfied with the cascade testing process but based this on a self-selected small sample size who responded reported to an online request and did not undertake a saturation analysis in the remainder of the study population; further refinement of pre-test counselling methods may be warranted. Our use of an apo(a) isoform sensitive immunoassay to quantitate Lp(a) mass, with potential bias from the effects of larger and smaller isoform sizes [23], is another limitation. However, a close correlation between this immunoassay and an isoform-independent LCMS method has been previously reported [24]. We did not adjust LDL-cholesterol concentration for Lp(a) cholesterol in our probands and relatives with elevated Lp(a), because there is no standard correction factor for making this adjustment [25]. We did not test the Lp(a) concentration of the spouses/partners of probands, because this is not part of usual practice in our clinic. However, among the progeny tested, we only found 7 individuals tested from 6 families with Lp(a) concentration greater than concentration of the affected parents, implying that at least 10% of spouses/partners would have Lp(a) ≥ 50 mg/dL. Hence, knowledge of elevated Lp(a) in spouses/partners of probands may provide further justification for testing for elevated Lp(a) in offsprings during cascade testing for elevated Lp(a). Nevertheless, as reported elsewhere [13], the expected prevalence of Lp(a) ≥ 50 mg/dL among relatives from index cases who do not have elevated Lp(a) is 17% [13]. This implies that, in the majority of patients detected in our program, elevated Lp(a) levels could be attributable to the penetrance of heritability of LPA genotype [smaller apo(a) isoform] from affected probands. Finally, we reported mainly on the outcomes of detection among adults with Lp(a), recommending that further studies should be carried out in children [26]. Our adult population was also mainly of Caucasian ancestry and further studies are also indicated in other ethnic groups, especially Southern Asian who may have a particularly high Lp(a) mediated risk of ASCVD [4,5,27].
5. Conclusion and clinical implications
Elevated Lp(a) is a common risk factor for ASCVD in the community, with one in 5 individuals having a plasma concentration ≥50 mg/dL [4,5]. Detecting affected people is a priority, as emphasized by current guidelines [[6], [7], [8], [9],27]. Selective, opportunistic, and universal screening approaches have been promulgated. By contrast to families with probands with FH and high Lp(a), in whom cascade testing would normally be carried out principally to detect FH, our study in patients without FH suggests that a separate model of care for identifying index cases with high Lp(a) to cascade test families needs to be developed. However, none of these approaches has hitherto been established and implemented. We show the potential value and cost of cascade testing from probands with high Lp(a) and how to make testing more effective. However, a formal cost utility evaluation of this approach to detecting high Lp(a) is required. The acceptability of screening for elevated Lp(a) needs to be confirmed with clear demonstration that interventions that lower elevated Lp(a) reduce clinical risk of ASCVD. Other than lipoprotein apheresis, there is no approved intervention for specifically lowering Lp(a) by a sufficient amount likely to have a major impact on major cardiovascular events [28]. However, a clinical trial is underway to test this hypothesis in high-risk individuals utilizing an anti-sense oligonucleotide targeted at the mRNA transcript of the apo(a) gene that decreases the hepatic production of Lp(a) particles (NCT04023552) [29]. A potent and more durable form of RNA therapeutics, utilizing the principle of small interfering RNA, has also recently been described [30]. At present, the value of screening for elevated Lp(a), including the cascade testing approach described in the present study, is to mitigate cardiovascular risk by addressing modifiable behavioural and clinical risk factors with established interventions, including use of statins, ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors [6,27].
Funding
The study was supported by a Raine Medical Research Foundation Grant. AC is supported by an Australian Government Research Training Program (RTP) Scholarship. JP is supported by National Health and Medical Research Council (NHMRC) Investigator Grant.
Author Contributions
GFW, DCC, KLE and JP designed the study. AC, JP, KLE, WB, AMW, MV and GFW conducted the study. AC, DCC, KLE, JP, RW, RN, EKM and GFW analysed the data. AC, DCC and GFW drafted the manuscript. All authors reviewed and approved the manuscript.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: GFW has received honoraria for advisory boards and research grants from Amgen, Arrowhead, Esperion, AstraZenca, Kowa, Novartis, Pfizer, Sanofi and Regeneron.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2022.100343.
Appendix. Supplementary materials
References
- 1.Kamstrup P.R., Tybjaerg-Hansen A., Steffensen R., et al. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 2.Erqou S., Kaptoge S., Perry P.L., et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamstrup P.R., Tybjærg-Hansen A., Nordestgaard B.G. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–477. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Reyes-Soffer G., Ginsberg H.N., Berglund L., et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42:e48–e60. doi: 10.1161/ATV.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis K.L., Boffa M.B., Sahebkar A., et al. The renaissance of lipoprotein(a): brave new world for preventive cardiology? Prog Lipid Res. 2017;68:57–82. doi: 10.1016/j.plipres.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Wilson D.P., Jacobson T.A., Jones P.H., et al. Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13:374–392. doi: 10.1016/j.jacl.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Grundy S.M., Stone N.J., Bailey A.L., et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2018;139:e1082–ee143. doi: 10.1161/CIR.0000000000000625. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cegla J., Neely R.D.G., France M., et al. HEART UK consensus statement on Lipoprotein(a): a call to action. Atherosclerosis. 2019;291:62–70. [Google Scholar]
- 9.Mach F., Baigent C., Catapano A.L., et al. ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;41:111–188. doi: 10.1093/eurheartj/ehz455. 2020. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt K., Noureen A., Kronenberg F., et al. Structure, function, and genetics of lipoprotein (a) J Lipid Res. 2016;57:1339–1359. doi: 10.1194/jlr.R067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ademi Z., Watts G.F., Pang J., et al. Cascade screening based on genetic testing is cost-effective: evidence for the implementation of models of care for familial hypercholesterolemia. J Clin Lipidol. 2014;8:390–400. doi: 10.1016/j.jacl.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Chakraborty A., Pang J., Chan D.C., et al. Cascade testing for elevated lipoprotein(a) in relatives of probands with familial hypercholesterolaemia and elevated lipoprotein(a) Atherosclerosis. 2021 doi: 10.1016/j.atherosclerosis.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Ellis K.L., Perez de Isla L., Alonso R., et al. Value of measuring lipoprotein(a) during cascade testing for familial hypercholesterolemia. J Am Coll Cardiol. 2019;73:1029–1039. doi: 10.1016/j.jacc.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 14.Utermann G., Menzel H.J., Kraft H.G., et al. Lp(a) glycoprotein phenotypes. Inheritance and relation to Lp(a)-lipoprotein concentrations in plasma. J Clin Invest. 1987;80:458–465. doi: 10.1172/JCI113093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis K.L., Pang J., Chan D.C., et al. Familial combined hyperlipidemia and hyperlipoprotein(a) as phenotypic mimics of familial hypercholesterolemia: frequencies, associations and predictions. J Clin Lipidol. 2016;10:1329–1337. doi: 10.1016/j.jacl.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Trinder M., Vikulova D., Pimstone S., et al. Polygenic architecture and cardiovascular risk of familial combined hyperlipidemia. Atherosclerosis. 2022;340:35–43. doi: 10.1016/j.atherosclerosis.2021.11.032. [DOI] [PubMed] [Google Scholar]
- 17.Sharifi M., Futema M., Nair D., et al. Polygenic hypercholesterolemia and cardiovascular disease risk. Curr Cardiol Rep. 2019;21:43. doi: 10.1007/s11886-019-1130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pylypchuk R., Wells S., Kerr A., et al. Cardiovascular disease risk prediction equations in 400 000 primary care patients in New Zealand: a derivation and validation study. Lancet. 2018;391:1897–1907. doi: 10.1016/S0140-6736(18)30664-0. [DOI] [PubMed] [Google Scholar]
- 19.Verbeek R., Sandhu M.S., Hovingh G.K., et al. Lipoprotein(a) improves cardiovascular risk prediction based on established risk algorithms. J Am Coll Cardiol. 2017;69:1513–1515. doi: 10.1016/j.jacc.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Mullan B., Chan D., Charlesworth J., et al. Novel behavioural approaches and implementation science for mitigating genetic risk of cardiovascular disease due to elevated lipoprotein(a) Curr Opin Endocrinol Diabetes Obes. 2021;28:174–180. doi: 10.1097/MED.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 21.Marston N.A., Giugliano R.P., Melloni G.E.M., et al. Association of apolipoprotein B-containing lipoproteins and risk of myocardial infarction in individuals with and without atherosclerosis: distinguishing between particle concentration, type, and content. JAMA Cardiol. 2021 doi: 10.1001/jamacardio.2021.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page M.M., Ellis K.L., Pang J., et al. Coronary artery disease and the risk-associated LPA variants, rs3798220 and rs10455872, in patients with suspected familial hypercholesterolaemia. Clin Chim Acta. 2020;510:211–215. doi: 10.1016/j.cca.2020.07.029. [DOI] [PubMed] [Google Scholar]
- 23.Marcovina S.M., Albers J.J. Lipoprotein (a) measurements for clinical application. J Lipid Res. 2016;57:526–537. doi: 10.1194/jlr.R061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golledge J., Rowbotham S., Velu R., et al. Association of serum lipoprotein (a) with the requirement for a peripheral artery operation and the incidence of major adverse cardiovascular events in people with peripheral artery disease. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeang C., Witztum J.L., Tsimikas S. Novel method for quantification of lipoprotein(a)-cholesterol: implications for improving accuracy of LDL-C measurements. J Lipid Res. 2021;62 doi: 10.1016/j.jlr.2021.100053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson D.P., Koschinsky M.L., Moriarty P.M. Expert position statements: comparison of recommendations for the care of adults and youth with elevated lipoprotein(a) Curr Opin Endocrinol Diabetes Obes. 2021;28:159–173. doi: 10.1097/MED.0000000000000624. [DOI] [PubMed] [Google Scholar]
- 27.Virani S.S., Koschinsky M.L., Maher L., et al. Global think tank on the clinical considerations and management of lipoprotein(a): the top questions and answers regarding what clinicians need to know. Prog Cardiovasc Dis. 2022 Jan 19 doi: 10.1016/j.pcad.2022.01.002. S0033-0620(22)00002-0Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Madsen C.M., Kamstrup P.R., Langsted A., et al. Lipoprotein(a)-lowering by 50 mg/dL (105 nmol/L) may be needed to reduce cardiovascular disease 20% in secondary prevention: a population-based study. Arterioscler Thromb Vasc Biol. 2020;40:255–266. doi: 10.1161/ATVBAHA.119.312951. [DOI] [PubMed] [Google Scholar]
- 29.Tsimikas S., Moriarty P.M., Stroes E.S. Emerging RNA therapeutics to lower blood levels of Lp(a) J Am Coll Cardiol. 2021;77:1576–1589. doi: 10.1016/j.jacc.2021.01.051. [DOI] [PubMed] [Google Scholar]
- 30.Koren M.J., Moriarty P.M., Baum S.J., et al. Preclinical development and phase 1 trial of a novel siRNA targeting lipoprotein(a) Nat Med. 2022;28:96. doi: 10.1038/s41591-021-01634-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.