Abstract
Purpose
To report a case of multilayered intraocular hemorrhage at the posterior pole as a complication of transorbital neuroendoscopic surgery.
Observations
Our patient underwent an uncomplicated endoscopic transorbital resection of a left sphenoid wing meningioma. In the immediate post-operative period, the patient reported blurred vision of her left eye, and dilated fundus examination demonstrated multilayered hemorrhages at the posterior pole. No intracranial hemorrhage was identified on post-operative imaging. Due to persistent subnormal visual acuity and non-clearing hemorrhage over several weeks of follow-up, a pars plana vitrectomy with peeling of the internal limiting membrane was performed to clear the hemorrhagic component obscuring the macula.
Conclusions and Importance
We report the first case of multilayered intraocular hemorrhages at the posterior pole, mimicking Terson syndrome, in the absence of intracranial hemorrhage or elevated intracranial pressure as a complication of transorbital surgery.
Keywords: Terson syndrome, Transorbital, Neuroendoscopic, Surgery, Sphenoid wing, Spheno-orbital, Meningioma
1. Introduction
Multilayered intraocular hemorrhages of the posterior pole occur in association with intracranial hemorrhage in Terson syndrome.1 Notably, similar ophthalmic presentations have been reported in the absence of intracranial hemorrhage, but in association with elevated intracranial pressure (ICP), suggesting that an acute rise in ICP may be involved in the pathogenesis of Terson syndrome.2, 3, 4, 5 However, the origin of intraocular blood and the role of ICP fluctuation in the pathophysiology of Terson syndrome remains a matter of debate.6
Over recent years, endoscopic transorbital approaches have emerged as minimally invasive alternatives to transcranial approaches to the middle fossa skull base, including the cavernous sinus, Meckel's cave and greater and lesser wings of the sphenoid bone.7 One of the more common indications has been removal of sphenoid wing and spheno-orbital meningiomas.8,9 As a relatively novel surgical approach, the limitations and adverse events associated with this surgical technique are still being established.9 Here, we report the case of a patient undergoing resection of a left sphenoid wing meningioma via the endoscopic transorbital eyelid approach, complicated by acute vision loss secondary to a multilayered intraocular hemorrhage, in the absence of either intracranial hemorrhage or elevated ICP.
2. Case report
A 60-year-old previously healthy female with no past ocular history was admitted to our hospital for planned resection of a small left sphenoid wing meningioma with associated edema and hyperostosis via the endoscopic transorbital eyelid approach (Fig. 1). A baseline ophthalmic examination was obtained at the time of admission, which demonstrated visual acuity of 20/20, no relative afferent pupillary defect, full confrontational visual fields, and normal ocular motility in both eyes. Slit lamp examination was notable solely for early nuclear sclerotic cataracts in both eyes, and a dilated fundus exam was unremarkable.
Fig. 1.
Pre-operative magnetic resonance imaging
T1 post-contrast MRI (left) and T2 FLAIR (middle) showing dural-based mass and surrounding edema (arrows). Preoperative non-contrast computerized tomography scan showing left sphenoid bone hyperostosis underlying the tumor (arrows).
2.1. Transorbital surgical technique
The upper eyelid was incised, and orbicularis oculi was divided along the length of the incision to expose the lateral orbital rim periosteum (Fig. 2). A malleable retractor was used to retract the eye and orbital contents medially. The periosteum was incised at the arcus marginalis, and a Freer elevator was used to elevate the periosteum off the lateral orbital wall, to the margin of the superior orbital and inferior orbital fissures. The sphenoid bone between the superior and inferior orbital fissures was exposed. Computerized tomography image guidance was employed. Decompression of hyperostotic bone was conducted using high-speed drills and Kerrison rongeurs. The dura of the middle fossa where the meningioma was attached was incised and the tumor was removed from the temporal pole. The dural defect was repaired using a two-layer button technique with Allomax.10, 11, 12 Throughout the case, retraction on the orbit was ceased every 20 minutes, and interval pupillary examination remained normal. No intraoperative complications were encountered. Notably, there was no hemodynamic Cushing response of bradycardia and hypertension during the procedure. During dural opening there was no evidence of elevated ICP, with no brain herniation or cerebrospinal fluid leak. Post-operative MRI scan revealed a radiographic gross total resection (Fig. 3).
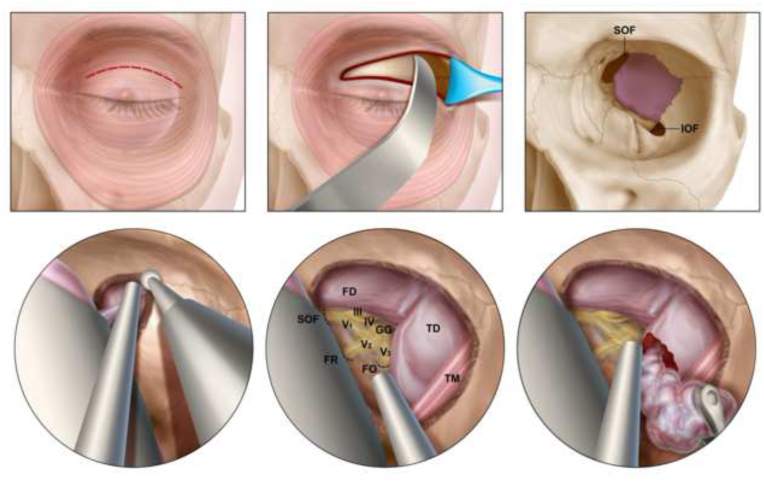
Fig. 2.
Illustration demonstrating the endoscopic transorbital eyelid approach for the resection of spheno-orbital meningiomas
The upper eyelid is incised, and the orbital contents are retracted medially (upper left and central panels). The sphenoid bone between the superior orbital fissure and inferior orbital fissures is exposed (upper right). Decompression of the hyperostotic bone is performed (bottom left). Key landmarks are identified including the superior orbital fissure, the foramen rotundum, the foramen ovale, the geniculate ganglion as well as the dura overlying the frontal and temporal lobes (bottom center). The dura is incised, and the tumor decompressed (bottom right). FD = frontal dura; TD = temporal dura; SOF = superior orbital fissure; FR = foramen rotundum; FO = foramen ovale; III = CN III (oculomotor nerve); IV = CN IV (trochlear nerve); GG = gasserian ganglion; V1 = ophthalmic branch of CN V (trigeminal nerve); V2 = maxillary branch of CN V; V3 = mandibular branch of CN V; TM = temporalis muscle.
Fig. 3.
Post-operative magnetic resonance imaging
3-month post-operative T1 post-contrast MRI (left) and T2 FLAIR (middle) showing resection of meningioma and resolution of surrounding edema. Postoperative day 1 non-contrast computerized tomography scan showing resection of hyperostotic component of sphenoid bone (right).
2.2. Post-operative course
Post-operatively, the patient complained of blurry central vision of the left eye, noting that she felt as if she were looking through a ‘red cloud’. Visual acuity in the left eye was 20/400. No relative afferent pupillary defect was observed. Intraocular pressure in the left eye was 16 mmHg. Confrontational visual fields were intact and ocular motility was full. Dilated fundus examination of the left eye revealed multilayered hemorrhage of the posterior pole, with a boat-shaped subhyaloid hemorrhage in the macula, and an area of subretinal hemorrhage extending inferotemporally underneath the inferior arcade. No intracranial hemorrhage was identified on post-operative magnetic resonance imaging. Fundus images and optical coherence tomography slices from the patient's first visit to the retina clinic ten days later are shown (Fig. 4). The patient was followed closely by our retina service over the ensuing weeks to monitor for spontaneous resolution, with visual acuity fluctuating between 20/100 and 20/400. Given persistent subnormal visual acuity at five weeks post-operatively, the patient elected to proceed with pars plana vitrectomy with peeling of the internal limiting membrane, with successful clearance of the component of the hemorrhage obscuring the macula (Fig. 5).
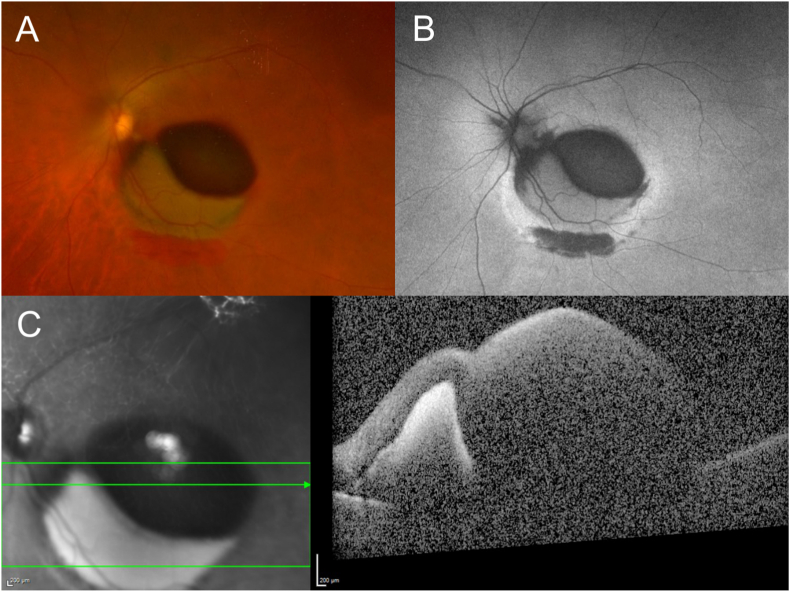
Fig. 4.
Multimodal imaging performed 10 days after neuroendoscopic surgery
Multilayered hemorrhages of the posterior pole are shown on Optos imaging and autofluorescence (A and B, respectively), as well as spectral-domain optical coherence tomography (C).
Fig. 5.
Multimodal imaging performed 1 week following pars plana vitrectomy with peeling of the internal limiting membrane
The component of the hemorrhage obscuring the macula is cleared after peeling the internal limiting membrane. Optos imaging and autofluorescence are shown (A and B, respectively), in addition to spectral-domain optical coherence tomography (C).
Six months following vitrectomy, the patient's left eye visual acuity was 20/50.
3. Discussion
An association between intraocular hemorrhage and intracranial hemorrhage has been recognized for well over a century.13 First reported by the German ophthalmologist Moritz Litten in 1881, it was the French ophthalmologist Albert Terson whose 1900 report resulted in the eponymous title Terson syndrome.13,14 Indeed, Terson syndrome is commonly observed in the setting of aneurysmal subarachnoid hemorrhage, in which vitreous hemorrhage has been reported to occur in 12–29% patients.1,15,16 However, the definition of Terson syndrome has shifted over the decades, and is now commonly used to describe the association of an acutely elevated ICP with intraocular hemorrhages, even in the absence of intracranial hemorrhage.2, 3, 4, 5 Here, we present a Terson-like clinical picture of multilayered hemorrhage at the posterior pole, as a complication of transorbital neuroendoscopic surgery, occurring in the absence of either elevated ICP or intracranial hemorrhage.
The leading theory to explain the pathophysiology of Terson syndrome posits that a rapid increase in ICP causes a pressure wave within the retrobulbar optic nerve sheath, thereby causing venous hypertension and the rupture of thin retinal capillaries.17,18 However, this theory is not without controversy.6 Alternative explanations include the direct transmission of subarachnoid blood forward through the optic nerve sheath19 and reflux of blood in subarachnoid cisterns through glymphatic channels into the globe.6 For clarity, and to distinguish the case presented here from those presentations with elevated ICP, we avoid referring to the reported case as Terson's syndrome, instead describing the fundoscopic findings of multilayered macular hemorrhages, noting that these mimic the exam findings in Terson's.
Two previous cases have been reported in the neurosurgical literature of iatrogenic Terson syndrome.20,21 Both cases were neuro-endoscopic procedures (a third ventriculostomy21 and a left frontal ventriculoscopy20) in which a hemodynamic Cushing response with severe bradycardia and hypertension was noted intra-operatively following initiation of irrigation, which resolved with cessation of irrigation and removal of the endoscope. However, intraventricular endoscopy bears little resemblance to transorbital endoscopy. In these other reported cases, both patients reported blurry vision post-operatively and were found to have subretinal, intraretinal and pre-retinal hemorrhages in both eyes. Our report differs from these cases as no hemodynamic issues were noted intra-operatively, with no evidence of raised ICP. Moreover, our case exhibits unilateral rather than bilateral involvement.
There are several possible explanations for the Terson-like presentation observed in our patient. Manipulation of the posterior globe or optic nerve during the case may have induced transient increases in venous pressure, resulting in retinal capillary rupture. Similarly, local pressure fluctuations at the orbital apex during bone decompression or tumor resection may have been transmitted anteriorly, along the optic nerve sheath, with the same effect. Although there was no evidence of raised ICP intraoperatively, either of these explanations would mimic the putative pathophysiology of Terson syndrome resulting from raised ICP causing a pressure wave in the retrobulbar optic nerve sheath to be transmitted anteriorly, causing rupture of retinal capillaries. Finally, sphenoid wing meningiomas may compress venous channels including the superficial sylvian veins, and resection could result in transient venous compression, with a resultant elevation in cavernous sinus and ophthalmic vein pressure.22 The case reported here is not consistent with either the theory of direct transmission of subarachnoid blood forward through the optic nerve sheath or reflux of blood in subarachnoid cisterns through glymphatic channels into the globe, given that there was no evidence of intracranial hemorrhage.
4. Conclusions
This case describes a patient developing multilayered macular hemorrhages following transorbital neuro-endoscopic resection of a left sphenoid wing meningioma. The purpose of reporting this case is twofold. Firstly, the report identifies intraocular hemorrhage as a potential complication of this surgical approach, one that has not yet to our knowledge been reported. Secondly, in describing a Terson-like syndrome occurring without intracranial hemorrhage, our case favors an understanding of Terson syndrome in which observed hemorrhage at the posterior pole is derived from an intraocular, rather than intracranial, source. Although there was no evidence of raised ICP in our case, there are plausible explanations for retrobulbar pressure fluctuations in the surgical approach described, which may have simulated the pressure fluctuations due to rapid effusion of cerebrospinal fluid into the optic nerve sheath during an acute elevation of ICP.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editor of this journal.
Funding
This project was supported in part by a department grant from the Research to Prevent Blindness Foundation.
Authors’ contributions
All authors attest that they meet the current ICMJE criteria for Authorship.
Ethics approval and consent to participate
No ethical approval required.
Availability of data and materials
All data generated and analyzed during this study are included in this article.
Declaration of competing interest
The following authors have no financial disclosures: WF, VSN, BWB, MJD, FH, KJG, DJD, THS, AO.
Acknowledgements
None.
References
- 1.Fountas K.N., Kapsalaki E.Z., Lee G.P., et al. Terson hemorrhage in patients suffering aneurysmal subarachnoid hemorrhage: predisposing factors and prognostic significance. J Neurosurg. 2008;109(3):439–444. doi: 10.3171/JNS/2008/109/9/0439. [DOI] [PubMed] [Google Scholar]
- 2.Naseri A., Blumenkranz M.S., Horton J.C. Terson's syndrome following epidural saline injection. Neurology. 2001;57(2):364. doi: 10.1212/wnl.57.2.364. [DOI] [PubMed] [Google Scholar]
- 3.Clark C.J., Whitwell J. Intraocular haemorrhage after epidural injection. Br Med J. 1961;2(5267):1612. doi: 10.1136/bmj.2.5267.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagani-Estévez G.L., Chen J.J., Watson J.C., Leavitt J.A. Acute vision loss secondary to epidural blood patch: Terson syndrome. Reg Anesth Pain Med. 2016;41(2):164–168. doi: 10.1097/AAP.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 5.Matsuoka T., Matsuda S., Harino S., et al. Subarachnoid hemorrhage-negative Terson syndrome after intracranial artery treatment with a flow diverter device. Am J Ophthalmol Case Reports. 2020;20 doi: 10.1016/j.ajoc.2020.100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumaria A., Gruener A.M., Dow G.R., Smith S.J., Macarthur D.C., Ingale H.A. An explanation for Terson syndrome at last: the glymphatic reflux theory. J Neurol. 2021 doi: 10.1007/s00415-021-10686-4. [DOI] [PubMed] [Google Scholar]
- 7.Moe K.S., Bergeron C.M., Ellenbogen R.G. Transorbital neuroendoscopic surgery. Neurosurgery. 2010;67(3 suppl Operative) doi: 10.1227/01.NEU.0000373431.08464.43. [DOI] [PubMed] [Google Scholar]
- 8.Almeida J.P., Omay S.B., Shetty S.R., et al. Transorbital endoscopic eyelid approach for resection of sphenoorbital meningiomas with predominant hyperostosis: report of 2 cases. J Neurosurg. 2018;128(6):1885–1895. doi: 10.3171/2017.3.JNS163110. [DOI] [PubMed] [Google Scholar]
- 9.Kong D.S., Kim Y.H., Hong C.K. Optimal indications and limitations of endoscopic transorbital superior eyelid surgery for spheno-orbital meningiomas. J Neurosurg. 2020;134(5):1472–1479. doi: 10.3171/2020.3.JNS20297. [DOI] [PubMed] [Google Scholar]
- 10.Singh H., Essayed W.I., Schwartz T.H. Endoscopic technology and repair techniques. Handb Clin Neurol. 2020;170:217–225. doi: 10.1016/B978-0-12-822198-3.00042-2. [DOI] [PubMed] [Google Scholar]
- 11.Luginbuhl A.J., Campbell P.G., Evans J., Rosen M. Endoscopic repair of high-flow cranial base defects using a bilayer button. Laryngoscope. 2010;120(5):876–880. doi: 10.1002/lary.20861. [DOI] [PubMed] [Google Scholar]
- 12.Youngerman B.E., Kosty J.A., Gerges M.M., et al. Acellular dermal matrix as an alternative to autologous fascia lata for skull base repair following extended endoscopic endonasal approaches. Acta Neurochir (Wien) 2020;162(4):863–873. doi: 10.1007/s00701-019-04200-z. [DOI] [PubMed] [Google Scholar]
- 13.Terson A. De l’hémorrhagie dans le corps vitre au cours de l’hémorrhagie cerebrale. Clin Ophthalmol. 1900;(6):309–312. [Google Scholar]
- 14.Litten M. Über Einige vom Allgemein-Klinischen Standpunkt aus Interessante Augenveränderungen. Berliner Klin Wochenschrift. 1881;(18):23–27. [Google Scholar]
- 15.Stienen M.N., Lücke S., Gautschi O.P., Harders A. Terson haemorrhage in patients suffering aneurysmal subarachnoid haemorrhage: a prospective analysis of 60 consecutive patients. Clin Neurol Neurosurg. 2012;114(6):535–538. doi: 10.1016/j.clineuro.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 16.Sung W., Arnaldo B., Sergio C., Juliana S., Michel F. Terson's syndrome as a prognostic factor for mortality of spontaneous subarachnoid haemorrhage. Acta Ophthalmol. 2011;89(6):544–547. doi: 10.1111/j.1755-3768.2009.01735.x. [DOI] [PubMed] [Google Scholar]
- 17.Garfinkle A.M., Danys I.R., Nicolle D.A., Colohan A.R.T., Brem S. Terson's syndrome: a reversible cause of blindness following subarachnoid hemorrhage. J Neurosurg. 1992;76(5):766–771. doi: 10.3171/jns.1992.76.5.0766. [DOI] [PubMed] [Google Scholar]
- 18.Gress D.R., Wintermark M., Gean A.D. A case of Terson syndrome and its mechanism of bleeding. J Neuroradiol. 2013;40(4):312–314. doi: 10.1016/j.neurad.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Manschot W.A. Subarachnoid hemorrhage intraocular symptoms and their pathogenesis. Am J Ophthalmol. 1954;38(4):501–505. [PubMed] [Google Scholar]
- 20.Reddy D., Rodriguez A.R., Alsunbul W., Ling E., Kosick T., Reddy K.K.V. Endoscopic associated iatrogenic Terson's syndrome. Can J Neurol Sci. 2013;40(2):265–266. doi: 10.1017/s0317167100013871. [DOI] [PubMed] [Google Scholar]
- 21.Hoving E.W., Rahmani M., Los L.I., Renardel De Lavalette V.W. Bilateral retinal hemorrhage after endoscopic third ventriculostomy: iatrogenic Terson syndrome: case report. J Neurosurg. 2009;110(5):858–860. doi: 10.3171/2008.6.17610. [DOI] [PubMed] [Google Scholar]
- 22.Silva CE da, Romero A.D.C.B., Freitas PEP de, Olijnyk L.D. Severe edema and venous congestion following sphenoorbital meningioma resection in a meningiomatosis case: importance of predicting venous disturbances. J Neurol Surg Reports. 2015;76(2):e239. doi: 10.1055/s-0035-1564058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study are included in this article.