Abstract
Bioinoculants provide better opportunity for ecological farming practices to improve the plant growth and enhanced crop productivity. Different types of bioinoculants containing single microbial culture and multiple microbial strains in single formulation could be used for agricultural sustainability. The different efficient microbial strain in single formulation as a consortium is an emerging trend in the present era. The present study deals with the isolation of nitrogen fixing, phosphorus and potassium solubilizing microbes from rhizospheric soil and root's internal tissues of different cereal/pseudocereal crops and their application as a microbial consortium for the growth of cereal crops. A total of 152 rhizospheric and endophytic bacteria were isolated and screened for the plant growth promoting (PGP) traits of nitrogen fixation, solubilization of phosphorus, and potassium. Among all the isolates, nine were found to fix nitrogen, fifteen and eleven exhibited phosphorus and potassium solubilization activity, respectively. Three selected efficient bacterial strains were identified using 16S rRNA gene sequencing as Erwinia sp. EU-B2SNL1 (N-fixer), Chryseobacterium arthrosphaerae EU-LWNA-37 (P-solubilizer), and Pseudomonas gessardii EU-MRK-19 (K-solubilizer). The inoculation of these three bacterial strains on barley crop as single inoculum and as microbial consortium enhanced the growth and physiological parameters including root/shoot length and biomass, chlorophyll, carotenoids, phenolics, flavonoids and soluble sugar content in comparison with untreated control. The microbial consortium was found to be more effective as compared to single inoculum. The microbial consortium of nitrogen fixing and mineral solubilizing microbes could be used as biofertilizer for plant growth and soil health.
Keywords: Bioinoculants, Microbial consortium, Nitrogen, Phosphorus, Potassium, Sustainability
Bioinoculants; Microbial consortium; Nitrogen; Phosphorus; Potassium; Sustainability.
1. Introduction
Macronutrients, nitrogen (N), phosphorus (P) and potassium (K) are the basic need for plant growth which play several significant roles in the entire life of the plants. These nutrients perform beneficial activities in the metabolism of plant and protect them from various abiotic and biotic factors exerted from outer environment. Macronutrients help in increasing the quality and quantity of crop grain (Tripathi et al., 2014). The fulfillment of nutrient requirements until the middle of the twentieth century was relied on organic manures, but the beginning of green revolution, the chemical fertilizers are used for high production and its consumption drastically increased with every progressed year worldwide. In India, during the 1950–1951, the consumption of N fertilizer (urea), P fertilizer (di-ammonium phosphate) and K fertilizer (potash) was 0.06, 0.01 million ton (Mt) and almost nil, respectively and by 2000–2001 the consumption of N, P, K fertilizer hiked to 10.8 Mt, 1.8 Mt and 0.81 Mt, respectively and which resulted an increment of crop the yield by 190 folds (Pathak et al., 2010). According to FAOSTAT 2021 report 27 billion tons of NPK fertilizer is being yearly used.
In earlier times, i.e. from 1950-1991 the use of the NPK chemical fertilizers increased the annual per capita food availability to 208 kg but after a decade it declined to 192 kg. The reason behind the stagnation rate of food availability was due to fertilizers harmful impact such as N fertilizer leads to acid deposition, nitrate leaching into ground water, eutrophication, loss of biodiversity and production of greenhouse gas that contributes to ozone depletion (Powlson 1993). Whereas, P fertilizer pollutes the surface as well as groundwater and on the other hand K-fertilizer increases the soil dispersion and infiltration that leads to the soil erodibility (Auerswald et al., 1996; Spiess 2011). This serious concern began the scientific interest for using the beneficial microbes as biofertilizer instead of chemical fertilizers to ensure the agrarian sustainability (Fasusi et al., 2021; Yadav and Sarkar 2019).
Microbes, the tiny miracle are increasingly gaining attention for agriculture sustainability due to cost-effective and eco-friendly nature. Various groups of plant associated microbial strains have been reported for improving plant growth in normal and stressed environmental conditions such as Bacillus, Pseudomonas, and Rhizobium (Afzal et al., 2017), Azospirillum brasilense (Di Salvo et al., 2018), Oceanobacillus (Albdaiwi et al., 2019), Streptomyces laurentii (Kour et al., 2020), and Pseudomonas alcaliphila (AlAli et al., 2021). Microbes living freely or in association with plant's different regions (rhizospheric, epiphytic and endophytic) promotes the growth of the host via direct and indirect PGP attributes such as fixation of atmospheric nitrogen; nutrients (P, K, Zn) solubilization and mobilization; production of siderophores (Fe chelating agent), plant growth regulators (auxin, abscisic acid, cytokinin, ethylene, gibberellins, jasmonic acid), ammonia, hydrogen cyanide, 1-aminocyclopropane-1-carboxylate (ACC), hydrolytic enzymes (protease, cellulase, amylase and chitinase) and various plant growth promoting bioactive compounds secondary metabolites (Kaur et al., 2021; Kour et al., 2019).
Microbes have capability to promote the growth of plants when applied either singly or collective in a mixture as microbial consortium. Microbial consortium is an emerging trend for agricultural sustainability. Microbial consortium are more effective for plant growth and enhanced crop yield as compare with single microbial inoculation (Jain et al., 2013). In a study, the inoculation of microbial consortium containing nitrogen fixer (Enterobacter sp.), phosphorus solubilizer (Microbacterium arborescens) and IAA producer (Serratia marcescens), on wheat was efficiently improving the growth, yield and nutrient uptake as compared to the single application of these bacteria (Kumar et al., 2017). Similarly, another study of Ghorchiani et al. (2018), microbial consortium is more efficient over single microbial culture by experimenting co-inoculation of arbuscular mycorrhiza fungi (Funneliformis mosseae) and phosphorus solubilizing bacterium (Pseudomonas fluorescens), on maize crop. In comparison to single microbial inoculation treatment and control, the microbial consortium was efficiently enhancing the growth and yield of the maize crops. In another report, PGP microbes, Burkholderia sp. Mesorhizobium sp., Pseudomonas sp. and arbuscular mycorrhiza fungi (Claroideoglomus claroideum, Funneliformis geosporum and Rhizophagus irregularis) on chickpea was reported for increasing growth, grain yield and protein content under the rainfed condition over single strain inoculation (Laranjeira et al., 2021). The present investigation aimed for examining microbial consortium of N-fixer, P and K solubilizer in both in-vitro and field trials. The study was concerned with the impact of microbial inoculum over individual microbial inoculation on barley growing in hilly region.
2. Materials and methods
2.1. Area of study and samples collection
The rhizospheric soil and plant samples were collected from the Baru Sahib Valley of Divine Peace (30.7537 N, 77.2965 E), District Sirmour, Himachal Pradesh, India. A total of 17 samples of cereal and pseudocereal crops (wheat, rice, maize, finger millet, and oats) were collected from Baru Sahib in sterilized plastic packets and stored at temperature 4 °C until further process.
2.2. Isolation of rhizospheric and endophytic microbes from crops
The culturable rhizospheric microbes were sorted out through enrichment methods using the standard technique of serial dilution and spread plate using different defined, undefined and selective growth media including nutrient agar (NA), tryptic soy agar, T3A agar, King's B agar, Luria-Bertani described by Verma et al. (2014). The endophytic microbes were isolated as per the method described by Conn and Franco (2004). The colonies that appeared on agar plate were purified by re-streaking using respective medium agar plates. The obtained pure cultures were maintained on nutrient agar slants and in 25% of glycerol stock at 4 °C and -80 °C, respectively for future experiments.
2.3. Screening of microbes for NPK plant growth prompting traits
2.3.1. Qualitative estimation
All the isolates were screened for PGP traits i.e. nitrogen fixation, P solubilization and K solubilization. Solubilization of P and K attributes was screened qualitatively using Pikovskaya and Aleksandrov agar plate assay as described by Pikovskaya (1948) and Hu et al. (2006), respectively. P solubilization was done using Pikovskaya agar supplemented with 0.5% each of tricalcium phosphate, rock phosphate, and apatite as an inorganic source of P whereas K solubilization was carried out using Aleksandrov's agar supplemented with 0.2% mica as of inorganic source of K.
2.3.2. Quantitative estimation
Biological nitrogen fixation: The nitrogen fixing capability of the isolates was estimated using the acetylene reduction assay (ARA) (Han and New 1998). The rhizospheric and endophytic isolates were inoculated in nitrogen free bromothymol blue (NFb) medium slants and incubated at 30 °C for 24–36h. After the growth, cotton plugs of the test tubes were replaced by Suba seal and gas phase inside the test tube was interchanged with 10% mixture of nitrogen gas, air, and acetylene (90:10:10, v/v). The tubes were re-incubated for 24 h at 30 °C and the produced ethylene was calculated by a Perkin Elmer F-11 gas chromatograph.
Solubilization of phosphorus: Quantitatively, P solubilization of the selected bacterial strains was estimated by method of Murphy and Riley (1962). The test was performed by the inoculation of bacterial culture (1 mL) in Pikovskaya broth (25 mL) containing 0.5% tricalcium phosphate. After the incubation of 7 days, the culture suspension was centrifuged for 15 min at 10,000g. The optical density (OD) of the supernatant was estimated at 600nm and P concentration was expressed in mg/L.
Solubilization of potassium: Quantitative estimation of the K solubilization of the selected bacterial strains was estimated as per the method of Sugumaran and Janarthanam (2007). The experiment was performed by the inoculation of bacterial culture in 50 mL of Aleksandrov broth containing mica as insoluble source of potassium. After 7 days of incubation, 10 mL of microbial suspension was centrifuged for 10 min at 10,000g. The K content of the supernatant was indicated by using flame spectrophotometry.
2.4. Identification of selected NPK bacterial strain and phylogenetic analysis
Genomic DNA (gDNA) of the selected bacterial strains having attributes of N-fixation, P, and K solubilization was carried out by the earlier described method by Yadav et al. (2015). 16S rRNA gene was amplified using set primers pA (5′AGAGTTTGATCCTGGCTCAG3′) and pH (5′AAGGAGGTGATCCAGCCGCA3′) to obtain nearly 1500-bp fragment. The PCR amplification of the gDNA was carried out in a 100 mL volume, and conditions of amplification were used as described earlier by Yadav et al. (2015). The amplified PCR products were purified with a QIA quick purification kit (Qiagen). The identity of bacteria was determined by BLASTn programme to those of closely related strains of bacteria based on a sequence similarity percentage (>97%) available at the GenBank database. The phylogenetic tree was constructed using neighbor joining (NJ) method on the aligned dataset implemented in the program MEGA 4.0.2. (Tamura et al., 2007).
2.5. Development of microbial consortium
The microbial consortium was developed using three efficient bacterial strains EU-B2SNL1, EU-LWNA-37, and EU-MRK-19 having nitrogen fixation, P and K solubilization PGP traits, respectively. The bacterial strains were checked for their compatibility using cross streaked assay onto nutrient agar medium. One of the bacterial strain was streaked in the center of the agar plate and other two bacterial strains were cross streaked. The plate was incubated at 28 °C for 2–3 days and compatibility was determined on the basis of no growth inhibition of colonies. After the compatibility check, the microbial consortium was prepared by growing bacterial cultures individually in 200 mL nutrient broth for 24 h at 28 °C. After the growth of bacterial cultures colony-forming units was recorded 1.23×107 cells/mL (EU-B2SNL1), 2.54×107 cells/mL (EU-LWNA-37) and 1.32×107 cells/mL (EU-MRK-19). The bacterial suspension of each strain was mixed in an equal amount and microbial consortium was prepared for further validation on barley crop.
2.6. Validation of microbial consortium under greenhouse and field conditions
The combined and individual effect of efficient N-fixer, and P and K solubilizer were studied under both greenhouse (pot experiment) and field condition on barley crop. A total of thirteen treatments viz. T1 (N-fixer + P-solubilizer + K-solubilizer); T2 (N-fixing bacteria); T3 (P-solubilizer bacteria); T4 (K-solubilizer bacteria); T5 (recommended full dose of NPK chemical fertilizer); T6 (half dose of NPK chemical fertilizer); T7 (recommended full dose of urea); T8 (half dose of urea); T9 (recommended full dose of DAP); T10 (half dose of DAP); T11 (recommended full dose of potash); T12 (half dose of potash); T13 (un-inoculated control) were replicated three times in both greenhouse and field conditions. In both greenhouse and field experiment randomized block design was followed. In greenhouse, the experiment was carried out in plastic pots (30 cm × 30 cm×26 cm) filled with 4 kg non-sterile soil. The pots were placed at equal distance from each other to reduce the cross contamination of the control. In each pot, 6 seeds were sown and after germination four plants were maintained in each pot till the harvesting. A field experiment was conducted at the Experimental Farm, Machher (30.7537 N, 77.2965 E), Eternal University, Baru Sahib, District Sirmour, Himachal Pradesh, India, in the plot size of 16.5 m2 (11 × 1.5 m2) in which each bed was 0.5 m apart. The seeds of barley treated with single culture and microbial consortium were coated with sugar solution in ration of 1:1 before the sowing. After the 90 days of sowing, barley's growth and physiological parameters were recorded.
2.7. Analysis of growth and physiological parameters
The growth parameters such as length, fresh/dry weight of shoot/root were studied. The content of chlorophyll and carotenoid was determined as per method of Lichtenthaler (1987). The soluble sugar content of barley was done as per the method of Irigoyen et al. (1992) and leaf phenolics and flavonoid content was determined by the method of Kim et al. (2003) and Park et al. (2008), respectively.
2.8. Statistical analysis
Data were subjected to statistical significance using a Student's t-test. Mean comparisons were conducted using the least significant difference (LSD) test (P = 0.05) and critical difference (5% and 1%). Standard error and LSD results were calculated.
3. Results
3.1. Isolation of rhizospheric and endophytic microbes from crops
The rhizobacterial and endophytic bacterial population was enumerated on different growth medium from cereal and pseudocereal crops grown in Baru Sahib region. A total of 152 bacterial strains (98 rhizospheric and 55 endophytic) were isolated. The population of rhizospheric and endophytic varied from 0.36×107 to 3.54 × 107 CFU/g of soil and 0.01×107 to 2.19 × 107 CFU/g of plant, respectively. The highest diversity was observed in nutrient agar medium from maize in case of rhizospheric soil samples and wheat in case of endophytic samples. The pure colonies were sorted out from each sample on different growth medium on the basis of colony and cultural characteristic.
3.2. Screening of microbes for NPK plant growth prompting traits
Among 152 bacterial isolates, 9 isolates exhibited nitrogenase activity, 15 and 11 showed P and K solubilization, respectively. Nine bacterial strains exhibited nitrogenase activity in range of 2.01–22.51 nmol C2H4 mg−1 protein hr−1 whereas fifteen isolates showed P-solubilization in the range of 41.7 ± 0.005 mg/L to 155.90 ± 0.005 mg/L. Among nine bacterial isolates exhibiting nitrogenase activity strain EU-B2SNL1 showed maximum nitrogenase activity of 22.51 nmol C2H4 mg−1 protein hr−1 whereas highest phosphorus was solubilized by EU-LWNA-37 (155.90 ± 0.005 mg/L) and strain EU-MRK-19 (32 ± 0.8 mg mL−1) was found to be an efficient K-solubilizer.
3.3. Identification of selected NPK bacterial strain and phylogenetic analysis
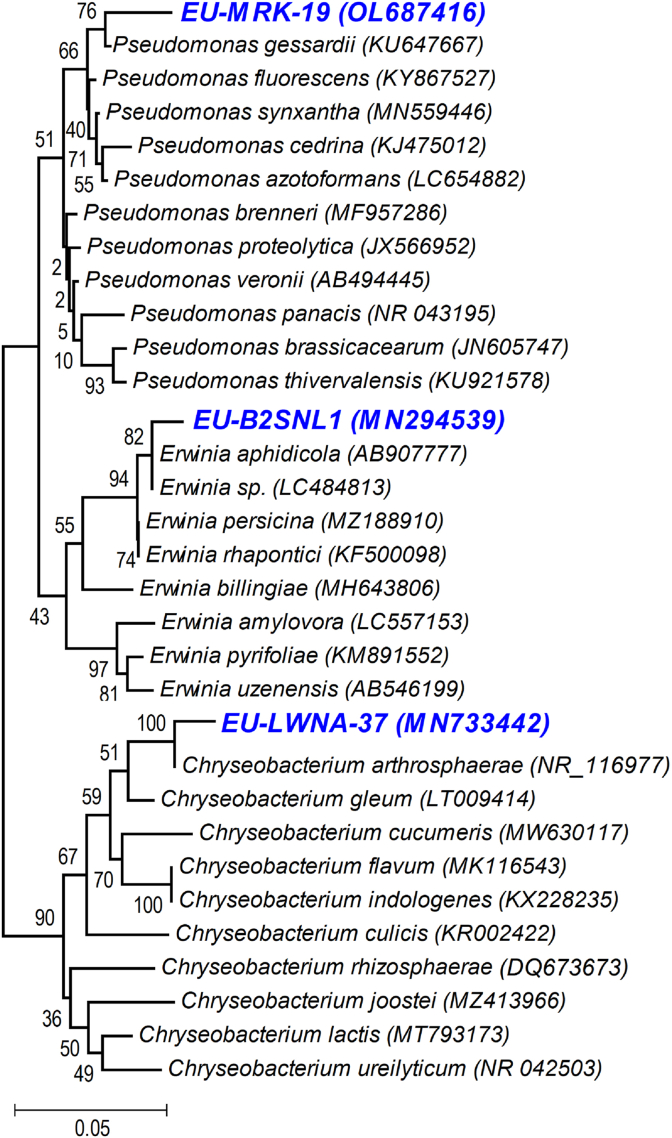
Partial 16S rRNA gene sequences obtained after sequencing were compared with those available in NCBI database using BLASTn algorithm. The phylogenetic tree was constructed to know the taxonomic affiliation of obtained strain (Figure 1). The BLASTn analysis of 16S rRNA gene sequence of three selected potential strains EU-B2SNL1, EU-LWNA-37 and EU-MRK-19 showed <99% similarity with Erwinia sp., Chryseobacterium arthrosphaerae and Pseudomonas gessardii, respectively. The partial sequence of 16S rRNA gene was submitted to online database NCBI GenBank and accession number were assigned as MN294539, MN733442 and OL687416. The bacterial strains EU-B2SNL1, EU-LWNA-37, and EU-MRK-19 were deposited at ICAR-National Bureau of Agriculturally Important Microorganisms (NBAIM) culture-collection facility, Mau Nath Bhanjan, Uttar Pradesh, India.
Figure 1.
Phylogenetic tree showing the relationship among nitrogen fixing, phosphorus and potassium solubilizing bacterial isolates, 16S rRNA gene sequences with reference sequences obtained through BLAST analysis. The trees were constructed using neighbor joining (NJ) with algorithm using MEGA 4 software (Tamura et al., 2007).
3.4. Validation of microbial consortium under greenhouse and field conditions
The efficient N2 fixer, P and K solubilizer, Erwinia sp. EU-B2SNL1, C. arthrosphaerae EU-LWNA-37, and P. gessardii EU-MRK-19 were evaluated as microbial consortium and individual inoculation for PGP of barley crop under greenhouse and field conditions.
3.5. Analysis of growth and physiological parameters
The combined treatment of Erwinia sp. EU-B2SNL1, C. arthrosphaerae EU-LWNA-37, P. gessardii EU-MRK-19 increased the growth parameters including shoot/root length, fresh/dry weight of the barley (Tables 1 and 2). The microbial consortium of Erwinia sp. EU-B2SNL1, C. arthrosphaerae EU-LWNA-37, and P. gessardii EU-MRK-19 showed 1.3, 1.3, 1.3 and 2.8 fold increment of the shoot length in greenhouse experiment and 1.2, 1. 2, 1.3 and 1.7 fold high in field trial in comparison to individual inoculation of Erwinia sp. EU-B2SNL1, C. arthrosphaerae EU-LWNA-37, P. gessardii EU-MRK-19 and uninoculated control, respectively. Root length was found to be enhanced by the microbial consortium in greenhouse and field experiment as compared to EU-B2SNL1, EU-LWNA-37, EU-MRK-19 and uninoculated control by 1.4, 1.8, 1.6, and 3.3 fold and 1.5, 1.2, 1.1 and 1.7 folds respectively. The fresh weight of the barley was also found to be positively affected by the microbial consortium in comparison to all the treatments in both greenhouse and field conditions. In greenhouse experiment, microbial consortium enhanced the barley fresh weight in comparison with EU-B2SNL1 (1.7 fold), EU-LWNA-37 (2.1 fold), EU-MRK-19 (1.6 fold), full recommended dose of NPK chemical fertilizer (2.3 fold), half recommended dose of NPK chemical fertilizer (2.8 fold), full dose of urea (1.8 fold), half dose of urea (1.9 fold), full dose of DAP (2.1 fold), half dose of DAP (1.9 fold), full dose of potash (3.6 fold), half dose of potash (3.5 fold) and uninoculated control (3.8 fold). Similarly, in field conditions the microbial consortium showed the increment in the fresh weight of barley by 1.5 fold (EU-B2SNL1), 1.9 fold (EU-LWNA-37), 1.4 fold (EU-MRK-19), 1.7 fold (full recommended dose of NPK chemical fertilizer), 2.9 fold (half dose of NPK chemical fertilizer), 2.5 fold (full dose of urea), 3.3 fold (half dose of urea), 2.1 fold (full dose of DAP), 3.8 fold (half dose of DAP), 2.8 fold (full dose of potash), 3.5 fold (half dose of potash), and 4.4 fold (uninoculated control).
Table 1.
Effect of microbial consortium on growth and physiological parameters of barley under greenhouse conditions.
| Treatments | Shoot length (cm) | Root length (cm) | Fresh weight (g) | Dry weight (g) | Chlorophyll (mg/g) | Carotenoids (g/L) | Phenolics (μg/g) | Flavonoid (μg/g) | Soluble sugar (μg/g) |
|---|---|---|---|---|---|---|---|---|---|
| MC | 60.90g∗∗±2.71 | 47.35i∗∗±1.15 | 12.34g∗±2.32 | 3.21h∗±1.54 | 68.76j∗∗±0.05 | 15.68i∗∗±0.10 | 1.14i∗∗±0.02 | 8.96j ± 0.01 | 62.65g∗∗±0.68 |
| N-culture | 44.25e∗±2.96 | 33.60g∗±2.71 | 7.19f ± 1.88 | 1.62e ± 0.11 | 40.72f∗±0.07 | 13.58h∗±0.12 | 0.85h∗∗±0.01 | 8.26i ± 0.36 | 45.41d∗∗±2.36 |
| P-culture | 46.00f∗±2.41 | 25.45d ± 0.95 | 5.76d ± 0.11 | 2.09f ± 1.19 | 34.25c ± 0.59 | 12.91g∗±6.05 | 0.85h∗∗±0.00 | 8.11h ± 0.14 | 48.82e∗∗±1.28 |
| K-culture | 45.25e∗±0.65 | 29.35f∗±0.55 | 7.40f ± 0.90 | 2.53g ± 0.28 | 55.10i∗∗±0.28 | 14.07h∗±0.96 | 0.67d∗∗±0.00 | 8.21i ± 0.28 | 50.76f∗∗±1.97 |
| NPK 100% | 41.75d∗±0.15 | 24.70d ± 1.71 | 5.18c ± 2.44 | 0.78a ± 0.08 | 28.58b ± 0.32 | 8.25e ± 0.22 | 0.65c∗∗±0.00 | 6.81b ± 0.88 | 43.15c∗∗±1.81 |
| NPK 50% | 34.20c ± 2.01 | 24.60d ± 2.11 | 4.37b ± 0.20 | 0.85b ± 0.04 | 24.36a ± 1.18 | 6.37d ± 0.17 | 0.77f∗∗±0.01 | 7.45e ± 0.49 | 38.76b∗∗±3.57 |
| N 100% | 41.70d∗±0.60 | 35.05h∗±1.76 | 6.66e ± 0.71 | 1.55e ± 0.14 | 37.88e∗±5.58 | 11.87f ± 1.07 | 0.80g∗∗±0.00 | 7.47e ± 0.12 | 44.24c∗∗±1.22 |
| N 50 % | 37.20c ± 1.10 | 23.15c ± 2.16 | 6.41e ± 0.54 | 1.47d ± 0.21 | 35.44d ± 3.83 | 13.43g∗±1.08 | 0.78f∗∗±0.01 | 7.30d ± 0.10 | 48.73e∗∗±2.84 |
| P 100% | 35.95c ± 4.16 | 22.50c ± 0.80 | 5.73d ± 0.46 | 1.44d ± 0.13 | 49.66h∗∗±0.16 | 8.54e ± 0.23 | 0.79g∗∗±0.01 | 7.92g ± 0.43 | 46.23d∗∗±1.86 |
| P 50% | 37.15c ± 2.26 | 15.90b ± 0.60 | 6.40e ± 0.04 | 1.31c ± 0.09 | 43.52g∗∗±0.48 | 5.00b ± 0.14 | 0.53b∗±0.01 | 7.50f ± 0.45 | 51.05f∗∗±3.61 |
| K 100% | 46.30f∗±1.71 | 33.05g∗±3.26 | 3.34a ± 0.03 | 1.51b ± 0.13 | 32.47c ± 5.27 | 5.77c ± 0.10 | 0.77f∗∗±0.01 | 7.56f ± 0.24 | 44.63c∗∗±2.50 |
| K 50% | 26.15b ± 0.85 | 26.65e ± 3.36 | 3.47a ± 0.47 | 0.89b ± 0.13 | 30.00b ± 0.40 | 4.88b ± 0.18 | 0.73e∗∗±0.01 | 6.92c ± 0.54 | 38.52b∗±5.59 |
| Control | 21.50a ± 3.61 | 14.00a ± 1.71 | 3.23a ± 1.74 | 0.75a ± 0.02 | 22.92a±0.79 | 4.26a ± 0.12 | 0.45a ± 0.00 | 6.73a ± 0.60 | 12.47a ± 0.69 |
| LSD | 1.67 | 1.22 | 0.33 | 0.08 | 1.85 | 0.58 | 0.01 | 0.07 | 1.62 |
| SE | 9.84 | 8.92 | 4.6 | 1.6 | 7.42 | 4.12 | 0.04 | 1.8 | 11.72 |
| CD 5 percent | 17.53 | 15.89 | 8.19 | 2.85 | 13.22 | 7.34 | 0.07 | 3.2 | 20.88 |
| CD 1 percent | 26.38 | 23.91 | 12.33 | 4.28 | 19.89 | 11.04 | 0.10 | 4.82 | 31.42 |
MC: Erwinia sp. EU-B2SNL1+C. arthrosphaerae EU-LWNA-37 + P. gessardii EU-MRK-19; N-culture: Erwinia sp. EU-B2SNL1; P culture: C. arthrosphaerae EU-LWNA-37; K-culture: P. gessardii EU-MRK-19.
[Numerical values are mean ± Standard deviation of mean (SD) of three independent observations].
Common superscript code on mean values indicate the non-significant differences among derivatives as based on unpaired Student t-test at p < 0.05. On the other hand, different superscript indicates significant differences among lines.
Table 2.
Effect of microbial consortium on the growth and physiological parameters of barley under field conditions.
| Treatments | Shoot length (cm) | Root length (cm) | Fresh weight (g) | Dry weight (g) | Chlorophyll (mg/g) | Carotenoids (g/L) | Phenolics (μg/g) | Flavonoid (μg/g) | Soluble sugar (μg/g) |
|---|---|---|---|---|---|---|---|---|---|
| MC | 48.00h∗∗±1.00 | 8.25i ± 0.75 | 4.02k ± 0.03 | 0.58h∗±0.01 | 72.37i∗∗±0.55 | 17.77j∗±1.15 | 1.60i∗∗±0.03 | 10.01j∗∗±0.01 | 42.93i∗∗±0.03 |
| N-culture | 38.25g ± 2.26 | 5.25c ± 0.25 | 2.57i ± 0.39 | 0.44f ± 0.09 | 30.72c ± 0.10 | 12.45e ± 0.29 | 1.12h∗∗±0.02 | 9.25i∗∗±0.15 | 32.46g∗∗±0.43 |
| P-culture | 37.00f ± 2.01 | 6.75g ± 0.25 | 2.06g ± 0.22 | 0.28d ± 0.01 | 37.24e∗∗±0.68 | 12.74e ± 0.63 | 1.04g∗∗±0.02 | 8.16h∗±0.35 | 33.09h∗∗±0.11 |
| K-culture | 36.75f ± 1.76 | 7.00h ± 0.00 | 2.78j ± 0.62 | 0.35e ± 0.02 | 43.10g∗∗±0.09 | 15.63h ± 0.76 | 0.62b ± 0.02 | 7.95g∗±0.05 | 23.66c∗∗±0.03 |
| NPK 100% | 35.50e ± 0.50 | 5.25c ± 0.25 | 2.33h ± 0.40 | 0.47g ± 0.08 | 34.40d∗∗±0.05 | 13.39f ± 2.28 | 0.74d∗±0.02 | 7.97g∗±0.02 | 33.17h∗∗±0.43 |
| NPK 50% | 36.25f ± 2.76 | 4.75a ± 0.25 | 1.37d ± 0.28 | 0.24c ± 0.07 | 25.55b ± 1.04 | 9.91d ± 0.25 | 0.75d∗±0.03 | 7.42e ± 0.04 | 22.31b∗∗±0.71 |
| N 100% | 35.25e ± 1.25 | 6.25e ± 0.25 | 1.59e±0.71 | 0.29d ± 0.08 | 39.45f∗∗±0.55 | 17.02i∗±2.37 | 0.79e∗±0.02 | 7.01d ± 0.25 | 28.35f∗∗±0.37 |
| N 50 % | 28.75b ± 2.76 | 6.00d ± 1.00 | 1.20c ± 1.28 | 0.17b ± 0.07 | 40.73f∗∗±0.00 | 12.38e ± 2.28 | 0.64b ± 0.01 | 6.53c ± 0.33 | 24.79d∗∗±0.42 |
| P 100% | 35.00e ± 1.00 | 7.00h ± 1.00 | 1.89f±0.43 | 0.16b ± 0.01 | 50.67h∗∗±0.07 | 14.16g ± 3.65 | 0.92f∗∗±0.02 | 8.03g∗±0.07 | 31.81g∗∗±0.20 |
| P 50% | 30.50c ± 0.50 | 5.00b ± 0.00 | 1.05b ± 0.37 | 0.23c ± 0.04 | 51.22h∗∗±2.39 | 12.34e ± 0.49 | 0.73d∗±0.03 | 7.72f∗±0.32 | 26.68e∗∗±1.76 |
| K 100% | 32.00d ± 1.00 | 6.50f ± 0.50 | 1.39d ± 0.06 | 0.16b ± 0.01 | 25.28b ± 0.71 | 8.14c ± 1.50 | 0.80e∗ ±0.02 | 6.25b ± 0.15 | 23.79c∗∗±0.79 |
| K 50% | 29.25b ± 0.25 | 5.00b ± 0.50 | 1.12b ± 0.42 | 0.17b ± 0.15 | 29.00c ± 0.16 | 6.98b ± 2.38 | 0.68c ± 0.01 | 7.36e ± 0.40 | 26.16e∗∗±0.06 |
| Control | 26.75a ± 1.76 | 4.75a ± 0.25 | 0.90a ± 0.03 | 0.13a ± 0.01 | 20.24a ± 0.06 | 3.95a ± 0.66 | 0.45a ± 0.05 | 6.09a ± 0.16 | 11.68a±0.06 |
| LSD | 0.77 | 0.15 | 0.12 | 0.02 | 1.98 | 0.52 | 0.04 | 0.15 | 1.05 |
| SE | 6.66 | 2.05 | 2.51 | 0.23 | 2.52 | 7.3 | 0.14 | 0.90 | 2.59 |
| CD 5 percent | 11.86 | 3.65 | 4.47 | 0.4 | 4.49 | 13 | 0.24 | 1.60 | 4.61 |
| CD 1 percent | 17.85 | 5.49 | 6.72 | 0.61 | 6.75 | 19.57 | 0.37 | 2.41 | 6.94 |
MC: Erwinia sp. EU-B2SNL1+C. arthrosphaerae EU-LWNA-37 + P. gessardii EU-MRK-19; N-culture: Erwinia sp. EU-B2SNL1; P culture: C. arthrosphaerae EU-LWNA-37; K-culture: P. gessardii EU-MRK-19.
[Numerical values are mean ± Standard deviation of mean (SD) of three independent observations].
Common superscript code on mean values indicate the non-significant differences among derivatives as based on unpaired Student t-test at p < 0.05. On the other hand, different superscript indicates significant differences among lines.
The dry weight of barley was shown to be increased as compared to other treatments and maximum increase was observed with uninoculated control i.e. by 3.6 folds in both greenhouse and field condition. Microbial consortium also positively affected the chlorophyll content in greenhouse conditions in barley by 1.6 fold as compared to EU-B2SNL1, 2.0 fold then EU-LWNA-37, 1.2 fold in comparison to EU-MRK-19, 2.4 fold then full dose of NPK chemical fertilizer, 2.8, 1.5, 2.2, and 3.1 folds then, half dose of NPK chemical fertilizer, half dose of DAP, half dose of potash and uninoculated control, respectively. In field experiment chlorophyll content also significantly enhanced as compared to EU-B2SNL1 (2.2 fold), EU-LWNA-37 (1.9 fold), EU-MRK-19 (1.8) full dose of NPK chemical fertilizer (2.1 fold), half dose of NPK chemical fertilizer (2.8 fold), full dose of urea (1.0 fold), half dose of urea (1.8 fold), full dose of potash (1.4 fold), half dose of potash (1.4 fold) and uninoculated control (3.6 fold fold). In case of carotenoids content, microbial consortium also showed increment in both greenhouse and field conditions. In greenhouse conditions the carotenoids was 3.3 fold higher as compared to uninoculated control, whereas in field experiment, carotenoids content was 4.4 fold higher in comparison with control.
The phenolics content in the barley leaves were also observed to be enhanced by the microbial consortium by 2.5 folds in greenhouse condition as compared to uninoculated control. In field conditions, the microbial consortium of N-fixer, P, and K-solubilizer increased the phenolic content in comparison with uninoculated control by 3.2 folds. In both greenhouse and field experiment, flavonoids content was too hiked by microbial consortium in comparison to all the treatments and by uninoculated control by 1.3 and 1.6 folds, respectively. The sugar content has been increased by the microbial consortium by 4.1 fold in greenhouse conditions and 1.9 fold in field conditions as compared to uninoculated control.
4. Discussion
Chemical fertilizers are one of the basic and important needs of the agricultural farmers. The application of chemical fertilizers in field cause problems like pollution, depletion of biodiversity, groundwater contamination, eutrophication, soil erodibility, acid deposition and production of greenhouse gases. Moreover the synthetic fertilizer has also led to accumulation of heavy metals like nickel, copper, cadmium, lead and mercury. All these environmental effects of chemical fertilizers have resulted in to 33% loss of cultivable land (Prashar and Shah 2016). Rhizospheric and endophytic plant growth promoting microbes are best alternative of the chemical fertilizer which promotes plant growth by availing required plant nutrients without affecting the environment. Microbes have begun to substitute the chemical fertilizer in agricultural fields (Kumari et al., 2019). Diverse group of microbes with potential of nitrogen fixation, P and K solubilization attributes have been reported such as Bacillus pumilus, Curtobacterium albidum, Pseudomonas libanensis, and P. azotoformans (Adhikari et al., 2021; Kour et al., 2019; Verma et al., 2016; Vimal et al., 2019). These PGP microbes possess better capabilities to enhance the growth of the plants then chemical fertilizer when applied singly to plants.
There are few reports available on microbial mixture exhibiting different PGP attributes and their application for plant growth promotion. In present study microbial consortium of Erwinia sp. EU-B2SNL1, C. arthrosphaerae EU-LWNA-37, and P. gessardii EU-MRK-19 were isolated from the plant interiors and rhizospsheric region. In a study, Erwinia sp. was reported as an endophye of coastal sand dune plants roots (Shin et al., 2007). In present study, C. arthrosphaerae EU-LWNA-37, and P. gessardii EU-MRK-19 were reported as rhizospheric bacteria. In a report, C. arthrosphaerae was sorted out from the rhizosphere of the cucumber (Jeong et al., 2017). El Kahkahi et al. (2019) reported P. gessardii from the carob tree (Ceratonia siliqua L.) rhizospheric region. In the present investigation, microbial strains Erwinia sp. EU-B2SNL1, C. arthrosphaerae EU-LWNA-37, and P. gessardii EU-MRK-19 were reported to fix nitrogen, solubilized the P and K, respectively and their single and combined inoculation in barley crop showed an increased growth. In a report, Erwinia sp. exhibiting PGP traits such as phosphorus solubilization, production of ammonia, siderophores, IAA and ACC deaminase activity was reported for enhancing the plant growth of wheat by elongating the root and shoot length as compared to the untreated control (Sagar et al., 2018). In another report, bacterium species belonging to genera Chryseobacterium was reported as a efficient solubilizer of P and reported to enhance the plant growth in horsegram in comparison with 100%, 50% and 30% recommended dose of N and P fertilizer (Singh et al., 2013). Heredia-Acuña et al. (2019) reported P. gessardii from coniferous trees rhizosphere for exhibiting PGP trait of solubilizing P. This strain was concluded as beneficial microbe for the forest nursery production.
The combination of N fixing (Erwinia sp. EU-B2SNL1), P solubilizing (C. arthrosphaerae EU-LWNA-37) and K solubilizing (P. gessardii EU-MRK-19) strain were shown to improve the growth parameters shoot/root length and fresh/dry biomass of the barley crop. Many studies have reported collective inoculation of different microbes for enhancing the plant growth parameters. In a report, the similar results have been reported in which co-inoculation of Rhziobium phaseoli and Pseudomonas sp. in common bean has increased shoot dry weight (Knezevic-vukcevic 2011). Similar finding was reported by the inoculation of microbial mixture Enterobacter asburiae, Bacillus amyloliquefaciens subsp. plantarum, Microbispora hainanensis and Streptomyces canus in sugarcane which showed the enhancement of shoot/root length and biomass (Kruasuwan and Thamchaipenet 2016). Etesami and Alikhani (2016) concluded, co-inoculation of rhizospheric and endophytic bacterium Pseudomonas putida and P. fluorescens, respectively in rice crop, enhanced the fresh dry weight, length of root and shoot in comparison to recommended dose N-fertilizer. The similar finding has shown that, the tri-inoculation of Arthrobacter chlorophenolicus, Enterobacter sp. and Pseudomonas aeruginosa in wheat, enhanced the crop biomass in comparison to control under both greenhouse and field conditions (Kumar et al., 2021).
The chlorophyll also showed an increment by the incoulation of microbial consortium in present study. A similar finding, has reported the inoculation of Azotobacter sp. in maize crop was enhancing the chlorophyll content and alleviating the salinity stress (Rojas-Tapias et al., 2012). Similarly, tetra-inoculation of Azotobacter chroococcum, Bacillus polymixa, Pseudomonas putida, and Glomus intraradices have also been reported for enhancing the chlorophyll content in the stevia plant (Vafadar et al., 2014). The content of the compounds flavonoid and phenolics have been reported to show increment by microbial consortium of Erwinia sp. EU-B2SNL1, C. arthrosphaerae EU-LWNA-37, and P. gessardii EU-MRK-19 in the present study. In a report, the phenolic and flavonoids have been enhanced by the co-inoculation of Delftia sp. and Sinorhizobium meliloti in alfalfa plant (Morel et al., 2015). The content of phenolics compounds content was reported to be enhanced by Bacillus amyloliquefaciens and Pseudomonas fluorescens in peppermint plant growing under drought stress (Chiappero et al., 2019). The present study also revealed that microbial consortium Erwinia sp. EU-B2SNL1, C. arthrosphaerae EU-LWNA-37 and P. gessardii EU-MRK-19 also enhanced the soluble sugar content as compared to single inoculation and untreated control in the barley plant. The similar finding have been reported by Upadhyay and Singh (2015) in which Bacillus aquimaris inoculated in salinity stressed wheat crop was having higher total soluble content in comparison with control. Similarly, the co-inoculation of the Pseudomonas koreensis and Microbacterium hydrothermale in red pepper growing in saline conditions was found to have higher accumulation of sugar.
In conclusion, the microbial consortium of N-fixing and P–K solubilizing microbes Erwinia sp. EU-B2SNL1, C. arthrosphaerae EU-LWNA-37, and P. gessardii EU-MRK-19 enhanced the growth of the barley crop more in comparison to single inoculation, chemical control, and uninoculated control. In recent days, reduction of NPK chemical fertilizers is in emergence due to sustainability of agriculture and environment. The use of microbial consortium could be an ideal bioformulations for fulfilling macronutrient nutritional requirement of plant. In future, the microbial consortium could be experimented on the different cereal and horticultural crops. The strains could be improved through the genetic engineering to the better performance to enhance the crop productivity and growth.
Declarations
Author contribution statement
Tanvir Kaur: Performed the experiments; Wrote the paper.
Rubee Devi; Sunil Kumar: Analyzed and interpreted the data.
Imran Sheikh: Contributed reagents, materials, analysis tools or data.
Divjot Kour; Ajar Nath Yadav: Conceived and designed the experiments.
Funding statement
This work was supported by the Department of Biotechnology, Dr. Khem Singh Gill Akal College of Agriculture, Eternal University, Baru Sahib, and Department of Environment, Science & Technology (DEST), Shimla, HP funded project “Development of microbial consortium as bio-inoculants for drought and low temperature growing crops for organic farming in Himachal Pradesh”.
Data availability statement
Data associated with this study has been deposited at MN294539, MN733442, and OL687416.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to the Department of Biotechnology, Akal College of Agriculture, Eternal University, Baru Sahib and Department of Environment, Science & Technology (DEST), Shimla, HP funded project “Development of microbial consortium as bio-inoculants for drought and low temperature growing crops for organic farming in Himachal Pradesh” for providing the facilities support, to undertake the investigations.
Footnotes
This article is a part of the "Use of Bioinoculants in Agriculture" Special issue.
References
- Adhikari P., Jain R., Sharma A., Pandey A. Plant growth promotion at low temperature by phosphate-solubilizing Pseudomonas spp. isolated from high-altitude Himalayan soil. Microb. Ecol. 2021;82:677–687. doi: 10.1007/s00248-021-01702-1. [DOI] [PubMed] [Google Scholar]
- Afzal I., Iqrar I., Shinwari Z.K., Yasmin A. Plant growth-promoting potential of endophytic bacteria isolated from roots of wild Dodonaea viscosa L. Plant Growth Regul. 2017;81:399–408. [Google Scholar]
- AlAli H.A., Khalifa A., Almalki M. Plant growth-promoting rhizobacteria from Ocimum basilicum improve growth of Phaseolus vulgaris and Abelmoschus esculentus. South Afr. J. Bot. 2021;139:200–209. [Google Scholar]
- Albdaiwi R.N., Khyami-Horani H., Ayad J.Y., Alananbeh K.M., Al-Sayaydeh R. Isolation and characterization of halotolerant plant growth promoting rhizobacteria from durum wheat (Triticum turgidum subsp. durum) cultivated in saline areas of the dead sea region. Front. Microbiol. 2019;10:1639. doi: 10.3389/fmicb.2019.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerswald K., Kainz M., Angermüller S., Steindl H. Influence of exchangeable potassium on soil erodibility. Soil Use Manag. 1996;12:117–121. [Google Scholar]
- Chiappero J., Cappellari L.d.R., Sosa Alderete L.G., Palermo T.B., Banchio E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crop. Prod. 2019;139:111553. [Google Scholar]
- Conn V.M., Franco C.M. Effect of microbial inoculants on the indigenous actinobacterial endophyte population in the roots of wheat as determined by terminal restriction fragment length polymorphism. Appl. Environ. Microbiol. 2004;70:6407–6413. doi: 10.1128/AEM.70.11.6407-6413.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Salvo L.P., Cellucci G.C., Carlino M.E., García de Salamone I.E. Plant growth-promoting rhizobacteria inoculation and nitrogen fertilization increase maize (Zea mays L.) grain yield and modified rhizosphere microbial communities. Appl. Soil Ecol. 2018;126:113–120. [Google Scholar]
- El Kahkahi R., Moustaine M., Channaoui S., Hafidi M., Zouhair R., Chitt M.A., et al. Characterization of plant growth promoting rhizobacteria isolated from the rhizosphere of carob tree (Ceratonia siliqua L.) in Morocco. EurAsian J. Biosci. 2019;13:921–930. [Google Scholar]
- Etesami H., Alikhani H.A. Co-inoculation with endophytic and rhizosphere bacteria allows reduced application rates of N-fertilizer for rice plant. Rhizosphere. 2016;2:5–12. [Google Scholar]
- Fasusi O.A., Cruz C., Babalola O.O. Agricultural sustainability: microbial biofertilizers in rhizosphere management. Agriculture. 2021;11:163. [Google Scholar]
- Ghorchiani M., Etesami H., Alikhani H.A. Improvement of growth and yield of maize under water stress by co-inoculating an arbuscular mycorrhizal fungus and a plant growth promoting rhizobacterium together with phosphate fertilizers. Agric. Ecosyst. Environ. 2018;258:59–70. [Google Scholar]
- Han S.O., New P.B. Variation in nitrogen fixing ability among natural isolates of Azospirillum. Microb. Ecol. 1998;36:193–201. doi: 10.1007/s002489900106. [DOI] [PubMed] [Google Scholar]
- Heredia-Acuña C., Almaraz-Suarez J.J., Arteaga-Garibay R., Ferrera-Cerrato R., Pineda-Mendoza D.Y. Isolation, characterization and effect of plant-growth-promoting rhizobacteria on pine seedlings (Pinus pseudostrobus Lindl.) J. For. Res. 2019;30:1727–1734. [Google Scholar]
- Hu X., Chen J., Guo J. Two phosphate- and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J. Microbiol. Biotechnol. 2006;22:983–990. [Google Scholar]
- Irigoyen J., Einerich D., Sánchez-Díaz M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plantarum. 1992;84:55–60. [Google Scholar]
- Jain A., Singh A., Singh S., Singh H.B. Microbial consortium-induced changes in oxidative stress markers in pea plants challenged with Sclerotinia sclerotiorum. J. Plant Growth Regul. 2013;32:388–398. [Google Scholar]
- Jeong J.-J., Lee D.W., Park B., Sang M.K., Choi I.-G., Kim K.D. Chryseobacterium cucumeris sp. nov., an endophyte isolated from cucumber (Cucumis sativus L.) root, and emended description of Chryseobacterium arthrosphaerae. Int. J. Syst. Evol. Microbiol. 2017;67:610–616. doi: 10.1099/ijsem.0.001670. [DOI] [PubMed] [Google Scholar]
- Kaur T., Devi R., Kour D., Yadav A., Yadav A.N., Dikilitas M., et al. Plant growth promoting soil microbiomes and their potential implications for agricultural and environmental sustainability. Biologia. 2021;76:2687–2709. [Google Scholar]
- Kim D.-O., Jeong S.W., Lee C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–326. [Google Scholar]
- Knezevic-vukcevic J. Improvement of common bean growth by co-inoculation with Rhizobium and plant growth-promoting bacteria. Rom. Biotechnol. Lett. 2011;16:5919–5926. [Google Scholar]
- Kour D., Rana K.L., Kaur T., Sheikh I., Yadav A.N., Kumar V., et al. Microbe-mediated alleviation of drought stress and acquisition of phosphorus in great millet (Sorghum bicolour L.) by drought-adaptive and phosphorus-solubilizing microbes. Biocatal. Agric. Biotechnol. 2020;23:101501. [Google Scholar]
- Kour D., Rana K.L., Sheikh I., Kumar V., Yadav A.N., Dhaliwal H.S., et al. Alleviation of drought stress and plant growth promotion by Pseudomonas libanensis EU-LWNA-33, a drought-adaptive phosphorus-solubilizing bacterium. Proc. Natl. Acad. Sci. India B Biol. Sci. 2019;90:785–795. [Google Scholar]
- Kruasuwan W., Thamchaipenet A. Diversity of culturable plant growth-promoting bacterial endophytes associated with sugarcane roots and their effect of growth by co-inoculation of diazotrophs and actinomycetes. J. Plant Growth Regul. 2016;35:1074–1087. [Google Scholar]
- Kumar A., Maurya B.R., Raghuwanshi R. The microbial consortium of indigenous rhizobacteria improving plant health, yield and nutrient content in wheat (Triticum aestivum) J. Plant Nutr. 2021;44:1942–1956. [Google Scholar]
- Kumar A., Maurya B.R., Raghuwanshi R., Meena V.S., Tofazzal Islam M. Co-inoculation with Enterobacter and rhizobacteria on yield and nutrient uptake by wheat (Triticum aestivum L.) in the alluvial soil under Indo-Gangetic plain of India. J. Plant Growth Regul. 2017;36:608–617. [Google Scholar]
- Kumari B., Mallick M.A., Solanki M.K., Solanki A.C., Hora A., Guo W. In: Plant Health under Biotic Stress: Volume 2: Microbial Interactions. Ansari R.A., Mahmood I., editors. Springer Singapore; Singapore: 2019. Plant growth promoting rhizobacteria (PGPR): modern prospects for sustainable agriculture; pp. 109–127. [Google Scholar]
- Laranjeira S., Fernandes-Silva A., Reis S., Torcato C., Raimundo F., Ferreira L., et al. Inoculation of plant growth promoting bacteria and arbuscular mycorrhizal fungi improve chickpea performance under water deficit conditions. Appl. Soil Ecol. 2021;164:103927. [Google Scholar]
- Lichtenthaler H.K. In: Methods in Enzymology. Packer L., Douce R., editors. Vol. 148. Academic Press; 1987. [34] Chlorophylls and carotenoids: pigments of photosynthetic biomembranes; pp. 350–382. [Google Scholar]
- Morel M., Cagide C., Minteguiaga M., Dardanelli M.S., Castro-Sowinski S. The pattern of secreted molecules during the co-inoculation of alfalfa plants with Sinorhizobium meliloti and Delftia sp. strain JD2: an interaction that improves plant yield. Mol. Plant Microbe Interact. 2015;28:134–142. doi: 10.1094/MPMI-08-14-0229-R. [DOI] [PubMed] [Google Scholar]
- Murphy J., Riley J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962;27:31–36. [Google Scholar]
- Park Y.S., Jung S.T., Kang S.G., Heo B.G., Arancibia-Avila P., Toledo F., et al. Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem. 2008;107:640–648. [Google Scholar]
- Pathak H., Mohanty S., Jain N., Bhatia A. Nitrogen, phosphorus, and potassium budgets in Indian agriculture. Nutrient Cycl. Agroecosyst. 2010;86:287–299. [Google Scholar]
- Pikovskaya R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya. 1948;17:362–370. [Google Scholar]
- Powlson D. Understanding the soil nitrogen cycle. Soil Use Manag. 1993;9:86–93. [Google Scholar]
- Prashar P., Shah S. In: Sustainable Agriculture Reviews. Lichtfouse E., editor. Vol. 19. Springer International Publishing; Cham: 2016. Impact of fertilizers and pesticides on soil microflora in agriculture; pp. 331–361. [Google Scholar]
- Rojas-Tapias D., Moreno-Galván A., Pardo-Díaz S., Obando M., Rivera D., Bonilla R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays) Appl. Soil Ecol. 2012;61:264–272. [Google Scholar]
- Sagar A., Thomas G., Rai S., Mishra R.K., Ramteke P.W. Enhancement of growth and yield parameters of wheat variety AAI-W6 by an organic farm isolate of plant growth promoting Erwinia species (KP226572) Int. J. Agric. Environ. Biotechnol. 2018;11:159–171. [Google Scholar]
- Shin D.S., Park M.S., Jung S.R., Lee M.S., Lee K.H., Bae K.S., et al. Plant growth-promoting potential of endophytic bacteria isolated from roots of coastal sand dune plants. J. Microbiol. Biotechnol. 2007;17:1361–1368. [PubMed] [Google Scholar]
- Singh A.V., Chandra R., Goel R. Phosphate solubilization by Chryseobacterium sp. and their combined effect with N and P fertilizers on plant growth promotion. Arch. Agron Soil Sci. 2013;59:641–651. [Google Scholar]
- Spiess E. Nitrogen, phosphorus and potassium balances and cycles of Swiss agriculture from 1975 to 2008. Nutrient Cycl. Agroecosyst. 2011;91:351–365. [Google Scholar]
- Sugumaran P., Janarthanam B. Solubilization of potassium containing minerals by bacteria and their effect on plant growth. World J. Agric. Sci. 2007;3:350–355. [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tripathi D.K., Singh V.P., Chauhan D.K., Prasad S.M., Dubey N.K. In: Improvement of Crops in the Era of Climatic Changes. Ahmad P., Wani M.R., Azooz M.M., Phan Tran L.S., editors. Vol. 2. Springer New York; New York, NY: 2014. Role of macronutrients in plant growth and acclimation: recent advances and future prospective; pp. 197–216. [Google Scholar]
- Upadhyay S., Singh D. Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015;17:288–293. doi: 10.1111/plb.12173. [DOI] [PubMed] [Google Scholar]
- Vafadar F., Amooaghaie R., Otroshy M. Effects of plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungus on plant growth, stevioside, NPK, and chlorophyll content of Stevia rebaudiana. J. Plant Interact. 2014;9:128–136. [Google Scholar]
- Verma P., Yadav A.N., Kazy S.K., Saxena A.K., Suman A. Evaluating the diversity and phylogeny of plant growth promoting bacteria associated with wheat (Triticum aestivum) growing in central zone of India. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:432–447. [Google Scholar]
- Verma P., Yadav A.N., Khannam K.S., Kumar S., Saxena A.K., Suman A. Molecular diversity and multifarious plant growth promoting attributes of bacilli associated with wheat (Triticum aestivum L.) rhizosphere from six diverse agro-ecological zones of India. J. Basic Microbiol. 2016;56:44–58. doi: 10.1002/jobm.201500459. [DOI] [PubMed] [Google Scholar]
- Vimal S.R., Patel V.K., Singh J.S. Plant growth promoting Curtobacterium albidum strain SRV4: an agriculturally important microbe to alleviate salinity stress in paddy plants. Ecol. Indicat. 2019;105:553–562. [Google Scholar]
- Yadav A.N., Sachan S.G., Verma P., Tyagi S.P., Kaushik R., Saxena A.K. Culturable diversity and functional annotation of psychrotrophic bacteria from cold desert of Leh Ladakh (India) World J. Microbiol. Biotechnol. 2015;31:95–108. doi: 10.1007/s11274-014-1768-z. [DOI] [PubMed] [Google Scholar]
- Yadav K.K., Sarkar S. Biofertilizers, impact on soil fertility and crop productivity under sustainable agriculture. Environ. Ecol. 2019;37:89–93. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has been deposited at MN294539, MN733442, and OL687416.