Abstract
Background
The incidence of venous thromboembolism (VTE) has gradually increased in the Korean population. This study aimed to evaluate the annual age- and sex-adjusted incidence rates (ASR) of VTE and anticoagulation trends between 2014 and 2018.
Methods
Using the Korean Health Insurance Review and Assessment Service database, we retrospectively identified VTE patients between 2014 and 2018 using both diagnostic and medication anticoagulant codes assigned within 6 months of the initial index event. Anticoagulant patterns were classified as follows: direct oral anticoagulants (DOAC), parenteral anticoagulants, warfarin, and mixed anticoagulation regimens.
Results
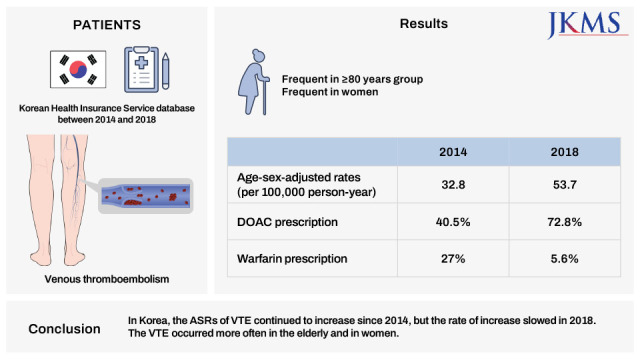
We identified 95,205 patients with VTE (female, 56.8%). The ASR for VTE per 100,000 person-years increased from 32.8 in 2014 to 53.7 cases in 2018 (relative risk of 1.63; 95% confidence interval, 1.6–1.67). The VTE incidence rates were 25 times higher in the ≥ 80 group than in the 30s group. VTE occurred 1.29 times more often in women than in men. The proportion of DOAC prescriptions increased from 40.5% to 72.8%, whereas warfarin prescriptions decreased from 27% to 5.6% in 2014 and 2018.
Conclusion
In Korea, the ASRs of VTE continued to increase since 2014, but the rate of increase slowed in 2018. The VTE occurred more often in the elderly and in women. Five years after the introduction of DOACs in 2013, they accounted for 73% of all anticoagulants used to treat VTE.
Keywords: Anticoagulants, Incidence, Pulmonary Embolism, Venous Thromboembolism
Graphical Abstract
INTRODUCTION
Venous thromboembolism (VTE) is associated with reduced survival rates.1 Asian studies have shown that the incidence of VTE is 13.8–57 per 100,000 person-years.1,2,3,4,5,6,7 However, this is still low compared to the 114–184 per 100,000 person-years in western studies.1,8,9,10,11,12,13,14 A second epidemiologic study of the Korean population covering 2008 to 2014 demonstrated an annual incidence rate of 23.4 per 100,000 person-years.2,15 Direct oral anticoagulants (DOAC) has been covered by the Korean insurance service since January 2013 and began to replace previously established anticoagulants.16 DOACs have major pharmacologic advantages over warfarin, including rapid onset/off set of action, few drug interactions, predictable pharmacokinetics and no need for INR monitoring. Increased awareness of VTE among clinicians and easy access to standard imaging methods for diagnosis has created a virtuous cycle of further diagnosis and optimal treatment. Moreover, older people are at increased risk of VTE because most acquired risk factors are more common in older individuals and constitute multifactorial causes of VTE.2 Therefore, we expected the further increase in incidence of VTE since 2013. This third nationwide population-based epidemiologic study primarily aimed to evaluate the annual age- and sex-adjusted incidence rates (ASR) of VTE between 2014 and 2018. A secondary aim was to describe the anticoagulation trends during the study period since the introduction of DOAC in 2013.
METHODS
Data acquisition
The Korean National Health Insurance (NHI) program, which is operated by the Ministry of Health and Welfare of Korea, is a compulsory system that covers approximately 97% of Korea’s population.17 This government-operated organization creates accurate systems for evaluating and assessing claims for the NHI. The Health Insurance and Review Assessment (HIRA) reviews the claim data for the remaining 3% of the population covered by the National Medical Aid program. According to the NHI data, the registered population was 52,272,755 in 2016.17,18 The NHI covers almost the entire population of Korea, enabling it to perform nationwide epidemiologic studies.
Previously studies have provided detailed information about the HIRA database.2,15,19 We had access to the HIRA database with the approval of the HIRA Data Access Committee in conformity with HIRA’s Rules for Data Exploration and Utilization. All information was processed anonymously. We used diagnostic codes from the Korean Classification of Disease, seventh edition (KCD-7), which is a modified version of the International Classification of Disease 10th edition (ICD-10).
Ethics statements
All method was performed in accordance with the relevant guidelines and regulations. The data is anonymized, and the database is publicly not available. This study was exempted from review by the Seoul National University Bundang Hospital Institutional Review Board (X-2008/628-901). The informed consent was waived by approving committee.
Definition and treatment of VTE
An index date is defined as VTE occurrence if both diagnostic (from the main to the fifth minor diagnostic code) and medication codes were identified simultaneously in a patient. We searched the data without distinguishing outpatients and inpatients. In this study, diagnostic codes for the term “VTE” include the following: 1) I80.2 or I80.3 (deep vein thrombosis [DVT] of lower extremity) and 2) I26 (pulmonary thromboembolism), I26.0 (pulmonary embolism [PE] with mention of acute cor pulmonale), or I26.9 (PE without mention of acute cor pulmonale) (Supplementary Table 1). If a patient had DVT in the lower extremity and PE, the patient was considered to have PE. Hereafter in this study, the term “DVT” denotes “DVT of the lower extremity without PE,” “PE” means “PE with or without DVT,” and “VTE” is the “combination of both DVT and PE.” The medication codes for unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), DOAC (rivaroxaban, dabigatran, apixaban, and edoxaban), and warfarin were used with coincident diagnostic codes to detect VTE (Supplementary Table 2). Treatment of VTE was classified to four groups 1) DOAC, DOAC with/without preceding parenteral anticoagulants (PAC), 2) PAC, UFH and/or low-molecular-weight heparin, 3) warfarin, warfarin with/without preceding PAC, and 4) mixed anticoagulants mean switching from one to the other regimen during the six months at least one time.
Case identification
We searched the HIRA database from July 1, 2013, to June 30, 2019, to identify inpatient and outpatient cases with both diagnostic and medication anticoagulant codes prescribed for VTE events. We considered the VTE diagnosis date as the first day of the simultaneous assignment of diagnostic and medication codes.
From 134,519 patients identified, we excluded 16,868 patients who had diagnostic codes for atrial fibrillation (AF) and anticoagulation codes for stroke prevention within the 12 months prior to VTE (Supplementary Fig. 1). AF was defined as having diagnostic codes I48.0-I48.4, I48.9, and diagnostic codes for valvular AF, such as mitral stenosis (I050, I052, and I059) or mechanical heart valves (Z952–Z954) (Supplementary Fig. 1).
We had wash-out period of six months. Thus, we excluded patients who had been diagnosed with VTE during the initial 6 months and last 6 months of the study period to exclude prevalent cases and cases with short term follow-up (n = 11,782 and 10,664, respectively). To count the index VTE case, we excluded subsequent VTE cases (n = 29,023) that were detected in other calendar years in each patient. Finally, 95,205 individuals with index VTE cases (Population A) were included (54,085 female, 56.8%) (Supplementary Fig. 1).
Analysis of the VTE incidence and pattern of VTE treatment
We analyzed the ASR and relative risk (RR) for VTE according to calendar year, age, and sex in Population B (Supplementary Fig. 1). We evaluated all medication codes for UFH, LMWH, DOAC (rivaroxaban, dabigatran, apixaban, and edoxaban), and warfarin prescribed within six months of VTE diagnosis. The types of LMWH were as follows: enoxaparin sodium (20 mg, 30 mg, 40 mg, 60 mg, 80 mg, and 100 mg), dalteparin sodium (2,500 IU, 7,500 IU, and 10,000 IU), and nadroparin calcium (2,850 IU, 3,800 IU, 5,700 IU, and 47,500 IU). Fondaparinux has not been approved for VTE treatment in Korea. We analyzed anticoagulation patterns and classified them as follows: 1) DOAC-based regimen, DOAC only or DOACs with UFH/LMWH but not with warfarin; 2) UFH/LMWH regimen, use of UFH and/or LMWH without DOAC and warfarin; 3) warfarin-based regimen, warfarin with UFH/LMWH; and 4) mixed anticoagulation regimen, use of both DOACs and warfarin within the initial 6 months of VTE index event in Population A.
Statistical analysis
Age- and sex-adjusted annual incidences of VTE, DVT, and PE were directly adjusted to the 2016 medical coverage population based on the HIRA database. The rate of incidence and confidence interval (CI) by age, sex, and year were estimated using the Poisson distribution.20 The average annual percent change in incidence was assessed as previously described.20 Statistical significance was set at P < 0.05. Statistical analyses were performed using SAS Enterprise version 6.1.
RESULTS
From January 2014 to December 2018, 124,228 annual index cases and 95,205 individuals with VTE (female, 56.8%) were identified (Supplementary Fig. 1). The ASR annual incidences of VTE, DVT, and PE were 32.8, 13.8, and 19.0 in 2014; 50.7, 23.1, and 27.7 in 2016; and 53.7, 22.8, and 30.9 in 2018, respectively (Supplementary Table 3). The RR for VTE, DVT, and PE per 100,000 person-years in 2018 were 1.63 (95% CI, 1.60–1.67; P < 0.001), 1.65 (95% CI, 1.60–1.70; P < 0.001), and 1.62 (95% CI, 1.58–1.67; P < 0.001), compared with those in 2014, respectively (Table 1). The incidence of VTE in 2018 increased by 1.8 times from 2013 and six times from 2004, compared with the results of a previous study.2,15 However, the ASRs for VTE are similar in 2017 and 2018 (Supplementary Fig. 2). The RRs for VTE incidence are 0.12 in the 10s (95% CI, 0.11–0.13; P < 0.001), 0.51 in the 20s (95% CI, 0.48–0.53; P < 0.001), but 3.03 in the 50s (95% CI, 2.94–3.13; P < 0.001), 7.66 in the 60s (95% CI, 7.44–7.90; P < 0.001), 18.48 in the 70s (95% CI, 17.95–19.04; P < 0.001), and 25.40 in the 80s or older (95% CI, 24.63–26.18; P < 0.001) age groups, compared with that of the 30s group, respectively (Table 1).
Table 1. Incidence rate ratio of VTE, DVT, and PE according to age, sex, and year.
| Variables | VTE | DVT | PE | ||||
|---|---|---|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | ||
| Age, yr | |||||||
| 0–9 | 0.02 (0.02–0.03) | < 0.001 | 0.03 (0.02–0.04) | < 0.001 | 0.02 (0.01–0.02) | < 0.001 | |
| 10–19 | 0.12 (0.11–0.13) | < 0.001 | 0.13 (0.11–0.14) | < 0.001 | 0.12 (0.1–0.14) | < 0.001 | |
| 20–29 | 0.51 (0.48–0.53) | < 0.001 | 0.47 (0.44–0.51) | < 0.001 | 0.54 (0.5–0.58) | < 0.001 | |
| 30–39 | 1 | 1 | 1 | ||||
| 40–49 | 1.62 (1.56–1.67) | < 0.001 | 1.61 (1.53–1.67) | < 0.001 | 1.62 (1.55–1.71) | < 0.001 | |
| 50–59 | 3.03 (2.94–3.13) | < 0.001 | 2.93 (2.80–3.04) | < 0.001 | 3.13 (3.00–3.28) | < 0.001 | |
| 60–69 | 7.66 (7.44–7.90) | < 0.001 | 6.99 (6.70–7.22) | < 0.001 | 8.37 (8.02–8.74) | < 0.001 | |
| 70–79 | 18.48 (17.95–19.04) | < 0.001 | 14.7 (14.09–15.17) | < 0.001 | 22.45 (21.53–23.40) | < 0.001 | |
| ≥ 80 | 25.40 (24.63–26.18) | < 0.001 | 18.44 (17.65–19.10) | < 0.001 | 32.67 (31.30–34.10) | < 0.001 | |
| Year | |||||||
| 2014 | 1 | 1 | 1 | ||||
| 2015 | 1.34 (1.32–1.37) | < 0.001 | 1.41 (1.37–1.46) | < 0.001 | 1.30 (1.26–1.33) | < 0.001 | |
| 2016 | 1.54 (1.52–1.57) | < 0.001 | 1.67 (1.62–1.72) | < 0.001 | 1.46 (1.42–1.49) | < 0.001 | |
| 2017 | 1.64 (1.6–1.67) | < 0.001 | 1.72 (1.67–1.77) | < 0.001 | 1.57 (1.53–1.61) | < 0.001 | |
| 2018 | 1.63 (1.6–1.67) | < 0.001 | 1.65 (1.6–1.7) | < 0.001 | 1.62 (1.58–1.67) | < 0.001 | |
| Sex | |||||||
| Male | 1 | 1 | 1 | ||||
| Female | 1.29 (1.28–1.31) | < 0.001 | 1.33 (1.30–1.35) | < 0.001 | 1.27 (1.25–1.29) | < 0.001 | |
VTE = venous thromboembolism, DVT = deep vein thrombosis, PE = pulmonary embolism with or without DVT, RR = relative risk, CI = confidence interval.
The risks for VTE, DVT, and PE in women are 1.29 (95% CI, 1.28–1.31; P < 0.001), 1.33 (95% CI, 1.30–1.35; P < 0.001), and 1.27 (95% CI, 1.25–1.29; P < 0.001), respectively, compared with those in men (Table 1). The ASR was depicted in each age group according to sex in 2014 and 2018 (Supplementary Fig. 3). The proportions of cumulative incidence of VTE in patients aged 60 years and older were 72.4%, 72.9%, and 73.7% in 2014, 2016, and 2018, respectively (Supplementary Fig. 4).
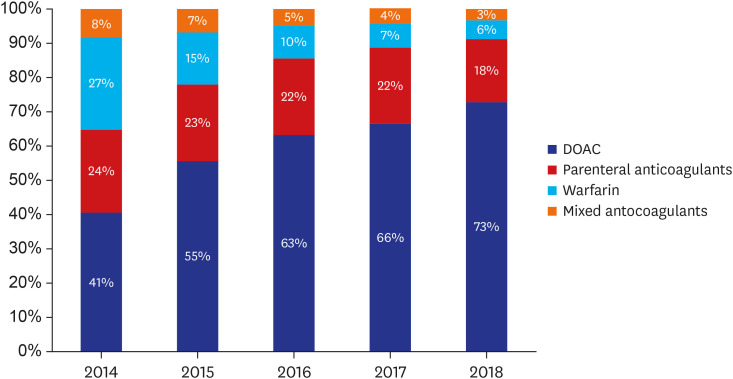
During the study period, DOAC are the most frequently used anticoagulant therapy, followed by PAC. The proportion of DOAC prescriptions increased 1.8 times from 40.5% to 72.8%. However, the prescriptions of PAC decreased from 24.3% to 18.4%, warfarin from 27% to 5.6%, and mixed anticoagulation from 8.2% to 3.2% in 2014 and 2018, respectively (Fig. 1).
Fig. 1. Anticoagulation trend for venous thromboembolism from 2014 to 2018.
DOAC = direct oral anticoagulants.
DISCUSSION
This study demonstrated that since the second epidemiologic study on VTE incidence in South Korea, the ASR of VTE has continuously risen between 2014 and 2018. The ASR of 53.66 per 100,000 person-years in 2018 is a six-fold increase since 2004, and it is comparable to Asia’s highest incidence rate in Singapore.4 However, the increase in VTE incidence rate has slowed to 53.69 and 53.66 per 100,000 person-years between 2017 and 2018 (Supplementary Fig. 2).
The incidence of VTE incidences in countries in Europe and North America (114–184 per 100,000)1,8,9,10,11,12,13,14,21 are much higher than those in Korea and Asian countries (13.8–57 per 100,000)2,3,4,5,6,15 (Table 2). Heit et al.13 showed that the ASR of annual VTE incidence over 1981–2010 did not change significantly despite the increased prevalence of obesity, surgery, active cancer, and leg paresis. It seems that VTE incidence in Western countries had already reached their peak. If ethnic differences exist, in Korea, the incidence of VTE appears to have reached the maximum level.
Table 2. Reported VTE incidence in studies conducted over the past two decades.
| Studies and Locations | Study period | Study design | Size of population | VTE | DVT | PE | ||
|---|---|---|---|---|---|---|---|---|
| Western countries | ||||||||
| Delluc et al.8 | Western France | Mar 2013–Feb 2014 | Prospective cohort | Among 367,911 inhabitants | 157 | 119.8 | 64.2 | |
| Bouée et al.9 | France | 2 years (2010–2011) | Population-based | Around 600,000 individuals | 184 | |||
| Alotaibi et al.10 | Alberta (Canada) | Apr 2002–Mar 2012 | Population-based | Over 4 million in 2014 | 138 | 100 | 38 | |
| Johansson et al.11 | Västerbotten (Sweden) | 2006 | Population-based | 204,836 residents | 167 | 76.6 | 78.6 | |
| Tagalakis et al.1 | Que´bec (Canada) | 2000–2009 | Population-based | Over 74 million person-years | 122 | 78 | 45 | |
| Spencer et al.12 | Worcester, MA (USA) | 1999, 2001, and 2003 | Population-based | 477,800 residents in 2000 | 114 | 95 | 34 | |
| Heit et al.13 | Olmsted, MN (USA) | 1981–2010 | Population-based | 493,000 individuals | 123 | 47 | 62 | |
| Lehnert et al.14 | Denmark | 2004–2014 | Population-based | 4,301,673 individuals | 83 | |||
| Asian countries | ||||||||
| Hwang et al.16 (2021) | Korea (this study) | 2014–2018 | Population-based | Around 52 million/year | 50.7 | 23.1 | 27.7 | |
| Hong et al.2 | Korea (our previous) | 2009–2013 | Population-based | Around 50 million/year | 23.4 | 9.4 | 14.1 | |
| Jang et al.15 | Korea (our previous) | 2004–2008 | Population-based | Around 49 million/year | 13.8 | 5.31 | 7.0 | |
| Sakuma et al.3 | Japan | Aug–Sep 2006 | Questionnaire | 6,135 replies | 11.6 | 6.2 | ||
| Molina et al.4 (2011) | Singapore | 2006 | Cohort (3 major hospital) | 98,121 admitted patients | 57 | 15 | ||
| Lee et al.5 | Taiwan | 2002 | Population-based | Around 22.5 million/year | 16.5 | 4.8 | ||
| Law et al.7 | Hong Kong | 2010–2011 | Population-based | 2,898,107 admissions | 41.7 | 30.0 | 11.7 | |
| Cheuk et al.6 | Hong Kong | 2000–2001 | Retrospective | 2,082,245 admissions | 21 | 17.1 | 3.9 | |
VTE = venous thromboembolism, DVT = deep vein thrombosis, PE = pulmonary embolism with or without DVT.
However, the elderly population is expected to have a further increase in the incidence of VTE, regardless of ethnic differences in Korea (Supplementary Fig. 4). The present study shows that the proportion of cumulative incidence of VTE in patients aged ≥ 60 years accounted for more than 70%. The ASR of VTE is also more common in patients aged ≥ 80 years than in the 30–39 years age group. Furthermore, the ASR in 2018 increased significantly compared to that in 2014 (Table 1 and Supplementary Fig. 3). In contrast, Park et al.22 reported that among patients under 30 years of age, bimodal age distribution of the VTE incidence with peaks at infancy and again after 16 years. In Korea, the old age group will grow more rapidly in the near future than in any other country, and the elderly will have an increased incidence of cancer-associated VTE (CAT) due to cancer-related surgeries or improved cancer survival rates with anticancer treatment. They also have the multiple morbidities needing admission, and the additional risk of long bone fractures. These will lead to a VTE burden on the public health in Korea.23,24,25
Female sex was associated with a significant increase in the incidence of VTE as compared to male sex in the second (2009–2013) (RR, 1.19; 95% CI, 1.17–1.21; P < 0.001) and third (2014–2018) (RR, 1.29; 95% CI, 1.28–1.31; P < 0.001) epidemiologic studies. However, at the distribution according to age, the incidence of VTE was low among women in the < 60 group, similar to that of women in their 60s group, and high only among women in the ≥ 70s group, as compared to men (Supplementary Table 3). Law et al.7 reported that in Hong Kong, the overall male-to-female ratio is 1:1.24, and age-specific incidences are higher among women over 55 years of age, who have PE with DVT, than in men. A population-based study in northern Sweden showed that the incidence of VTE per 100,000 person-years in women over 75 years of age exceeds that of men.11 In contrast, White et al. reported that there were no differences in the incidence of VTE between men and women.26
In all likelihood, long-lived women are more likely to be exposed to the risk of thrombosis than men. Boardman et al.27 reported that both PE and VTE events were increased among postmenopausal women receiving hormone replacement therapy as compared to the control (RR, 1.81; 95% CI, 1.32–2.48; RR, 1.92; 95% CI, 1.36–2.69 respectively). Because of limited information in the HIRA data, this study was unable to determine which factors increase the incidence of VTE in women versus in men.
This study shows that since the introduction of DOAC to Korea in 2013, its prescription rate has increased from 41% to 73% by 2018 (Fig. 1). In the GARFIELD-VTE study, 24.5% of cancer patients and 50.6% of non-cancer patients received DOACs. These patients were enrolled in a similar period (2014–2017).28 It seems that the convenience of use for patients, physician preference, and active marketing of new anticoagulants contributed to the increased use of DOACs globally. In particular, insurance coverage and easy access to medical institutions seem to be related to greater DOAC use in Korea than in other countries.
On the other hand, the proportion of UFH/LMWH use decreased from 24% in 2014 to 18% in 2018. The GARFIELD VTE study reported that parenteral anticoagulation was prescribed in some proportion of patients with active cancer, history of cancer, or no cancer (57.8%, 18.9%, and 12.1%, respectively).28 Although LMWH may play a role in the real world for patients with CAT or for severely ill patients, the proportion of DOAC use in CAT will increase further given that recent trials showed evidence of the efficacy of DOACs in the treatment of VTE in such patients.29,30,31 Warfarin prescriptions also decreased by 79%, from 27% to 5.6%. This means that despite the hassle of monitoring prothrombin time, the need for warfarin still remains in some patients with renal failure or mechanical heart valves.
This study has some limitations given that it was a retrospective, population-based study. First, the HIRA database provided limited information about the patients. The operational definition of VTE was not based on imaging studies, but on diagnostic codes and medication codes. Therefore, we did not validate specific information by reviewing the medical records or imaging studies. Second, we could not classify the patients as inpatients, outpatients, or by provoking factors such as cancer, surgery, bone fracture, or pregnancy. We are planning the subsequent analyses for CAT and pregnancy associated VTE.
Third, because we defined VTE if diagnostic and medication code were identified simultaneously, therefore there is the possibility of not recognizing those few patients for whom anticoagulation is contraindicated or not initiating it in cases of asymptomatic distal DVT or subsegmental PE. We identified all types of DOACs for which reimbursement was claimed during study period. However, there is possibility that the claim was omitted if it was prescribed at their own expense, but it is considered to be very small. Thus, the actual incidence of VTE may be slightly higher than that calculated in this study. Fourth, there is concern about duplicated prescription for anticoagulants. But treatment patterns allow for some overlapping (parenteral anticoagulation and warfarin or some DOACs) and overlapping date in the bill with other combination of anticoagulants does not mean a duplicate of the patient’s medication. Therefore, we did not analyze it separately.
In Korea, the ASR of VTE continued to increase since 2014, but the rate of increase slowed in 2018. The incidence of VTE was significantly higher in the elderly population than in the middle-aged group. VTE also occurred more often in women than in men. Prescribing DOAC has become a major option, accounting for 73% of anticoagulants used in treating VTE five years after their introduction in 2013.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.co.kr) for English language editing.
Footnotes
Funding: This work was supported by a grant (KSTH 2017-001) from the Korean Society on Thrombosis and Hemostasis. This research was supported by the Soonchunhyang University Research Fund (SURF-20160740). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure: All authors have no conflicts of interest to disclose.
- Conceptualization: Hwang HG, Kim YK, Yhim HY, Hong J, Bang SM.
- Data curation: Lee JH.
- Formal analysis: Lee JH, Kim SA.
- Funding acquisition: Hwang HG.
- Methodology: Hwang HG, Kim SA, Hong J, Bang SM.
- Writing - original draft preparation: Hwang HG, Bang SM.
- Writing - review & editing: Hwang HG, Lee JH, Kim SA, Kim YK, Yhim HY, Hong J, Bang SM.
SUPPLEMENTARY MATERIALS
Diagnostic code and definition
Drug code for anticoagulants
Age- and sex-adjusted annual incidence per 100,000 population in patients with VTE between 2014 and 2018
Overall flow of analysis.
Trend of sex- and age-adjusted annual incidence per 100,000 population in Korea between the 1st and 3rd study periods (2004–2018).
Sex- and age-adjusted annual incidence of VTE per 100,000 cases of VTE in 2014 and 2018.
Cumulative incidence cases of VTE in each group in 2014 and 2018.
References
- 1.Tagalakis V, Patenaude V, Kahn SR, Suissa S. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126(9):832.e13–832.e21. doi: 10.1016/j.amjmed.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 2.Hong J, Lee JH, Yhim HY, Choi WI, Bang SM, Lee H, et al. Incidence of venous thromboembolism in Korea from 2009 to 2013. PLoS One. 2018;13(1):e0191897. doi: 10.1371/journal.pone.0191897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakuma M, Nakamura M, Yamada N, Ota S, Shirato K, Nakano T, et al. Venous thromboembolism: deep vein thrombosis with pulmonary embolism, deep vein thrombosis alone, and pulmonary embolism alone. Circ J. 2009;73(2):305–309. doi: 10.1253/circj.cj-08-0372. [DOI] [PubMed] [Google Scholar]
- 4.Molina JA, Jiang ZG, Heng BH, Ong BK. Venous thromboembolism at the National Healthcare Group, Singapore. Ann Acad Med Singapore. 2009;38(6):470–478. [PubMed] [Google Scholar]
- 5.Lee CH, Cheng CL, Lin LJ, Tsai LM, Yang YH. Epidemiology and predictors of short-term mortality in symptomatic venous thromboembolism. Circ J. 2011;75(8):1998–2004. doi: 10.1253/circj.cj-10-0992. [DOI] [PubMed] [Google Scholar]
- 6.Cheuk BL, Cheung GC, Cheng SW. Epidemiology of venous thromboembolism in a Chinese population. Br J Surg. 2004;91(4):424–428. doi: 10.1002/bjs.4454. [DOI] [PubMed] [Google Scholar]
- 7.Law Y, Chan YC, Cheng SW. Epidemiological updates of venous thromboembolism in a Chinese population. Asian J Surg. 2018;41(2):176–182. doi: 10.1016/j.asjsur.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Delluc A, Tromeur C, Le Ven F, Gouillou M, Paleiron N, Bressollette L, et al. Current incidence of venous thromboembolism and comparison with 1998: a community-based study in Western France. Thromb Haemost. 2016;116(5):967–974. doi: 10.1160/TH16-03-0205. [DOI] [PubMed] [Google Scholar]
- 9.Bouée S, Emery C, Samson A, Gourmelen J, Bailly C, Cotté FE. Incidence of venous thromboembolism in France: a retrospective analysis of a national insurance claims database. Thromb J. 2016;14(1):4. doi: 10.1186/s12959-016-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alotaibi GS, Wu C, Senthilselvan A, McMurtry MS. Secular trends in incidence and mortality of acute venous thromboembolism: the AB-VTE population-based study. Am J Med. 2016;129(8):879.e19–879.e25. doi: 10.1016/j.amjmed.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 11.Johansson M, Johansson L, Lind M. Incidence of venous thromboembolism in northern Sweden (VEINS): a population-based study. Thromb J. 2014;12(1):6. doi: 10.1186/1477-9560-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer FA, Emery C, Joffe SW, Pacifico L, Lessard D, Reed G, et al. Incidence rates, clinical profile, and outcomes of patients with venous thromboembolism. The Worcester VTE study. J Thromb Thrombolysis. 2009;28(4):401–409. doi: 10.1007/s11239-009-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heit JA, Ashrani A, Crusan DJ, McBane RD, Petterson TM, Bailey KR. Reasons for the persistent incidence of venous thromboembolism. Thromb Haemost. 2017;117(2):390–400. doi: 10.1160/TH16-07-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehnert P, Lange T, Møller CH, Olsen PS, Carlsen J. Acute pulmonary embolism in a National Danish Cohort: increasing incidence and decreasing mortality. Thromb Haemost. 2018;118(3):539–546. doi: 10.1160/TH17-08-0531. [DOI] [PubMed] [Google Scholar]
- 15.Jang MJ, Bang SM, Oh D. Incidence of venous thromboembolism in Korea: from the Health Insurance Review and Assessment Service database. J Thromb Haemost. 2011;9(1):85–91. doi: 10.1111/j.1538-7836.2010.04108.x. [DOI] [PubMed] [Google Scholar]
- 16.Hwang HG, Lee JH, Hong J, Kim SA, Kim YK, Kim MS, et al. Recurrence of cancer-associated venous thromboembolism between 2009 and 2013: a nationwide Korean study. Clinical & Experimental Thrombosis and Hemostasis. 2021;7(1):14–19. [Google Scholar]
- 17.Kim L, Kim JA, Kim S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol Health. 2014;36:e2014008. doi: 10.4178/epih/e2014008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Utilization of Medical Services by Region in 2014. The 2014 Statistical Yearbook on Utilization of Medical Services by Region 2015. Volume 1 [Google Scholar]
- 19.Jang MJ, Bang SM, Oh D. Incidence of pregnancy-associated venous thromboembolism in Korea: from the Health Insurance Review and Assessment Service database. J Thromb Haemost. 2011;9(12):2519–2521. doi: 10.1111/j.1538-7836.2011.04518.x. [DOI] [PubMed] [Google Scholar]
- 20.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raskob GE, Angchaisuksiri P, Blanco AN, Büller H, Gallus A, Hunt BJ, et al. Thrombosis: a major contributor to global disease burden. Semin Thromb Hemost. 2014;40(7):724–735. doi: 10.1055/s-0034-1390325. [DOI] [PubMed] [Google Scholar]
- 22.Park ES, Choi HS, Lee KS, Kim SW, Lee JM. Venous thromboembolism in children and young adults in Korea: analysis of the Korean Health Insurance Review and Assessment Service Database. J Korean Med Sci. 2019;34(49):e316. doi: 10.3346/jkms.2019.34.e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park TY, Jung JW, Choi JC, Shin JW, Kim JY, Choi BW, et al. Epidemiological trend of pulmonary thromboembolism at a tertiary hospital in Korea. Korean J Intern Med. 2017;32(6):1037–1044. doi: 10.3904/kjim.2016.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SA, Yhim HY, Bang SM. Current management of cancer-associated venous thromboembolism: focus on direct oral anticoagulants. J Korean Med Sci. 2019;34(6):e52. doi: 10.3346/jkms.2019.34.e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens JA, Olson S. Reducing falls and resulting hip fractures among older women. MMWR Recomm Rep. 2000;49(RR-2):3–12. [PubMed] [Google Scholar]
- 26.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23) Suppl 1:I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 27.Boardman HM, Hartley L, Eisinga A, Main C, Roqué i Figuls M, Bonfill Cosp X, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3):CD002229. doi: 10.1002/14651858.CD002229.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weitz JI, Haas S, Ageno W, Goldhaber SZ, Turpie AG, Goto S, et al. Cancer associated thrombosis in everyday practice: perspectives from GARFIELD-VTE. J Thromb Thrombolysis. 2020;50(2):267–277. doi: 10.1007/s11239-020-02180-x. [DOI] [PubMed] [Google Scholar]
- 29.Agnelli G, Becattini C, Meyer G, Muñoz A, Huisman MV, Connors JM, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599–1607. doi: 10.1056/NEJMoa1915103. [DOI] [PubMed] [Google Scholar]
- 30.Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D) J Clin Oncol. 2018;36(20):2017–2023. doi: 10.1200/JCO.2018.78.8034. [DOI] [PubMed] [Google Scholar]
- 31.Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diagnostic code and definition
Drug code for anticoagulants
Age- and sex-adjusted annual incidence per 100,000 population in patients with VTE between 2014 and 2018
Overall flow of analysis.
Trend of sex- and age-adjusted annual incidence per 100,000 population in Korea between the 1st and 3rd study periods (2004–2018).
Sex- and age-adjusted annual incidence of VTE per 100,000 cases of VTE in 2014 and 2018.
Cumulative incidence cases of VTE in each group in 2014 and 2018.