Abstract
The in vitro activities of ABT-773, erythromycin, clarithromycin, and azithromycin were compared. ABT-773 was the most active compound against macrolide-susceptible Streptococcus pneumoniae, Streptococcus pyogenes, Staphylococcus aureus, Staphylococcus epidermidis, Listeria monocytogenes, and Enterococcus spp. and multidrug-resistant Streptococcus pneumoniae. It also had good activity against gram-negative and atypical respiratory tract pathogens and Helicobacter pylori.
Macrolide antibiotics are used for treating community-acquired respiratory tract infections (1). Ketolides, erythromycin analogs in which a ketone functionality replaces the three-position cladinose, demonstrate in vitro antibacterial activity and in vivo efficacy in animal models of infection (3, 11). In addition, many ketolides retain potency against macrolide-resistant strains of streptococci (2, 3, 11) due to dimethylation of the 23S rRNA by Erm methylases or by macrolide-specific efflux pumps (3, 14, 15).
In addition to the ketone group at position 3, the novel ketolide ABT-773 is modified by an O-allyl-3-quinoline at the 6 position and a cyclized carbamate group between the 11 and 12 positions (11). This study evaluated the potency and spectrum of ABT-773. The MIC ranges and MICs inhibiting 50 (MIC50) and 90% (MIC90) of the tested strains are presented in Tables 1 and 2.
TABLE 1.
Comparative in vitro activity of ABT-773 by agar dilution methods
| Species (resistance) [n] and antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| S. pyogenes (macrolide S) [17] | |||
| ABT-773 | ≤0.015–0.03 | 0.03 | 0.03 |
| Clarithromycin | 0.03 | 0.03 | 0.03 |
| Azithromycin | 0.12–0.25 | 0.12 | 0.25 |
| Erythromycin | 0.03–0.06 | 0.03 | 0.06 |
| Clindamycin | 0.03–0.06 | 0.03 | 0.06 |
| S. pyogenes (MefA) [13] | |||
| ABT-773 | ≤0.015–0.25 | 0.12 | 0.25 |
| Clarithromycin | 2–16 | 4 | 16 |
| Azithromycin | 2–8 | 8 | 8 |
| Erythromycin | 2–16 | 8 | 16 |
| Clindamycin | ≤0.015–0.06 | 0.06 | 0.06 |
| S. pyogenes (inducible ErmA) [11] | |||
| ABT-773 | ≤0.015–>1 | 0.03 | 0.12 |
| Clarithromycin | 2–>64 | 8 | 8 |
| Azithromycin | 8–>128 | 32 | 128 |
| Erythromycin | 2–128 | 16 | 16 |
| Clindamycin | 0.03–0.5 | 0.12 | 0.5 |
| S. pyogenes (constitutive ErmB) [6] | |||
| ABT-773 | 0.5–8 | ||
| Clarithromycin | >128 | ||
| Azithromycin | >128 | ||
| Erythromycin | >128 | ||
| Clindamycin | 128–>128 | ||
| S. aureus (macrolide S) [16] | |||
| ABT-773 | 0.03 | 0.03 | 0.03 |
| Clarithromycin | 0.12–0.25 | 0.12 | 0.25 |
| Azithromycin | 0.25–1 | 0.5 | 1 |
| Erythromycin | 0.25 | 0.25 | 0.25 |
| Clindamycin | 0.06–0.12 | 0.12 | 0.12 |
| S. aureus (inducible ErmA) [11] | |||
| ABT-773 | 0.03–0.25 | 0.03 | 0.25 |
| Clarithromycin | 2–>128 | >128 | >128 |
| Azithromycin | 8–>128 | >128 | >128 |
| Erythromycin | 4–>128 | >128 | >128 |
| Clindamycin | 0.06–0.12 | 0.06 | 0.06 |
| S. aureus (constitutive ErmA, ErmC) [18] | |||
| ABT-773 | >128 | >128 | >128 |
| Clarithromycin | >128 | >128 | >128 |
| Azithromycin | >128 | >128 | >128 |
| Erythromycin | >128 | >128 | >128 |
| Clindamycin | >128 | >128 | >128 |
| S. epidermidis (macrolide S) [13] | |||
| ABT-773 | ≤0.015–0.03 | 0.03 | 0.03 |
| Clarithromycin | ≤0.015–0.25 | 0.12 | 0.25 |
| Azithromycin | ≤0.12–1 | 0.5 | 1 |
| Erythromycin | ≤0.03–0.25 | 0.25 | 0.25 |
| Clindamycin | 0.06–0.12 | 0.06 | 0.12 |
| S. epidermidis (constitutive MLSB) [12] | |||
| ABT-773 | >128 | >128 | >128 |
| Clarithromycin | >128 | >128 | >128 |
| Azithromycin | >128 | >128 | >128 |
| Erythromycin | >128 | >128 | >128 |
| Clindamycin | >128 | >128 | >128 |
| Enterococcus spp. (macrolide S) [12] | |||
| ABT-773 | ≤0.015–0.03 | 0.03 | 0.03 |
| Clarithromycin | 0.12–0.5 | 0.25 | 0.5 |
| Azithromycin | 0.25–2 | 1 | 2 |
| Erythromycin | 0.06–0.5 | 0.25 | 0.5 |
| Clindamycin | 0.25–32 | 16 | 16 |
| Enterococcus spp. (macrolide R) [30] | |||
| ABT-773 | 1–>128 | 16 | 128 |
| Clarithromycin | >128 | >128 | >128 |
| Azithromycin | >128 | >128 | >128 |
| Erythromycin | >128 | >128 | >128 |
| Clindamycin | >128 | >128 | >128 |
| Corynebacterium spp. [18] | |||
| ABT-773 | 0.002–0.5 | 0.015 | 0.5 |
| Clarithromycin | 0.008–>64 | 4 | 64 |
| Azithromycin | 0.03–>128 | >128 | >128 |
| Erythromycin | 0.004–>128 | 8 | 128 |
| Clindamycin | 0.12–>128 | >128 | >128 |
| L. monocytogenes [24] | |||
| ABT-773 | 0.03 | 0.03 | 0.03 |
| Clarithromycin | 0.12 | 0.12 | 0.12 |
| Azithromycin | 1 | 1 | 1 |
| Erythromycin | 0.12 | 0.12 | 0.12 |
| E. coli [28] | |||
| ABT-773 | 2–64 | 16 | 64 |
| Clarithromycin | 32–>128 | 128 | >128 |
| Azithromycin | 2–16 | 4 | 16 |
| Erythromycin | 32–>128 | 128 | >128 |
| M. catarrhalis [17] | |||
| ABT-773 | 0.06–0.12 | 0.12 | 0.12 |
| Clarithromycin | 0.12–0.25 | 0.12 | 0.25 |
| Azithromycin | 0.015–0.06 | 0.03 | 0.06 |
| Erythromycin | 0.12–0.25 | 0.12 | 0.25 |
| Legionella spp. [11] | |||
| ABT-773 | 0.5–1 | 0.5 | 1 |
| Clarithromycin | 0.06–0.12 | 0.12 | 0.12 |
| Azithromycin | 0.12–2 | 0.5 | 2 |
| Erythromycin | 0.25–1 | 1 | 1 |
| N. gonorrhoeae [11] | |||
| ABT-773 | ≤0.008–0.5 | 0.015 | 0.25 |
| Clarithromycin | 0.015–2 | 0.06 | 1 |
| Azithromycin | 0.03–0.25 | 0.03 | 012 |
| Erythromycin | 0.015–4 | 0.12 | 1 |
| H. pylori (macrolide S) [15] | |||
| ABT-773 | 0.008–0.06 | 0.03 | 0.06 |
| Clarithromycin | 0.008–0.06 | 0.008 | 0.015 |
| Azithromycin | 0.06–0.5 | 0.25 | 0.5 |
| Erythromycin | 0.06–0.25 | 0.12 | 0.25 |
| H. pylori (macrolide R) [15] | |||
| ABT-773 | 4–64 | 32 | 64 |
| Clarithromycin | 4–128 | 32 | 128 |
| Azithromycin | 128–>128 | >128 | >128 |
| Erythromycin | 64–>128 | 128 | >128 |
| M. avium (macrolide S) [13] | |||
| ABT-773 | 0.5–128 | 32 | 64 |
| Clarithromycin | 0.25–32 | 4 | 16 |
| Azithromycin | 16–>256 | 64 | >256 |
| Erythromycin | 4–>256 | 64 | 256 |
| M. avium (macrolide R) [15] | |||
| ABT-773 | >256 | >256 | >256 |
| Clarithromycin | >256 | >256 | >256 |
| Azithromycin | >256 | >256 | >256 |
| Erythromycin | >256 | >256 | >256 |
TABLE 2.
Comparative in vitro activity of ABT-773 by broth dilution techniquesa
| Species (resistance) [n] and antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| S. pneumoniae (macrolide S) [30] | |||
| ABT-773 | ≤0.001– 0.008 | ≤0.001 | 0.004 |
| Clarithromycin | 0.002–0.06 | 0.015 | 0.03 |
| Azithromycin | 0.004–0.25 | 0.06 | 0.12 |
| Erythromycin | 0.002–0.06 | 0.03 | 0.03 |
| Clindamycin | 0.008–0.06 | 0.03 | 0.03 |
| Penicillin | 0.004–4 | 0.03 | 4 |
| S. pneumoniae (Mef) [23] | |||
| ABT-773 | ≤0.002–0.12 | 0.06 | 0.06 |
| Clarithromycin | 0.5–8 | 4 | 4 |
| Azithromycin | 2–16 | 8 | 8 |
| Erythromycin | 2–8 | 4 | 8 |
| Clindamycin | 0.015–0.12 | 0.03 | 0.03 |
| Penicillin | 0.015–>1 | 1 | >1 |
| S. pneumoniae (ErmB) [31] | |||
| ABT-773 | 0.004–2 | 0.008 | 0.25 |
| Clarithromycin | 2–>128 | >128 | >128 |
| Azithromycin | 4–>128 | >128 | >128 |
| Erythromycin | 2–>128 | >128 | >128 |
| Clindamycin | 16–>128 | 128 | >128 |
| Penicillin | ≤0.008–4 | 1 | 4 |
| H. influenzae [25] | |||
| ABT-773 | 0.5–4 | 1 | 4 |
| Clarithromycin | 2–32 | 4 | 16 |
| Azithromycin | 0.25–2 | 0.5 | 2 |
| Erythromycin | 1–16 | 4 | 8 |
| M. pneumoniae [8] | |||
| ABT-773 | ≤0.0005 | ||
| Clarithromycin | 0.001–0.004 | ||
| Azithromycin | ≤0.0005 | ||
| Erythromycin | 0.004–0.008 | ||
| B. burgdorferi [2] | |||
| ABT-773 | ≤0.001 | ||
| Clarithromycin | 0.004–0.008 | ||
| Azithromycin | ≤0.001 | ||
| Erythromycin | 0.008 | ||
Broth microdilution was used for S. pneumoniae, H. influenzae, and M. pneumoniae. Broth macrodilution was used for B. burgdorferi.
ABT-773, azithromycin, and clarithromycin were prepared at Abbott Laboratories, Abbott Park, Ill. Erythromycin, clindamycin, and penicillin reference powders were purchased from U.S. Pharmacopeial Convention, Inc., Rockville, Md.
Clinical isolates or reference strains obtained from the American Type Culture Collection (Manassas, Va.) were tested. The molecular mechanisms of macrolide resistance were identified by PCR amplification of the mef and erm genes (14, 15) and in Helicobacter pylori and Mycobacterium avium by DNA sequence analysis for mutations in 23S rRNA (8, 16).
MICs were determined by agar dilution or broth microdilution as described by the National Committee for Clinical Laboratory Standards (NCCLS) (9). Mueller-Hinton agar was supplemented with 5% sheep blood for testing Streptococcus pyogenes, Listeria monocytogenes, and Moraxella catarrhalis; the plates were incubated in an atmosphere containing 5% CO2 for tests of S. pyogenes and M. catarrhalis. Quality control results met NCCLS standards (9, 10). The susceptibilities of Legionella spp., M. avium, Mycoplasma pneumoniae, Borrelia burgdorferi, and Chlamydia trachomatis were determined as described previously (5, 6, 7, 13, 18).
ABT-773 was at least fourfold more potent than the three comparator macrolides against macrolide-susceptible strains of gram-positive species, including Streptococcus pneumoniae, S. pyogenes, Staphylococcus aureus, Staphylococcus epidermidis, L. monocytogenes, and Enterococcus spp; the strains were inhibited by <0.06 μg of ABT-773/ml. ABT-773 was as potent as azithromycin against the gram-negative pathogens Haemophilus influenzae, M. catarrhalis, Legionella spp., and Neisseria gonorrhoeae with MIC90s of 4, 0.12, 1, and 0.25 μg/ml, respectively. ABT-773 had little to no activity against Escherichia coli, other enterobacteriaceae, and Pseudomonas aeruginosa (MICs, ≥8 μg/ml) [data not shown]. ABT-773 was highly active against M. pneumoniae, demonstrating MICs of <0.015 μg/ml. The MIC for ABT-773 against a single strain of C. trachomatis was 0.015 μg/ml, similar to the activity against Chlamydia pneumoniae (17).
The MIC90s for ABT-773 were 0.25 and 0.03 μg/ml for inducible ErmA and susceptible strains of S. aureus and S. pyogenes. In contrast, macrolide MICs were more than 100-fold higher against inducible ErmA strains than against susceptible strains. Moreover, ABT-773 did not induce resistance to clindamycin in these strains by the disk approximation test; erythromycin induced resistance to both ABT-773 and clindamycin (data not shown; 12). These results suggest that ABT-773 does not induce 23S rRNA methylation in inducible ErmA strains.
Although the macrolides and clindamycin were inactive against constitutive ErmB strains of S. pneumoniae and S. pyogenes, the ABT-773 MIC90 for ErmB strains of S. pneumoniae was 0.25 μg/ml, and all ErmB strains of S. pyogenes were inhibited by ≤8 μg/ml. The difference in activity of ABT-773 was likely due to the use of agar dilution with incubation in CO2 to test S. pyogenes and broth microdilution to test S. pneumoniae and not to inherent species-dependent differences in susceptibility to ABT-773, since other studies show that the MIC90s for ErmB strains of S. pneumoniae and S. pyogenes are similar when the susceptibilities of both species are determined by broth microdilution (V. D. Shortridge, unpublished data). The potent activity of ABT-773 against constitutive ErmB streptococci may be due to its affinity for methylated ribosomes, and ABT-773 may not effectively induce higher levels of ribosome methylation in ErmB strains (4). This may also explain the activity of ABT-773 against other constitutive macrolide-lincosamide-streprogramin B (MLSB) resistant species, such as Corynebacterium spp. However, ABT little to no vitro activity against constitutive MLSB-resistant staphylococci and enterococci. The reason for different susceptibilities in various species with constitutive MLSB resistance is unknown.
The MIC90s for MefA strains of S. pneumoniae and S. pyogenes were 0.06 and 0.25 μg/ml, respectively. ABT-773 accumulates rapidly in cells of S. pneumoniae containing the Mef efflux pump because the high affinity of ABT-773 for ribosomes may overcome export (4) or ABT-773 may have poor affinity for the pump.
Penicillin susceptibility had no effect on susceptibility to ABT-773 for pneumococci. For macrolide-susceptible strains, the ABT-773 MIC90 was 0.002 μg/ml for 16 penicillin-susceptible strains and ≤0.002 μg/ml for 14 penicillin-nonsusceptible strains. The ABT-773 MIC90s were 0.06, 0.06, and 0.12 μg/ml for 27 penicillin-susceptible, 19 penicillin-intermediate, and 37 penicillin-resistant strains, respectively. Each group comprised macrolide-susceptible, MefA, and ErmB isolates. The MIC90s of ABT-773 in a study of 1,601 pneumococci are similar: ≤0.008, 0.03, and 0.12 μg/ml for penicillin-susceptible, -intermediate, and -resistant strains, respectively (2).
ABT-773 was fourfold less active than clarithromycin against macrolide-susceptible strains of H. pylori and M. avium, with MIC90s of 0.06 and 64 μg/ml, respectively. Point mutations in the 23S rRNA at residues A2058 and A2059 (E. coli numbering) reduce macrolide binding to ribosomes and result in resistance in H. pylori and M. avium (8, 16). ABT-773 was significantly less active against H. pylori strains having A2058G or A2059G mutations and against M. avium strains having mutations at A2058 than against the corresponding susceptible strains. These results confirmed the importance of A2058 and A2059 for ABT-773 binding to ribosomes (4).
Minimum bacterial concentrations (MBCs) were determined in conjunction with broth microdilution MICs for four strains (5, 9). ABT-773 is 87 to 96% bound to human plasma proteins. The MICs and MBCs were also done in medium containing 50% (vol/vol) human serum (Scantibodies Laboratory, Inc., Santee, Calif.) which had been heated for 1 h at 56°C (Table 3). ABT-773 was bactericidal for single strains of H. influenzae, S. pneumoniae, and M. catarrhalis in medium alone or in medium containing 50% human serum, since the MBCs were no more than twofold higher than the corresponding MICs. In contrast, ABT-773 was bacteriostatic for a strain of S. aureus.
TABLE 3.
Minimum bactericidal activities of ABT-773a
| Species | ABT-773
|
Erythromycin
|
Ciprofloxacin
|
|||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| S. pneumoniae ATCC 6303 | ||||||
| No serum | 0.002 | 0.004 | 0.03 | 0.12 | 1 | 1 |
| Serum | 0.004 | 0.008 | 0.06 | 2 | 1 | 2 |
| H. influenzae 1882 | ||||||
| No serum | 0.5 | 1 | 4 | 8 | 0.004 | 0.008 |
| Serum | 0.5 | 1 | 1 | 2 | 0.004 | 0.004 |
| M. catarrhalis 2604 | ||||||
| No serum | 0.25 | 0.5 | 0.25 | 0.5 | 0.12 | 0.12 |
| Serum | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 |
| S. aureus ATCC 6538P | ||||||
| No serum | 0.03 | >1 | 0.25 | >8 | 0.12 | 0.25 |
| Serum | 0.015 | >1 | 0.03 | >1 | 0.12 | 0.5 |
In micrograms per milliliter. The serum was 50% human serum.
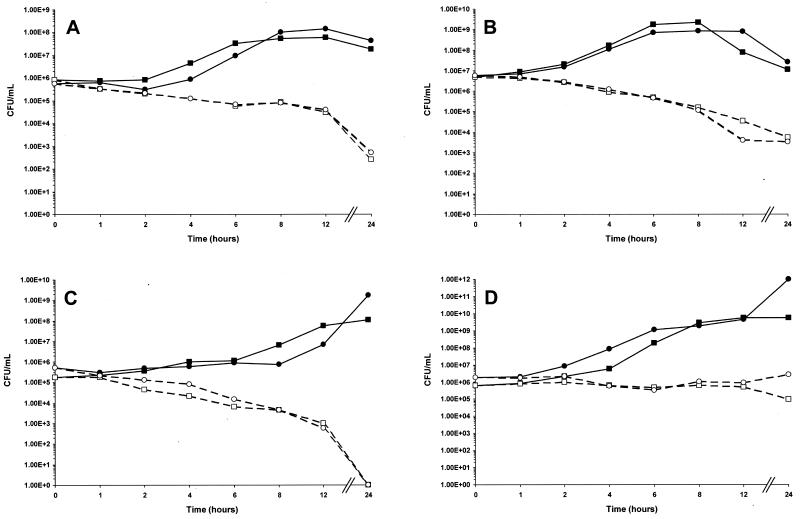
MBC results were confirmed by time-kill analysis using ABT-773 at four times the MIC (Fig. 1) (5). For H. influenzae, S. pneumoniae, and M. catarrhalis in medium alone or in medium containing 50% human serum, the number of viable cells remaining after 24 h of incubation with ABT-773 was reduced by at least 99.9% from the number of viable cells present in the initial inocula. For S. aureus, ABT-773 caused less than a 10-fold loss of viable cells after 24 h of incubation.
FIG. 1.
Time-kill analysis of ABT-773 at antibiotic concentrations equal to four times the MIC against S. pneumoniae ATCC 6303 (A), H. influenzae 1882 (B), M. catarrhalis 2604 (C), and S. aureus ATCC 6538P (D); MICs are presented in Table 4. ■, drug-free control in medium; □, ABT-773 at four times the MIC in medium; ●, drug-free control in medium containing 50% human serum, ○, ABT-773 at four times the MIC in medium containing 50% human serum.
The overall antibacterial activity of ABT-773 was more potent and included a broader spectrum of key respiratory tract pathogens, including multidrug-resistant, atypical, and intracellular pathogens, than those of clarithromycin, azithromycin, and erythromycin. In addition, ABT-773 had comparable or improved activity against non-respiratory-tract pathogens. The improved potency is due to the greater affinity of ABT-773 to macrolide-susceptible ribosomes, resulting in rapid accumulation in bacterial cells and slower dissociation from the ribosome (4). ABT-773 retained potent antibacterial activity against macrolide-resistant pneumococcal isolates. The MIC90 for the 53 macrolide-resistant pneumococci tested was 0.12 μg/ml, and this activity derives from affinity for methylated streptococcal ribosomes, no or poor induction of ErmB, and the ability to overcome Mef efflux (4).
REFERENCES
- 1.Bartlett J G, Breiman R F, Mandell L A, File T M., Jr Community acquired pneumonia in adults: guidelines for management. Clin Infect Dis. 1998;26:811–838. doi: 10.1086/513953. [DOI] [PubMed] [Google Scholar]
- 2.Brueggemann A B, Doern G V, Huynh H K, Wingert E M, Rhomberg P R. In vitro activity of ABT-773, a new ketolide, against recent clinical isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob Agents Chemother. 2000;44:447–449. doi: 10.1128/aac.44.2.447-449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryskier A. New research in macrolides and ketolides since 1997. Exp Opin Investig Drugs. 1999;8:1171–1194. doi: 10.1517/13543784.8.8.1171. [DOI] [PubMed] [Google Scholar]
- 4.Capobianco J O, Cao Z S, Shortridge V D, Ma Z, Flamm R K, Zhong P. Studies of the novel ketolide ABT-773: transport, binding to ribosomes, and inhibition of protein synthesis in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2000;44:1562–1567. doi: 10.1128/aac.44.6.1562-1567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clement J J, Tanaka S K, Alder J, Vojtko C, Beyer J, Hensey D, Ramer N, McDaniel D, Chu D T W. In vitro and in vivo evaluations of A-80556, a new fluoroquinolone. Antimicrob Agents Chemother. 1994;38:1071–1078. doi: 10.1128/aac.38.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gump D W. Antimicrobial susceptibility testing for some atypical microorganisms: Chlamydiae, Mycoplasmas, Rickettsia, and spirochetes. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: Williams and Wilkins; 1996. pp. 212–229. [Google Scholar]
- 7.Johnson S E, Klein G C, Schmid G P, Feeley J C. Susceptibility of the Lyme disease spirochete to seven antimicrobial agents. Yale J Biol Med. 1984;57:549–553. [PMC free article] [PubMed] [Google Scholar]
- 8.Nash K A, Inderlied C B. Genetic basis of macrolide resistance in Mycobacterium avium isolated from patients with disseminated disease. Antimicrob Agents Chemother. 1995;39:2625–2630. doi: 10.1128/aac.39.12.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; ninth informational supplement. Approved standard M100–S9. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 11.Or Y S, Clark R F, Wang S, Chu D T W, Nilius A M, Flamm R K, Mitten M, Ewing P, Alder J, Ma Z. Design, synthesis, and antimicrobial activity of 6-O-substituted ketolides active against resistant respiratory tract pathogens. J Med Chem. 2000;43:1045–1049. doi: 10.1021/jm990618n. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez M L, Flint K K, Jones R N. Occurrence of Macrolide-Lincosamide-Streptogramin resistances among staphylococcal clinical isolates at a university medical center. Diagn Microbiol Infect Dis. 1993;16:205–213. doi: 10.1016/0732-8893(93)90111-j. [DOI] [PubMed] [Google Scholar]
- 13.Segreti J, Kessler H A, Kapell K S, Trenholme G M. In vitro activity of A-56268 (TE-031) and four other antimicrobial agents against Chlamydia trachomatis. Antimicrob Agents Chemother. 1989;31:100–101. doi: 10.1128/aac.31.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shortridge V D, Doern G V, Brueggemann A B, Beyer J M, Flamm R K. Prevalence of macrolide resistance mechanisms in Streptococcus pneumoniae isolates from a multicenter antibiotic resistance surveillance study conducted in the United States in 1994–1995. Clin Infect Dis. 1999;29:1186–1188. doi: 10.1086/313452. [DOI] [PubMed] [Google Scholar]
- 15.Shortridge V D, Flamm R K, Ramer N, Beyer J, Tanaka S K. Novel mechanism of macrolide resistance in Streptococcus pneumoniae. Diagn Microbiol Infect Dis. 1996;26:73–78. doi: 10.1016/s0732-8893(96)00183-6. [DOI] [PubMed] [Google Scholar]
- 16.Stone G G, Shortridge D, Flamm R K, Versalovic J, Beyer J, Idler K, Zulawinski L, Tanaka S K. Identification of a 23S rRNA gene mutation in clarithromycin-resistant Helicobacter pylori. Helicobacter. 1996;1:227–228. doi: 10.1111/j.1523-5378.1996.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 17.Strigl S, Roblin P M, Reznik T, Hammerschlag M R. In vitro activity of ABT-773, a new ketolide antibiotic, against Chlamydia pneumoniae. Antimicrob Agents Chemother. 2000;44:1112–1113. doi: 10.1128/aac.44.4.1112-1113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swenson J M, Thomsberry C, Silcox V A. Rapidly growing mycobacteria: testing of susceptibility to 34 antimicrobial agents by broth microdilution. Antimicrob Agents Chemother. 1982;22:186–192. doi: 10.1128/aac.22.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]