Abstract
Suggestions that Lyme disease exists in Greece remain controversial and no study to date has definitively identified the presence of a Borrelia species that infects humans. We examined patients throughout Greece suspected for Lyme disease by enzyme-linked immunosorbent assay (ELISA) and by western blotting for Borrelia burgdorferi sensu lato species. We found one patient positive for Borrelia burgdorferi and two patients positive for Borrelia afzelii specific IgG antibodies. Both B. afzelii patients were suffering by neurological manifestations and had never traveled abroad. We provide serological evidence of two autochthonous Lyme disease cases in Greece, possibly caused by B. afzelii.
Keywords: Borrelia afzelii, Greece, Lyme borrelia, lyme disease
Introduction
Lyme disease is caused by spirochetes within the Borrelia burgdorferi sensu lato species complex that are transmitted by specific Ixodes spp. Ticks [1]. In Europe, at least five species of Lyme borrelia (Borrelia afzelii, Borrelia garinii, Borrelia burgdorferi, Borrelia spielmanii, and Borrelia bavariensis) can cause the disease, leading to a wider variety of possible clinical manifestations [1]. The most common clinical manifestations in Europe are erythema migrans and Lyme neuroborreliosis, whereas B. afzelii is considered to be the predominant genospecies [1,2]. The incidence of Lyme disease has been increasing across the globe and it is estimated that more than 200,000 cases of Lyme disease occur in Europe each year [3]. Suggestions that Lyme disease exists in Greece remain controversial and no study to date has definitively identified the presence of a Borrelia spp. that infects humans. In addition, few serological studies have been conducted in animals from Greece, suggesting the possibility of Borrelia spp. presence in the country [[4], [5], [6]]. To our knowledge, physicians in Greece associate Lyme disease only with B. burgdorferi infection, as studies in the 1990s, based on enzyme-linked immunosorbent assay (ELISA), suggested the existence of B. burgdorferi [7]. However, since then, this hypothesis has never been confirmed and to date Lyme disease is not considered endemic. To determine this, we retrospectively examined for B. burgdorferi sensu lato species complex patients throughout Greece suspected for B. burgdorferi infection.
Ethics statement
This study is based on routine diagnosis samples. All collected data were anonymized in standardized forms according to the Ethic and Scientific Committee of the Hellenic Pasteur Institute under registration number EIP-GDPR-E01.01.
Materials
Patients
We studied serum and blood samples obtained from hospitalized patients and outpatients throughout Greece that were sent to our laboratory from January to December 2019. As a referent laboratory, we routinely receive specimens from patients with suspected zoonotic infections throughout Greece. Inclusion criteria for participation in this study included age above 18 years and suspicion of zoonotic infection. Exclusion criteria was diagnosis by an agent other than Borrelia spp. Clinical data, medical history and complications during illness, habitat and environmental characteristics, presence of pets, livestock, or rodents, daily activities before symptom onset and travel during the previous one year were documented for patients with a positive western blotting (WB) result thanks to phone calls.
Serological assays
A two-stage serodiagnostic (screening and confirmation) testing strategy was applied and firstly blood serum samples were screened by ELISA using the anti-Borrelia IgM and IgG ELISA (EUROIMMUN) kit to determine the presence of IgG and IgM B. burgdorferi sensu lato antibodies. ELISA results were classified as negative (< 16 RU/mL), equivocal (16–22 RU/ml) or positive (> 22 RU/mL) according to the manufacturer's instructions.
All ELISA-positive and equivocal serum samples were analyzed by WB using the anti- Borrelia spp. WB IgM and IgG (EUROIMMUN EUROLINE-RN-AT) for confirmation. The sum of the points attributed to each antigenic (OspA, OspC, p100, VlsE, p39, p58, p18, p41) band revealed on the strip according to their intensity was calculated and interpreted by the test strip analysis software, and scored as negative, positive, or equivocal. A sample was considered positive only if the positive or equivocal ELISA result in IgG or IgM was confirmed by a positive WB result.
Molecular assays
Total genomic DNA was extracted (Biorobot EZ1 Workstation; QIAGEN, Courtaboeuf, France) from blood and serum samples of Borrelia spp. seropositive patients and were used as a template in a previously described real-time reverse transcription–PCR (RT-PCR) targeting the internal transcribed spacer (ITS) and a 16S rRNA gene sequence-based system, as previously described [8,9]. Two sets of negative controls (DNA of blood from a nonfebrile patient and sterile water) and a positive control (B. recurrentis DNA) were also analyzed in each run.
Results
We totally studied 294 serum samples obtained from 294 different patients (56% females) and we identified seven (2%) samples positive and 21 (7%) samples with equivocal antibody index values by ELISA. All these samples were further tested by WB and a positive B. burgdorferi sensu lato result was obtained for three patients (Table 1). In addition, all blood samples remained negative by molecular assays for Borrelia. Detailed histories for each patient are described below.
Table 1.
B. burgdorferi sensu lato species seropositive patients
| Patient | ELISA (RU/mL) |
Western blotting |
Real time PCR |
|||
|---|---|---|---|---|---|---|
| IgM | IgG | IgM | IgG | ITS | 16S rRNA | |
| 1 | 18 | 77 | Negative | B. burgdorferi | Negative | Negative |
| 2 | 14 | 45 | Negative | B. afzelii | Negative | Negative |
| 3 | 11 | 95 | Negative | B. afzelii | Negative | Negative |
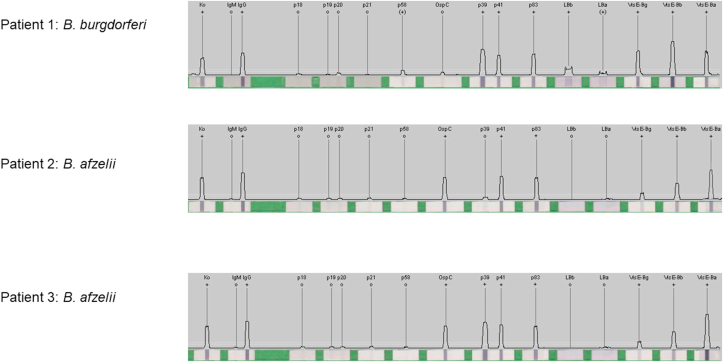
The first patient was a 56-year-old male patient with an expanded erythema migrans on the right foot and epitrochlear adenopathy. WB was positive for B. burgdorferi specific IgG antibodies as was reactive for the p39, p41, p58, and p83 antigens and for B. burgdorferi VlsE specific antigen (Fig. 1). The patient reported a recent travel to Sweden, where he recalled a tick bite and a diagnosis of B. burgdorferi infection was given.
Fig. 1.
IgG Western blotting results for Borrelia burgdorferi sensu lato species complex positive patients.
Patient 2, was a 43-year-old woman suffering by severe neurological manifestations, chronic encephalomyelitis, and encephalopathy. All tests for rheumatoid arthritis, systemic lupus erythematosus, myasthenia gravis, and other autoimmune diseases were negative for this patient. She presented specific IgG antibodies reactive for B. afzelii VlsE, OspC (p25), p41, and lipid B. afzelii antigens (Fig. 1). A diagnosis of possible B. afzelii infection was given but further investigation was not possible because she passed away. However, investigation after she passed away revealed that she presented a persistent erythema after an insect bite in the city of Athens one year before the symptoms' onset.
Patient 3 was a 35-year -old woman with neurological manifestations associated with persistent myalgia and arthritis of the large joints, especially the knee. She mentioned frequent outdoor activities, animal contacts and a cutaneous lesion after an insect bite close to the city of Larissa before the symptoms' onset. WB was reactive for IgG antibodies of B. afzelii VlsE, OspC (p25), p41, and lipid B. afzelii antigens (Fig. 1). A diagnosis of possible B. afzelii infection was given and a course of 200 mg of oral doxycycline once per day for three months was introduced.
Discussion
We present two patients with autochthonous B. afzelii infection in Greece, based on their ELISA and WB serological reactivity. We believe that our serological results are accurate, as both our ELISA and WB assays have previously been evaluated and are routinely used for the diagnosis of Lyme disease. However, a limitation is that we did not detect B. afzelii by molecular assays. Both patients reported epidemiological risk factors and presented clinical manifestations that are commonly presented in patients suffering by Lyme neuroborreliosis or Lyme arthritis [1,2].
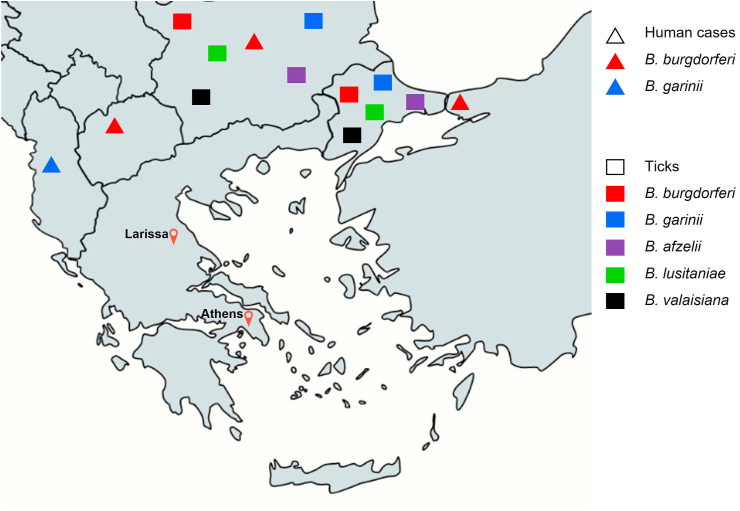
The principal tick vector for Borrelia species in Europe is Ixodes ricinus. Although I. ricinus is prevalent in Greece, epidemiological studies have not detected Borrelia in it [10]. In contrast, B. burgdorferi sensu lato species complex is prevalent in ticks collected from countries close to Greece and B. afzelii was found to be the predominant species in I. ricinus ticks collected from Bulgaria [11]. Lyme disease pathogens, including B. garinii and B. afzelii, have also been detected in ticks collected in the Marmara Region of Turkey [[12], [13], [14]]. Additionally, Lyme disease has been known to be endemic in Albania, North Macedonia, Bulgaria and Turkey [[15], [16], [17]]; the reported incidence of Lyme borreliosis in Bulgaria is about 6/100,000 of the population [18], whereas in Turkey 75 cases of Lyme disease have been reported [19] (Fig. 2). However, the true incidence of the disease in these countries is probably much higher, because of the fact that the disease is often self-limited and mild cases remain unrecognized. Additionally, serological studies conducted in animals from Turkey and Bulgaria have reported seropositivity against Borrelia burgdorferi sensu lato in rodents [20,21], cats [22,23], dogs [[24], [25], [26], [27]], and horses [24,28,29]. In Greece, serological studies have reported a low seroprevalence (up to 2.2%) in dogs [5,6] but a significant seropositivity of 23.6% in sheep as reported in a study from 318 tick-infested sheep [4].
Fig. 2.
Human and tick presence of Borrelia burgdorferi sensu lato species to countries close to Greece.
In conclusion, we demonstrate the possibility of the presence of B. afzelii in Greece. Therefore, we need to raise awareness of Lyme disease among healthcare providers and ensure that B. afzelii, and possibly also other Lyme borrelia species must be taken into account. The true underlying rate of Lyme disease in Greece remains unknown. The rarity of tick-borne diseases creates a surveillance and diagnostic challenge and the need to determine the true burden of Lyme disease in Greece in order to improve public health prevention messaging to healthcare providers and the public.
Funding
None.
Authors' contributions
I.K.: investigation, M.K.: Writing, I.P.: collect clinical data, A.V.K.: investigation, S.M.: Writing, EA: Writing, Supervision.
Transparency declaration
There is no conflict of interest.
Acknowledgments
No.
References
- 1.Steere A.C., Strle F., Wormser G.P., Hu L.T., Branda J.A., Hovius J.W., et al. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanek G., Wormser G.P., Gray J., Strle F. Lyme borreliosis. Lancet. 2012;379(9814):461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 3.Sykes R.A., Makiello P. An estimate of Lyme borreliosis incidence in Western Europedagger. J Public Health (Oxf) 2017;39(1):74–81. doi: 10.1093/pubmed/fdw017. [DOI] [PubMed] [Google Scholar]
- 4.Athanasiou L.V., Spanou V.M., Katsogiannou E.G., Katsoulos P.D. Hematological features in sheep with IgG and IgM antibodies against Borrelia burgdorferi sensu lato. Pathogens. 2021;10(2) doi: 10.3390/pathogens10020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angelou A., Gelasakis A.I., Verde N., Pantchev N., Schaper R., Chandrashekar R., et al. Prevalence and risk factors for selected canine vector-borne diseases in Greece. Parasit Vectors. 2019;12(1):283. doi: 10.1186/s13071-019-3543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Athanasiou L.V., Kontos V.I., Kritsepi Konstantinou M., Polizopoulou Z.S., Rousou X.A., Christodoulopoulos G. Cross-sectional serosurvey and factors associated with exposure of dogs to vector-borne pathogens in Greece. Vector Borne Zoonotic Dis. 2019;19(12):923–928. doi: 10.1089/vbz.2019.2471. [DOI] [PubMed] [Google Scholar]
- 7.Chatzipanagiotou S., Papandreou-Rakitzis P., Malamou-Ladas H., Antoniou P. vol. 11. 1992. Determination of antibody titres for Borrelia burgdorferi in the serum of gipsies living in Attika, Greece; pp. 477–478. (European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology). 5. [DOI] [PubMed] [Google Scholar]
- 8.Fotso Fotso A., Angelakis E., Mouffok N., Drancourt M., Raoult D. Blood-Borne candidatus Borrelia algerica in a patient with prolonged fever in oran, Algeria. Am J Trop Med Hyg. 2015;93(5):1070–1073. doi: 10.4269/ajtmh.15-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Socolovschi C., Reynaud P., Kernif T., Raoult D., Parola P. Rickettsiae of spotted fever group, Borrelia valaisiana, and Coxiella burnetii in ticks on passerine birds and mammals from the Camargue in the south of France. Ticks Tick-Borne Dis. 2012;3(5–6):355–360. doi: 10.1016/j.ttbdis.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Diakou A., Norte A.C., Lopes de Carvalho I., Nuncio S., Novakova M., Kautman M., et al. Ticks and tick-borne pathogens in wild birds in Greece. Parasitol Res. 2016;115(5):2011–2016. doi: 10.1007/s00436-016-4943-3. [DOI] [PubMed] [Google Scholar]
- 11.Schouls L.M., Van De Pol I., Rijpkema S.G., Schot C.S. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37(7):2215–2222. doi: 10.1128/jcm.37.7.2215-2222.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karasartova D., Gureser A.S., Gokce T., Celebi B., Yapar D., Keskin A., et al. Bacterial and protozoal pathogens found in ticks collected from humans in Corum province of Turkey. PLoS Negl Trop Dis. 2018;12(4) doi: 10.1371/journal.pntd.0006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guner E.S., Hashimoto N., Takada N., Kaneda K., Imai Y., Masuzawa T. First isolation and characterization of Borrelia burgdorferi sensu lato strains from Ixodes ricinus ticks in Turkey. J Med Microbiol. 2003;52(Pt 9):807–813. doi: 10.1099/jmm.0.05205-0. [DOI] [PubMed] [Google Scholar]
- 14.Christova I., Van De Pol J., Yazar S., Velo E., Schouls L. vol. 22. 2003. Identification of Borrelia burgdorferi sensu lato, Anaplasma and Ehrlichia species, and spotted fever group Rickettsiae in ticks from Southeastern Europe; pp. 535–542. (European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology). 9. [DOI] [PubMed] [Google Scholar]
- 15.di Meo N., Stinco G., Nan K., Pinzani C., Trevisan G. Parry-Romberg syndrome: a case with a possible association with Lyme disease. Acta Dermatovenerol Alp Pannonica Adriat. 2015;24(4):77–79. [PubMed] [Google Scholar]
- 16.Meloska I.H.-P., Jankijevic A.H.-P. Project management of Lyme Disease through monitoring of seroprevalence of anti-Borrelia antibodies in Macedonian patients. EconPapers. 2014;10:245–254. [Google Scholar]
- 17.Myrseli T., Schönberg A., Simaku A., Çomo N., Pipero P., Bërxholi K., et al. The current situation of Lyme borreliosis and the first seroepidemiology survey in dogs in Albania. Albanian Med J. 2018;2 [Google Scholar]
- 18.Ermenlieva N., Tsankova G., Todorova T.T. Epidemiological study of Lyme disease in Bulgaria. Cent Eur J Public Health. 2019;27(3):235–238. doi: 10.21101/cejph.a5007. [DOI] [PubMed] [Google Scholar]
- 19.Onal U., Aytac Erdem H., Uyan Onal A., Resat Sipahi O. Systematic review of Lyme disease in Turkey. Trop Doct. 2019;49(3):165–170. doi: 10.1177/0049475519843387. [DOI] [PubMed] [Google Scholar]
- 20.Guner E.S., Watanabe M., Kadosaka T., Polat E., Gargili A., Gulanber A., et al. Seroepidemiology of Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in wild mice captured in northern Turkey. Epidemiol Infect. 2005;133(2):331–336. doi: 10.1017/s0950268804003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christova I., Gladnishka T. Prevalence of infection with Francisella tularensis, Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in rodents from an endemic focus of tularemia in Bulgaria. Ann Agric Environ Med. 2005;12(1):149–152. [PubMed] [Google Scholar]
- 22.Muz M.N., Erat S., Mumcuoglu K.Y. Protozoan and microbial pathogens of house cats in the province of tekirdag in western Turkey. Pathogens. 2021;10(9) doi: 10.3390/pathogens10091114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsachev I., Baymakova M., Zlateva N., Kundurzhiev T., Solano-Gallego L. Seroprevalence rates of tick-borne pathogens in cats from southern Bulgaria. Vector Borne Zoonotic Dis. 2020;20(11):864–867. doi: 10.1089/vbz.2020.2628. [DOI] [PubMed] [Google Scholar]
- 24.Bhide M., Yilmaz Z., Golcu E., Torun S., Mikula I. Seroprevalence of anti-Borrelia burgdorferi antibodies in dogs and horses in Turkey. Ann Agric Environ Med. 2008;15(1):85–90. [PubMed] [Google Scholar]
- 25.Manev I. Serological survey of vector-borne pathogens in stray dogs from Sofia area, Bulgaria. Vet Parasitol Reg Stud Rep. 2020;21:100441. doi: 10.1016/j.vprsr.2020.100441. [DOI] [PubMed] [Google Scholar]
- 26.Pantchev N., Schnyder M., Vrhovec M.G., Schaper R., Tsachev I. Current surveys of the seroprevalence of Borrelia burgdorferi, ehrlichia canis, anaplasma phagocytophilum, Leishmania infantum, Babesia canis, Angiostrongylus vasorum and Dirofilaria immitis in dogs in Bulgaria. Parasitol Res. 2015;114(Suppl. 1):S117–S130. doi: 10.1007/s00436-015-4518-8. [DOI] [PubMed] [Google Scholar]
- 27.Iliev P.T., Kirkova Z.T., Tonev A.S. Preliminary study on the prevalence of endoparasite infections and vector-borne diseases in outdoor dogs in Bulgaria. Helminthologia. 2020;57(2):171–178. doi: 10.2478/helm-2020-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsachev I., Pantchev N., Marutsov P., Petrov V., Gundasheva D., Baymakova M. Serological evidence of Borrelia burgdorferi, anaplasma phagocytophilum and Ehrlichia spp. Infections in horses from southeastern Bulgaria. Vector Borne Zoonotic Dis. 2018;18(11):588–594. doi: 10.1089/vbz.2018.2293. [DOI] [PubMed] [Google Scholar]
- 29.Tsachev I., Baymakova M., Pantchev N. Seroprevalence of Anaplasma phagocytophilum, Ehrlichia spp. and Borrelia burgdorferi infections in horses: first report from Northern Bulgaria - short communication. Acta Vet Hung. 2019;67(2):197–203. doi: 10.1556/004.2019.021. [DOI] [PubMed] [Google Scholar]