Abstract
Background
Knee osteoarthritis (OA) is a widespread and debilitating disease that continues to plague patients. Over the past decade, neuromuscular electrical stimulation (NMES) therapy has shown promise in alleviating knee OA-related symptoms. This study sought to evaluate the efficacy and safety of a home-based NMES therapy for reduction of pain, stiffness, and function associated with knee OA.
Material and methods
A randomized, sham-controlled, double-blind, multicenter trial was conducted with 12-week follow-up in 156 knee OA patients receiving either home-based NMES therapy or a modified low-voltage NMES therapy. Outcome measures including knee pain, stiffness, and functionality were collected at baseline through week 12 after the therapy. The primary endpoint was the percentage change from baseline (PCFB) in the Visual Analog Scale (VAS) pain for a patient-nominated physical activity. Secondary endpoints included VAS for general knee pain, Western Ontario and McMaster Universities Osteoarthritis Index, Knee Injury and Osteoarthritis Outcome Score Joint Replacement, and isometric quadriceps strength test.
Results
A clinically meaningful reduction for VAS Nominated Activity was higher in the per-protocol treatment-compliant NMES group than that in the sham low-voltage NMES group at week 12 (PCFB of 42.8% vs 38.6%, P = .562). This was similarly true for the Western Ontario and McMaster Universities Osteoarthritis Index pain subscale (PCFBs of 36.8% vs 26.6%, P = .038). Similar trends and reductions of pain were observed for VAS General, Knee Injury and Osteoarthritis Outcome Score Joint Replacement Pain subscale, and isometric quadriceps strength.
Conclusion
Home-based NMES treatment resulted in a clinically meaningful reduction of knee pain, stiffness, and knee functional improvements at week 12 compared with sham NMES treatment.
Keywords: NMES, knee OA, osteoarthritis, home-based therapy, conservative
Introduction
Knee osteoarthritis (OA) is a leading cause of disability and continues to pose substantial health and economic burden [1,2]. The prevalence of symptomatic knee OA is expected to increase as a sequelae of improved life-expectancy and growing obesity in the United States (U.S.) [3,4]. Nonoperative treatments constitute the initial approach for knee OA and serve to delay definitive modalities like total knee arthroplasty (TKA). Current practice guidelines strongly recommend participation in physical activity, weight loss, and use of nonsteroid anti-inflammatory drugs [5]. However, these nonoperative measures have resulted in variable success [6]. Providers routinely encourage exercise to offset and mitigate the effects of knee OA; however, patients often have trouble adhering to these lifestyle changes. The demand for effective long-term treatments with few side-effects has led to a search for innovative solutions to reduce knee pain and restore function. In addition, based on the growth in the aging population, a rapid increase in development of knee OA disease rate, and opioid addiction risks associated with the treatment of chronic pain, the need for alternative therapies to address knee OA becomes crucial.
Home-based Neuromuscular Electrical Stimulation (NMES) therapy has recently emerged as an affordable and practical option to address quadriceps muscle dysfunction, a culprit of knee cartilage deterioration [[7], [8], [9], [10], [11]]. The quadriceps muscles are critical to dynamic joint stability and function to relieve compressive loads, thereby accelerating progression toward knee OA. NMES functions to send an electrical impulse to alter motor recruitment by preferentially activating a greater proportion of larger type II muscle fibers in the target muscle group than volitional exercises at comparable intensities [[12], [13], [14], [15]]. Multiple studies have suggested improved knee pain and function utilizing NMES therapy following total knee procedures [13,[16], [17], [18]]. A recent randomized trial found significant knee pain reduction and improved functional scores following the use of NMES therapy within 6 weeks of TKA [17]. High-level studies evaluating NMES use for the management of knee OA has been limited although a recent regulatory clearance has been received for this indication from the United States Food and Drug Administration. A review of electrical stimulation modalities for OA-related knee pain demonstrates safety and success [19]. However, the included studies are limited by small sample sizes, significant heterogeneity, and lack of reported data related to the patients’ compliance with the actual applied NMES treatment dose during the studies. Despite reported success with NMES, there is a paucity of high-quality studies identifying innovative treatments targeting quadriceps weakness-related OA. Further examination is warranted to identify its utility for patients presenting with knee OA.
A home-based NMES as a noninvasive, nonsurgical, and nonpharmacological therapy has the potential to play a significant role in management of knee OA in the comfort of a patient’s home. This system utilizes a waveform pulse generator incorporated into a light knee wrap and includes digital health features to facilitate the reporting of the actual applied NMES therapy data by the patients. The purpose of this study was to evaluate the effects of this NMES modality compared with a sham treatment group for the treatment of OA symptoms: knee pain, joint stiffness, and joint mobility.
Material and methods
Study design
This study was a multicenter randomized, sham-controlled, double-blind, parallel-group trial (NCT04128618) [20] conducted between October 2019 and June 2020 at 7 centers across the United States. Central or local institutional review board approvals were obtained for each site in accordance with the International Council for Harmonisation Guidelines for Good Clinical Practice. All patients provided written informed consent prior to participation.
Patients
Adults with degenerative knee OA, radiographically defined by a Kellgren-Lawrence grade [21] II, III, and IV, were randomized to receive either the active, original, or modified low-voltage version of NMES therapy. Randomization schedule was created using a random permuted block methodology stratified by site. In order to assist with recruitment and retention, assignment was in a 2:1 ratio of NMES treatment therapy to sham treatment. Intent-to-treat population was defined as all patients randomized (treatment NMES N = 106 and sham low-voltage NMES N = 50). Per Protocol Therapy Compliant (PPTC) population was defined as all randomized patients with at least 1 session of study therapy applied, no major protocol deviations, and any patient in the treatment NMES group who applied the NMES therapy at least for 800 minutes per month (PPTC treatment NMES, N = 69 at week 4, N = 61 at week 8, N = 45 at week 12).
Treatment
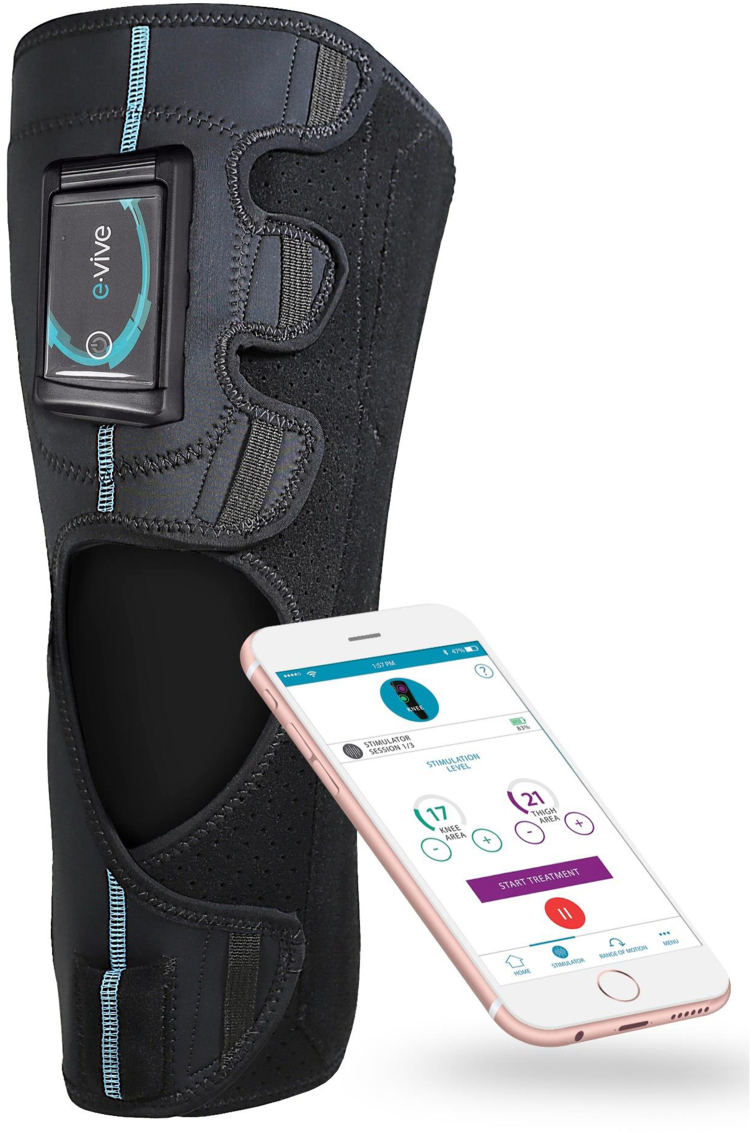
A NMES therapy device (CyMedica Orthopedics NMES therapy, Scottsdale, AZ) was provided to the patients and consisted of a conductive knee garment with a controller (pulse generator), docking receptacle, 2 range-of-motion sensors, and 3 electrodes. The electrodes were designed to be placed on the vastus medialis oblique and rectus femoris muscles of the quadriceps.
Trial interventions
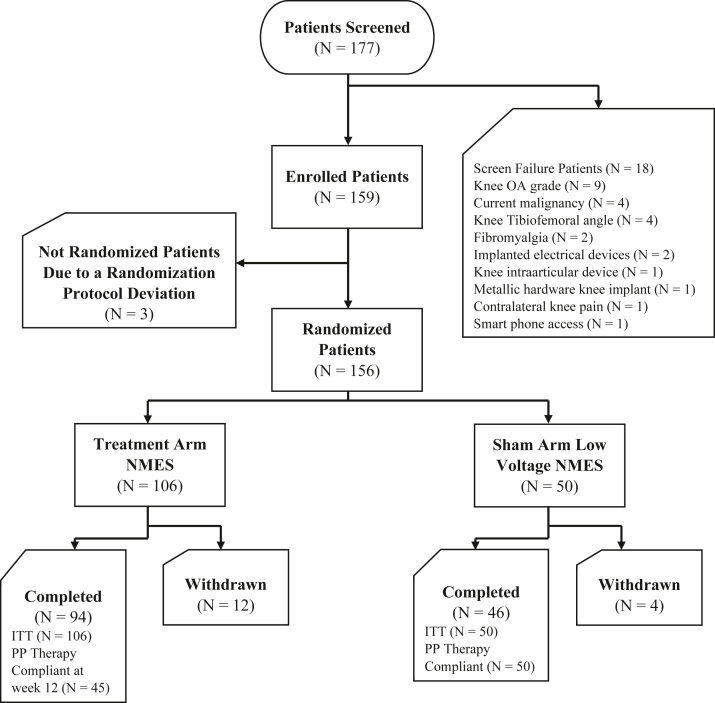
A total of 177 potential patients were screened, and application of selection criteria yielded 159 eligible for enrollment (Fig. 1). A complete list of inclusion and exclusion criteria can be found on the clinicaltrials.gov listing [20]. Two study groups were defined: treatment NMES group that received the original NMES therapy and a sham low-voltage NMES group as a control group and received a modified low-voltage NMES therapy (Fig. 2). The applied NMES intensities were controlled by the patients on the device mobile app and could range from an incremental level of 0 (no voltage) to 100 (maximum available voltage). The patients in the sham low-voltage NMES group were given a modified version of the NMES therapy with a limited maximum applied intensity of level 5. Both groups were instructed to apply the NMES treatment twice a day (each NMES session was 20 minutes), 5 days a week for 12 weeks (a minimum of 800 minutes of therapy per month). Overall compliance for groups is listed in Table 1, Table 2.
Figure 1.
Patient disposition flowchart.
Figure 2.
CyMedica e-vive system.
Table 1.
Summary of the actual applied NMES therapy duration (minutes) in the ITT population.
| Parameter | Treatment NMES-ITT population |
Sham low-voltage NMES-ITT population |
||||
|---|---|---|---|---|---|---|
| Week 4 | Week 8 | Week 12 | Week 4 | Week 8 | Week 12 | |
| n | 106 | 96 | 88 | 49 | 44 | 40 |
| Mean | 850.3 | 872.8 | 766.5 | 871.0 | 873.9 | 803.2 |
| SD | 396.2 | 497.3 | 448.6 | 479.3 | 537.0 | 427.8 |
| Median | 907.5 | 894.5 | 810.0 | 862.0 | 872.5 | 780.0 |
| Min. | 21 | 20 | 20 | 2 | 20 | 40 |
| Max. | 2146 | 3551 | 2161 | 3005 | 2945 | 2292 |
| Missing | - | 10 | 18 | - | 5 | 9 |
SD, standard deviation.
Table 2.
Summary of the actual applied NMES therapy duration (minutes) in the PPTC population.
| Parameter | Treatment NMES-PPTC population |
Sham low-voltage NMES-PP population |
||||
|---|---|---|---|---|---|---|
| Week 4 | Week 8 | Week 12 | Week 4 | Week 8 | Week 12 | |
| n | 69 | 61 | 45 | 49 | 44 | 40 |
| Mean | 1079.4 | 1139.7 | 1110.8 | 871.0 | 873.9 | 803.2 |
| SD | 231.4 | 425.4 | 298.2 | 479.3 | 537.0 | 427.8 |
| Median | 1060.0 | 1022.5 | 1020.0 | 862.0 | 872.5 | 780.0 |
| Min. | 800 | 800 | 800 | 2 | 20 | 40 |
| Max. | 2146 | 3551 | 2161 | 3005 | 2945 | 2292 |
| Missing | - | - | - | - | 5 | 9 |
BMI, body mass index; SD, standard deviation; PP, per protocol; SD, standard deviation.
Study visits and outcome measures
The study visits included screening, baseline, week 4, week 8, and week 12. Several patient-reported outcome measures were collected at baseline and each follow-up visit. Visual Analog Scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) [22], Knee Injury and Osteoarthritis Outcome Score Joint Replacement (KOOS JR) [23], and Patient Global Impression of Change were utilized. VAS was assessed for evaluation of pain for a patient-nominated activity (VAS Nominated Activity) [24] and a general knee pain (VAS General). VAS Nominated Activity studied by Parkes et al. [24] was defined as a physical activity that caused the worst knee pain for the patient at the baseline visit. Isometric quadriceps strength test as an indicator for muscle strength was conducted and analyzed. Using a handheld dynamometer (model 01 163; Lafayette Instrument Company, Lafayette, IN), peak torque (lb.ft) exerted from patients’ lower leg when pushed against the dynamometer at 90 degrees of flexion was measured to represent the patient’s isometric quadriceps strength. The primary endpoint was VAS Nominated Activity at week 12. The primary endpoint was defined as either success or failure (binary endpoint) where the patient experienced at least a 30% or greater improvement from baseline at week 12 in the VAS Nominated Activity or did not experience this defined improvement. Secondary endpoints were patient reported outcomes and isometric quadriceps strength at week 12.
Statistical analysis
Sample size calculations were performed using a 2-group Fisher’s exact test of equal proportions, with unequal sample sizes, and a total of 99 patients were found to be required for this study. This was assuming a two-sided test with an alpha of 0.05, 90% power, and a 2:1 ratio of NMES study treatment compared with sham treatment when estimating 80% of patients compared with 45% of patients would reach success in the NMES and sham treatment, respectively (a power of 80% and an alpha of 0.05 would require 80 patients). The study enrolled a total of 159 patients to account of potential attrition (106 to the NMES study treatment and 53 to the sham treatment). nQuery Advisor (Statsols, San Diego, CA) was used for all sample size calculations. Descriptive statistics were conducted for primary and secondary endpoints at baseline and weeks 4 through 12. Chi-square tests compared the number and proportion of patients who achieved 30% or greater improvement from baseline in knee pain as measured by VAS Nominated Activity and other endpoints as point estimates with 95% confidence intervals. Logistic regression models provided sensitivity analysis. In addition, analysis of change over time from week 4 through 12 (percentage change from baseline or PCFB) for the primary and secondary endpoints and the statistical comparison against the sham low-voltage NMES were conducted using Shapiro-Wilk test or Mann-Whitney U test. Statistical analysis was conducted via SAS version 9.4 (SAS Institute Inc., Cary, NC).
Patient demographic and baseline characteristics
A summary of demographic and baseline characteristics in the treatment NMES and sham low-voltage NMES for the ITT and PPTC populations is provided (Table 3).
Table 3.
Patient demographic and baseline characteristics.
| Variable | Treatment NMES (N = 106, ITT) | Sham low-voltage NMES (N = 50, ITT) | Treatment NMES (N = 69 at week 4, PPTC) | Treatment NMES (N = 61 at week 8, PPTC) | Treatment NMES (N = 45 at week 12, PPTC) | All patients (N = 156) |
|---|---|---|---|---|---|---|
| Age (y) | ||||||
| Mean (SD) | 61.8 (11.0) | 59.6 (10.0) | 62.1 (10.6) | 63.1 (10.0) | 63.3 (8.7) | 61.1 (10.4) |
| Median | 64 | 61 | 64 | 64 | 64 | 62 |
| Range | 27-82 | 38-79 | 27-82 | 27-82 | 44-79 | 27-82 |
| Gender, N (%) | ||||||
| Male | 42 (39.6) | 19 (38.0) | 28 (40.6) | 18 (29.5) | 17 (37.8) | 47 (30.1) |
| Female | 64 (60.4) | 31 (62.0) | 41 (59.4) | 43 (70.5) | 28 (62.2) | 72 (46.2) |
| Race, N (%) | ||||||
| White | 76 (71.7) | 37 (74.0) | 51 (73.9) | 44 (72.1) | 32 (71.1) | 88 (56.4) |
| Black or African American | 25 (23.6) | 11 (22.0) | 14 (20.3) | 13 (21.3) | 10 (22.2) | 25 (16.0) |
| Asian | 4 (3.8) | 2 (4.0) | 3 (4.3) | 3 (4.9) | 2 (4.4) | 5 (3.2) |
| American Indian | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 1 (0.9) | 0 | 1 (1.4) | 1 (1.6) | 1 (2.2) | 1 (0.6) |
| BMI (kg/m2) | ||||||
| Mean (SD) | 31.8 (6.1) | 31.3 (5.8) | 31.9 (6.1) | 32.7 (6.4) | 32.9 (6.7) | 31.6 (6.0) |
| Median | 30.5 | 31.3 | 31.3 | 30.7 | 32.6 | 31.3 |
| Kellgren-Lawrence classification, N (%) | ||||||
| Grade II | 45 (42.5) | 24 (48.0) | 28 (40.6) | 25 (41.0) | 19 (42.2) | 69 (44.2) |
| Grade III | 32 (30.2) | 14 (28.0) | 21 (30.4) | 18 (29.5) | 15 (33.3) | 46 (29.5) |
| Grade IV | 29 (27.4) | 12 (24.0) | 20 (29.0) | 18 (29.5) | 11 (24.4) | 41 (26.3) |
BMI, body mass index; SD, standard deviation.
PP, per protocol; SD, standard deviation.
Results
Knee pain in ITT patient population
A 39% (PCFB) reduction of knee pain was observed for VAS Nominated Activity in the treatment NMES group at week 12. However, the differences against the sham group did not reach significance due to a mix of different treatment compliance levels in the ITT treatment NMES group, which ranged from 20 minutes to 2161 minutes of actual applied therapy at week 12. The outcomes for the therapy-compliant treatment NMES group (PPTC) who applied a minimum of 800 minutes of therapy at week 12 exceeded the MCID criteria of 30% and demonstrated a significant difference against the sham group for the majority of the endpoints.
Knee pain in PPTC population
A clinically meaningful reduction for VAS Nominated Activity was higher in the PPTC treatment NMES group than that in the sham low-voltage NMES group at week 12 (PCFB of −42.8 vs −38.6%, P = .562). (Table 4) A greater proportion of the patients in the PPTC treatment NMES group experienced at least a 30% reduction of VAS Nominated Activity compared with the sham patients at week 12 (treatment responder rates: 71% vs 62%). (Table 5) A clinically meaningful reduction in the WOMAC pain subscale was higher in the PPTC treatment NMES group than in the sham low-voltage NMES group at week 12 (PCFBs of −36.8% vs −26.6%, P = .038). A greater proportion of the patients in the PPTC treatment NMES group experienced at least a 30% reduction in WOMAC pain subscale compared with the sham patients at week 12 (treatment responder rates: 64% vs 42%, P = .029). Similar trends and reductions of pain were observed for VAS General and KOOS JR Pain subscale (Table 4, Table 5).
Table 4.
Comparison of percentage change from baseline or PCFBs at week 12.
| Parameter | Treatment NMES (N = 45, PPTC) PCFB, mean (SD) | Sham low-voltage NMES (N = 50, ITT & PP) PCFB, mean (SD) | P value (t-test) |
|---|---|---|---|
| VAS Nominated Activity | −42.8% (37.3) | −38.6% (33.5) | .562 |
| WOMAC pain subscale | −36.8% (54.7) | −26.6% (32.7) | .038 |
| KOOS JR pain subscale | −43.2% (40.1) | −27.7% (29.9) | .010 |
| WOMAC stiffness subscale | −44.7% (35.5) | −17.4% (41.3) | .002 |
| KOOS JR stiffness subscale | −39.8% (40.0) | −14.5% (49.0) | .010 |
| WOMAC function subscale | −40.1% (34.3) | −24.5% (34.2) | .029 |
| KOOS JR function subscale | −39.3% (38.0) | −19.7% (47.4) | .029 |
| WOMAC total | −41.4% (33.1) | −24.9% (32.5) | .016 |
| KOOS JR total | −41.4% (36.8) | −26.0% (31.4) | .018 |
SD, standard deviation.
Table 5.
Comparison of treatment responder rates at week 12.
| Parameter | Treatment NMES (N = 45, PPTC) treatment responder rate | Sham low voltage NMES (N = 50, ITT & PP) treatment responder rate | P value (chi-square test) |
|---|---|---|---|
| VAS Nominated Activity | 71% | 62% | .348 |
| VAS General | 67% | 46% | .043 |
| WOMAC pain subscale | 64% | 42% | .029 |
| KOOS JR pain subscale | 56% | 44% | .076 |
| WOMAC stiffness subscale | 62% | 56% | .011 |
| KOOS JR stiffness subscale | 64% | 46% | .071 |
PP, per protocol.
Knee stiffness in PPTC population
A clinically meaningful reduction in WOMAC stiffness subscale was higher in the PPTC treatment NMES group than in the sham low-voltage NMES group at week 12 (PCFBs of −44.7 vs −17.4%, P = .002). (Table 4) A greater proportion of the patients in the PPTC treatment NMES group experienced at least a 30% reduction in WOMAC stiffness subscale compared with the sham patients at week 12 (treatment responder rates: 62% vs 56%, P = .011) (Table 5). Similar trends and reduction of knee stiffness were observed for KOOS JR stiffness subscale (Table 4, Table 5).
Knee functionality in PPTC population
A clinically meaningful improvement in WOMAC functional subscale was higher in the PPTC treatment NMES group than in the sham low-voltage NMES group at week 12 (PCFBs of −40.1 vs −24.5%, P = .029) (Table 4, Table 5). Similar trends and improvements of knee functionality were observed for KOOS JR function subscale (PCFBs of −39.3% vs −19.7%, P = .029).
Isometric quadriceps strength test
The isometric quadriceps strength improvement was more pronounced in the PPTC treatment NMES than in the ITT treatment NMES patients at week 12 (PCFBs of 81.5% vs 64.6% at week 12) (Table 6). The PCFBs at week 12 compared with the baseline reached significance within the ITT and PPTC treatment NMES groups (P = .0006 and P = .0001, respectively). The isometric quadriceps strength also demonstrated improvements in the sham group at week 12 (PCFB of 56%).
Table 6.
Isometric quadriceps strength (peak torque) endpoints change from baseline at week 12.
| Parameter | Isometric quadriceps strength PCFB at week 12 | P value (t-test) |
|---|---|---|
| Treatment NMES-PPTC, N = 38 | 81.5 (116.2) | .0006 |
| Treatment NMES-ITT, N = 106 | 64.7 (101.7) | .0001 |
| Sham low-voltage NMES-ITT, N = 50 | 56.3 (103.3) | .0643 |
Patient Global Impression of Change survey
A larger proportion of the patients in the PPTC treatment NMES group responded either “very much improved” or “much improved” in their health status than those in the sham low-voltage NMES group (64.4% vs 44%, P = .046).
Discussion
Knee OA continues to have a substantial impact on individuals and hospital systems and is expected to worsen in the coming years [1]. Quadriceps muscle dysfunction is thought to expedite physiologic deterioration leading to OA; however, current treatment options directly influencing this muscle group are limited [[7], [8], [9], [10], [11],[25], [26], [27]]. This study sought to examine OA-related knee pain, stiffness, and function utilizing an innovative treatment modality: home-based NMES therapy. We found clinically meaningful improvements in knee pain, joint stiffness, and joint mobility among patients randomized to the NMES treatment group compared with the sham low-voltage group. The improvements were more pronounced for the patients who adhered to the full daily treatment dose of 2 sessions per day (20 minutes each) and 5 days of the week, and the results were different than those in the sham low-voltage NMES group. Even in the overall (ITT) population, the treatment NMES group including less-compliant patients demonstrated a clinically meaningful reduction of knee pain.
This study is not without its limitations. Starting in March 2020, the U.S. encountered a novel coronavirus disease (COVID-19). This interrupted trial protocols due to temporary site closures and out-of-window visits; however, to reduce the risk of missing data at the 8- and 12-week visits, an alternative option of a telephone visit and mailed-in study questionnaire was provided. Treatment and sham group results may have been confounded by the placebo effect, which may be more pronounced in the modified low-voltage NMES group. Patients in the low-voltage sham NMES group may have experienced a strong placebo response, which is well established in OA studies [28] and could have obscured differences between groups. Patients in the sham low-voltage group were also given a modified version of the NMES therapy and can arguably not be considered a “true” placebo absent of any effect. In fact, our placebo group achieved moderate levels of improvement compared with baseline pain, coinciding with previously published rates [29]. Finally, patients were required to nominate an activity that elicited the most knee pain. The type of activity differed among patients; however, we were able to objectively trend each patient’s knee pain as scored by the VAS at each study visit using this methodology. These study limitations should not be disqualifying given the sample size and meaningful clinical changes reported between the 2 study groups to address OA related knee pain, stiffness, and functionality.
Pham et al. [30] reported a set of knee pain and function treatment responders’ criteria in knee OA studies for oral nonsteroid anti-inflammatory drug and knee oral specific drug treatments. A “moderate improvement” was defined as a knee pain relative change of 30% and knee function relative change of 20%. A “high improvement” was defined as a knee pain relative change of 55% and knee function relative change of 50% according to the OMERACT-OARSI set of responder criteria. The effectiveness for all pain, stiffness, and functionality endpoints in the NMES therapy treatment group of our study can be categorized as “moderate improvement” after 12 weeks of use based on these criteria. The observed gradual improvements in the isometric quadriceps strength and improvements in the pain and function outcomes are supported by prior findings in other NMES-therapy-related studies [[25], [26], [27]]. Concomitantly, the study-safety-related results demonstrated an excellent safety profile for the NMES therapy with only 3 device-related adverse events that were not serious and did not result in study discontinuation. Together, these results demonstrate efficacy and safety using NMES as a treatment modality for knee OA. Given patient preference to limit in-office physician or physical therapy visits [19], this home-based and practical NMES therapy may be of increasing utility for patients suffering from knee OA symptoms.
Conclusions
The home-based NMES therapy as a nonsurgical treatment used in this study has shown efficacy in the treatment of OA-related knee pain, stiffness, and function. This treatment has the potential to provide suffering patients with a bridge between conservative management and definitive total knee procedures. Given the increasing incidence of patients with knee OA due to improved longevity, the health-care implications are clear. Moreover, as more providers embrace the bundled payment model, the cost-saving potential is imminent with TKA sparing measures like these. Future studies should further explore the low-voltage NMES effects on knee OA as seen in our sham group.
Conflicts of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: V. Dasa is paid consultant and paid presenter or speaker for bioventus; receives research support from cartiheal; is a paid consultant for, receives research support from, and has stock or stock options in cymedica; has stock or stock options in Doc Social, Goldfinch consulting, Grand Care, mymedicalimages.com, Ortho Lazer, SIGHT Medical, and MEND; is in the editorial or governing board of the Journal of Orthopedic Experience and Innovation; and is paid presenter or speaker and paid consultant for pacira bioscience. N. Skrepnik is a paid consultant for Adamis; receives research support from Biomimetic, Flexion, Genzyme, Johnson & Johnson, Pfizer, Regeneration Technologies, Inc., SamuMed, Sanofi-Aventis, Smith & Nephew, Stryker, Wright Medical Technology, Inc., Zimmer, and Medtronic Sofamor Danek; is a paid consultant for Genzyme, Orthofix, Inc., and SamuMed; is a paid presenter or speaker for Pfizer and Regeneron. R. E. Delanois receives research support from Biocomposites Inc., Cymedica Orthopedics, DePuy Synthes Product Inc., Flexion Therapeutics, Microport Orthopedics, Orthofix Inc., Patient-Centered Outcomes Research Institute, Smith & Nephew, Tissue Gene, and United Orthopedic Corporation and also serves as a board member for the Baltimore City Medical Society.
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2022.03.007.
Footnotes
Funding: CyMedica Orthopedics provided research support for this study; however, they played no role in editing or writing.
Appendix A. Supplementary data
References
- 1.Arthritis Foundation . 2018. Arthritis by the numbers/book of trusted facts & figures.https://www.arthritis.org/getmedia/e1256607-fa87-4593-aa8a-8db4f291072a/2019-abtn-final-march-2019.pdf [accessed 01.10.22] [Google Scholar]
- 2.Barbour K.E., Helmick C.G., Boring M., Brady T.J. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation — United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2017;66:246. doi: 10.15585/mmwr.mm6609e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Caspian J Intern Med. 2011;2:205. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y., Jordan J.M. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355. doi: 10.1016/j.cger.2010.03.001. Erratum in: Clin Geriatr Med. 2013 May;29(2):ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jevsevar D.S. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21:571. doi: 10.5435/JAAOS-21-09-571. [DOI] [PubMed] [Google Scholar]
- 6.Lespasio M.J., Piuzzi N.S., Husni M.E., et al. Knee osteoarthritis: a primer. Perm J. 2017;21:16. doi: 10.7812/TPP/16-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alnahdi A.H., Zeni J.A., Snyder-Mackler L. Muscle impairments in patients with knee osteoarthritis. Sports Health. 2012;4:284. doi: 10.1177/1941738112445726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewek M.D., Rudolph K.S., Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22:110. doi: 10.1016/S0736-0266(03)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petterson S.C., Barrance P., Buchanan T., Binder-Macleod S., Snyder-Mackler L. Mechanisms underlying quadriceps weakness in knee osteoarthritis. Med Sci Sports Exerc. 2008;40:422. doi: 10.1249/MSS.0b013e31815ef285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slemenda C., Brandt K.D., Heilman D.K., et al. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127:97. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Al-Johani A.H., Kachanathu S.J., Ramadan Hafez A., et al. Comparative study of hamstring and quadriceps strengthening treatments in the management of knee osteoarthritis. J Phys Ther Sci. 2014;26:817. doi: 10.1589/jpts.26.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walls R.J., McHugh G., O’Gorman D.J., Moyna N.M., O’Byrne J.M. Effects of preoperative neuromuscular electrical stimulation on quadriceps strength and functional recovery in total knee arthroplasty. A pilot study. BMC Musculoskelet Disord. 2010;11:119. doi: 10.1186/1471-2474-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werner S., Arvidsson H., Arvidsson I., Eriksson E. Electrical stimulation of vastus medialis and stretching of lateral thigh muscles in patients with Patello-femoral symptoms. Knee Surg Sports Traumatol Arthrosc. 1993;1:85. doi: 10.1007/BF01565458. [DOI] [PubMed] [Google Scholar]
- 14.Lake D.A. Neuromuscular electrical stimulation: an overview and its application in the treatment of sports injuries. Sports Med. 1992;13:320. doi: 10.2165/00007256-199213050-00003. [DOI] [PubMed] [Google Scholar]
- 15.Kittelson A.J., Stackhouse S.K., Stevens-lapsley J.E. Neuromuscular Electrical Stimulation after total joint arthroplasty: a critical review of recent controlled studies. Eur J Phys Rehabil Med. 2013;49(6):909. [PubMed] [Google Scholar]
- 16.Hauger A.V., Reiman M.P., Bjordal J.M., et al. Neuromuscular electrical stimulation is effective in strengthening the quadriceps muscle after anterior cruciate ligament surgery. Knee Surg Sports Traumatol Arthrosc. 2018;26:399. doi: 10.1007/s00167-017-4669-5. [DOI] [PubMed] [Google Scholar]
- 17.Delanois R., Sodhi N., Acuna A., et al. Use of home neuromuscular electrical stimulation in the first 6 weeks improves function and reduces pain after primary total knee arthroplasty: a matched comparison. Ann Transl Med. 2019;7(Suppl 7):S254. doi: 10.21037/atm.2019.09.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chughtai M., Piuzzi N., Yakubek G., et al. Use of an app-controlled neuromuscular electrical stimulation system for improved self-management of knee conditions and reduced costs. Surg Technol Int. 2017;31:221. [PubMed] [Google Scholar]
- 19.Zeng C., li H., Yang T., et al. Electrical stimulation for pain relief in knee osteoarthritis: systematic review and network meta-analysis. Osteoarthritis Cartilage. 2015;23:189. doi: 10.1016/j.joca.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Evaluation of a Home-based NMES Therapy for Knee Osteoarthritis Pain - Full Text View - ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT04128618?term=cymedica&type=Intr&cond=Osteoarthritis%2C+Knee&draw=2&rank=1 [accessed 10.11.20]
- 21.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellamy N., Buchanan W.W., Goldsmith C.H., Campbell J., Stitt L.W. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833. [PubMed] [Google Scholar]
- 23.Lyman S., Lee Y.Y., Franklin P.D., et al. Validation of the KOOS, JR: a short-form knee arthroplasty outcomes survey. Clin Orthop Relat Res. 2016;474(6):1461. doi: 10.1007/s11999-016-4719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parkes M.J., Callaghan M.J., O’Neill T.W., et al. Sensitivity to change of patient-preference measures for pain in patients with knee osteoarthritis: data from two trials. Arthritis Care Res. 2016;68(9):1224. doi: 10.1002/acr.22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imoto A.M., Peccin M.S., Teixeira L.E. P.d.P., et al. Is neuromuscular electrical stimulation effective for improving pain, function and activities of daily living of knee osteoarthritis patients? A randomized clinical trial. Sao Paulo Med J. 2013;131:80. doi: 10.1590/S1516-31802013000100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruce-Brand R.A., Walls R.J., Ong J.C., et al. Effects of home-based resistance training and neuromuscular electrical stimulation in knee osteoarthritis: a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:118. doi: 10.1186/1471-2474-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laufer Y., Elboim-Gabyzon M., Shtarker H. The effects of exercise and neuromuscular electrical stimulation in subjects with knee osteoarthritis: a 3-month follow-up study. Clin Interv Aging. 2014;9:1153. doi: 10.2147/CIA.S64104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Z., Chen J., Hu Q.S., et al. Meta-analysis of pain and function placebo responses in pharmacological osteoarthritis trials. Arthritis Res Ther. 2019;21(1):173. doi: 10.1186/s13075-019-1951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abhishek A., Doherty M. Mechanisms of the placebo response in pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21:1229. doi: 10.1016/j.joca.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Pham T., Van Der Heijde D., Lassere M., et al. Outcome variables for osteoarthritis clinical trials: the OMERACT-OARSI set of responder criteria. J Rheumatol. 2003;30:1648. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.