Abstract
Synthesis of new polyesters by acyclic diene metathesis (ADMET) polymerization of α,ω-diene, 4-allyl-2-methoxyphenyl 10-undecenoate (M1), prepared from bio-renewable eugenol and castor oil (undecenoate), have been demonstrated. Ruthenium-carbene (called second generation Grubbs) catalyst afforded polymers with unimodal molecular weight distributions (Mn = 12 700, Mw/Mn = 1.85). The polymerization in the presence of a triarm cross-linker, 5-formylbenzene-1,2,3-triyl tris(undec-10-enoate), also afforded polymers with certain uniform network structures.
Synthesis of high molecular weight polymers by acyclic diene metathesis (ADMET) polymerization of α,ω-diene prepared from bio-renewable eugenol and castor oil (undecenoate) has been demonstrated.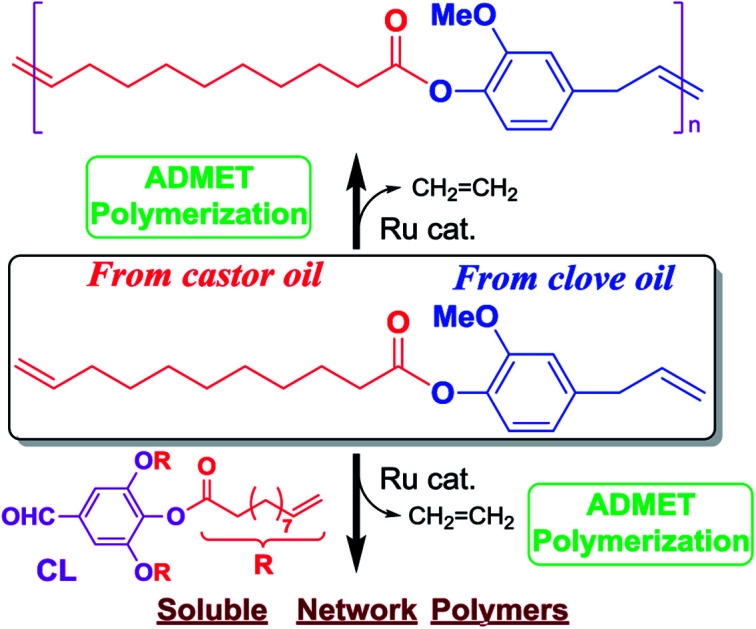
Introduction
Polyesters are widely used in our daily life and attract considerable attention due to their tunable mechanical properties and potential biodegradability.1 Most polyesters are currently prepared from compounds derived from petroleum-based resources (especially from fossil resources). Synthesis of polyesters from bio-derived monomers is thus of great interest for research and development on high performance and sustainable materials.2 In this context, various raw materials from renewable resources such as plant oils,3 lignin,4 polysaccharides,5 sugars,6 and terpenes7 have been investigated. Among them, plant oils exemplified by castor oil,8 which generally convert to fatty acids or fatty acid methyl esters by chemical modifications, are useful feedstock for synthesis of polymers.9 Acyclic diene metathesis (ADMET) polymerization is very useful for the synthesis of a wide variety of linear polymers and polymer architectures that are not available using the other polymerization methods.10 Recently, numerous studies have concentrated on the ADMET polymerization of fatty acids and its derivatives to obtain polyesters.11 Castor oil derivative, ω-undecenoate, containing both olefinic double bond and carboxylate at the termini is an ideal substance for synthesis of high purity α,ω-dienes as valuable monomers for preparing polyesters via ADMET polymerization. For instance, ADMET polymerizations of castor oil-based α,ω-dienes (undecenyl-undecenoate) using second generation Grubbs-type ruthenium catalysts resulted in high molecular weight unsaturated polyesters have been investigated by Meier et al.12 The ADMET polymerization of α,ω-dienes prepared from castor-oil derivative with hydroxyl-bearing unsaturated compounds, diols and diamine have also been studied.13
Eugenol (UG, 4-allyl-2-methoxyphenol) is an interesting renewable resource obtained from clove oil. UG has been widely used not only in the production of cosmetics, perfumes, soaps, and even food, but also in the medical and pharmaceutical fields as antibiotic and antihypertensive agents.14 Owing to its functional groups [hydroxyl (–OH), methoxy (OCH3) and allyl groups], UG can be used as starting material for synthesis of new compounds exemplified in designing polymer networks via thiol–ene coupling15 or bismaleimide networks.16 Recently, study on UG mainly focused on Ru-catalysed olefin metathesis reactions such as self-metathesis17 and cross-metathesis with either electron-deficient olefins18 or unsaturated fatty acid methyl esters19 due to presence of the terminal olefin group. Therefore, it is possible to prepare different types of functionalized phenol derivatives to develop new routes for the production of different multifunctional products from UG.20 Additionally, UG structure, which contains an aromatic ring, has the potential alternative to petroleum-based phenolic monomers, which are widely used nowadays in the field of polyesters. For instance, a wide variety of polyesters based on UG and α,ω-diols have been prepared by polycondensation and thiol–ene click reactions.21 However, there are no reports had been published till date about using UG as the precursor for the synthesis of novel α,ω-dienes monomers incorporating with aliphatic chain (undecenoate) to produce polyesters via ADMET polymerization, although there is one report for ADMET polymerization of dieugenol derived from UG affording amorphous polymer with high molecular weight.22
In this paper, we wish to present a simple preparation of α,ω-diene monomer M1, derived from bio-renewable UG and castor oil, for synthesis of polyesters P1 by ADMET polymerization. The ADMET polymerization conditions in term of catalyst loading, type of catalyst and reaction time, have been investigated in detail. The ADMET polymerization of M1 in the presence of triarm cross-linker, 5-formylbenzene-1,2,3-triyl tris(undec-10-enoate) was also studied. Two aliphatic polyesters with linear aliphatic α,ω-dienes were also prepared for comparison.
Results and discussion
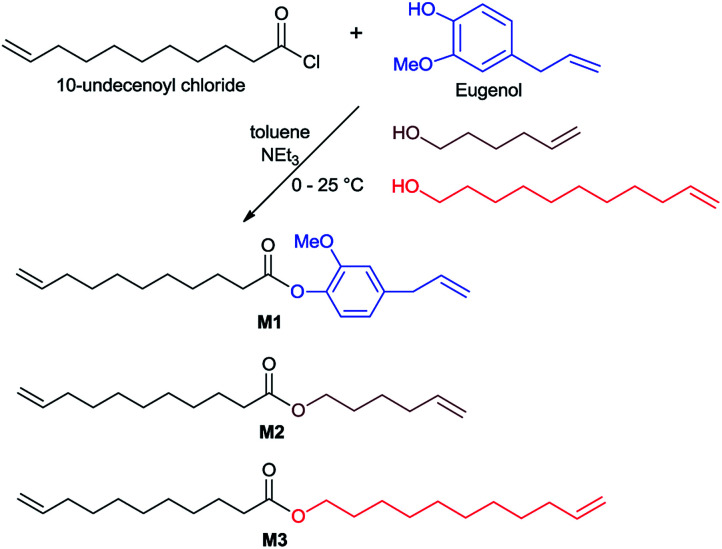
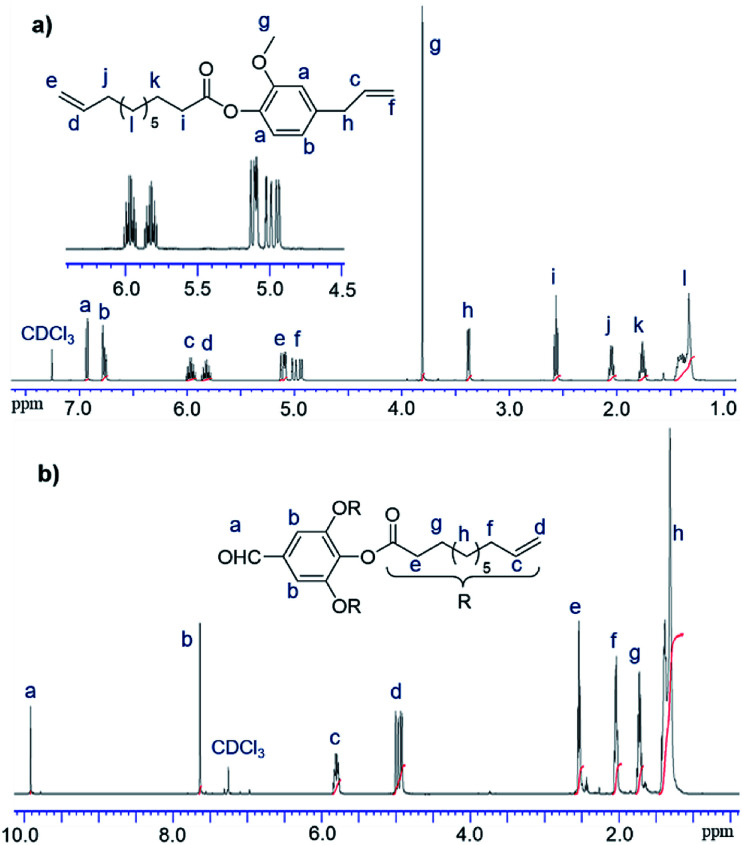
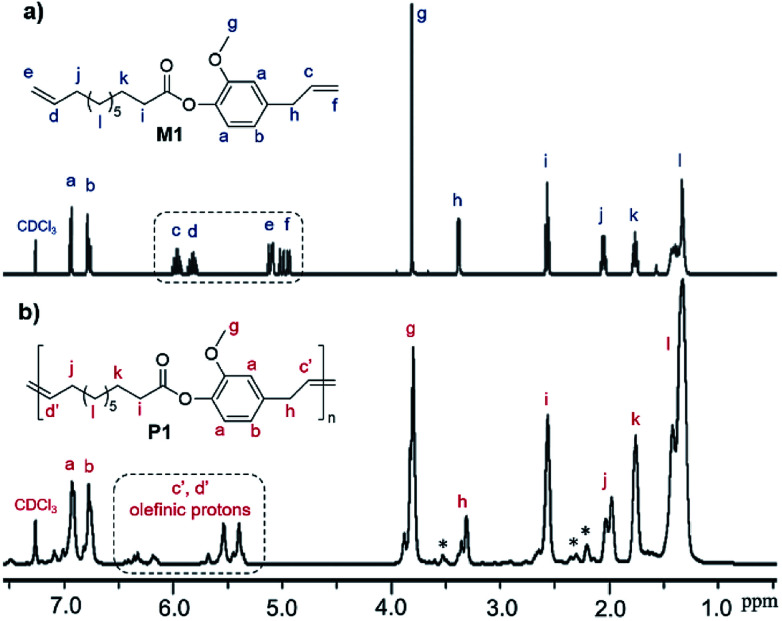
In this study, 10-undecenoyl chloride (a derivative commercially available from undecenoic acid that can be obtained as a major component from castor oil)8 has been chosen to prepare α,ω-diene monomer with eugenol (UG, obtained from clove oil). 5-Hexen-1-ol and 10-undecen-1-ol12 instead of UG were also chosen for comparison. These monomers (expressed as M1–M3 in Scheme 1) were prepared in toluene in the presence of triethylamine according to the reported procedure,23 and the resultant compounds were purified by column chromatography, and were identified by NMR spectra and APCI mass spectrometry (shown in the Experimental section, and the selected spectra are also shown in the ESI,†).241H NMR spectrum of the monomer, 4-allyl-2-methoxyphenyl 10-undecenoate (M1, Fig. 1a), shows characteristic resonances at 4.92–5.02 and 5.92–6.0 ppm, and 5.08–5.13 and 5.78–5.86 ppm ascribed to olefinic protons in terminal position; corresponding resonances ascribed to the carbons are also observed at 114.3 and 116.2 ppm, 139.3 and 137.2 ppm.24 Similarly, reaction of 3,4,5-trihydroxybenzaldehyde with 10-undecenoyl chloride in THF in the presence of excess triethylamine afforded the corresponding ester, 5-formylbenzene-1,2,3-triyl tris(undec-10-enoate) (CL), employed as the cross-linker in this study. CL was also identified by NMR spectra (Fig. 1b)24 and APCI mass spectrometry.
Scheme 1. Synthesis of monomers (M1–M3).
Fig. 1. 1H NMR spectra (in CDCl3 at 25 °C) of (a) 4-allyl-2-methoxyphenyl 10-undecenoate (M1) and (b) 5-formylbenzene-1,2,3-triyl tris(undec-10-enoate) (CL) with their peak assignments.24.
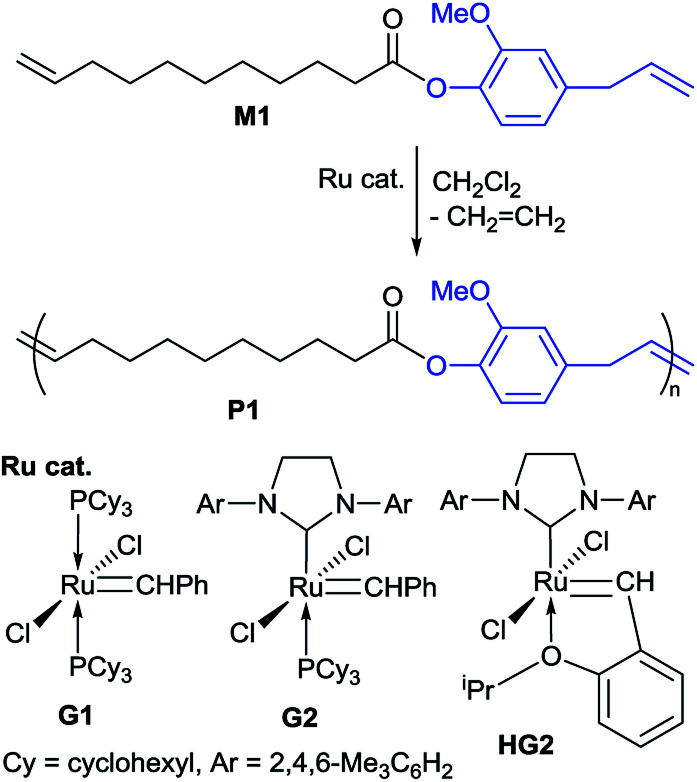
Acyclic diene metathesis (ADMET) polymerizations of M1 were conducted in CH2Cl2 using a sealed Schlenk tube equipped with a high-vacuum valve in the presence of ruthenium catalyst (Scheme 2).24 The reactions were conducted in an oil bath heated at 50 °C under nitrogen atmosphere initially for a certain period (30 min), and the mixture was then placed in vacuo to remove ethylene formed in this condensation polymerization (experimental details are described in the Experimental section),25 as conducted in synthesis of all-trans poly(9,9-n-alkyl fluorene-2,7-vinylene)s by the ADMET polymerization.26 The results are summarized in Table 1.24
Scheme 2. Acyclic diene metathesis (ADMET) polymerization of M1 in the presence of Ru-carbene catalysts.
ADMET polymerization of 4-allyl-2-methoxyphenyl 10-undecenoate (M1) by ruthenium catalystsa.
| Run | Ru cat. (mol%)b | Time/h | M n c | M w/Mnc | Yieldd/% |
|---|---|---|---|---|---|
| 1 | G2 (1.0) | 12 | 7100 | 1.74 | 86 |
| 2e | G2 (1.0) | 12 | 8100 | 2.05 | 88 |
| 3 | G2 (0.2) | 12 | 3200 | 3.63 | 55 |
| 4 | G2 (0.5) | 12 | 7500 | 2.09 | 79 |
| 5 | G2 (1.0) | 12 | 8100 | 2.05 | 88 |
| 6 | G2 (1.5) | 3 | 9400 | 1.97 | 87 |
| 7 | G2 (1.5) | 6 | 10 300 | 1.95 | 87 |
| 8 | G2 (1.5) | 12 | 12 700 | 1.85 | 91 |
| 9 | G2 (1.5) | 18 | 12 400 | 1.64 | 87 |
| 10 | G1 (1.5) | 12 | 5500 | 1.87 | 81 |
| 11 | HG2 (1.5) | 12 | 4500 | 1.63 | 79 |
| 12 | G2 (2.0) | 12 | 12 500 | 1.84 | 91 |
| 13 | G2 (3.0) | 12 | 7900 | 1.93 | 81 |
Conditions: Ru catalyst (shown in Scheme 2), monomer M1 (330 mg, 1.0 mmol), CH2Cl2 0.4 mL (initial monomer concentration 2.50 M), 50 °C. Detailed procedure is shown in the Experimental section.
Mol% based on monomer M1.
GPC data in THF vs. polystyrene standards.
Isolated yield by precipitation as the methanol insoluble fraction.
The tube was placed in vacuo twice at the first time (30 min).
It was revealed that the ADMET polymerization of M1 using called second generation Grubbs catalyst (G2) afforded polymers with unimodal molecular weight distributions (Mn = 7100, 8100; Mw/Mn = 1.74, 2.05, respectively, runs 1, 2). Efficient removal of ethylene formed in this condensation polymerization seems beneficial for obtainment of high molecular weight polymers, because the Mn value seemed decreasing with increasing the initial reaction time under nitrogen [Mn, Mw/Mn = 7100, 1.87 (1.0 h under N2 instead of 30 min); Mn, Mw/Mn = 6900, 1.96 (4.0 h under N2)] or the Mn value increased when ethylene was removed repeatedly after the initial reaction under nitrogen [Mw/Mn = 8100, 2.05, run 2].
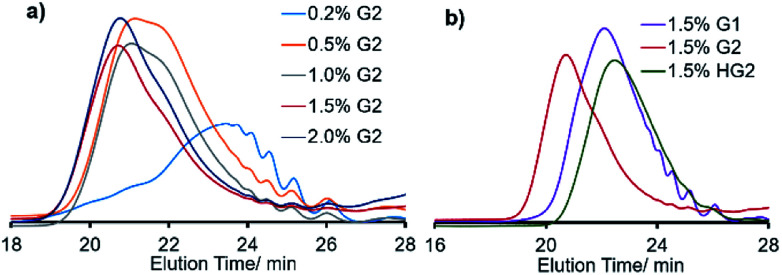
It was also revealed that the Mn value was also affected by the amount of ruthenium catalyst loaded (G2, runs 3–5, 8, 12, 13, Fig. 2a), and the polymerization in the presence of low Ru loading (0.2 mol%) afforded low molecular weight oligomer (run 3, Fig. 2a). Moreover, the Mn value in the resultant polymer increased by increasing the Ru loading [Mn = 7500 (0.5 mol%, run 4) vs. Mn = 12 700 (1.5 mol%, run 8)], and the GPC traces became unimodal upon increasing the Ru loading as clearly demonstrated in Fig. 2a. It thus turned out that the optimized conditions concerning the amount of Ru are 1.5 or 2.0 mol% (based on M1, runs 8 and 12) for obtainment of high molecular weight polymers with unimodal molecular weight distributions. The Mn value was also affected by the reaction time (runs 6–9), and no significant increase in the Mn value was observed after 12 h (runs 8, 9). It also turned out, under the above optimized conditions (run 8), that the other ruthenium catalysts (expressed as G1, and HG2 in Scheme 2) afforded polymers with rather low molecular weights (runs 10, 11, respectively, Fig. 2b). This would be probably because of low reactivity of G1 toward olefins in this ADMET polymerization.27 It also seems likely that rather low catalyst efficiency by HG2 compared to G2 might be considered for the explanation under these conditions. Therefore, G2 seems to be the most suitable in terms of synthesis of higher molecular weight polymers with unimodal molecular weight distributions.
Fig. 2. GPC traces of polymers (P1) in ADMET polymerization of M1 under (a) effect of different G2 loading, and (b) effect of Ru catalysts. Detailed data are shown in Table 1.
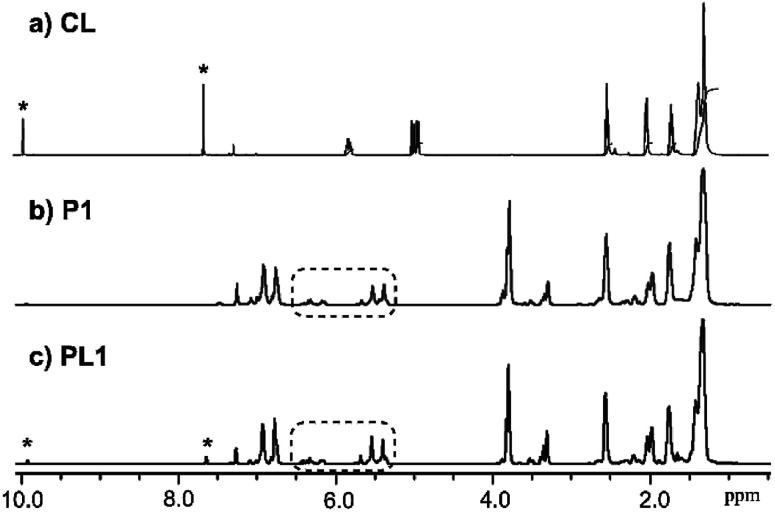
Fig. 3 shows 1H NMR spectrum (in CDCl3 at 25 °C) for 4-allyl-2-methoxyphenyl 10-undecenoate (M1, Fig. 3a) and the resultant polymer (P1, Fig. 3b) prepared by ADMET polymerization (sample run 8). Resonances ascribed to protons of terminal olefins (at 4.92–5.02 and 5.92–6.0 ppm, and 5.08–5.13 and 5.78–5.86 ppm) disappeared and resonances ascribed to protons assigned to internal olefins (at 5.35–5.67 and 6.15–6.45 ppm, placed as dashed circle in Fig. 3b) were observed, whereas the other resonances were remained. The results thus clearly indicate formation of polymers by the ADMET polymerization.25,27 As also suggested by the NMR spectra (broad and several resonances ascribed to olefinic protons), the resultant polymers do not have regular structures in almost certainly but are probably a mixture of head-to-head, head-to-tail, and tail-to-tail arrangement of the repeat unit containing cis and trans double bonds. This can also be suggested by 13C NMR spectrum in P1 (resonances ascribed to internal olefinic carbons at 128–141 ppm, Fig. S10 in ESI†).24 The fact might also explain additional resonances in the aliphatic region (marked with *) compared to M1 in Fig. 3b. The resultant polymer (P1) possesses glass transition temperature (Tg) at −9.6 °C by the DSC thermogram (sample run 8, shown in ESI†),24 and no melting temperature was observed, suggesting that P1 is amorphous material with uniform composition.
Fig. 3. 1H NMR spectrum (in CDCl3 at 25 °C) for (a) 4-allyl-2-methoxyphenyl 10-undecenoate (M1), and (b) the resultant polymer (P1) prepared by the ADMET polymerization (run 8).
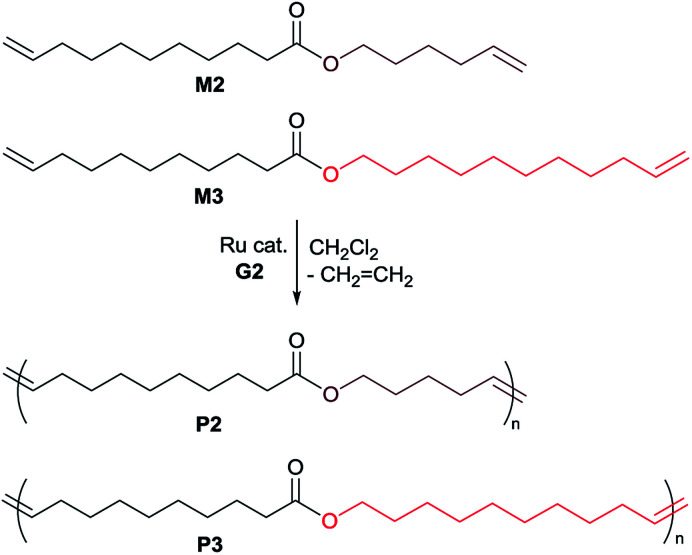
Table 2 summarizes the results for ADMET polymerizations of monomers M2 and M312 in the presence of ruthenium-carbene catalyst (G2, Scheme 3). It was revealed that the polymerizations of M2 afforded polymers with unimodal molecular weight distributions (e.g. Mn = 8300 Mw/Mn = 1.52, run 14) under optimized conditions. As observed in the ADMET polymerization of M1, in the polymerization of M2, the Mn value was affected by the amount of ruthenium catalyst charged, and the Mn value in the resultant polymer (P2) increased by increasing the Ru loading [Mn = 8300 (2.0 mol%, run 14) vs. Mn = 7300 (0.5 mol%, run 16)]. In contrast, in the polymerization of M3,12 the optimized conditions seem 0.5 mol% (run 19) for obtainment of rather high molecular weight polymer (P3) with unimodal molecular weight distribution. Both 1H and 13C NMR spectra clearly support formation of polymers by the ADMET polymerization (disappearance of resonances ascribed to terminal olefins and observed resonances assigned to internal olefins, the spectra are shown in the ESI,†).24 DSC thermograms in the resultant polymers (P2, P3) show a melting temperature (Tm) at 14.3 °C (P2, sample run 14), 51.5 °C (P3, sample run 19), respectively, as observed in long-chain aliphatic polyesters.28,29 In general the melting temperature (Tm value) in the polyester increases with increasing hydrocarbon chain length,30 and the Tm value in the polyester consisting of two types of methylene units also affected the distribution.31 Therefore, increase in the Tm value from P2 to P3 would be due to increase of methylene chain length. However, observed Tm values might be rather low probably due to that microstructure in the resultant polymers are a mixture of head-to-tail, head-to-head and tail-to-tail repeat units. In contrast, placing phenyl group into the polymer main chain in P1 afforded the amorphous materials.
ADMET polymerization of M2 and M3 by G2 catalysta.
| Run | Monomer | G2/mol%b | M n c | M w/Mnc | Yieldd/% |
|---|---|---|---|---|---|
| 14 | M2 | 2.0 | 8300 | 1.52 | 71 |
| 15 | M2 | 1.0 | 7700 | 1.49 | 71 |
| 16 | M2 | 0.5 | 7300 | 1.44 | 72 |
| 17 | M3 | 2.0 | 6200 | 1.50 | 78 |
| 18 | M3 | 1.0 | 6900 | 1.53 | 78 |
| 19 | M3 | 0.5 | 8500 | 1.64 | 84 |
| 20 | M3 | 0.2 | 7200 | 1.55 | 83 |
Conditions: monomer M2 (266 mg, 1.0 mmol) or M3 (336 mg, 1.0 mmol), CH2Cl2 0.4 mL (initial monomer concentration 2.50 M), 50 °C, 12 h. Detailed procedure is shown in the Experimental section.
Mol% based on monomer.
GPC data in THF vs. polystyrene standards.
Isolated yield by precipitation as the methanol insoluble fraction.
Scheme 3. Acyclic diene metathesis (ADMET) polymerization of M2 and M3 in the presence of Ru-carbene catalyst (G2).
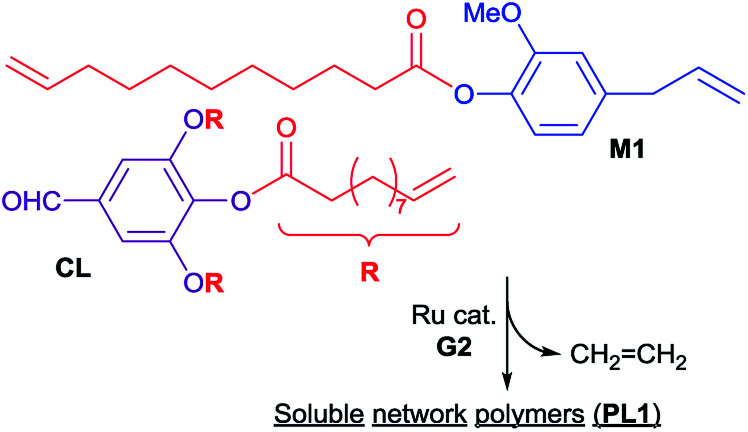
In order to demonstrate a possibility of synthesis of cross-linked polymers (often employed to improve mechanical properties etc.), ADMET polymerizations of 4-allyl-2-methoxyphenyl 10-undecenoate (M1) were conducted in the presence of cross-linker (CL), prepared by reaction of 3,4,5-trihydroxybenzaldehyde with 10-undecenoyl chloride in this study (Scheme 4). The results are summarized in Table 3.24
Scheme 4. Acyclic diene metathesis (ADMET) polymerization of 4-allyl-2-methoxyphenyl 10-undecenoate (M1) in the presence of 5-formylbenzene-1,2,3-triyl tris(undec-10-enoate) (CL).
ADMET polymerization of M1 using G2 in the presence of cross-linker (CL)a.
| Run | CLb/mol% | Time/h | M n c | M w/Mnc | Yieldd/% |
|---|---|---|---|---|---|
| 8 | — | 12 | 12 700 | 1.85 | 91 |
| 21 | 2.5 | 12 | 13 300 | 2.58 | 88 |
| 22 | 2.5 | 18 | 13 600 | 2.28 | 89 |
| 23 | 5.0 | 12 | 11 500 | 3.95 | 88 |
| 24 | 5.0 | 18 | 13 500 | 3.48 | 88 |
| 25e | 5.0 | 18 | 13 800 | 2.80 | 90 |
| 26f | 5.0 | 18 | 10 200 | 2.05 | 81 |
| 27f | 5.0 | 24 | 11 800 | 2.59 | 86 |
Conditions: monomer M1 (330 mg, 1.0 mmol), ruthenium catalyst (G2) 1.5 mol%, cross-linker (CL), CH2Cl2 0.4 mL (initial monomer concentration 2.50 M), 50 °C. Detailed procedure is shown in the Experimental section.
Mol% based on monomer M1.
GPC data in THF vs. polystyrene standards.
Isolated yield by precipitation as the methanol insoluble fraction.
Ru 2.0 mol%.
Cross-linker (CL, 5.0 mol%) was added after 30 min.
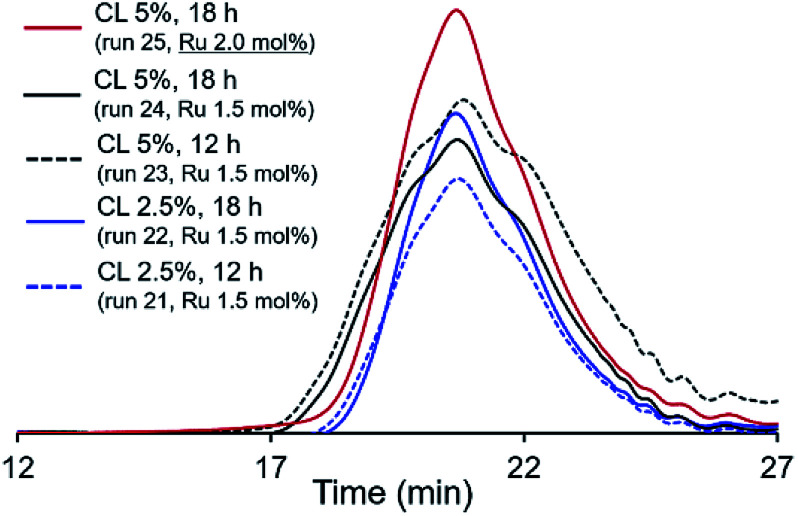
It was revealed that Mn value in the resultant polymer (P1) slightly increased in the presence of CL [Mn = 12 700 (run 8) vs. 13 300 (run 21), reaction 12 h, CL 2.5 mol%], and further stirring afforded polymer with low PDI (Mw/Mn) value (run 22, reaction 18 h). Increasing the amount of CL (from 2.5 to 5.0 mol%) afforded the polymers with rather broad molecular weight distributions even after 18 h (runs 23, 24), and no significant increase in the Mn values were observed (runs 21–24). However, as shown in Fig. 4, GPC traces in the resultant polymers, it seems that the molecular weight distributions became unimodal after 18 h; the PDI value became low upon increasing the Ru loading (run 25). It turned out that increase of Ru loading (run 25), addition of CL after the initial ADMET polymerization did not affect the increase of Mn value in the resultant polymers.
Fig. 4. GPC traces polymers (PL1) in ADMET polymerization of M1 using G2 in the presence of cross-linker (CL). Detailed data are shown in Table 3.
Fig. 5 shows 1H NMR spectra (in CDCl3 at 25 °C) of P1 (sample run 8) and PL1 (sample run 24). As observed in P1 (Fig. 3b), protons assigned to terminal olefins in M1 and CL were disappeared and resonances ascribed to protons in the internal olefins were observed (placed as dashed circle in Fig. 5b and c). Moreover, resonances ascribed to CL (in particular protons corresponding to aldehyde and aromatic proton marked as * in Fig. 5a and c) were clearly observed.24 DSC thermograms in the resultant polymer (PL1) show a glass transition temperature (Tg) at −10.5 °C, which is relatively close to that in P1 (Tg = −9.6 °C).24 These results thus probably suggest that PL1 possesses a certain network structure consisting of P1 and CL (with low degree of cross-linking) with uniform composition. The resultant polymer sample (PL1) was hardly soluble in CDCl3 (30 mg/2.0 mL) at room temperature but became completely soluble overnight, whereas the sample is easily soluble in THF for GPC measurement.
Fig. 5. 1H NMR spectra (in CDCl3 at 25 °C) for (a) cross-linker (CL), (b) the resultant polymer (P1) prepared by ADMET polymerization of M1 (run 8), and (c) resultant polymer (PL1) prepared by the ADMET polymerization of M1 in the presence of CL (5.0 mol%, sample run 24).
Conclusions
We have shown that synthesis of new polyesters by ADMET polymerization of α,ω-dienes, 4-allyl-2-methoxyphenyl 10-undecenoate (M1), prepared from bio-renewable eugenol (obtained from clove oil) and 10-undecenoic acid derivative (obtained from castor oil). Ruthenium-carbene (called second generation Grubbs) catalyst afforded polymers with unimodal molecular weight distributions (Mn = 12 700, Mw/Mn = 1.85). The polymerization of M1 in the presence of triarm cross-linker, 5-formylbenzene-1,2,3-triyl tris(undec-10-enoate), also afforded certain network polymers, suggested by NMR spectra and DSC thermogram. Since 9-decenoate obtained from the other vegetable oil (e.g. methyl oleate) by ethenolysis, should be used in place of 10-undecenoate, also since, as described in the introductory, further chemical modification can be possible from functional group (methoxy group) in the resultant polymers (P1, PL1), we thus believe that the present approach is promising and should be applicable for synthesis of polyesters from monomers prepared from bio-renewable resources.
Experimental section
General experimental considerations
All experiments were carried out under nitrogen atmosphere or using standard Schlenk techniques unless otherwise specified. Anhydrous grade dichloromethane and toluene (>99.5%, Kanto Chemical Co., Inc.) were transferred into a bottle containing molecular sieves (mixture of 3A 1/16, 4A 1/8 and 13X 1/16) in the drybox. RuCl2(PCy3)2(CHPh) (called 1st generation Grubbs catalyst (G1); Cy = cyclohexyl), RuCl2(PCy3)(H2IMes)(CHPh) [called 2nd generation Grubbs catalyst (G2); IMesH2 = 1,3-bis(2,4,6-trimethylphenyl)imidazolin-2-ylidene] and RuCl2(IMesH2)(CH-2-OiPr-C6H4) [called 2nd generation Hoveyda–Grubbs catalyst (HG2)] were purchased from Aldrich Chemical Co., and were used as received. Chemicals of reagent grades such as Eugenol (UG, >99.0%, Tokyo Chemical Industry, Co., Ltd.), 10-undecenoyl chloride (UDC, >99%), 10-undecen-1-ol (UDO, >98%), 5-hexene-1-ol (Hex), 3,4,5-trihydroxybenzaldehyde (>98%) and triethylamine (NEt3, >99%) were obtained from Tokyo Chemical Industry, Co., Ltd., and were used as received. Ethyl vinyl ether (>98%) and Celite were purchased from Wako Pure Chemical Industries, Ltd.
All 1H and 13C NMR spectra were recorded using a Bruker AV500 spectrometer (500.13 MHz for 1H, 125.77 MHz for 13C). All chemical shifts were reported in parts per million (ppm) with referenced to SiMe4 (TMS) at 0.00 ppm. Obvious multiplicities and routine coupling constants are usually not listed, and all spectra were obtained in the solvent indicated at 25 °C unless otherwise noted. Molecular weights and the molecular weight distributions of the resultant polymers were measured by gel-permeation chromatography (GPC). GPC measurements were performed at 40 °C on a Shimadzu SCL-10A using a RID-10A detector (Shimadzu Co., Ltd.) in THF (containing 0.03 wt% of 2,6-di-tert-butyl-p-cresol, flow rate 1.0 mL min−1). HPLC grade THF (Wako Pure Chemical Ind., Inc.) was used as the eluent with a flow rate of 1.0 mL min−1, and was degassed prior to use. GPC columns (ShimPAC GPC-806, 804 and 802, 30 cm × 8.0 mm diameter, spherical porous gel made of styrene/divinylbenzene copolymer, ranging from < 102 to 2 × 107 MW) were calibrated versus polystyrene standard samples. Differential scanning calorimetric (DSC) data for polymer were measured using a Hitachi DSC 7020 analyzer. Nitrogen was used as purge gas and all samples (5–7 mg) were placed in standard aluminium pans. Polymer samples were first heated from 25 to 250 °C then cooled to −100 °C. The glass transition (Tg) and melting (Tm) temperature were determined upon second heating cycle. All runs were performed at a rate of 10 °C min−1. Atmospheric pressure chemical ionization (APCI) mass spectrometry was performed on Bruker MicroTOF II-SDT1.
Synthesis of 4-allyl-2-methoxyphenyl undec-10-enoate (M1)
10-undecenoyl chloride (3.04 g, 15 mmol) was added dropwise into a toluene solution (15.0 mL) containing eugenol (2.46 g, 15 mmol) and triethylamine (2.51 mL, 18 mmol) over 30 min at 0 °C. The mixture was then warmed to room temperature and was stirred for 4 h. After the reaction was reached to completion, by confirmation of consumption of 10-undecenoyl chloride by TLC, the reaction mixture was then neutralized with 2 N HCl, and was washed with 5% NaHCO3 (15 mL × 3), deionized water (15 mL × 4), was then with brine (15 mL × 2). The solution was dried over anhydrous MgSO4, and was then filtered through a Celite pad, and the filtrate was evaporated under reduced pressure. The crude product was purified by column chromatography using hexane/ethyl acetate (9/1) as an eluent to yield M1 as colorless oil (3.87 g, 78% yield). 1H NMR (CDCl3): δ 1.33 (s, 10H, 5CH2) 1.75–1.79 (quint, J = 7.5 Hz, 2H, CH2), 2.03–2.07 (quart, J = 7.5 Hz, 2H, CH2CH CH2), 2.55–2.58 (t, J = 7.5 Hz, 2H, –CH2COO–), 3.37–3.38 (d, J = 6.8 Hz, 2H, Ar–CH2), 3.81 (s, 3H, OCH3), 4.92–5.02 (m, 2H, Ar–CH2CH CH2), 5.08–5.13 (2H, –CH CH2), 5.78–5.86 (m, J = 6.68, 6.68 and 6.77 Hz, 1H, –CH CH2), 5.92–6.0 (m, J = 6.75, 6.75 and 6.79 Hz, 1H, Ar–CH2CH CH2), 6.75–6.79 (m, 1HAr, CH), 6.93–6.94 ppm (m, 2HAr, 2CH). 13C{1H} NMR (CDCl3): δ 25.1 (CH2), 29.0 (CH2), 29.1 (CH2), 29.3 (CH2), 29.4 (CH2), 33.9 (CH2COO–), 34.1 (CH2), 40.2 (Ar–CH2), 55.9 (OCH3), 112.8 (CH), 114.3 (CH CH2), 116.2 (Ar–CH2CH CH2), 120.7 (CH), 122.6 (CH), 137.2 (Ar–CH2CH CH2), 138.2 (Ar), 138.9 (Ar), 139.3 (CH CH2), 151.0 (Ar), 172.1 ppm (–COO–). APCI-MS: calculated for C21H30O3 [M + H]+ 331.2; found 331.2.
Synthesis of 5-hexen-1-yl 10-undecenoate (M2)
10-undecenoyl chloride (3.04 g, 15 mmol) was added dropwise into a toluene solution (20.0 mL) containing 5-hexen-1-ol (1.50 g, 15 mmol) and triethylamine (2.51 mL, 18 mmol) over 30 min at 0 °C. The mixture was then warmed to room temperature and was stirred for 2.5 h. After the reaction was reached to completion, by confirmation of consumption of 10-undecenoyl chloride by TLC, the reaction mixture was then neutralized with 2 N HCl, and was washed with 5% NaHCO3 (15 mL × 3), deionized water (15 mL × 4), was then with brine (15 mL × 2). The solution was dried over anhydrous MgSO4, and was then filtered through a Celite pad, and the filtrate was evaporated under reduced pressure. The crude product was purified by column chromatography using hexane/ethyl acetate (9/1) as an eluent to yield M2 as a colorless oil (3.24 g, 81% yield). 1H NMR (500 MHz, CDCl3, ppm): δ 1.28–1.36 (s, 10H, 5CH2), 1.44 (m, 2H, CH2), 1.61–1.64 (m, 4H, 2CH2), 2.02–2.08 (m, J = 15.2 and 16.9 Hz, 4H, 2CH2CH CH2), 2.26–2.29 (t, J = 7.4 Hz, 2H, –CH2COO–), 4.06 (t, J = 6.5 Hz, 2H, –COOCH2–), 4.91–5.02 (m, J = 8.8 and 14.0 Hz, 4H, 2CH2 CH–), 5.77–5.80 ppm (m, J = 1.4 and 11.6 Hz, 2H, 2CH2 CH–). 13C{1H} NMR (125 MHz, CDCl3, ppm): δ 25.1 (CH2), 25.3 (CH2), 28.2 (CH2), 29.0 (CH2), 29.2 (CH2), 29.3 (CH2), 29.4 (CH2), 33.4 (CH2), 33.9 (CH2), 34.5 (CH2), 64.2 (–COOCH2–), 114.3 (CH CH2), 139.3 (CH CH2), 174.1 (–COO–). APCI-MS: calculated for C17H30O2 [M + H]+ 267.2; found 267.2.
Synthesis of 10-undecen-1-yl 10-undecenoate (M3)
10-undecenoyl chloride (3.04 g, 15 mmol) was added dropwise into a toluene solution (20.0 mL) containing 10-undecen-1-ol (2.55 g, 15 mmol) and triethylamine (2.51 mL, 18 mmol) over 30 min at 0 °C. The mixture was then warmed to room temperature and was stirred for 2.5 h. After the reaction was reached to completion, by confirmation of consumption of 10-undecenoyl chloride by TLC, the reaction mixture was then neutralized with 2 N HCl, and was washed with 5% NaHCO3 (15 mL × 3), deionized water (15 mL × 4), was then with brine (15 mL × 2). The solution was dried over anhydrous MgSO4, and was then filtered through a Celite pad, and the filtrate was evaporated under reduced pressure. The crude product was purified by column chromatography using hexane/ethyl acetate (9/1) as an eluent to yield M3 as a colorless oil (4.26 g, 85% yield). 1H NMR (500 MHz, CDCl3, ppm): δ 1.27–1.36 (s, 22H, 11CH2), 1.60 (s, 4H, 2CH2), 2.02–2.03 (d, J = 6.4 Hz, 4H, 2CH2CH CH2), 2.26–2.29 (t, J = 7.4 Hz, 2H, –CH2COO–), 4.03–4.05 (t, J = 6.6 Hz, 2H, –COOCH2–), 4.90–4.99 (m, J = 9.3 and 17.1 Hz, 4H, 2CH2 CH–), 5.76–5.81 ppm (d, J = 6.8 Hz, 2H, 2CH2 CH–). 13C{1H} NMR (125 MHz, CDCl3, ppm): δ 26.0 (CH2), 28.8 (CH2), 29.0 (CH2), 29.1 (CH2), 29.2 (CH2), 29.3 (CH2), 29.4 (CH2), 29.5 (CH2), 29.6 (CH2), 33.9 (CH2), 34.5 (CH2), 64.5 (–COOCH2–), 114.2 (CH CH2), 139.2 (CH CH2), 174.0 ppm (–COO–). APCI-MS: calculated for C22H40O2 [M + H]+ 337.3; found 337.3.
Synthesis of 5-formylbenzene-1,2,3-triyl tris(undec-10-enoate) (CL)
10-undecenoyl chloride (1.98 g, 9.78 mmol) was added dropwise into a THF solution (15.0 mL) containing 3,4,5-trihydroxybenzaldehyde (508 mg, 3.30 mmol) and triethylamine (2.1 mL, 14.6 mmol) over 20 min at −30 °C. The mixture was then warmed to room temperature and was stirred for 14 h. After the reaction was completed, the reaction mixture was passed through a Celite pad (two times), and the filtrate was evaporated under reduced pressure to yield CL as a yellow oil (2.0 g, 94% yield). 1H NMR (500 MHz, CDCl3, ppm): δ 1.32–1.40 (s, 30H, 15CH2) 1.70–1.76 (quint, J = 7.5 Hz, 6H, 3CH2), 2.02–2.06 (quart, J = 7.0 Hz, 6H, 3CH2CH CH2), 2.53–2.56 (t, J = 7.5 Hz, 6H, 3CH2COO–), 4.92–5.01 (m, J = 10.2 and 17.1 Hz, 6H, 3CH CH2), 5.77–5.85 (m, J = 16.7, 16.8 and 17.0 Hz, 3H, 3CH CH2), 7.64 (s, 2HAr, 2CH), 9.92 ppm (s, 1H, –COH). 13C{1H} NMR (125 MHz, CDCl3, ppm): δ 24.9 (CH2), 29.0 (CH2), 29.1 (CH2), 29.2 (CH2), 29.3 (CH2), 29.4 (CH2), 33.8 (CH2), 114.3 (CH CH2), 121.7 (CH), 134.0 (Ar), 139.2 (CH CH2), 139.9 (Ar), 144.5 (Ar), 170.5 (–COO–), 189.5 ppm (–CHO). APCI-MS: calculated for C40H60O7 [M + H]+ 652.43; found 652.4.
General procedure for synthesis of polymers by acyclic diene metathesis (ADMET) polymerization using ruthenium catalysts
A typical procedure for synthesis of polymer (P1) by ADMET polymerization (Table 1, run 8) is as follows. The monomer (M1, 330 mg, 1.0 mmol) was loaded into a 25 mL sealed Schlenk-type tube. The 2nd generation Grubbs catalyst G2, RuCl2(PCy3)(H2IMes)(CHPh) (0.0127 g, 1.5 mol%) was dissolved in 0.4 mL of dichloromethane and transferred into a sealed Schlenk-type tube. The reaction mixture was magnetically stirred in an oil bath set at 50 °C under nitrogen atmosphere for 30 min (the valve was opened and connected to the vacuum/nitrogen line). The mixture was then placed into a liquid nitrogen bath to remove ethylene gas from the reaction medium by opening the valve connected to the vacuum line for a short period (less than 1 min), and the valve was then closed and the tube was placed into the oil bath to continue the reaction. This is the similar procedure for synthesis of all-trans poly(9,9-dialkyl-fluorene-2,7-vinylene)s by the ADMET polymerization,26 and the apparatus was shown in ref. 26a. The procedure removing ethylene was repeated with a certain period (30 min for the first time then every 1.0 h). The polymerization mixture was then cooled to room temperature and was quenched with excess ethyl vinyl ether while stirring for 1.0 h. The resultant solution was then dissolved in chloroform (2.0 mL) for dilution, and the solution was added dropwise into the cold methanol (50 mL). The solution was stirred for 1.0 h, and the precipitates were then collected by filtration and dried in vacuo to yield P1 as rubbery solids (0.299 g, 91% yield). A similar polymerization protocol was used for polymerization of M2 (266 mg, 1.0 mmol) and M3 (336 mg, 1.0 mmol) to yield polymers P2 and P3, respectively. The 1H NMR and 13C NMR spectra of obtained polymers with detailed peak assignments are shown in the ESI.†23
P1 (sample run 8)
1H NMR (CDCl3): δ 1.33, 1.75–1.79, 2.03–2.07, 2.55–2.58, 3.37–3.38, 3.81, 5.39–5.67, 6.15–6.44, 6.75–6.79, 6.93–6.94.13C{1H} NMR (CDCl3): δ 25.1, 26.8, 29.0, 29.1, 29.3, 29.4, 32.6, 33.9, 34.1, 39.1, 55.9, 112.8, 120.7, 122.6, 128.6, 132.5, 138.2, 138.9, 151.0, 172.1.
PL1 (sample run 24)
1H NMR (CDCl3): δ 1.32, 1.75, 1.97–2.03, 2.56, 3.31–3.35, 3.80, 5.36–5.68, 6.18–6.44, 6.77, 6.92, 7.64, 9.92. 13C{1H} NMR (CDCl3): δ 25.2, 26.8, 29.0, 29.1, 29.3, 29.4, 32.6, 33.9, 34.1, 39.1, 55.9, 112.8, 120.7, 122.6, 128.6, 132.5, 138.2, 138.9, 144.5, 151.2, 170.5, 172.2, 189.5.
P2 (sample run 14)
1H NMR (CDCl3): δ 1.27–1.28, 1.38–1.41, 1.60–1.63, 1.95–2.04, 2.26–2.29, 4.06, 5.33–5.42. 13C{1H} NMR (CDCl3): δ 25.2, 25.9, 28.3, 29.2, 29.3, 29.4, 29.5, 32.3, 32.7, 32.8, 34.5, 64.3, 130.4, 174.1.
P3 (sample run 19)
1H NMR (CDCl3): δ 1.29, 1.61, 1.96, 2.28, 4.05, 5.38. 13C{1H} NMR (CDCl3): δ 25.2, 26.0, 28.3, 29.3, 29.4, 29.5, 29.6, 29.7, 29.8, 29.9, 32.7, 34.5, 64.5, 130.5, 174.1.
Conflicts of interest
There are no conflicts of interest to declare.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the financial support provided by Thammasat University Research Fund under the TU Research Scholar, Contract No. 2/14/2560, and the international collaboration research program sponsored by Tokyo Metropolitan University (TMU). The project is partly supported by Grant-in-Aid for Challenging Exploratory Research (18K18981), Japan Society for the Promotion of Science (JSPS).
Electronic supplementary information (ESI) available: (i) Selected 1H and 13C NMR spectra for monomers, cross-linker and polymers synthesized by ADMET polymerization; (ii) atmospheric pressure chemical ionization (APCI) mass spectra for monomers and cross-linker; (iii) selected GPC traces and DSC thermograms of polymers. See DOI: 10.1039/c9ra01065c
Notes and references
- (a) Lillie L. M. Tolman W. B. Reineke T. M. Polym. Chem. 2017;8:3746–3754. doi: 10.1039/C7PY00575J. [DOI] [Google Scholar]; (b) Nasiri M. Reineke T. M. Polym. Chem. 2016;7:5233–5240. doi: 10.1039/C6PY00700G. [DOI] [Google Scholar]; (c) Gallagher J. J. Hillmyer M. A. Reineke T. M. ACS Sustainable Chem. Eng. 2015;3:662–667. doi: 10.1021/sc5008362. [DOI] [Google Scholar]; (d) Hillmyer M. A. Tolman W. B. Acc. Chem. Res. 2014;47:2390–2396. doi: 10.1021/ar500121d. [DOI] [PubMed] [Google Scholar]; (e) Coulembier O. Degée P. Hedrick J. L. Dubois P. Prog. Polym. Sci. 2006;31:723–747. doi: 10.1016/j.progpolymsci.2006.08.004. [DOI] [Google Scholar]
- Mülhaupt R. Macromol. Chem. Phys. 2013;214:159–174. doi: 10.1002/macp.201200439. [DOI] [Google Scholar]
- (a) Meier M. A. R. Metzger J. O. Schubert U. S. Chem. Soc. Rev. 2007;36:1788–1802. doi: 10.1039/B703294C. [DOI] [PubMed] [Google Scholar]; (b) Biermann U. Bornscheuer U. Meier M. A. R. Metzger J. O. Schäfer H. J. Angew. Chem., Int. Ed. 2011;50:3854–3871. doi: 10.1002/anie.201002767. [DOI] [PubMed] [Google Scholar]
- Zakzeski J. Bruijnincx P. C. A. Jongerius A. L. Weckhuysen B. M. Chem. Rev. 2010;110:3552–3599. doi: 10.1021/cr900354u. [DOI] [PubMed] [Google Scholar]
- Kobayashi H. Fukuoka A. Green Chem. 2013;15:1740–1763. doi: 10.1039/C3GC00060E. [DOI] [Google Scholar]
- (a) Shearouse W. C. Lillie L. M. Reineke T. M. Tolman W. B. ACS Macro Lett. 2015;4:284–288. doi: 10.1021/acsmacrolett.5b00099. [DOI] [PubMed] [Google Scholar]; (b) Fenouillot F. Rousseau A. Colomines G. Saint-Loup R. Pascault J. P. Prog. Polym. Sci. 2010;35:578–622. doi: 10.1016/j.progpolymsci.2009.10.001. [DOI] [Google Scholar]
- Wilbon P. A. Chu F. Tang C. Macromol. Rapid Commun. 2013;34:8–37. doi: 10.1002/marc.201200513. [DOI] [PubMed] [Google Scholar]
- Mutlu H. Meier M. A. R. Eur. J. Lipid Sci. Technol. 2010;112:10–30. doi: 10.1002/ejlt.200900138. [DOI] [Google Scholar]
- Gandini A. and Lacerda T. M., Monomers and Polymers from Chemically Modified Plant Oils and their Fatty Acids, Polymers from Plant Oils, John Wiley & Sons, Inc., Hoboken, NJ, USA, and Scrivener Publishing LLC, Beverly, MA, USA, 2nd edn, 2019, pp. 33–82 [Google Scholar]
- (a) Schwendeman J. E. Church A. C. Wagener K. B. Adv. Synth. Catal. 2002;344:597–613. doi: 10.1002/1615-4169(200208)344:6/7<597::AID-ADSC597>3.0.CO;2-P. [DOI] [Google Scholar]; (b) Gandini A. and Lacerda T. M., Metathesis Reactions Applied to Plant Oils and Polymers Derived from the Ensuing Products, Polymers from Plant Oils, John Wiley & Sons, Inc., Hoboken, NJ, USA, and Scrivener Publishing LLC, Beverly, MA, USA, 2nd edn, 2019, pp. 83–108 [Google Scholar]
- Dannecker P. Biermann U. Sink A. Bloesser F. R. Metzger J. O. Meier M. A. R. Macromol. Chem. Phys. 2019;220:1800400. [Google Scholar]
- Rybak A. Meier M. A. R. ChemSusChem. 2008;1:542–547. doi: 10.1002/cssc.200800047. [DOI] [PubMed] [Google Scholar]
- (a) Dannecker P. Biermann U. Sink A. Bloesser F. R. Metzger J. O. Meier M. A. R. Macromol. Chem. Phys. 2019;220:1800400. [Google Scholar]; (b) Lebarbé T. Neqal M. Grau E. Alfos C. Cramail H. Green Chem. 2014;16:1755–1758. doi: 10.1039/C3GC42280A. [DOI] [Google Scholar]; (c) Mutlu H. Meier M. A. R. Macromol. Chem. Phys. 2009;210:1019–1025. doi: 10.1002/macp.200900045. [DOI] [Google Scholar]
- Rojo L. Vazquez B. Parra J. López Bravo A. Deb S. San Roman J. Biomacromolecules. 2006;7:2751–2761. doi: 10.1021/bm0603241. [DOI] [PubMed] [Google Scholar]
- (a) Yoshimura T. Shimasaki T. Teramoto N. Shibata M. Eur. Polym. J. 2015;67:397–408. doi: 10.1016/j.eurpolymj.2014.11.013. [DOI] [Google Scholar]; (b) Guzmán D. Ramis X. Fernández-Francos X. De la Flor S. Serra A. Eur. Polym. J. 2017;93:530–544. doi: 10.1016/j.eurpolymj.2017.06.026. [DOI] [Google Scholar]
- (a) Shibata M. Tetramoto N. Imada A. Neda M. Sugimoto S. React. Funct. Polym. 2013;73:1086–1095. doi: 10.1016/j.reactfunctpolym.2013.05.002. [DOI] [Google Scholar]; (b) Neda M. Okinaga K. Shibata M. Mater. Chem. Phys. 2014;148:319–327. doi: 10.1016/j.matchemphys.2014.07.050. [DOI] [Google Scholar]
- (a) Taber D. F. Frankowski K. J. J. Org. Chem. 2003;68:6047–6048. doi: 10.1021/jo030005p. [DOI] [PubMed] [Google Scholar]; (b) Alexander K. A. Paulhus E. A. Lazarus G. M. L. Leadbeater N. E. J. Organomet. Chem. 2016;812:74–80. doi: 10.1016/j.jorganchem.2015.09.018. [DOI] [Google Scholar]
- (a) Moïse J. Arseniyadis S. Cossy J. Org. Lett. 2007;9:1695–1698. doi: 10.1021/ol0703940. [DOI] [PubMed] [Google Scholar]; (b) Bilel H. Hamdi N. Zagrouba F. Fischmeister C. Bruneau C. RSC Adv. 2012;2:9584–9589. doi: 10.1039/C2RA21638H. [DOI] [Google Scholar]
- Le D. Samart C. Tsutsumi K. Nomura K. Kongparakul S. ACS Omega. 2018;3:11041–11049. doi: 10.1021/acsomega.8b01695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Thirukumaran P. Shakila A. Muthusamy S. RSC Adv. 2014;4:7959–7966. doi: 10.1039/C3RA46582A. [DOI] [Google Scholar]; (b) Harvey B. G. Sahagun C. M. Guenthner A. J. Groshens T. J. Cambrea L. R. Reams J. T. Mabry J. M. ChemSusChem. 2014;7:1964–1969. doi: 10.1002/cssc.201400019. [DOI] [PubMed] [Google Scholar]
- (a) Hu K. Zhao D. Wu G. Ma J. Polym. Chem. 2015;6:7138–7148. doi: 10.1039/C5PY01075F. [DOI] [Google Scholar]; (b) Hu K. Zhao D. Wu G. Ma J. RSC Adv. 2015;5:85996–86005. doi: 10.1039/C5RA17457K. [DOI] [Google Scholar]
- Llevot A. Grau E. Carlotti S. Grelier S. Cramail H. Polym. Chem. 2015;6:7693–7700. doi: 10.1039/C5PY01232E. [DOI] [Google Scholar]
- Nomura K. Takahashi S. Imanishi Y. Macromolecules. 2001;34:4712–4723. doi: 10.1021/ma0102013. [DOI] [Google Scholar]
- Selected NMR spectra of monomers (M1–M3), cross-linker (CL) and polymers (P1–P3, PL1) are shown in the ESI.† Selected DSC thermograms in the resultant polymers are also shown in the ESI.†
- For example, selected review in ADMET polymerization ; (a) Berda E. B. and Wagener K. B., in Polymer Science: A Comprehensive Reference, ed. K. Matyjaszewski and K. Müllen, Elsevier BV, Amsterdam, 2012, vol. 5, pp. 195–216 [Google Scholar]; (b) Berda E. B. and Wagener K. B., in Synthesis of Polymers; New Structures and Methods, ed. D. Schluter, C. Hawker and J. Sakamoto, Wiley-VCH, Weinheim, Germany, 2012, pp. 587–600 [Google Scholar]
- Related experimental procedure for synthesis of all-trans poly(9,9′-dialkyl-fluorene-2,7-vinylene)s by ADMET polymerization, see: ; (a) Nomura K. Morimoto H. Imanishi Y. Ramhani Z. Geerts Y. J. Polym. Sci., Part A: Polym. Chem. 2001;39:2463. doi: 10.1002/pola.1223. [DOI] [Google Scholar]; (b) Yamamoto N. Ito R. Geerts Y. Nomura K. Macromolecules. 2009;42:5104. doi: 10.1021/ma900775x. [DOI] [Google Scholar]
- For example ; (a) Handbook of Metathesis, ed. R. H. Grubbs, Wiley-VCH, Weinheim, Germany, 2003 [Google Scholar]; (b) Olefin Metathesis: Theory and Practice, ed. K. Grela, John Wiley & Sons, Inc., Hoboken, New Jersey, USA, 2014 [Google Scholar]; (c) Handbook of Metathesis, ed. R. H. Grubbs and A. G. Wenzel, Wiley-VCH, Weinheim, Germany, 2nd edn, 2015, vol. 1 [Google Scholar]
- For examples: ; (a) Dannecker P. Biermann U. Sink A. Bloesser F. R. Metzger J. O. Meier M. A. R. Macromol. Chem. Phys. 2019;220:1800400. [Google Scholar]; (b) Gaines T. W. Nakano T. Chujo Y. Trigg E. B. Winey K. I. Wagener K. B. ACS Macro Lett. 2015;4:624–627. doi: 10.1021/acsmacrolett.5b00258. [DOI] [PubMed] [Google Scholar]; (c) Parkhurst R. R. Balog S. Weder C. Simon Y. C. RSC Adv. 2014;4:53967–53974. doi: 10.1039/C4RA08788G. [DOI] [Google Scholar]; (d) Türünç O. Meier M. A. R. Green Chem. 2011;13:314–320. doi: 10.1039/C0GC00773K. [DOI] [Google Scholar]; (e) Rybak A. Meier M. A. R. ChemSusChem. 2008;1:542–547. doi: 10.1002/cssc.200800047. [DOI] [PubMed] [Google Scholar]
- Synthesis of long-chain aliphatic polyesters: ; (a) Stempfle F. Ritter B. S. Mülhaupt R. Mecking S. Green Chem. 2014;16:2008–2014. doi: 10.1039/C4GC00114A. [DOI] [Google Scholar]; (b) Rybak A. Meier M. A. R. ChemSusChem. 2008;1:542–547. doi: 10.1002/cssc.200800047. [DOI] [PubMed] [Google Scholar]
- Stempfle F. Ortmann P. Mecking S. Chem. Rev. 2016;116:4597–4641. doi: 10.1021/acs.chemrev.5b00705. [DOI] [PubMed] [Google Scholar]
- Korshak V. V. and Vinogradova S. V., Polyesters, Pergamon Press, Oxford, UK, 1965 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.